Abstract
In the enteric nervous system (ENS) excitatory nicotinic cholinergic transmission is mediated by neuronal nicotinic acetylcholine receptors (nAChR) and is critical for the regulation of gastric motility. nAChRs are ligand-gated pentameric ion channels found in the central and peripheral nervous systems. The expression of heteromeric nAChR and receptor subunit mRNAs was investigated in the neonatal rat ENS using receptor autoradiography with the radiolabeled ligand 125I-Epibatidine, and in situ hybridization with subtype specific probes for ligand binding alpha (α2, α3, α4, α5, α6) and structural beta (β2, β3, β4) subunits. The results showed strong nicotine sensitive binding of 125I-Epibatidine around the stomach, and small and large intestines. The binding was partially displaced by A85380, a nicotinic ligand which differentiates between different heteromeric nAChR subtypes, suggesting a mixed receptor population. Radioactive in situ hybridization detected expression of α3, α5, α7, β2 and β4 mRNA in the myenteric plexus of the stomach, and small and large intestines. In the submucosal plexus of the small and large intestines expression of α3, α5 and β4 was found in some ganglia. There was no signal for α4, α6 and β3 in the ENS but positive hybridization signal for α2 transcripts was seen in some areas of the small intestines. However, the signal was not associated with any ganglion cells. The results confirm the presence of heteromeric nAChRs in the ENS similar to those found in the peripheral nervous system, with the majority being composed of α3(α5)β4, and a few α3β2 nAChRs. In addition, homomeric α7 nAChRs could be present.
Keywords: gastrointestinal, nicotine, myenteric plexus, submucosal, in situ hybridization, epibatidine
INTRODUCTION
The enteric nervous system (ENS) can control gastrointestinal functions independent of the central nervous system (CNS) via integrated activation of two interconnected ganglionated plexi, the myenteric plexus and submucosal plexus. The myenteric plexus controls contractions and relaxation of gastrointestinal (GI) smooth muscles, the submucosal plexus controls secretory and absorptive functions of the GI epithelium, local blood flow and neuroimmune responses (Kunze and Furness, 1999). There are two types of neuron in these plexi; S neurons which are enteric interneurons or motor neurons, and AH neurons which are enteric sensory neurons (Bornstein et al., 2004, Furness et al., 1998). Acetylcholine, derived from intrinsic cholinergic neurons or parasympathetic vagal projections (Schemann et al., 1993; Schemann and Grundy, 1992, Porter et al., 1996), is the main excitatory neurotransmitter regulating motility and mucosal function (Kosterlitz and Lees, 1964, Galligan and North, 2004). In the myenteric plexus most of the myenteric neurons receive fast excitatory postsynaptic potentials (fEPSPs) via nAChRs (Browning et al., 1996) which are completely or partially blocked by nAChR antagonists (Bian et al., 2003). In the submucosal plexus, fEPSPs on S-neurons can be completely inhibited by nAChR antagonists (Evans and Surprenant, 1992), whereas most AH neurons do not receive fast excitatory synaptic input, but nevertheless express functional nAChRs (Schneider and Galligan, 2000). Thus, nicotinic receptors play a major regulatory role in the ENS, and there is evidence for the presence of both heteromeric and homomeric nAChRs.
Nicotinic acetylcholine receptors (nAChR) are heteromeric or homomeric cation channels, which exist as pentameric complexes of various combinations of alpha and non-alpha subunits (Sargent 1993, Le Novère and Changeux 1995; Lukas 1995; Dani and Bertrand 2007). In the mammalian nervous system eight ligand binding α (α2-α7, α9-α10) and three structural β (β2-β4) subunits have been identified, and different subunit combinations result in specific nAChR subtypes, with α7 and α9 being the only subunits capable of forming functional homomeric receptors. Neuronal nAChRs are widely expressed in the central (CNS) and peripheral (PNS) nervous systems in adults and during development. In the CNS the majority of nAChRs are either heteromeric α4β2 containing nAChRs or homomeric α7 nAChRs, whereas in the PNS heteromeric α3β4-type nAChRs, with or without α5, are the predominant receptors (Mao et al., 2006). However, the expression of nAChRs in the ENS is less well established.
Most studies have used guinea pigs to characterize nAChR in the ENS (Mandl and Kiss, 2007, Kirchgessner and Liu, 1998, Zhou et al., 2002, Glushakov et al., 2004, Obaid et al., 2005, Obaid et al., 1999). In this species, immunohistochemical studies have revealed that a large number of enteric neurons are labeled by monoclonal antibodies directed against α3, α5, β2, and β4 subunit proteins which form different heteromeric nAChR subtypes, whereas the presence of α7 subunit protein is more controversial (Kirchgessner and Liu, 1998, Obaid et al., 1999 Glushakov et al., 2004, Obaid et al., 2005). However, the specificity of the antibodies used is not clear (Herber et al., 2004, Moser et al., 2007). Furthermore, rats are a prominent animal model to study nicotinic receptors and nicotinic responses, but our knowledge about nAChRs in rat ENS is very limited. Therefore, in this study we used receptor autoradiography with the iodinated ligand epibatidine to determine the spatial distribution of high affinity heteromeric nAChR binding sites, and in situ hybridization for the detection of subunit mRNAs involved in the formation of heteromeric nAChRs, to confirm the presence of nAChRs in the GI tract of the rat.
