Abstract
Approximately 27% of North American cancer deaths are attributable to cancer of the lung. Many lung cancers are found at an advanced stage, rendering the tumours inoperable and the patients palliative. Common symptoms associated with palliative lung cancer include cough, hemoptysis, and dyspnea, all of which can significantly debilitate and diminish quality of life (qol). In studies of the effects of cancer therapies, the frequent evaluative endpoints are survival and local control; however, it is imperative that clinical trials with palliative patients also have a qol focus when a cure is unattainable. We conducted a literature review to investigate the use of qol instrument tools in trials studying qol or symptom palliation of primary lung cancer or lung metastases through the use of radiotherapy. We identified forty-three studies: nineteen used a qol tool, and twenty-four examined symptom palliation without the use of a qol instrument. The European Organization for Research and Treatment of Cancer (eortc) qlq-C30 survey was the most commonly used qol questionnaire (in thirteen of twenty trials). Of those thirteen studies, eight also incorporated the lung-specific qol survey eortc qlq-LC13 (or the eortc qlq-LC17). A second lung-specific survey, the Functional Assessment of Cancer Therapy–Lung (fact-L) was used in only two of the twenty trials. In total, only ten of forty-three trials (23%) used a lung-specific qol tool, suggesting that qol was of low priority as an endpoint and that measures created for lung cancer patients are underused. We encourage investigators in future trials to include specific qol instruments such as the eortc qlq-LC13 or the fact-L for studies in palliative thoracic radiotherapy because those instruments provide a measure of qol specific to patients with lung cancer or lung metastases.
Keywords: Lung cancer, quality of life, qol instrument, review, fact-L, eortc qlq-LC13, eortc qlq-C30
1. INTRODUCTION
Lung cancer is a rising epidemic and remains the leading cause of cancer death in both men and women in Canada1. In general, 500 Canadians are diagnosed with and 400 Canadians die of lung cancer every week1. Such high morbidity and mortality in patients with primary lung cancer emphasizes the need for palliative treatment intent.
Morbidity from lung cancer or lung metastases often presents as troublesome thoracic symptoms such as hemoptysis, cough, chest pain, and dyspnea. Palliative radiotherapy has been effective in ameliorating these symptoms 2–4 and improves or preserves the quality of life (qol) remaining in approximately one third of affected patients5.
In the past, clinical trials in patients with lung cancer have focused on traditional endpoints such as overall survival, disease-free survival, or local control 6. Given the relatively poor prognosis of patients with locally advanced lung cancer or lung metastases, the inclusion of qol as a primary endpoint of treatment becomes increasingly important. Quality of life encompasses the minimization of risks and maximization of benefits of a treatment, including physical and psychosocial effects on the well-being of patients7. Studying qol is particularly relevant in the field of palliative radiotherapy because of known treatment-related side effects and toxicities.
Few studies focus on qol and symptom palliation as primary endpoints. The purpose of the present review was to accurately assess the recent use of qol tools in trials that evaluated the efficacy of palliative radiotherapy in patients with lung cancer or lung metastases.
2. METHODS
2.1 Search Strategy
We conducted a literature review using the medline (Ovid) database for 1950 to February 2008. Key terms such as “lung cancer,” “lung neoplasms,” or “lung metastases” were combined with the terms “radiotherapy,” “radiation,” “external-beam irradiation,” or “palliative radiotherapy.” This search was then combined with “quality of life” or “qol” and also “symptom palliation.” Relevant articles and abstracts were reviewed, and references from those sources were also manually searched for additional relevant publications.
2.2 Inclusion Criteria
To be included in the present literature review, articles had to meet these criteria:
Population: patients with a histologic, cytologic, or radiologic diagnosis of primary lung cancer or lung metastases
Intervention: external beam radiotherapy or endobronchial brachytherapy in at least one study arm, with palliative intent
Types of studies: randomized trials, prospective or retrospective cohort studies
Endpoints: qol or symptom palliation as a primary or secondary endpoint or measured outcome
2.3 Exclusion Criteria
Articles were excluded if they met any of these criteria:
Article type: individual case report or review article
Language: publication in a language other than English
Intervention: no evaluation, in at least one arm, of external beam irradiation to the thorax or endobronchial brachytherapy; or studies of interventions with curative intent
Types of studies: focus on populations other than those with primary lung cancer or lung metastases
Endpoints: use of the Karnofsky performance status (kps) or other similar prognostic tools, correlation of qol with cost–utility, or test of the reliability or validity of a qol instrument
2.4 Data Extraction
We extracted the following information from the studies:
Primary and secondary outcomes
Radiotherapy treatment details
Type and number of qol, symptom palliation, and additional tools, if any, used
Number of patients in each study arm
Median age and male: female ratio of the patients enrolled in the study
Median survival in each study arm
3. RESULTS
We identified a total of forty-three trials that evaluated, in at least one study arm, the use of palliative radiotherapy to the thorax, and that assessed qol or symptom palliation as a primary or secondary endpoint. Thirty studies (Table I) evaluated the treatment of patients with non-small-cell lung cancer (nsclc). Four studies (Table II) involved patients who were treated with endobronchial brachytherapy alone or in addition to external-beam radiation. Brachytherapy differs from external-beam radiation in that it is a more localized form of radiation that limits toxicity in healthy tissue to the immediate vicinity of the radiated region5. Another nine trials (Table III) evaluated the use of palliative radiotherapy in patients with lung cancer of a histologic type other than nsclc. The four identified studies that measured the difference in efficacy between endobronchial brachytherapy and external beam radiation 37–40 used both symptom palliation and qol scores as a primary outcome.
