Abstract
Background.
ST-elevation myocardial infarction (STEMI) is associated with increased inflammation and oxidative stress, enhancing the formation of advanced glycation endproducts (AGEs). These encompass a characteristic fluorescence pattern, which can be non-invasively measured as skin autofluorescence (AF). In this study we investigate whether skin AF is elevated in STEMI, its association with inflammatory and glycaemic stress and its predictive value for future events.
Methods.
Skin AF was measured in 88 STEMI patients (mean age 64±13 years) within 72 hours and around six months after discharge, in 81 stable coronary artery disease (sCAD) patients (64±10 years), and in 32 healthy controls (63±11 years). The cumulative one-year incidence of all-cause mortality and hospitalisation for myocardial infarction or heart failure was documented.
Results.
Skin AF was significantly higher in STEMI compared with sCAD and controls, irrespective of confounders, and was associated with HbA1c and C-reactive protein. Skin AF decreased significantly in STEMI patients, when measured >200 days after discharge. In STEMI patients, skin AF above the median was predictive of future events (hazard ratio 11.6, 95% CI 1.5 to 90.8, p=0.019).
Conclusion.
Skin AF is elevated in STEMI, is associated with inflammation and glycaemic stress, and predicts future major adverse cardiac events. (Neth Heart J 2009;17:162-8.Neth Heart J 2009;17:162–8.)
Keywords: myocardial infarction, oxidative stress, inflammation, autofluorescence, advanced glycation endproducts
Oxidative stress plays a pivotal role in the inflammatory process leading to atherosclerotic plaque formation.1 In clinical studies, several novel markers of oxidative stress have been found to be associated with acute coronary syndromes (ACS) and to predict future cardiovascular events, independent of traditional cardiovascular risk factors.2–6 However, since the measurement of most biomarkers requires sophisticated techniques, clinical applicability is limited.
Oxidative modification of carbohydrates and lipids enhances the formation of advanced glycation endproducts and advanced lipoxidation endproducts, generally referred to as AGEs. Although classically associated with diabetes and renal failure,7 these compounds are also implicated in the pathophysiology of atherosclerosis.8 We developed a device to quickly, easily and non-invasively assess skin accumulation of AGEs by measuring skin autofluorescence (AF). It has been validated with glycaemic and oxidative stress derived AGEs measured in skin biopsies and independently predicts cardiovascular mortality in patients with renal failure and in patients with type 2 diabetes.9,10 Furthermore, we have recently demonstrated that skin AF is elevated in patients with stable coronary artery disease (sCAD) and is associated with neopterin, a serum monocyte activation marker and with serum levels of the soluble isoform of the receptor for AGEs (sRAGE), making it a potentially non-invasive marker for the inflammatory process that characterises atherosclerosis. 11
In this study we investigate whether skin AF is elevated in acute ST-elevation myocardial infarction (STEMI) compared with sCAD and healthy controls. Furthermore, we investigate whether skin AF is associated with clinically available markers of inflammation and glycaemic stress in these patients, and whether it predicts the one-year incidence of major adverse cardiac events (MACE) in STEMI.
Patients and Method
Subjects
This observational study was performed between May 2004 and August 2005, including 88 patients with acute STEMI admitted to our coronary care unit and 81 patients with sCAD admitted for elective coronary angiography (CAG). Diagnostic criteria for STEMI were typical chest pain of >30 minutes duration and significant ST-segment elevation of >1 mm (0.1 mV) in more than two adjacent leads on electrocardiography. Stable CAD was defined as typical chest pain, or a history of an ACS, or vascular intervention, combined with the presence of at least one coronary artery with mild stenosis (>30% luminal narrowing) on CAG. Additionally, 31 healthy age- and sex-matched controls without a history of cardiovascular disease (CVD), with <2 vascular risk factors, and with normal carotid and femoral arteries on ultrasound examination, were recruited by advertisement in a local newspaper. Exclusion criteria for patients were recent ACS <3 months before admission, known renal disease or serum creatinine >1.7 mg/dl at admission, severe inflammatory or current malignant disease, and skin photo type V/VI (i.e. a coloured skin). The study was approved by the local ethics committee and all patients gave written informed consent.