MATERIALS AND METHODS
Tissue preparation and fixation
Animal procedures were approved by the institutional Laboratory Animal Care Committee according to the rules of the Texas A&M University, and consistent with National Institute of Health guidelines. Pregnant Sprague-Dawley rat dams purchased from Harlan (Houston, TX) were housed at the Texas A&M Health Science Center, College of Medicine. One day old rat pups were deeply anesthetized with inhaled isoflurane, frozen at -30°C in isopentane for 60 s and stored at -80°C.
For this study neonatal rat pups were used which allowed us to process whole body sections. Although, the developing gastrointestinal tract adapts to the increase in body size and thickness of the muscle layers (Schäfer et al., 1999), a previous study had shown that there are no differences in the qualitative expression of nAChRs between adult ENS neurons and cultured ENS neurons derived from embryos, indicating that there is very little age-related change with regard to nAChRs (Zhou et al., 2002). Twenty μm thick sagittal and coronal tissue sections were cut on a cryostat at -18°C, and mounted onto superfrost-plus microscope slides. For binding studies the slides were kept on ice during cutting, then desiccated overnight at 4°C and stored at -80°C until use. For in situ hybridization slides were kept at -18°C during cutting, and then fixed in 4% para-formaldehyde for one hour at room temperature, washed in phosphate buffer, dried and stored at -20°C until use.
Receptor autoradiography
Before processing, sections were warmed to room temperature and briefly fixed with para-formaldehyde vapor at 37°C for 15 min in a vacuum-sealed desiccator as described (Huang and Winzer-Serhan, 2006). Tissue sections were pre-incubated in fresh Tris-HCl buffer solution (50 mM Tris-HCl base, 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, and 1 mM MgCl2, adjusted to pH 7.4 with 10 N HCl) for 5 min. For total binding, sections were incubated with 0.4 nM [125I]-Epibatidine ([125I]-Epi) (PerkinElmer Life Science NEX358, Boston, MA, specific activity: 2200 Ci/mmol) in the same Tris-HCl buffer. Adjacent sections designated for nonspecific binding were processed in the presence of 100 μM (-)nicotine hydrogen tartrate which was added to pre-incubation and incubation buffers. For displacement of [125I]-Epi binding from heteromeric α4β2-type nAChRs, 0.4 nM A-85380 dihydrochloride (Sigma) was added to pre-incubation and incubation buffers. Sections were incubated at room temperature for 60 min and washed in ice-cold incubation buffer twice for 5 min each, followed by ice-cold ddH20 for 30 seconds, dried under constant airflow for 1 h at room temperature and exposed to film (Kodak BioMax MR Film) along with [125I]-standards of known radioactivity. After film exposure for one or two days the films were developed in D19 Kodak developer for 4 min, rinsed in water, and fixed in Kodak Rapid Fixer for 5 min. The slides were then exposed to vapor fixation and stained in Cresyl-Violet solution to assist the anatomical analysis.
cRNA probe synthesis
Plasmids containing full-length sequences for the nAChR subunits α2 (1931 bp), α3 (1858 bp), α4 (2110 bp), α5 (1667 bp), α6 (1760 bp),α7 (2100 bp),β2 (2196 bp), β3 (1780 bp), and β4 (2522 bp) (kindly provided by Dr. Jim Boulter, University of California, Los Angeles, USA) were linearized via restriction enzyme digest and antisense probes were reversed transcribed (MAXIscript™, Ambion, Austin, TX) from the cDNA templates in the presence of [35S]-UTP (PerkinElmer Boston, MA). Except for α2, the full-length probes were hydrolyzed into 600 bp fragments by alkaline hydrolysis, and diluted in hybridization buffer to a radioactive concentration of 107 cpm/ml.
In Situ Hybridization
Prior to hybridization, sections were treated with proteinase K for 30 min, rinsed in 0.1 M TEA at pH 8 for 3 min, acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min, rinsed twice in 2x standard sodium citrate (SSC) for 2 min each, dehydrated in increasing concentrations of ethanols and blow-dried for 30 min. In situ hybridization was performed as described (Winzer-Serhan et al., 1999). After an overnight hybridization at 60°C, sections were treated with RNase A at 37°C for 30 min, and washed in 4, 2, 1 and 0.5x SSC and a hot wash with 0.1x SSC at 65°C for 30 min. Slides were dehydrated through graded series of ethanols and air dried. Sections were apposed to BioMax MR film (Kodak, Rochester, NY) for three to five day. After developing the films, the slides were coated with liquid Kodak NTB emulsion and exposed for 4 to 5 weeks. The slides were developed in Kodak D-19 developer, fixed, counter-stained with Cresyl-Violet solution, and cover-slipped.