TABLE I.
Patients with inoperable non-small-cell lung cancer (nsclc) treated with palliative radiotherapy
| Reference | Type | Study Purpose | Arms | Pts (n) | Median survival | qol | Assessment tools Performance | Other | Measures of qol (n) |
|---|---|---|---|---|---|---|---|---|---|
| Simpson et al., 1985 8 | rct (multicentre) | To evaluate 3 xrt schedules and determine the most efficient | A: 40 Gy split course in 4 weeks
B: 30 Gy continuous for 2 weeks C: 40 Gy continuous for 4 weeks |
316 | A: 6.2 months
B: 6.4 months C: 6.9 months |
None | kps | Study designed: self-report either complete relief or relative relief by patient | 0 |
| Kaasa et al., 1988 9 | rct | qol of patients with radiation therapy and chemotherapy | A: Combination chemotherapy
B: 42 Gy/15 fr |
95 | Not stated | Study designed: 29 variables; only psychosocial well-being and global qol reported | who | None | 0 |
| Teo et al., 1988 10 | rct | To compare a hypofractionated scheme with traditional fractionation | A: 45 Gy/18 fr
B: 31.2 Gy/4 fr |
291 | Not stated | None | kps | Study designed: subjective responses in thoracic symptoms to changes | 0 |
| mrc Lung Cancer Working Party, 1991 11 | Randomized prospective | To determine if a shorter treatment course of xrt provides equally good symptom palliation | A: 17 Gy/2 fr
B: 30 Gy/10 fr |
369 | A: 179 days
B: 177 days |
None | who | Study designed: 4-point scale to rate symptoms | 0 |
| Regan et al., 1991 12 | Prospective | Correlate physician rating of xrt response to patient views of treatment | A: 30 Gy/10 fr
B: 17 Gy/2 fr |
40 | 30 Days | eortc qlq-C30 | ecog | mrc physician questionnaire | 1 |
| mrc Lung Cancer Working Party, 1992 13 | rct | Investigate whether a single fraction can provide palliation as good as that provided by 2 fractions | A: 17 Gy/2 fr
B: 10 Gy/1 fr |
233 | A: 100 days
B: 122 days |
None | who | Study designed: daily dairy for first 6 months: 4-point scale to rate symptoms | 0 |
| Omand and Meredith, 1994 14 | Prospective | To assess frequency of acute side effects of short-term xrt | A: 10 Gy/1 fr
B: 17Gy/ 2 fr |
61 | Not stated | None | None | Study designed: percentage improvement in symptoms | 0 |
| Abratt et al., 1995 15 | Randomized prospective | To evaluate the dose–response effect on survival of patients with good performance status | A: 35 Gy/10 fr
B: 45 Gy/15 fr |
84 | A: 8.5 months
B: 8.5 months |
None | who | Study designed: physician graded symptom improvements | 0 |
| Macbeth et al., 199616 | Randomized (multicentre) | To compare palliative with more-intensive xrt with respect to survival and qol | A: 17 Gy/2 fr
B: 39 Gy/13 fr |
509 | A: 7 months
B: 9 months |
None | who | hads, rscl mrc patient diary card | 0 |
| Ball et al., 199717 | Prospective | To assess the effect of adding continuous-infusion fluorouracil to palliative xrt | A: 20 Gy/5 fr
B: 20 Gy/5 fr with fluorouracil for 5 days |
200 | A: 6 months
B: 6.8 months |
Study-designed questionnaire | who | Study-designed questionnaire to detect symptom palliation | 1 |
| Gava et al., 199718 | Prospective (multicentre) | To assess the indications for xrt, compliance with treatment plans, and qol | A: Radical range: 30Gy–70Gy
B: Palliative range: <30 Gy to 70 Gy |
A: 109
B: 73 |
Not stated | Study designed | kps | None | |
| Lutz et al., 199719 | Retrospective | To measure symptom palliation in patients treated with xrt | 30 Gy/10–12 fr | 54 | 4 Months | None | swog performance status | lcss | 1 |
| Vyas et al., 199820 | Retrospective | To evaluate response in patients receiving palliative xrt in 2 large fractions | 17 Gy/2 fr | 37 | Not stated | None | Not stated | Study designed: patients asked to grade percentage improvement in symptoms | 0 |
| Donato et al., 1999 21 | Prospective | To examine the results obtained with a fractionated rt regimen | A: 20 Gy/5 fr (1 treatment)
B: 40 Gy/10 (2 treatments) |
52 | Not stated | None | ecog, kps | Study designed: subjective patient assessment of symptoms | 0 |
| Langendijk et al., 2000 5 | Prospective | To see the association between prognostic factors and qol and the impact of symptoms on qol | A: Curative schedule: 70 Gy in 7 weeks B: Radical schedule: 60 Gy in 6 weeks C: Palliative schedule: 30 Gy in 4 weeks | 262 | A: 19.1 months