Baseline and follow-up measurement and event scoring
In STEMI patients, skin AF was measured within 72 hours following symptom onset. After discharge, STEMI patients were routinely prescribed statins, β-blocking agents, aspirin, and clopidogrel. In a subgroup of 29 STEMI patients a second skin AF measurement was performed around six months after discharge. Since most patients were transferred back to a regional hospital <3 days after percutaneous coronary intervention (PCI), it was not possible to perform this measurement in all initial STEMI patients. Additionally, for all STEMI patients the one-year incidence of all-cause mortality and hospitalisation for myocardial infarction or heart failure was documented (MACE). Event scoring was performed blinded for patient characteristics and skin AF levels, obtained from questionnaires and verified with patient's hospital records. Non-responders were contacted by telephone or their status was verified with the family doctor. Three patients were lost to follow-up. Myocardial infarction was defined as typical clinical presentation, combined with troponin levels above normal or typical ECG changes, and heart failure was defined as hospitalisation for New York Heart Association (NYHA) class III or IV heart failure.
Skin autofluorescence
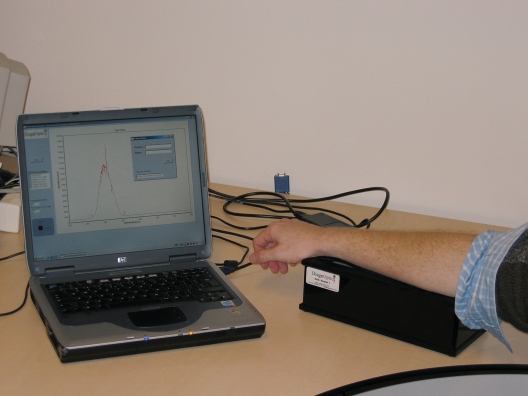
Skin AF was assessed on the ventral site of the lower arm with a prototype of the current AGE-Reader (DiagnOptics BV, Groningen, the Netherlands, see figure 1) as described elsewhere.12,13 This instrument is quick and easy to operate on any computer with an USB connection and the measurement takes approximately 30 seconds to complete. In short, the AGEReader consists of a tabletop box, containing a black light excitation light source (peak wavelength ~360 nm). Light emitted from the skin is measured with an integrated spectrometer. Measurement is fully automated, giving an average value over 50 individual scans. Skin AF is calculated by dividing the mean value of the emitted light intensity per nm between 420 and 600 nm by the mean value of the excitation light intensity per nm between 300 and 420 nm, expressed as arbitrary units (AU). The intra-individual Altman error percentage is 5.0% on a single day and 5.9% for seasonal changes.12
Figure 1 .
A picture of the AGE-Reader. This device was used to non-invasively measure skin autofluorescence in the study.
Laboratory assessments
Laboratory parameters were routinely measured at the local clinical laboratory and collected at admission, prior to CAG/PCI in sCAD and prior to or shortly after PCI in STEMI. However, since no admission blood was collected from approximately 20% of sCAD patients, the most recent (<3 months before study entry) values were used. C-reactive protein (CRP) was measured on a normal sensitivity machine with detection limit of 4 mg/dl (Vitros 250; Ortho Clinical Diagnostics, Rochester, USA). Creatinine clearance was estimated with the Cockcroft-Gault formula.