Data analysis
Images from autoradiograms derived from three different animals (n=3) were quantitatively analyzed using a PC-based image analysis system (MCID Basic, Imaging Research Inc., Canada, now InterFocus Imaging Ltd, UK). A calibration curve of radioactivity nCi/mg tissue or nCi/g tissue versus optical density was generated using [125I]- or [14C]-standards for receptor ligand binding and in situ hybridization, respectively. Non-specific binding or non-specific hybridization were negligible. Data are expressed as mean ± SEM and were analyzed by student’s t-test, p<0.05 was considered as significant.
RESULTS
Binding of [125I]Epibatidine
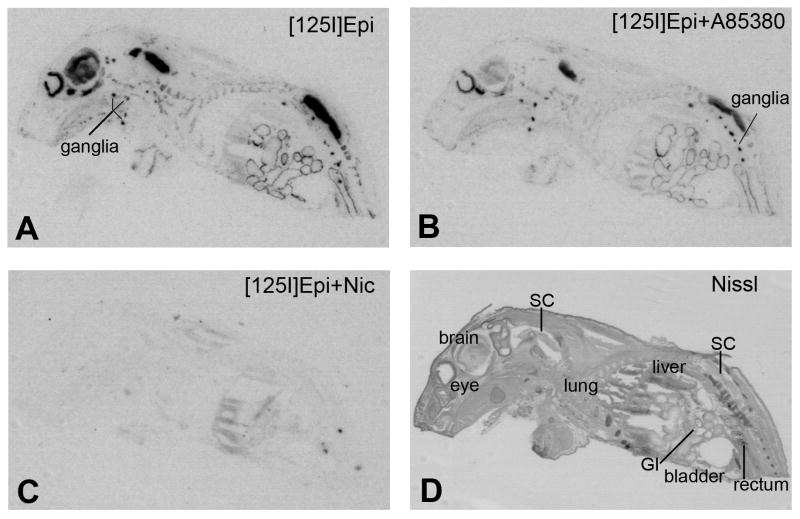
Strong binding of [125I]Epi was detected in brain, spinal cord, sympathetic and parasympathetic ganglia, retina and around the lining of the stomach, small intestines and large intestines of rat pups (Fig. 1A). Addition of 0.4 nM A85380 during the incubation with 0.4 nM [125I]Epi, which prevents binding of [125I]Epi to heteromeric β2-containing nAChRs (Fig. 1B), resulted in strongly reduced [125I]Epi binding in several brain areas (cortex, 88.27% ±4.56%; thalamus, 76.17% ±0.62%; brainstem 72.27% ±0.71%; p<0.001 for all). In peripheral ganglia, [125I]Epi binding was only reduced by 38.0 % (±9.48%) in the presence of A85380 but due to high variability the change was not statistically significant. In ganglia of the stomach and intestines [125I]Epi binding in the presence of A85380 was reduced by only 10.0% (±1.08%) and 13.62% (±0.85%, p<0.01), respectively. Thus, it appeared that [125I]Epi binding sites in the ganglia of the GI tract are the least sensitive to A85380, suggesting that the majority of heteromeric nAChRs contain β4 subunits. An excess of nicotine completely blocked specific binding of [125I]Epi in the brain, spinal cord, peripheral ganglia, ganglia of the stomach and GI tract (Fig. 1C) confirming that the [125I]Epi binding sites are sensitive to nicotine (Perry et al., 2002). A section adjacent to those used for binding was stained with Cresyl Violet as an anatomical reference (Fig. 1D).
Figure 1.
Distribution of [125I]-Epibatidine binding sites in sagittal whole body sections from neonatal rat pups. (A) binding of 0.4 nM [125I]-Epibatidine, (B) binding of 0.4 nM [125I]-Epibatidine in the presence 0.4 nM A85380, (C) binding of 0.4 nM [125I]-Epibatidine in the presence of 100 μM (-)nicotine hydrogen tartrate (nonspecific binding), (D) Nissl stained adjacent section. Abbreviations: [125I]Epi, [125I]-Epibatidine; Nic, nicotine; GI, gastrointestinal tract; SC, spinal cord.
Expression of nAChR subunit mRNA
Using sagittal whole body sections, the pattern of nAChR subunit mRNA distribution resembled the known expression pattern in the CNS and PNS, with widespread mRNA expression of α4, α7 and β2 in the CNS and PNS, strong expression of α3, α5, β3 and β4 mostly in the PNS, and restricted expression of α2 and α6 in both CNS and PNS suggesting high specificity for the probes used in this study (data not shown). In the GI tract positive hybridization signal was only detected for α2, α3, α5, α7, β2 and β4 which was further investigated in coronal body sections (Fig. 2).
Figure 2.