B: 8.5 months C: 4.1 months |
eortc-qlq-C30
eortc qlq-LC13 |
who | None | 2 |
| Langendijk et al., 2000 22 | Prospective | To investigate changes in symptoms and qol in patients receiving xrt | 30 Gy/in 4 weeks | 65 | Not stated |
eortc qlq-C30
eortc qlq-LC13 |
who | None | 2 |
| Nestle et al., 2000 23 | Randomized prospective | To see if there is a difference between palliative and more intensive treatment | A: 60 Gy/30 fr
B: 32 Gy/20 fr |
152 | A: 8.3 months
B: 8.4 months |
None | kps | Study designed: mrc daily diary card | 0 |
| Schaafsma and Coy, 2000 24 | Prospective | To estimate the effect of high-dose xrt on qol and computer qald gained | 30 Gy/10 fr | 54 | 266 Days | eortc qlq-C30 | kps | None | 1 |
| Auchter et al., 2001 25 | Prospective | To evaluate qol of patients before, at completion, and after accelerated fractionation of xrt | 57.6 Gy/36 fr over 15 days | 30 | 13 Months | fact-L | ecog | None | 1 |
| BCentingoz et al. , 2001 26 | Retrospective | To retrospectively evaluate the treatment effects of xrt | Median dose: 30 Gy/1–23 fr | 115 | 30 Weeks | None | kps | Study designed: subjective palliation rates in one of three groups: near total response, improvement, or no response | 0 |
| Langendijk et al., 2001 27 | Prospective | To evaluate changes in qol and symptoms after xrt | 60 Gy total dose | 164 | 8.5 Months |
eortc qlq-C30
eortc qlq-LC13 |
who | None | 2 |
| Bejzak et al., 2002 28 | rct (multicentre) | Comparison of 2 fractionation schedules on palliation of symptoms | A: 10 Gy/1 fr
B: 20 Gy/5 fr |
230 | A: 4.2 months
B: 6 months |
eortc qlq-C30 | ecog | lcss (1 item) | 1 |
| Falk et al., 2002 29 | rct (multicentre) | To determine if patients should be given palliative xrt immediately or as needed for symptom relief | A: 17 Gy/2 fr
B: 10 Gy/1 fr |
230 | A: 240 days
B: 253 days |
None | who | hads, rscl | 0 |
| Nihei et al., 2002 30 | Retrospective | To investigate the outcome of xrt for airway stenosis | 30 Gy/10 fr | 24 | Responders: 192 days
Non-responders: 43 days |
None | None | Study designed:
Patient subjective report of symptoms |
0 |
| Borthwick et al., 2003 31 | Prospective | To gain an understanding of fatigue in patients receiving xrt | A: Radical:
55 Gy/20 fr B: Palliative: 39 Gy/13 fr |
53 | Not stated | None | Not stated | Study designed: daily card with 9 questions relating to fatigue | 0 |
| Kramer et al., 2005 32 | rct (multicentre) | Compare various fractions of xrt on palliation of thoracic symptoms | A: 16 GY/2 fr
B: 30 GY/10 fr |
297 | Not stated | None | ecog | rscl | 0 |
| Senkus–Konefka et al., 2005 33 | Randomized prospective | To compare two palliative xrt schedules | A: 20 Gy/5 fr
B: 16 Gy/2 fr |
100 | A: 5.3 months
B: 8.0 months |
None | who | Study designed: patient-reported symptom relief on a 4-point scale | 0 |
| Sundstrøm et al., 2005 34 | Randomized prospective | To compare the course of symptoms and hr qol after immediate thoracic rt between symptomatic (Sym) and non-Sym (NSym) patients | 17 Gy/2 fr
42 Gy/15 fr 50 Gy/25 fr |
395 | NSym: 11.8 months
Sym: 6.0 months |
eortc qlq-C30
eortc qlq-LC13 |
kps | None | 2 |
| Sundstrøm et al., 2006 35 | Randomized | To examine the predictive value of baseline hr qol data in patients receiving xrt in comparison with demographic, clinical, and treatment variables | A: 17 Gy/2 fr
B: 42 Gy/15 fr C: 50 Gy/25 fr |
301 | A: 9.2 Months
B: 7.5 Months C: 7.5 Months |
eortc qlq-C30
eortc qlq-LC13 |
kps | None | 2 |
| Temel et al ., 2007 36 | Prospective | To assess the feasibility of early palliative care in patients with newly diagnosed nsclc | Not stated | 51 | 9.0 Months |
fact-G
fact-L |
ecog | hads | 2 |
Pts = patients; qol = quality of life; rct = randomized clinical trial; xrt = external-beam radiotherapy; kps = Karnofsky performance status; fr = fractions; who = World Health Organization; mrc = Medical Research Council; eortc = European Organization for Research and Treatment of Cancer; ecog = Eastern Cooperative Oncology Group; hads = Hospital Anxiety and Depression Scale; rscl = Rotterdam symptom checklist; swog = Southwest Oncology Group; lcss = Lung Cancer Symptom Scale; rt = radiotherapy; qald = quality-adjusted life-day; hr = health-related.
TABLE II.