Statistical analysis
Based on a pilot study of 37 STEMI patients, we determined that a sample size of 85 STEMI and sCAD patients would have 80% power to detect a difference of at least 10% at α=0.05 between STEMI and sCAD patients.14 Normal distribution of variables was tested with the Kolmogorov-Smirnov test. For comparison between groups, continuous variables were analysed by analysis of variance with correction for multiple comparisons (Bonferroni). In case of categorical variables the Fisher's exact test was used. Descriptive statistics are presented as mean values ± SD, as median (interquartile range) for skewed variables, or as percentages. To test whether between-group differences in skin AF could be explained by differences in potential confounders, one-way analysis of covariance (ANCOVA) was performed and F statistic and estimation of effect size (eta-squared, η2) were calculated. Skewed variables (i.e. glucose, HbA1c, triglycerides, and peak cardiac enzymes) were log transformed for a better linear fit. CRP was analysed as a dichotomous variable with separation by values above and below 4 mg/day. The Pearson correlation or Mann-Whitney test were used as appropriate for unadjusted associations. Stepwise, forward selection was used to construct multivariate models and variables with p values <0.05 which were retained in the model. The one-year survival period after STEMI until the occurrence of the composite endpoint was assessed by the Kaplan-Meier method and comparison was made between STEMI patients with skin AF values above and below the median, using the log-rank test. For assessment of the influence of confounding factors on skin AF as a predictor of the composite endpoint, the Cox proportional hazard method was used. Due to the small sample size and thus a low number of events, we could only correct for one potential confounder at a time. The following covariates were corrected for separately: age, sex, smoking, peak troponin levels, anterior infarct location, previous myocardial infarction, and admission blood pressure, glucose, and serum creatinine. Since CRP and HbA1c were measured in only 64 and 74 STEMI patients respectively, these values were not included in this analysis.
Tabel 1.
Clinical characteristics of study groups.
| STEMI (n=88) | sCAD (n=81) | Controls (n=32) | |
|---|---|---|---|
| Age (years) | 64±13 | 64±10 | 63±11 |
| Men | 67 (76) | 60 (74) | 23 (72) |
| Current smokers | 36 (44)*† | 15 (20)¶ | 0 (0) |
| Diabetes mellitus | 25 (28)*† | 12 (15)¶ | 0 (0) |
| Known | 11 (13)† | 12 (15)¶ | 0 (0) |
| New onset | 14 (16)*† | 0 (0) | 0 (0) |
| CrCl (ml/min/1.73 m2) | 80±28 | 74±25 | 81±21 |
| Glucose (mg/dl) | 130 (111–160)*† | 92 (83–106)¶ | 86 (83–95) |
| Cholesterol (mg/dl) | 215±39* | 183±39¶ | 222±31 |
| BMI (kg/m2) | 27±3 | 27±4 | 25±3 |
| Vascular history | |||
| Previous ACS | 6 (7)* | 27 (34) | 0 (0) |
| Previous PTCA | 3 (3)* | 35 (44)¶ | 0 (0) |
| Previous CABG | 2 (2)* | 19 (24)¶ | 0 (0) |
| Previous stroke | 3 (3) | 4 (5) | 0 (0) |
| Previous PAD | 4 (5) | 10 (13) | 0 (0) |
| Statin use | 11 (13)* | 67 (85)¶ | 1 (3) |
| Use of antihypertensive agents | |||
| Beta-blocking agents | 20 (23)*† | 63 (80) | 0 (0) |
| Diuretics | 8 (9) | 9 (11) | 1 (3) |
| ACE inhibitors | 7 (8)* | 28 (35)¶ | 2 (6) |
| ARBs | 5 (6) | 10 (13) | 0 (0) |
| Calcium antagonists | 5 (6)* | 38 (48)¶ | 0 (0) |
| Aspirin use | 7 (8)* | 62 (78)¶ | 0 (0) |
Data are mean ± SD, medium (quartiles), or number of subjects (%). Between-group differences were tested with Student's t-test, or Fisher's exact test when appropriate. * p<0.05 for STEMI vs. sCAD; †STEMI vs. controls; ¶sCAD vs. controls. Medication use indicates situation before admission. STEMI=ST-elevation myocardial infarction, sCAD=stable coronary artery disease; CrCl=estimated creatinine clearance, BMI=body mass index, ACS=acute coronary syndrome, PTCA=percutaneous transluminal coronary angioplasty; CABG=coronary artery bypass graft; PAD=peripheral artery disease; ACE=angiotensin-converting enzyme; ARB=angiotensin-receptor blocker.