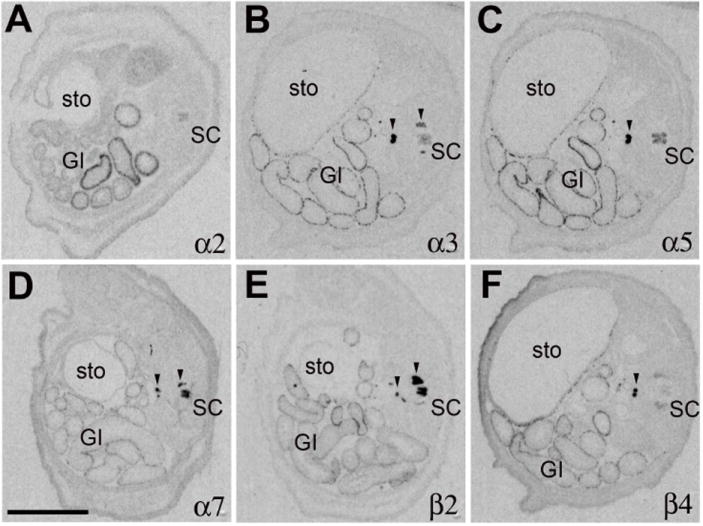
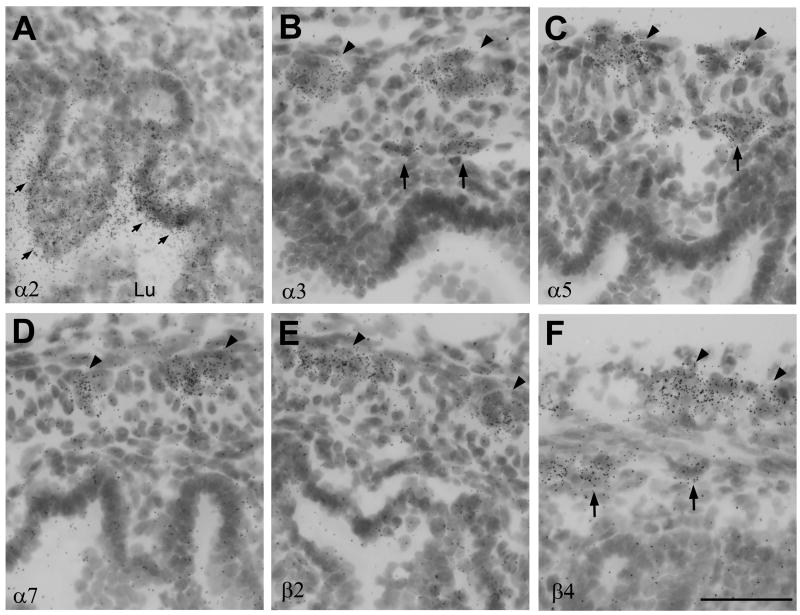
Expression pattern of nAChR subunit mRNAs in coronal lower body sections from neonatal rat pups. In situ hybridization pattern derived with antisense probes to (A) α2, (B) α3, (C) α5, (D) α7, (E) β2, (F) β4. Abbreviations: GI, gastrointestinal tract; SC, spinal cord; sto, stomach. Black arrow heads point to expression in peripheral ganglia. Size bar: 5 mm.
In the enteric ganglia moderate mRNA expression of α3, α5 and β4 was found in the lining of the stomach, and small and large intestines (Fig. 2B, C, F). Hybridization signals for α7 and β2 mRNAs were low, especially in comparison to their strong expression in spinal cord (Fig. 2D, E). Expression of α2 mRNA was detected in some regions of the intestines but not in the stomach (Fig. 2A). Microscopic analysis indicated that the expression of α2 was not associated with ganglion cells in either the myenteric or submucosal plexus but was located in the inner mucosal layer closest to the lumen, and appeared almost detached from any cell structure (Fig. 6A). Although, the sense probe did not exhibit any signal, it was not clear that this hybridization signal was specific, or perhaps reflected non-specific interaction of the antisense probe with a bacterial sequences, since prokaryotes also express nAChR-like proteins (Bocquet et al., 2007).
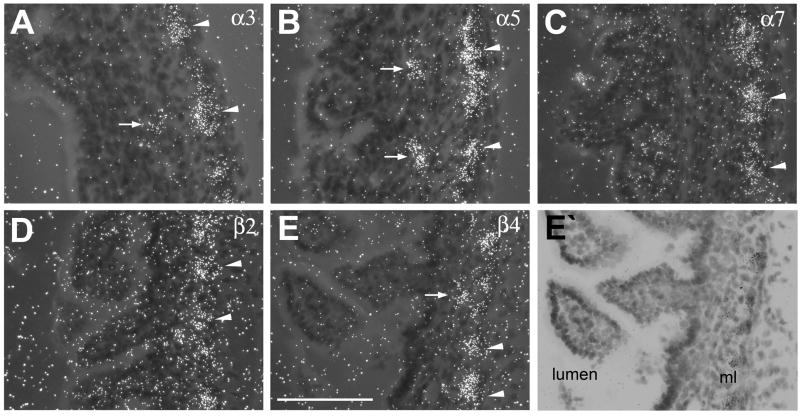
Figure 6.
High power lightfield photomicrographs of nAChR subunit mRNA expression in small intestines. (A) α2, (B) α3, (C) α5, (D) α7, (E) β2 (E) and (F) β4 hybridization signal. Black arrowheads point to expression in the myenteric plexus. Large black arrows point to expression in the submucosal plexus, small black arrows point to α2 mRNA expression near the lumen. Lu, lumen. Size bar: 50 μm.