Patients with symptomatic lung cancer treated with endobronchial brachytherapy (ebb) as compared with external-beam radiotherapy (xrt) with or without ebb
| Reference | Type | Study Purpose | Arms | Pts (n) | Median survival | qol | Assessment tools Performance | Other (n) | Measures of qol |
|---|---|---|---|---|---|---|---|---|---|
| Stout et al., 2000 37 | rct | To compare ebb and xrt for symptom palliation and the effect on functional status and qol of patients | A: 30 Gy/8 fr xrt B: 15 Gy/1 fr ebb |
99 | A: 287 days
B: 250 days |
None | who | Study designed: 4-point scoring system to monitor performance status and 9 key symptoms hads rscl modified for lung cancer | 2 |
| Langendijk et al., 200138 | rct | To test that the addition of ebb to xrt provides higher levels of palliation of dyspnea and increases qol | A: xrt alone: 60 Gy/24 fr or 30 Gy/10 fr
B: xrt (60 Gy/24 fr or 30 Gy/10 fr) plus ebb (15 Gy/2 fr) |
95 | A: 8.5 months
B: 7.0 months |
eortc qlq-C30
eortc qlq-LC13 |
who | None | 2 |
| Mallick et al., 2006 39 | Prospective | To test the hypothesis that palliative ebb treatment with or without xrt can reduce endobronchial symptoms for a prolonged period and also improve qol | A: 30 Gy/10 fr with ebb on days 6 and 13: 8Gy/1fr
B: 30 Gy/10 fr with ebb on day 13: 10 Gy/1 fr C: ebb 15 Gy/1 fr |
95 | A: 10 months
B: 10 months C: 10 months |
eortc qlq-C30
eortc qlq-LC13 |
kps | None | 2 |
| Mallick et al., 2007 40 | Prospective | To compare the subjective and objective responses to 3 regimens for duration, qol outcomes, and complications | A: 30 Gy/10 fr with ebb on days 6 and 13: 8 Gy/1 fr
B: 30 Gy/10 fr with ebb on day 13: 10 Gy/1 fr C: ebb 15 Gy/1 fr |
45 | Not stated |
eortc qlq-C30
eortc qlq-LC13 |
kps | None | 2 |
Pts = patients; qol = quality of life; rct = randomized clinical trial; fr = fractions; who = World Health Organization; hads = Hospital Anxiety and Depression Scale; rscl = Rotterdam symptom checklist; eortc = European Organization for Research and Treatment of Cancer; kps = Karnofsky performance status.
TABLE III.
Patients with inoperable lung cancer [other than non-small-cell lung cancer (nsclc)] treated with palliative radiotherapy
| Reference | Type | Study Purpose | Arms | Pts (n) | Median survival | qol | Assessment tools Performance | Other | Measures of qol (n) |
|---|---|---|---|---|---|---|---|---|---|
| Berry et al., 197741 | Prospective | Compares xrt alone and with chemotherapy | A: 40 Gy/20 fr or 36 Gy/12 fr
B: Single-agent continuous chemotherapy C: Intermittent quadruple chemotherapy |
A: 48
B: 49 C: 51 |
125 Days | None | None | Study designed: physicians recorded changes in patient symptoms | 0 |
| Collins et al., 1988 42 | Prospective | To determine whether palliative rt should be given to a patient with inoperable carcinoma of the bronchus | Range: 18 Gy/5 fr–48 Gy/10 fr (split course) | 96 | 38 Weeks | None | who | Study designed: symptom response questions | 0 |
| MRC Lung Cancer Working Party, 1989 43 | rct (multicentre) | To compare two policies of treatment | A: Combination chemotherapy and xrt (40 Gy/15 fr)
B: selective treatment: chemotherapy with or without xrt; treatment given as required to control symptoms |
151 | A: 32 weeks
B: 16 weeks |
None | who | Study designed: treatment reports and daily diary chart | 0 |
| Devereux et al., 1997 44 | Prospective | To assess the incidence and severity of the immediate side effects of palliative rt for bronchial carcinoma | Range: 8 Gy/1 fr–60 Gy/30 fr | 118 | Not stated | None | None | Study designed: questionnaire to determine occurrence of symptoms 24 hours post treatment | 0 |
| Rees et al., 1997 45 | Randomized prospective | To compare the symptomatic effects of two regimens of xrt | A: 17 Gy/2 fr
B: 22.5 Gy/5fr |
A: 111
B: 105 |
Not stated | None | who | Study designed: questionnaire to rate severity of symptoms | 0 |
| Ampil et al., 200146 | To see the effects of palliative xrt on patients with synchronous bilateral lung cancers | Range: 5–58 Gy (mean dose: 35 Gy) | 32 | 7 Months | None | swog | Study designed: subjective response | 0 | |
| Erridge et al., 2005 47 | rct | To determine whether palliation of chest symptoms was the same in two fractionation schedules | A: 10 Gy/1 fr
B: 30 Gy/10 fr |
149 | A: 28.3 Weeks
B: 22.7 Weeks |
Spitzer’s qol Index | who |
hads ecog |
1 |
| Turner et al., 2005 48 | Prospective | To see if older people benefit from xrt treatment, both in control of symptoms and improvement in qol (nsclc, sclc, and unknown types) | A: “High dose”: (36/39 Gy in 12/13 fr)
B: ‘‘low dose’’: (10 Gy in 1 fr, 17 Gy in 2 fr or 20 Gy in 5 fr) |
Elderly (>75 years): 83
Younger (<65 years): 49 |
A: 9 months
B: 7 months |
eortc qlq-C30
eortc qlq-LC17 |
who, Barthel adl Scale | hads Concerns Checklist | 2 |
| Hicsönmez et al., 2007 49 | Evaluate efficacy of palliative xrt in terms of qol and how ecog correlates with eortc qlq-C30 | Not stated | 88 | Not stated | eortc qlq-C30 | ecog | None | 1 |
Pts = patients; qol = quality of life; xrt = external-beam radiotherapy; fr = fractions; rt = radiotherapy; who = World Health Organization; mrc = Medical Research Council; rct = randomized clinical trial; swog = Southwest Oncology Group; hads = Hospital Anxiety and Depression Scale; ecog = Eastern Cooperative Oncology Group; sclc = small-cell lung cancer; eortc = European Organization for Research and Treatment of Cancer.
In twenty of the identified studies, symptom palliation was used as a primary outcome 8,10,11,13, 14,17,19,20,21,23,26,28–33,44,45,47. Ten trials used qol as a primary outcome 5,9,16,18,24,25,34,35,39,49, and six studies used both symptom palliation and qol together as a primary endpoint 22,27,37,38,40,48. Seven of the studies used neither symptom palliation nor qol as primary endpoints, but rather incorporated them as secondary outcomes 12,15,36,41–43,46. The four identified studies that measured the difference in efficacy between endobronchial brachytherapy and external beam radiation37–40 used both symptom palliation and qol scores as primary outcomes.