A two-sided p value <0.05 was considered statistically significant. All statistical analyses were carried out with the Statistical Package for Social Science (SPSS, version 12.0.2, 24 March 2005).
Results
Clinical data
Subject characteristics
Subject characteristics are shown in table 1. In 5% of STEMI patients no primary PCI was performed because the coronary occlusion had resolved spontaneously. Median myocardial ischaemia time was 3.2 (2.5 to 4.3) hours for STEMI.
Differences in skin AF between groups
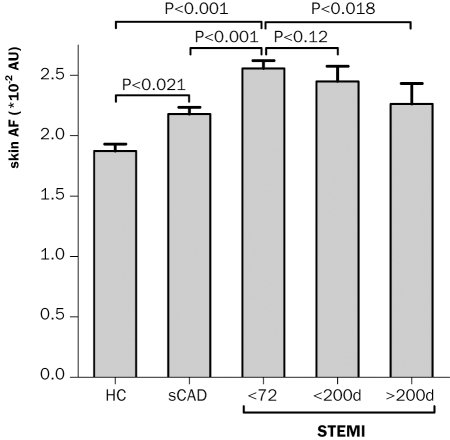
Skin AF was measured at a median of 21.2 (12.3 to 28.2) hours after symptom onset (63% <24 h, 27% 24 h to 48 h, 11% >48 h) and 16.6 (8.8 to 16.6) hours after PCI in STEMI patients. Figure 2 demonstrates that skin AF was significantly higher in STEMI compared with sCAD and controls. Also in sCAD, skin AF was significantly greater than in controls. These between-group differences in skin AF remained significant after correction for diabetes, smoking, cholesterol, and serum creatinine (F=5.2, η2=0.035, p=0.024 for STEMI compared with sCAD and F=8.9, η2=0.079, p=0.004 for STEMI compared with controls).
Skin AF and markers for glycaemic and inflammatory stress within STEMI group
Skin AF correlated positively with age (r=0.44, p<0.001), HbA1c (r=0.43, p<0.001), and glucose (r=0.24, p=0.027), negatively with systolic blood pressure (r=−0.23; p=0.034), and was higher in subjects with CRP >4 mg/l (p=0.007) or with previous CVD (p<0.001). Skin AF was not significantly associated with lipid parameters, peak cardiac enzymes, or gender. After multivariate adjustment for glucose, blood pressure, lipid parameters, peak cardiac enzymes, and gender, age (β 0.24, 95% CI 0.02 to 0.46, p=0.033), CRP (β 0.26, 95% CI 0.05 to 0.48, p=0.018), HbA1c (β 0.36, 95% CI 0.14 to 0.58, p=0.002), and previous CVD (β 0.24, 95% CI 0.01 to 0.44, p=0.042) were independently associated with skin AF (R2=0.45, p<0.001).
Figure 2 .
Bar charts represent mean skin autofluorescence (AF) + standard error in healthy age- and sex-matched controls (HC) and patients with stable coronary artery disease (sCAD) and <72 hours following ST-elevation myocardial infarction (STEMI). Comparisons between groups were corrected for multiple comparisons. Two last bars represent follow-up measurements of STEMI patients, <200 days (n=15; non-significant decrease) and >200 days (d) (n=14; significant decrease (p=0.018)) after discharge. AU=arbitrary units.
Figure 3 .