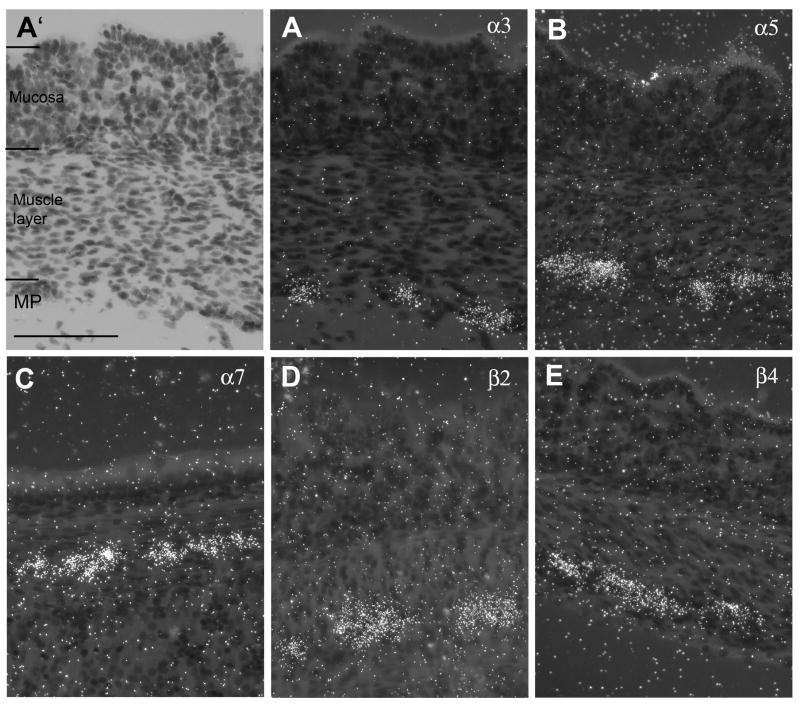
The spatial expression of nAChR subunits was further analyzed using higher power darkfield microscopy. In the stomach, the myenteric plexus is clearly delineated but there are divergent opinions about the existence of the submucosal plexus. The analysis revealed expression of α3, α5, β2, β4 and α7 subunit mRNAs in the myenteric plexus of the stomach (Fig. 3A, B, C, D, E) with no indication of expression in a submucosal plexus. However, expression of α7 mRNA was found only in a few ganglia shown in Fig. 3C. Fig. 3A’ represents a lightfield image of Fig. 3A and indicates the layers of the stomach wall for orientation.
Figure 3.
Darkfield photomicrographs of nAChR subunit mRNA expression in the stomach wall. (A) α3, (A’) lightfield image of A, (B) α5, (C) α7, (D) β2 (E) β4 mRNA expression. Size bar: 100 μm. MP, myenteric plexus.
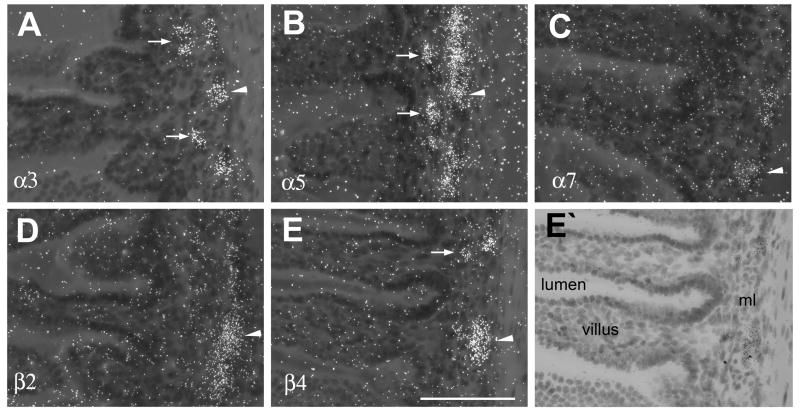
In small and large intestines expression of nAChR subunits was detected in both the myenteric and submucosal plexus, although, expression was more pronounced in the myenteric plexus (Fig. 4, Fig. 5). In the small and large intestines, moderate mRNA expression of α3, α5 and β4 (Fig. 4A, B, E, Fig. 5A, B, E), and low expression of α7 and β2 mRNA (Fig. 4C, D, Fig, 5C, D) was found in neurons of the myenteric plexus. In contrast, in the submucosal plexus hybridization signal was only detected for α3, α5 and β4 mRNAs, but signal intensities were low and ganglia exhibiting positive hybridization were infrequent in contrast to the stronger and more regular expression seen in the ganglia of the myenteric plexus (Fig. 6).
Figure 4.
Darkfield photomicrographs of nAChR subunit mRNA expression in the small intestines. (A) α3, (B) α5, (C) α7, (D)β2 (E) β4 mRNA expression. (E’) lightfield image of E. White arrowheads point to expression in the myenteric plexus. White arrows point to expression in the submucosal plexus; ml, muscle layer. Size bar: 100 μm.
Figure 5.
Darkfield photomicrographs of nAChR subunit mRNA expression in the large intestines. (A) α3, (B) α5, (C) α7, (D) β2 (E) β4 mRNA expression. (E’) lightfield image of E. White arrowheads point to expression in the myenteric plexus. White arrows point to expression in the submucosal plexus; ml, muscle layer. Size bar: 100 μm.