3.1 QOL and Symptom Palliation Tools Used
A total of 11 tools were used to assess either qol or palliation of lung cancer–related symptoms; the frequency of use of each tool is presented in Table IV. The most common qol tool used was the European Organization for Research and Treatment of Cancer (eortc) qlq-C30, a questionnaire that was created and validated to assess qol in individuals with any form of cancer. It has been translated into 81 languages and consists of 30 questions that encompass 5 functional scales: physical, role, cognitive, emotional, and social functioning49. The eortc qlq-C30 also incorporates 3 symptom scales: fatigue, pain, and nausea and vomiting. The remaining items on the questionnaire cover other symptom-related events that are often described by cancer patients, including dyspnea, diarrhea, and loss of appetite, among others48.
TABLE IV.
Frequency of instruments used in clinical trials measuring quality of life (qol) in patients with locally advanced lung cancer or lung metastases
| Instrument | Frequency |
|---|---|
| European Organization for Research and Treatment of Cancer (eortc) | |
| General cancer questionnaire (eortc qlq-C30) | 13 |
| Lung cancer questionnaire (eortc qlq-LC13) | 7 |
| Lung cancer questionnaire (eortc qlq-LC17) | 1 |
| Functional Assessment of Cancer Therapy (fact) | |
| General questionnaire (fact-G) | 1 |
| Lung questionnaire (fact-L) | 2 |
| Spitzer qlq Index | 1 |
| Hospital Anxiety and Depression Scale (hads) | 5 |
| Rotterdam Symptom Checklist (rscl) | 4 |
| Study-designed qlq questionnaire | 3 |
| Lung Cancer Symptom Scale (lcss) | 2 |
| Study-designed symptom palliation questionnaire | 19 |
The eortc qlq-C30 was used in fourteen of the forty-three studies identified in the search (32%), eight of which also used the lung cancer supplement, eortc qlq-LC13. The eortc qlq-LC13 is the latest version of a lung cancer specific questionnaire that consists of questions concerning lung cancer symptoms and the side effects of conventional treatments used for lung cancer49. One trial used an older version of the lung-specific module, the eortc qlq-LC17, in addition to the general questionnaire48
The Functional Assessment of Cancer Therapy (fact) qol tools constituted a second group used in the identified studies. Both the general questionnaire (fact-G) and the lung-specific questionnaire (fact-L) were used. Like the eortc qlq-C30, the fact-G is a general questionnaire that was developed for patients with any type of cancer. The fact-G covers 4 dimensions of qol: physical, social, emotional, and functional well-being50. The fact-L is similar to the eortc qlq-LC13 because it includes additional questions that relate specifically to qol in patients with lung cancer. The fact-L was used in two studies, and the fact-G in one.
A third validated qol tool was used in one trial: the Spitzer qol Index. The Spitzer Index covers 5 dimensions of qol: activity, daily living, health, support of family and friends, and outlook51. It is not a lung cancer–specific questionnaire, however; and thus it does not incorporate questions directly related to the lung-cancer-specific patient population.
Study-designed questionnaires were the most prevalent tool used in the forty-three identified studies. A study-specific method of determining qol was used in three trials, and nineteen trials attempted to evaluate symptom palliation using a study-designed questionnaire. Table V shows a breakdown of the proportion of studies using a validated qol or symptom palliation tool as compared with a study-designed tool. Study-designed instruments present a difficulty: drawing comparisons across studies is harder because the methods of measurement vary.
TABLE V.
Use of validated or study-designed tools in forty-three studies
| Questionnaire typea | ||||
|---|---|---|---|---|
| Symptom palliation | Quality of life | |||
| (n) | (%) | (n) | (%) | |
| Validated | 9 | 21 | 16 | 37 |
| Study-designed | 21 | 49 | 3 | 7 |
| Total | 30 | 70 | 19 | 44 |
Six studies used both a qol and a symptom palliation tool.
In five studies, a validated symptom palliation tool was used (the frequency of use can be seen in Table IV). The two general symptom tools used were the Hospital Anxiety and Depression Scale and the Rotterdam Symptom Checklist. The Rotterdam Symptom Checklist measures psychological and physical distress in cancer patients through the use of 38 items52. The Hospital Anxiety and Depression Scale is a tool used to measure anxiety and depression levels using 14 statements based on a patient’s experience over the preceding week53. One lung-specific symptom tool the Lung Cancer Symptom Scale was used. The Lung Cancer Symptom Scale is a tool designed to measure 6 lung-specific symptoms and their effects on symptomatic distress, functional burden, and global quality of life54,55.
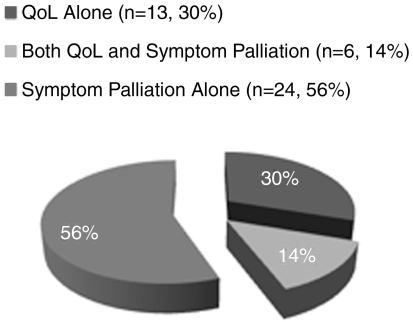
Figure 1 outlines the overall picture of questionnaire use in the identified trials. Most of the trials (54%) measured symptom palliation alone; some measured both symptom palliation and qol (14%). The remaining trials measured qol only.
FIGURE 1.
Questionnaire use in all identified studies.