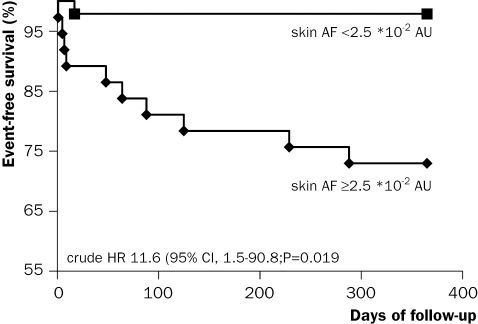
Kaplan-Meier estimates of survival during one-year follow-up with regard to the composite endpoint of all-cause mortality and hospitalisation for myocardial infarction or heart failure in patients with ST-elevation myocardial infarction in relation to skin autofluorescence (AF) below and above the median. Skin AF was <2.5 *102 AU in 46 and ≥2.5 AU *102 in 42 patients. CI=confidence interval, AU=arbitrary units, HR=hazard ratio.
Follow-up
Measurement and one-year incidence of composite endpoint in STEMI
Patients measured within 200 days following STEMI (n=15) did not show a significant decrease in skin AF; however, in patients measured >200 days after STEMI (n=14) skin AF did decrease significantly (p=0.018). Figure 3 demonstrates that during one-year followup, the composite endpoint occurred in 11 patients; four patients died, four were hospitalised for a new myocardial infarction and three for heart failure and that patients with skin AF values above the median (2.5 *10 to 2 AU) had a hazard ratio of 11.6 (95% CI 1.5 to 90.8; p=0.019) for developing MACE. Partial corrections for potential confounders revealed that skin AF predicted MACE independent of age (hazard ratio 8.7, 95% CI 1.0 to 74.4, p=0.048), sex (10.3, 1.3 to 83.7, p=0.030), current smoking (10.5, 1.3 to 84.1, p=0.027), peak troponin levels (12.3, 1.6 to 97.3, p=0.017), anterior infarct location (11.1, 1.4 to 87.7, p=0.022), previous myocardial infarction (12.4, 1.6 to 97.8, p=0.017), admission systolic (11.7, 1.5 to 92.0, p=0.019) or diastolic (11.5, 1.5 to 90.5, p=0.020) blood pressure, glucose (10.3, 1.3 to 82.7; p=0.028), or serum creatinine (11.2, 1.4 to 88.0, p=0.022).
Discussion
The measurement of skin AF is a novel method to noninvasively measure advanced glycation endproducts in the human skin. In this study we demonstrate that skin AF is elevated in acute ST-elevation myocardial infarction compared with stable coronary artery disease and healthy controls. Even after multivariable adjustment for known confounders, skin AF remained significantly higher in STEMI, and was age independently related to CRP and HbA1c. The elevation of skin AF within 72 hours following STEMI appeared to be partly of a transient nature, showing a tendency to decrease after 200 days. Furthermore, STEMI patients with an elevated skin AF were substantially more likely to die or to be hospitalised for a new myocardial infarction or heart failure in the following one year, which seemed to be independent of other risk factors such as age or infarction size. Our data suggest that skin AF may serve as a simple non-invasive measure of hyperglycaemia and inflammation derived oxidative stress involved in the development of ACS and may identify those patients with a potentially adverse outcome.
Comparison with previous clinical studies using skin AF
In previous reports we have already demonstrated that skin AF is strongly related to skin accumulation of AGEs, as evidenced by a high correlation with specific AGEs measured from skin biopsy homogenates.12 Since these measured AGEs included both exclusively carbohydrate-derived AGEs (pentosidine), but also mainly lipid-derived AGEs (carboxymethyllysine and carboxyethyllysine), we concluded that skin AF may be a non-invasive marker for both glycation and oxidative stress.15 This was also supported by the observation that skin AF was inversely related to plasma vitamin C levels, a strong antioxidant, in patients with renal failure.16 Furthermore, subjects with diabetes, especially those with evidence of micro- or macrovascular disease, and subjects with renal failure had significantly higher levels of skin AF12,16,17 and skin AF was an independent predictor of CVD-related mortality in these patients.10,18 The data from the present study are in agreement with our previous observations, and suggest that skin AF may also be of potential use in euglycaemic patients without impaired kidney function.