DISCUSSION
Neuronal nAChRs play a major role in the regulation of gut motility where they mediate fast synaptic transmission and serve secretory functions (Galligan, 2002). However, despite the importance of nAChRs in the ENS, the expression and spatial distribution of different nAChR subunit mRNAs remained largely unknown. This study presents the first anatomical description of the distribution of nAChR subunit mRNAs and receptor binding sites in the rat ENS. In here, we revealed the presence of high affinity nAChR binding sites in the walls of the GI tract, and were able to determine the expression of α3, α5, α7, β2, and β4 nAChR subunit mRNAs in plexi of the stomach and small and large intestines.
Expression of heteromeric nAChRs in the myenteric plexus
Epibatidine which has low affinity for homomeric α7 receptors, but very high affinity for heteromeric α4β2 and α3β2 nAChRs, and moderate affinity for α3β4 nAChRs (Perry et al. 2002), was used to determine the presence and distribution of heteromeric nicotinic binding sites. Nicotine displaceable [125I]Epi-binding was detected in the lining of the stomach, and small and large intestines suggesting the presences of nicotine-sensitive heteromeric nAChRs. This binding was only partially sensitive to low concentrations of A85380, a nicotinic agonists with preference for β2 containing nAChRs (Perry et al., 2002, Whiteaker et al., 2000), indicating two different types of heteromeric nAChRs in the ENS, an A85380-sensitive, perhaps α4β2 or α3β2 type, and an A85380-insensitive, perhaps α3β4 type which represents the majority of [125I]Epi-binding.
In situ hybridization revealed moderate mRNA expression of α3, α5, and β4 and low expression of β2 in neurons of the myenteric plexus in the stomach, and small and large intestines (Table 1 for summary of nAChR subunit expression). Supported by the results derived from [125I]Epi-binding, this strongly suggest that the majority of heteromeric nAChRs in the ENS are composed of α3β4 and/or α3α5β4 subunits, similar to the predominant nAChR subtype found in PNS neurons of sympathetic and parasympathetic ganglia (Sargent, 1993). However, the data also suggest the possibility of additional heteromeric α3β2 nAChRs being expressed in neurons of the ENS.
Table 1.
Expression intensities of nAChR subunit mRNAs in the enteric nervous system relative to their expression in peripheral ganglia (set as 100% for each subunit, derived from the analysis of autoradiograms, n=3).
| NAChR Subunit | Stomach MP | Small intestine MP | Small intestine SMP | Large intestine MP | Large intestine SMP |
|---|---|---|---|---|---|
| α2 | - | - | ++* | - | ++* |
| α3 | ++ | ++ | + | ++ | + |
| α4 | - | - | - | - | - |
| α5 | ++ | ++ | + | ++ | + |
| α6 | - | - | - | - | - |
| α7 | + | + | - | + | - |
| β2 | + | + | - | + | - |
| β3 | - | - | - | - | - |
| β4 | ++ | ++ | + | ++ | + |
- no detectable expression; + low (less than 15%); ++ moderate (20 to 35%); +++ high expression (> 35% of expression in PNS ganglia);
=α2 mRNA located in submucosal lining near the lumen. MP, myenteric plexus; SMP, submucosal plexus.
Note: The expression intensities are not compared between but within subunits to estimate their relative expression. Hybridization intensity in a single layer of neurons (ganglion cells in plexi) will appear lower in autoradiograms than in an area of tightly packed neurons (PNS ganglia), therefore, expression intensities might be underestimated.
Our findings corroborate results from immunohistochemistry studies using antibodies raised against specific nAChR subunits. Although the results have to be taken with caution, studies in guinea pig revealed immunoreactivity for α3, α5, β2 and β4 but not α4 in neurons of the myenteric plexus (Kirchgessner and Liu, 1998, Obaid et al., 2005, Zhou et al., 2002).
In addition, these anatomical findings are supported by functional pharmacological studies demonstrating that the predominant nAChR subtype are heteromeric receptors composed of α3α5β4 (or α3β4) and α3β2 which mediate the fast electrophysiological synaptic activity in enteric neurons of the myenteric plexus. This conclusion was based on agonist and antagonist rank order potencies, and on the finding that cytisine, which is a full agonist for β4, but only a partial agonist for β2 containing nAChRs, discriminated between these two types of heteromeric nAChRs (Zhou et al., 2002, Schneider and Galligan, 2000). Thus, data from receptor binding, in situ hybridization, immunohistochemistry and functional experiments all suggest that two or perhaps three types of heteromeric nAChR are present in neurons of the myenteric plexus, one would be an α3β4 and another an α3β2 subtype, with or without an α5 subunit.