3.2 Performance Assessment
In forty studies (91%), the performance status of the subjects was measured in addition to qol or symptom palliation. Performance status was measured primarily as a prognostic factor (twenty of forty trials, 50%) or as part of the exclusion criteria (fourteen of forty trials, 35%). Only six studies used a performance scale as part of the assessment. The 3 most predominant performance status tools used were the World Health Organization performance status, the Eastern Cooperative Oncology Group scale, and the Karnofsky performance status (kps). Although performance scales are useful to determine the functional status of a patient, they are not adequate tools for measuring symptom palliation or qol.
4. DISCUSSION
In patients with terminal cancer, qol is a significant concept, and it is influencedby many factors, including symptoms, functional level, coping strategies, and support systems51. Common symptoms that influence a lung cancer patient’s qol include anxiety, depression, pain, fatigue, dyspnea, and cough52. Because lung cancer is the leading cause of cancer death in men and the second-leading cause in women globally2, it is important that qol is considered when caring for these patients.
Meaningful palliation refers to symptom relief and prolongation of good-quality survival in lung cancer patients26. When treating a patient with palliative intent, it is necessary to use tools that measure the intent of the treatment. For 86% of doctors from the United Kingdom, the United States, and Canada, the treatment of choice for patients with inoperable report of a lung cancer is palliative radiotherapy33. It is therefore important that, when considering the side effects of palliative radiotherapy as compared with the side effects of the lung cancer itself, trials investigating the use of palliative radiotherapy use a qol measure to determine the benefit of the treatment.
A total of twenty identified trials considering palliative radiotherapy for lung cancer included an evaluation of qol. Of these trials, eleven used a tool that was specific to patients with lung cancer; the remaining nine used general qol questionnaires for cancer patients or a study-designed questionnaire. In thirty-one identified studies, the level of symptom palliation, one aspect that contributes to a qol measure, was assessed. This finding suggests that more trials should use a validated lung-specific tool when evaluating the outcome of palliative thoracic radiotherapy. Use of a validated, lung-specific tool will allow for comparisons between trials and will also increase the internal validity of individual studies. Two recommended lung-specific validated tools that would be beneficial for the measurement of qol in trials evaluating palliative thoracic radiotherapy are the fact-L and the eortc qlq-LC13.
5. ACKNOWLEDGMENT
This project was generously supported by the Michael and Karyn Goldstein Cancer Research Fund. We thank Ms. Stacy Lue for secretarial service.
6. REFERENCES
- 1.Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics. Toronto: Canadian Cancer Society; 2007. 2007. [Google Scholar]
- 2.Okawara G, Mackay JA, Evans WK, Ung YC on behalf of the Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Management of unresected stage III non-small cell lung cancer: a systematic review. J Thorac Oncol. 2006;1:377–93. [PubMed] [Google Scholar]
- 3.Brundage MD, Bezjak A, Dixon P, et al. The role of palliative thoracic radiotherapy in non-small cell lung cancer. Can J Oncol. 1996;6(suppl 1):25–73. [PubMed] [Google Scholar]
- 4.Sirzén F, Kjellén E, Sörenson S, Cavallin–Ståhl E. A systematic overview of radiation therapy effects in non-small cell lung cancer. Acta Oncol. 2003;42:493–515. doi: 10.1080/02841860310014453. [DOI] [PubMed] [Google Scholar]
- 5.Langendijk JA, Aaronson NK, ten Velde GP, de Jong JM, Muller MJ, Wouters EF. Pretreatment quality of life of inoperable non-small cell lung cancer patients referred for primary radiotherapy. Acta Oncol. 2000;39:949–58. doi: 10.1080/02841860050215936. [DOI] [PubMed] [Google Scholar]
- 6.Movsas B, Scott C. Quality-of-life trials in lung cancer: past achievements and future challenges. Hematol Oncol Clin North Am. 2004;18:161–86. doi: 10.1016/s0889-8588(03)00147-3. [DOI] [PubMed] [Google Scholar]
- 7.Yancik R, Edwards BK, Yates JW. Assessing the quality of life of cancer patients: practical issues in study implementation. J Psychosoc Oncol. 1989;7:59–74. [Google Scholar]
- 8.Simpson JR, Francis ME, Perez–Tamayo R, Marks RD, Rao DV. Palliative radiotherapy for inoperable carcinoma of the lung: final report of a rtog multi-institutional trial. Int J Radiat Oncol Biol Phys. 1985;11:751–8. doi: 10.1016/0360-3016(85)90307-4. [DOI] [PubMed] [Google Scholar]
- 9.Kaasa S, Mastekaasa A, Naess S. Quality of life of lung cancer patients in a randomized clinical trial evaluated by a psychosocial well-being questionnaire. Acta Oncol. 1988;27:335–42. doi: 10.3109/02841868809093551. [DOI] [PubMed] [Google Scholar]
- 10.Teo P, Tai TH, Choy D, Tsui KH. A randomized study on palliative radiation therapy for inoperable non small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1988;14:867–71. doi: 10.1016/0360-3016(88)90007-7. [DOI] [PubMed] [Google Scholar]