Oxidative stress and AGEs in CVD
Data on the potential value of oxidative stress markers in endothelial dysfunction and clinically overt CAD are extensive.1–6,19–22 Since most markers for oxidative stress require specialised laboratories, they are not readily available for clinical practice.23 AGEs are stable and non-invasively quantifiable using skin AF and may, therefore, serve as a potential new marker in CVD. Data from other clinical studies support this hypothesis. Plasma AGEs have been shown to be associated with the number of stenotic coronary arteries in non-diabetic subjects,24 and to predict the long-term incidence of cardiovascular mortality in non-diabetic women.25
Basic research has shown that in atherosclerotic plaques AGEs interact with their receptor (RAGE), which results in increased production of inflammatory mediators and proteolytic enzymes, rendering plaques more vulnerable to rupture.26 Recently, the key role of RAGE in the generation of oxidative stress and subsequent cellular damage was pointed out in an animal model of ischaemia/reperfusion injury after myocardial infarction. It was demonstrated that lipoxidationderived AGEs are generated by ischaemia/reperfusion and activate RAGE, thereby augmenting vascular and inflammatory cell activation. Animals lacking cellular expression of RAGE were less susceptible to ischaemia/reperfusion damage.27 This supports previous animal intervention studies showing that lowering AGE levels or antagonising their receptors may attenuate the atherosclerotic process.28,30 We recently reported that skin AF is strongly associated with serum levels of sRAGE in patients with sCAD, thus making the link between inflammation and oxidative stress and skin AF by means of the AGE-RAGE axis.26 In clinical studies it has been shown that lowering AGE in the circulation by dietary intake low in glycotoxins results in marked decreases in serum levels of inflammatory mediators such as CRP and vascular adhesion molecule-1.31 Because in the present study skin AF was related to CRP, a marker of inflammation known to be strongly associated with CAD,32 and predicted future events, our data are in agreement with the abovementioned reports.
Study limitations
Due to the limited number of events in the one-year follow-up period, it was not possible to differentiate between the individual contributions of all-cause mortality, myocardial infarction, and heart failure. CRP was measured with a normal sensitivity kit with a detection limit of 4 mg/dl. Because a significant number of patients had CRP levels <4 mg/dl it could only be analysed as a dichotomous variable. CRP and HbA1c could not be included in the Cox regression analysis either, because these were only measured in a subset of STEMI patients. More importantly, the small sample size did not allow for multivariable correction for more than one potentially confounding covariate in the Cox regression analysis. Additionally, because we measured skin AF after PCI, whether the elevation in skin AF was related to atherosclerotic plaque formation, ischaemia, or reperfusion, or a combination of those, cannot be determined from these data. Also, the observation that skin AF decreased after 200 days was based on a small number of patients, and should be considered as preliminary. However, we hypothesise that there may be a quick rise in skin AF during the oxidative stress related event of an acute STEMI, produced by AGEs. Since these compounds are slowly degraded, it takes a much longer time to decrease. In case of repeated acute oxidative stress related events during lifetime, this may result in a chronic accumulation of AGEs and other oxidative stress derived products. This may be one of the explanations why skin AF after STEMI does not decrease to the levels of the healthy control group. The objection that skin AF was performed within a wide time range is valid; however, we have seen that skin AF does not actually change in the first few days following STEMI (data not shown). In the current study, we did not measure circulating AGEs or other markers for oxidative stress. In order to determine the independent prognostic value of skin AF over these markers, a larger study in STEMI patients is warranted. This would also make multivariable correction possible in the Cox proportional hazard model. For it to be of clinical use in STEMI patients, it should add incremental information to currently available risk scores such as the Thrombolysis In Myocardial Infarction (TIMI), Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT), and Global Registry of Acute Coronary Events (GRACE) risk score. Finally, we are currently studying its value in patients with unstable angina or non-ST-elevation myocardial infarction. These patients are more of a challenge, since it is currently very difficult to select those patients that are at the highest risk of an adverse outcome. From previous investigations we have learned that skin AF cannot be reliably measured in subjects with skin photo type V-VI.33 Therefore, for this study we excluded these skin types and further development for improving the measurement in dark skin types is ongoing and a newer version of the instrument is capable of reliably measuring skin AF in darker skin types. Since not all AGEs encompass fluorescent properties, skin AF is only representative of part of the total AGE burden. However, in our validation study we found that skin AF also correlated strongly with non-fluorescent AGEs.12 Additionally, two major lipid peroxidation products, 4-hydroxynonenal and malondialdhyde – after binding to free aminogroups of protein – also encompass characteristic fluorescent properties.34 Since the AGE-Reader covers a wide excitation/emission spectrum, skin AF measured with the AGE-Reader may therefore have a miscellaneous origin.
