Abstract
Drug-eluting stents (DES) significantly reduce the risk of restenosis after percutaneous coronary revascularisation, but an increased risk of late stent thrombosis (LST) has been put forward as a major safety concern. Meta-analysis of clinical trials, however, does not support this caveat. Even so, many interventional cardiologists think that LST is associated with DES and related to delayed endothelialisation. This hypothesis is based on autopsy studies and clinical intracoronary angioscopy. In autopsy studies, differences between endothelialisation of DES and baremetal stents (BMS) have been reported. Most preclinical studies, however, have failed to show any significant differences in endothelialisation between DES and BMS. Our own studies, using the porcine coronary artery model, also suggest that DES show no differences in re-endothelialisation. However, DES do delay vascular healing and induce endothelial dysfunction. This paper will review clinical and animal studies which consider re-endothelialisation and LST. (Neth Heart J 2009;17:177-81.19421365)
Keywords: drug-eluting stents, stent thrombosis, endothelium, endothelial function
Polymer-based sirolimus (SES) and paclitaxel (PES) eluting coronary stents have reduced rates of restenosis and late lumen loss compared with bare-metal stents (BMS), resulting in a significant reduction in the need for target vessel revascularisation. 1,2 Delayed healing and more specifically delayed re-endothelialisation after drug-eluting stent (DES) implantation have been suggested as the cause of late stent thrombosis (LST, defined as thrombosis ≥30 days after stent deployment). 3 This concept is based on clinical autopsy studies and clinical intracoronary angioscopy studies.4,5 However we feel this concept is still open to debate. Our hypothesis is that delayed re-endothelialisation alone is not adequate to explain LST. The pathogenesis of LST is still not completely understood, and may be affected by a combination of factors. The present review discusses possible pathophysiological mechanisms of stent thrombosis in DES.
DES induced late stent thrombosis, clinical studies
Recent reports from randomised trials suggest that some DES may be associated with increased rates of LST as compared with BMS. Stone et al. reported that both SES and PES were associated with a significant increase in the incidence of LST between one and four years after implantation (0.6 and 0.7%), as compared with BMS (0.2%).6 Stettler and colleagues suggested that there is no evidence of an overall increase in definite stent thrombosis associated with SES between one and four years (0.3 vs. 0.2% in BMS).7 However, the risk of LST seemed to be increased with PES (0.6%) with a significantly increased hazard ratio (2.1, p=0.017 vs. BMS between day 0 and 4 years). Garg and colleagues reported a small increase in DES thrombosis compared with BMS after one year (>0.14%/year).8 This rate is, however, considerably lower than that reported by Daemen and Wenaweser and colleagues, who found that LST occurs in both DES at a steady rate of 0.5 % per year during follow-up up to four years, but in this report BMS were not included.9,10 Their overall results suggest that LST was slightly, but significantly more frequent with PES than with SES (cumulative incidence at four years of 3.6 vs. 2.7% respectively, p=0.02), but this was mainly due to a different outcome during the first year (2.0 vs. 1.3% respectively). This difference between SES and PES is concordant with the study by Stettler.7 SES seems to be clinically better than PES, but only during the first year. However, not all publications support this observation as Mauri (with a relatively small study) actually reports that within the first year BMS fare worse than SES (1.3 vs. 0.9% respectively) whereas PES and BMS are similar (0.9 vs. 0.8%).11 It seems clear that more clinical data with longer follow-up are needed to provide more consistent estimates of DES LST safety.
Angioscopy studies
Several clinical angioscopy studies have described delayed endothelialisation after DES implantation. Awata and colleagues showed that serial angioscopic findings up to two years after SES implantation were markedly different from those after BMS.5,12 Another study compared two different platforms of DES with angioscopy. In this study zotarolimuseluting stents (ZES) showed greater neointimal coverage grades than SES.13 We doubt whether angioscopy can really discriminate between complete and incomplete endothelial coverage. Data from experimental implantation of stents in the porcine coronary model show that stents that look non-covered as seen by macroscopy appeared completely covered by endothelium as assessed by scanning electron microscopy (figure 1). Available clinical intravascular imaging modalities such as angioscopy, optical coherence tomography and intravascular ultrasound (IVUS) are incapable of evaluating endothelialisation because the resolution of all these techniques is not sufficient to detect a thin layer of endothelium. In that respect, histology is a more powerful tool.