- 11.Lung Cancer Working Party, Medical Research Council. Inoperable non-small-cell lung cancer (nsclc): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer. 1991;63:265–70. doi: 10.1038/bjc.1991.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan J, Yarnold J, Jones PW, Cooke NT. Palliation and life quality in lung cancer; how good are clinicians at judging treatment outcome? Br J Cancer. 1991;64:396–400. doi: 10.1038/bjc.1991.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medical Research Council Lung Cancer Working Party. A Medical Research Council (mrc) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (nsclc) and poor performance status. Br J Cancer. 1992;65:934–41. doi: 10.1038/bjc.1992.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omand M, Meredith C. A study of acute side-effects related to palliative radiotherapy treatment of lung cancer. Eur J Cancer Care (Engl) 1994;3:149–52. doi: 10.1111/j.1365-2354.1994.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 15.Abratt RP, Shepherd LJ, Salton DG. Palliative radiation for stage 3 non-small cell lung cancer a prospective study of two moderately high dose regimens. Lung Cancer. 1995;13:137–43. doi: 10.1016/0169-5002(95)00487-4. [DOI] [PubMed] [Google Scholar]
- 16.Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Clin Oncol (R Coll Radiol) 1996;8:167–75. doi: 10.1016/s0936-6555(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 17.Ball D, Smith J, Bishop J, et al. A phase iii study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75:690–7. doi: 10.1038/bjc.1997.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gava A, Bertossi L, Zorat PL, et al. Radiotherapy in the elderly with lung carcinoma: the experience of the Italian “Geriatric Radiation Oncology Group. Rays. 1997;22:61–5. [PubMed] [Google Scholar]
- 19.Lutz ST, Huang DT, Ferguson CL, Kavanagh BD, Tercilla OF, Lu J. A retrospective quality of life analysis using the Lung Cancer Symptom Scale in patients treated with palliative radiotherapy for advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 1997;37:117–22. doi: 10.1016/s0360-3016(96)00406-3. [DOI] [PubMed] [Google Scholar]
- 20.Vyas RK, Suryanarayana U, Dixit S, et al. Inoperable non-small cell lung cancer: palliative radiotherapy with two weekly fractions. Indian J Chest Dis Allied Sci. 1998;40:171–4. [PubMed] [Google Scholar]
- 21.Donato V, Zurlo A, Bonfili P, et al. Hypofractionated radiation therapy for inoperable advanced stage non-small cell lung cancer. Tumori. 1999;85:174–6. doi: 10.1177/030089169908500305. [DOI] [PubMed] [Google Scholar]
- 22.Langendijk JA, ten Velde GP, Aaronson NK, de Jong JM, Muller MJ, Wouters EF. Quality of life after palliative radiotherapy in non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2000;47:149–55. doi: 10.1016/s0360-3016(99)00540-4. [DOI] [PubMed] [Google Scholar]
- 23.Nestle U, Nieder C, Walter K, et al. A palliative accelerated irradiation regimen for advanced non-small-cell lung cancer vs. conventionally fractionated 60 Gy: results of a randomized equivalence study. Int J Radiat Oncol Biol Phys. 2000;48:95–103. doi: 10.1016/s0360-3016(00)00607-6. [DOI] [PubMed] [Google Scholar]
- 24.Schaafsma J, Coy P. Response of global quality of life to high-dose palliative radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2000;47:691–701. doi: 10.1016/s0360-3016(00)00439-9. [DOI] [PubMed] [Google Scholar]
- 25.Auchter RM, Scholtens D, Adak S, Wagner H, Cella DF, Mehta MP on behalf of the Eastern Cooperative Oncology Group. Quality of life assessment in advanced non-small-cell lung cancer patients undergoing an accelerated radiotherapy regimen: report of ECOG study 4593. Int J Radiat Oncol Biol Phys. 2001;50:1199–206. doi: 10.1016/s0360-3016(01)01604-2. [DOI] [PubMed] [Google Scholar]
- 26.Cetingoz R, Kentli S, Uruk O, Demirtas E, Sen M, Kinay M. The role of palliative radiotherapy in locally advanced non-small cell lung cancer. Neoplasma. 2001;48:506–10. [PubMed] [Google Scholar]
- 27.Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58:257–68. doi: 10.1016/s0167-8140(00)00345-5. [DOI] [PubMed] [Google Scholar]
- 28.Bezjak A, Dixon P, Brundage M, et al. on behalf of the Clinical Trials Group of the National Cancer Institute of Canada. Randomized phase iii trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (ncic ctg sc.15) Int J Radiat Oncol Biol Phys. 2002;54:719–28. doi: 10.1016/s0360-3016(02)02989-9. [DOI] [PubMed] [Google Scholar]
- 29.Falk SJ, Girling DJ, White RJ, et al. on behalf of the Medical Research Council Lung Cancer Working Party. Immediate versus delayed palliative thoracic radiotherapy in patients with unresectable locally advanced non-small cell lung cancer and minimal thoracic symptoms: randomised controlled trial. BMJ. 2002;325:465. doi: 10.1136/bmj.325.7362.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nihei K, Ishikura S, Kawashima M, Ogino T, Ito Y, Ikeda H. Short-course palliative radiotherapy for airway stenosis in non-small cell lung cancer. Int J Clin Oncol. 2002;7:284–8. doi: 10.1007/s101470200041. [DOI] [PubMed] [Google Scholar]
- 31.Borthwick D, Knowles G, McNamara S, Dea RO, Stroner P. Assessing fatigue and self-care strategies in patients receiving radiotherapy for non-small cell lung cancer. Eur J Oncol Nurs. 2003;7:231–41. doi: 10.1016/s1462-3889(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 32.Kramer GW, Wanders SL, Noordijk EM, et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol. 2005;23:2962–70. doi: 10.1200/JCO.2005.01.685. [DOI] [PubMed] [Google Scholar]