Expression of homomeric α7 nAChRs in the myenteric plexus
The presence of homomeric α7 nAChRs in the ENS has been more controversial because of lack of evidence for functional α7 nAChR mediated responses which contrasts with positive immunoreactivity seen in anatomical studies. The data presented in here clearly demonstrate that transcripts for α7 mRNA are found in the myenteric plexus of the stomach, and small and large intestines, but not in the submucosal plexus, confirming earlier immunohistochemistry studies in guinea pig (Zhou et al., 2002, Obaid et al., 2000, Kirchgessner et al., 1998). Although expression intensity was low when compared to the strong expression of α7 mRNA in the PNS and CNS, α7 nAChRs could be present. However, based on the physiological and pharmacological evidence, nicotinic responses do not seem to be mediated via homomeric α7 nAChRs (Zhou et al., 2002), and therefore, functional homomeric α7 nAChRs in the ENS would be at best rare. This conclusion is supported by a lack of evidence for [125I]α-bungarotoxin binding, a marker for neuronal homomeric α7 nAChRs, in the neonatal rodent GI tract (Winzer-Serhan, unpublished results). It is difficult to reconcile these contradicting findings. However, possible explanations are that α7 mRNA is not translated into adequate amounts of subunit protein, resulting in low numbers of homomeric receptors, not enough to be detected in functional or binding studies; or the detection of positive immunoreactivity could be misleading because of non-specific false positive results obtained with monoclonal α7 antibodies (Herber et al., 2004, Moser et al., 2007). Another possible explanation is that α7 subunits form heteromeric instead of homomeric, receptors which could yield ion channels with different physiology and pharmacology. Heteromeric α7β2 nAChRs have been found in cholinergic neurons in the rodent basal forebrain (Liu et al. 2007), and cholinergic neurons are also present in the myenteric plexus (Nakajima et al., 2000, Schemann et al., 1993, Schemann et al., 2001). Thus, it is possible that heteromeric α7β2* nAChRs could be found in these cholinergic neurons. This could explain the discrepancy between anatomical and functional studies, because little is known about the pharmacological and physiological properties of heteromeric α7*nAChRs.
Expression of nAChRs in submucosal plexus
In the submucosal plexus mRNA expression for α3, α5 and β4 was detected, although the expression intensities appeared to be lower than in the myenteric plexus, and expression in submucosal ganglia seemed infrequent. However, unless further experiments determine single cell expression levels, it is difficult to compare signal intensities between plexis. Nevertheless, these results are in general agreement with studies that reported expression of heteromeric nAChR subunits and functional nicotinic response in this layer. In ganglion cells of the submucous plexus from guinea pig small intestine Obaid et al. (1999) reported strong immunoreactivity for α3/5-containing nAChR, and demonstrated the presence of functional α3-containing nAChRs. Other studies reported nAChR-positive immunoreactivity for α3 and α5 in some ganglia in the submucosal plexus (Glushakov et al., 2004, Kirchgessner and Liu 1998). Thus, there is general agreement that heteromeric α3β4-containing nAChRs are present in neurons of the submucosal plexus.
Again, the presence of homomeric α7 receptors is controversial. We did not find any evidence for the expression of α7 mRNA in ganglion cells of the submucosal plexus in rat. However, immunohistochemistry studies suggested the presence of α7 receptors in guinea pigs (Obaid et al., 1998, Glushakov et al., 2004). This discrepancy could reflect presynaptic or extrasynaptic locations of α7 receptors which could be indicate by positive immunoreactivity for α7 protein located on projections to the submucosal plexus but would not be detected by in situ hybridization (Mandl and Kiss, 2007).
Other subunits
There was no evidence from in situ hybridization studies for mRNA expression of α4, α6 and β3, which would limit their expression to the PNS and CNS, where they have been described previously (Le Novère eta l., 1996, Wada et al., 1989, Deneris et al., 1989, Boyd et al., 1991). However, hybridization signal for α2 subunit mRNA was detected in the inner lining of the small intestines but it was not clear to us that the signal was specific. No other subunit exhibited a similar expression pattern in the gut. However, strong expression of α2, but not of other subunits, was also detected in the liver, and it remains to be seen if α2 has a functional role, perhaps related to secretory functions, in the liver or in the gut.
CONCLUSIONS
In conclusion, binding sites for heteromeric nAChRs and expression of several subunit mRNAs are detected in ENS. The predominantly expressed subunits are α3, α5 and β4 which most likely form functional heteromeric nAChRs in neurons of the myenteric and submucosal plexus throughout the GI tract, similar to the nAChR subtype found in peripheral ganglia. Cells in the myenteric but not submucosal plexus also expressed mRNA transcripts for α7 and β2, giving rise to the possibility that there are at least two types of heteromeric nAChRs and perhaps homomeric nAChRs in the myenteric plexus. An understanding of the different nAChR subtypes in the ENS is important in the development of new drugs for the regulation of GI tract motility, and in evaluating the possible effects of smoking related nicotine exposure in the ENS during development and in adults.