Conclusion
To the best of our knowledge, this is the first study to demonstrate an association of a non-invasive marker for cumulative hyperglycaemia and inflammation-related oxidative stress with acute myocardial infarction. Despite the small sample size of this study, our prospective data do indicate that skin AF may potentially provide an easily applicable tool to identify those patients at risk of developing major adverse cardiac events after myocardial infarction. However, since this was a relatively small study, these observations should be confirmed in a larger follow-up study in patients with ACS to establish the usefulness of skin AF in this clinical setting.
Conflict of interest
Drs Smit and Graaff are founders of Diagnoptics, the Netherlands, which develops the ‘AGE Reader’, a prototype of which was used in this study.
References
- 1.Stocker R, Keaney J. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004;84:1381–478. [DOI] [PubMed] [Google Scholar]
- 2.Walter M, Jacob R, Jeffers B, Ghadanfar M, Preston G, Buch J, et al. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol 2004;44:1996–2002. [DOI] [PubMed] [Google Scholar]
- 3.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001;104: 2673–8. [DOI] [PubMed] [Google Scholar]
- 4.Holvoet P, Collen D, Van de Werf F. Malondialdehyde-modified LDL as a marker of acute coronary syndromes. JAMA 1999;281: 1718–21. [DOI] [PubMed] [Google Scholar]
- 5.Shimomura H, Ogawa H, Takazoe K, Soejima H, Miyamoto S, Sakamoto T, et al. Comparison of urinary biopyrrin levels in acute myocardial infarction (after reperfusion therapy) versus stable angina pectoris and their usefulness in predicting subsequent cardiac events. Am J Cardiol 2002;90:108–111. [DOI] [PubMed] [Google Scholar]
- 6.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brummer J, et al. Urinary 8-iso-Prostaglandin F2{alpha} as a risk marker in patients with coronary heart disease: A matched casecontrol study. Circulation 2004; 109:843–8. [DOI] [PubMed] [Google Scholar]
- 7.Smit A, Lutgers H. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem 2004;11:2767–84. [DOI] [PubMed] [Google Scholar]
- 8.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med 2000;28:1708–16. [DOI] [PubMed] [Google Scholar]
- 9.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 2005;16:3687–93. [DOI] [PubMed] [Google Scholar]
- 10.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, et al. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 2007;30:107–12. [DOI] [PubMed] [Google Scholar]
- 11.Mulder DJ, van Haelst PL, Gross S, de LK, Bijzet J, Graaff R, et al. Skin autofluorescence is elevated in patients with stable coronary artery disease and is associated with serum levels of neopterin and the soluble receptor for advanced glycation end products. Atherosclerosis 2007;197:217–23. [DOI] [PubMed] [Google Scholar]
- 12.Meerwaldt R, Oomen PHN, Links TP, Gans ROB, Smit AJ, Graaff R, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004;47:1324–30. [DOI] [PubMed] [Google Scholar]
- 13.Meerwaldt R, Links T, Graaff R, Thorpe SR, Baynes JW, Hartog J, et al. Simple noninvasive measurement of skin autofluorescence. Ann N Y Acad Sci 2005;1043:290–8. [DOI] [PubMed] [Google Scholar]
- 14.Mulder DJ, van Haelst PL, Graaff R, Smit AJ, Gans RO, Zijlstra F. Skin autofluorescence is an independent marker for acute myocardial infarction. Circulation 2005;112:U427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl) lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 1996;271:9982–6. [DOI] [PubMed] [Google Scholar]