- 33.Senkus–Konefka E, Dziadziuszko R, Bednaruk–Mlyńnski E, et al. A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (nsclc) Br J Cancer. 2005;92:1038–45. doi: 10.1038/sj.bjc.6602477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundstrøm S, Bremnes R, Brunsvig P, et al. on behalf of the Norwegian Lung Cancer Study Group. Immediate or delayed radiotherapy in advanced non-small cell lung cancer (nsclc)? Data from a prospective randomised study. Radiother Oncol. 2005;75:141–8. doi: 10.1016/j.radonc.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Sundstrøm S, Bremnes RM, Brunsvig P, Aasebø U, Kaasa S on behalf of the Norwegian Lung Cancer Study Group. Palliative thoracic radiotherapy in locally advanced non-small cell lung cancer: can quality-of-life assessments help in selection of patients for short- or long-course radiotherapy. J Thorac Oncol. 2006;1:816–24. [PubMed] [Google Scholar]
- 36.Temel JS, Jackson VA, Billings JA, et al. Phase ii study: integrated palliative care in newly diagnosed advanced non-small-cell lung cancer patients. J Clin Oncol. 2007;27:2377–82. doi: 10.1200/JCO.2006.09.2627. [DOI] [PubMed] [Google Scholar]
- 37.Stout R, Barber P, Burt P, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol. 2000;56:323–7. doi: 10.1016/s0167-8140(00)00252-8. [DOI] [PubMed] [Google Scholar]
- 38.Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58:257–68. doi: 10.1016/s0167-8140(00)00345-5. [DOI] [PubMed] [Google Scholar]
- 39.Mallick I, Sharma SC, Behera D, Ghoshal S, Oinam AS. Optimization of dose and fractionation of endobronchial brachytherapy with or without external radiation in the palliative management of non-small cell lung cancer: a prospective randomized study. J Can Res Ther. 2006;2:119–25. doi: 10.4103/0973-1482.27586. [DOI] [PubMed] [Google Scholar]
- 40.Mallick I, Sharma SC, Behera D. Endobronchial brachytherapy for symptom palliation in non-small cell lung cancer analysis of symptom response, endoscopic improvement and quality of life. Lung Cancer. 2007;55:313–18. doi: 10.1016/j.lungcan.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Berry RJ, Laing AH, Newman CR, Peto J. The role of radiotherapy in treatment of inoperable lung cancer. Int J Radiat Oncol Biol Phys. 1977;2:433–9. doi: 10.1016/0360-3016(77)90154-7. [DOI] [PubMed] [Google Scholar]
- 42.Collins TM, Ash DV, Close HJ, Thorogood J. An evaluation of the palliative role of radiotherapy in inoperable carcinoma of the bronchus. Clin Radiol. 1988;39:284–6. doi: 10.1016/s0009-9260(88)80536-1. [DOI] [PubMed] [Google Scholar]
- 43.Lung Cancer Working Party, Medical Research Council. Survival, adverse reactions and quality of life during combination chemotherapy compared with selective palliative treatment for small-cell lung cancer. Report to the Medical Research Council by its Lung Cancer Working Party. Respir Med. 1989;83:51–8. doi: 10.1016/s0954-6111(89)80060-5. [DOI] [PubMed] [Google Scholar]
- 44.Devereux S, Hatton MQ, Macbeth FR. Immediate side effects of large fraction radiotherapy. Clin Oncol (R Coll Radiol) 1997;9:96–9. doi: 10.1016/s0936-6555(05)80447-9. [DOI] [PubMed] [Google Scholar]
- 45.Rees GJ, Devrell CE, Barley VL, Newman HF. Palliative radiotherapy for lung cancer: two versus five fractions. Clin Oncol (R Coll Radiol) 1997;9:90–5. doi: 10.1016/s0936-6555(05)80446-7. [DOI] [PubMed] [Google Scholar]
- 46.Ampil FL, Chin HW. Palliative radiotherapy for synchronous bilateral lung cancers. Am J Clin Oncol. 2001;24:385–7. doi: 10.1097/00000421-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Erridge SC, Gaze MN, Price A, et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005;17:61–7. doi: 10.1016/j.clon.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Turner NJ, Muers MF, Haward RA, Mulley GP. Do elderly people with lung cancer benefit from palliative radiotherapy? Lung Cancer. 2005;49:193–202. doi: 10.1016/j.lungcan.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Hicsönmez A, Köse K, Andrieu MN, Güney Y, Kurtman C. The European Organization for Research and Treatment of Cancer core quality of life questionnaire (qlq-C30 version 3.0 Turkish) in cancer patients receiving palliative radiotherapy. Eur J Cancer Care (Engl) 2007;16:251–7. doi: 10.1111/j.1365-2354.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 50.Di Lorenzo G, Autorino R, Ciardiello F, et al. External beam radiotherapy in bone metastatic prostate cancer: impact on patients’ pain relief and quality of life. Oncol Rep. 2003;10:399–404. [PubMed] [Google Scholar]
- 51.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: a concise ql-index for use by physicians. J Chronic Dis. 1981;34:585–97. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 52.Vinholes JJ, Purohit OP, Abbey ME, Eastell R, Coleman RE. Relationships between biochemical and symptomatic response in a double-blind randomised trial of pamidronate for metastatic bone disease. Ann Oncol. 1997;8:1243–50. doi: 10.1023/a:1008238422151. [DOI] [PubMed] [Google Scholar]
- 53.Henoch I, Bergman B, Gustafsson M, Gaston–Johansson F, Danielson E. The impact of symptoms, coping capacity, and social support on quality of life experience over time in patients with lung cancer. J Pain Symptom Manage. 2007;34:370–9. doi: 10.1016/j.jpainsymman.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Bergman B, Aaronson NK. Quality-of-life and cost-effectiveness assessment in lung cancer. Curr Opin Oncol. 1995;7:138–43. doi: 10.1097/00001622-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Quality of Life Research Associates. Lung Cancer Symptom Scale. Charlottesville, VA: Quality of Life Research Associates; 2004. [Google Scholar]