Acknowledgments
This study was supported by NIH grant# DA 106487. Alexandra Garza was supported by the Texas A&M HSC SURF program. We are grateful to Drs. Louise Abbott (TAMU), Gerry Frye (TAMU/HSC), and Gregg Wells (TAMU/HSC) for their suggestions in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bian XC, Bornstein JC, Bertrand PP. Nicotinic transmission at functionally distinct synapses in descending reflex pathways of the rat colon. Neurogastroenterol Motil. 2003;15:161–171. doi: 10.1046/j.1365-2982.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil. 2004;16(Suppl 1):34–38. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- Boyd RT, Jacob MH, McEachern AE, Caron S, Berg DK. Nicotinic acetylcholine receptor mRNA in dorsal root ganglion neurons. J Neurobiol. 1991;22:1–14. doi: 10.1002/neu.480220102. [DOI] [PubMed] [Google Scholar]
- Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience. 1996;73:1029–1047. doi: 10.1016/0306-4522(96)00118-2. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14:611–623. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, North RA. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 1):64–70. doi: 10.1111/j.1743-3150.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- Glushakov AV, Voytenko LP, Skok MV, Skok V. Distribution of neuronal nicotinic acetylcholine receptors containing different alpha-subunits in the submucosal plexus of the guinea-pig. Auton Neurosci. 2004;110:19–26. doi: 10.1016/j.autneu.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Herber DL, Severance EG, Cuevas J, Morgan D, Gordon MN. Biochemical and histochemical evidence of nonspecific binding of alpha7 nAChR antibodies to mouse brain tissue. J Histochem Cytochem. 2004;52:1367–1376. doi: 10.1177/002215540405201013. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Winzer-Serhan UH. Effects of paraformaldehyde fixation on nicotinic acetylcholine receptor binding in adult and developing rat brain sections. J Neurosci Methods. 2006;153:312–317. doi: 10.1016/j.jneumeth.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol. 1998;390:497–514. [PubMed] [Google Scholar]
- Kosterlitz HW, Lees GM. Pharmacological analysis of intrinsic intestinal reflexes. Pharmacol Rev. 1964;16:301–339. [PubMed] [Google Scholar]
- Kunze WAA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chang Y, Zhang J, Xue F, Dechon J, Lukas RJ, WU J. A novel α7β2-nicotinic acetylcholine receptor in forebrain cholinergic neurons is highly sensitive to amyloid beta peptides. Neuroscience Abstract. 2007;39.1 [Google Scholar]
- Lukas RJ. Diversity and patterns of regulation of nicotinic receptor subtypes. Ann N Y Acad Sci. 1995;757:153–168. doi: 10.1111/j.1749-6632.1995.tb17471.x. [DOI] [PubMed] [Google Scholar]
- Mandl P, Kiss JP. Role of presynaptic nicotinic acetylcholine receptors in the regulation of gastrointestinal motility. Brain Res Bull. 2007;72:194–200. doi: 10.1016/j.brainresbull.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose Ganglia. Mol Pharmacol. 2006;70:1693–1699. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- Moser N, Mechawar N, Jones I, Gochberg-Sarver A, Orr-Urtreger A, Plomann M, Salas R, Molles B, Marubio L, Roth U, Maskos U, Winzer-Serhan U, Bourgeois JP, Le Sourd AM, De Biasi M, Schröder H, Lindstrom J, Maelicke A, Changeux JP, Wevers A. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J Neurochem. 2007;102:479–492. doi: 10.1111/j.1471-4159.2007.04498.x. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat. 2000;18:31–40. doi: 10.1016/s0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Obaid AL, Koyano T, Lindstrom J, Sakai T, Salzberg BM. Spatiotemporal patterns of activity in an intact mammalian network with single-cell resolution: optical studies of nicotinic activity in an enteric plexus. J Neurosci. 1999;19:3073–3093. doi: 10.1523/JNEUROSCI.19-08-03073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Nelson ME, Lindstrom J, Salzberg BM. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. J Exp Biol. 2005;208(Pt 15):2981–3001. doi: 10.1242/jeb.01732. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M. Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology. 1996;111:401–408. doi: 10.1053/gast.1996.v111.pm8690205. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schäfer KH, Hänsgen A, Mestres P. Morphological changes of the myenteric plexus during early postnatal development of the rat. Anat Rec. 1999;256:20–28. doi: 10.1002/(SICI)1097-0185(19990901)256:1<20::AID-AR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Schemann M, Reiche D, Michel K. Enteric pathways in the stomach. Anat Rec. 2001;262:47–57. doi: 10.1002/1097-0185(20010101)262:1<47::AID-AR1010>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol. 1992;263(5 Pt 1):G709–718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- Schemann M, Sann H, Schaaf C, Mäder M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993;265(5 Pt 1):G1005–1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Galligan JJ. Nicotinic acetylcholine receptors on nerve terminals releasing slow synaptic transmitters in the myenteric plexus of guinea pig ileum. Am J Physiol. 2000;279:G528–G535. doi: 10.1152/ajpgi.2000.279.3.G528. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic acetylcholine receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [(125)I]-epibatidine. Br J Pharmacol. 2000;131:729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect alpha 2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protoc. 1999;3:229–241. doi: 10.1016/s1385-299x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ren J, Brown E, Schneider D, Caraballo-Lopez Y, Galligan JJ. Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. J Pharmacol Exp Ther. 2002;302:889–897. doi: 10.1124/jpet.102.033548. [DOI] [PubMed] [Google Scholar]