- 16.Hartog J, de Vries A, Lutgers H, Meerwaldt R, Huisman R, van Son W, et al. Accumulation of advanced glycation end products, measured as skin autofluorescence, in renal disease. Ann N Y Acad Sci 2005;1043:299–307. [DOI] [PubMed] [Google Scholar]
- 17.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, et al. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 2006;29:2654–9. [DOI] [PubMed] [Google Scholar]
- 18.Meerwaldt R, Graaff R, Links T, Baynes JW, Navis G, Huisman R, et al. Skin autofluorescence, a noninvasive measure of advanced glycation end product accumulation, is a predictor of mortality in hemodialysis patients. Ann N Y Acad Sci 2005;1043:911 [Google Scholar]
- 19.Valgimigli M, Merli E, Malagutti P, Soukhomovskaia O, Cicchitelli G, Macri G, et al. Endothelial dysfunction in acute and chronic coronary syndromes: evidence for a pathogenetic role of oxidative stress. Arch Biochem Biophys 2003;420:255–61. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi M, Tsutsui M, Tasaki H, Morishita T, Suda O, Nakata S, et al. Upregulation of vascular extracellular superoxide dismutase in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol 2004;24:106–11. [DOI] [PubMed] [Google Scholar]
- 21.Wang XL, Adachi T, Sim AS, Wilcken DEL. Plasma extracellular superoxide dismutase levels in an Australian population with coronary artery disease. Arterioscler Thromb Vasc Biol 1998;18: 1915–21. [DOI] [PubMed] [Google Scholar]
- 22.Vassalle C, Petrozzi L, Botto N, Andreassi MG, Zucchelli GC. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. J Intern Med 2004;256:308–15. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004;109:IV6–19. [DOI] [PubMed] [Google Scholar]
- 24.Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care 2001;24:1620. [DOI] [PubMed] [Google Scholar]
- 25.Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Birkeland KI, et al. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol 2005;25: 815–20. [DOI] [PubMed] [Google Scholar]
- 26.Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc Res 1998;37: 586–600. [DOI] [PubMed] [Google Scholar]
- 27.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation 2006; 113:1226–34. [DOI] [PubMed] [Google Scholar]
- 28.Forbes J, Yee L, Thallas V, Lassila M, Candido R, Jandeleit-Dahm K, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 2004;53:1813–23. [DOI] [PubMed] [Google Scholar]
- 29.Park L, Raman K, Lee K, Lu Y, Ferran L, Chow W, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4:1025–31. [DOI] [PubMed] [Google Scholar]
- 30.Wolffenbuttel BHR, Crijns FRL, Huijberts MSP, Swennen GNM, Boulanger CM, Poitevin P et al. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A 1998;95:4630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A 2002;99:15596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liuzzo G, Biasucci LM, Rebuzzi AG, Maseri A, Grillo RL, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med 1994;331:417–24. [DOI] [PubMed] [Google Scholar]
- 33.Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, et al. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther 2006;8:523–35. [DOI] [PubMed] [Google Scholar]
- 34.Odetti P, Pronzato MA, Noberasco G, Cosso L, Traverso N, Cottalasso D, et al. Relationships between glycation and oxidation related fluorescences in rat collagen during aging. An in vivo and in vitro study. Lab Invest 1994;70:61–7. [PubMed] [Google Scholar]