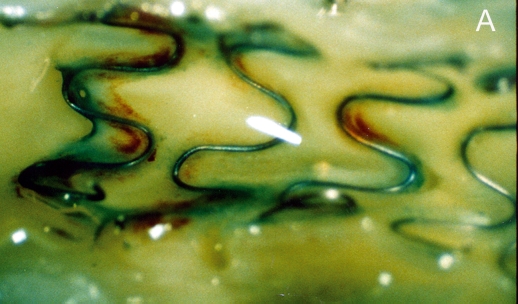
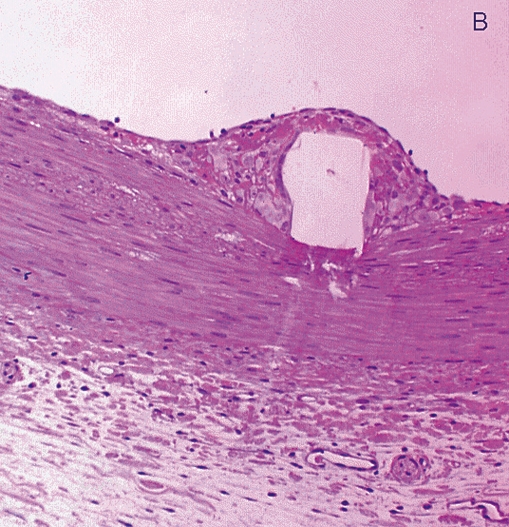
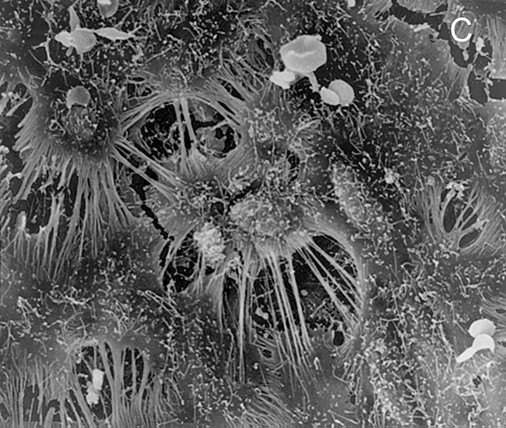
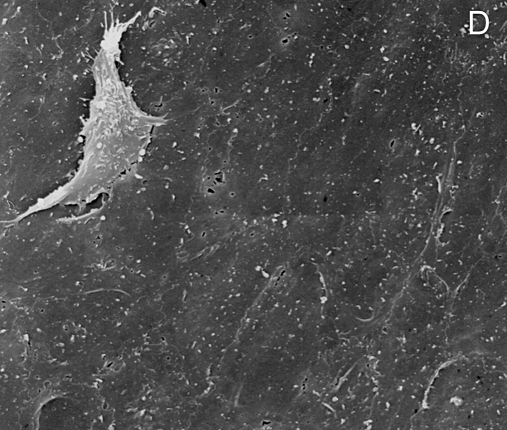
Figure 1 .
Stent struts have the macroscopic appearance of being not covered by tissue at five days after placement (panel A: angioscopic grade 0). Histology shows complete coverage by tissue (B), while scanning electron microscopy shows that the struts are covered by leaky endothelium (C) and that the vessels between the struts are completely endothelialised (D) (Modified from references 14 and 15).
Pathology of stent thrombosis
Postmortem analysis of human coronary artery stents demonstrated delayed endothelialisation after DES implantation, and thereby suggested a link between LST and delayed endothelialisation.4,16,17 Indeed, Finn and colleagues reported incomplete neointimal coverage of stent struts as the most important morphometric predictor of LST.4 However, the unexplained variation was high (1−r2=81.5%), and the results may not be representative of patients who receive DES and survive. Farb and colleagues reported several other procedural and pathological risk factors for LST in BMS such as stenting across branch ostia, disruption of adjacent vulnerable plaques and extensive plaque prolapse, indicating other parameters are needed to predict or explain LST.16 We studied the histology of in-stent restenosis tissue obtained by atherectomy from BMS and DES. In-stent restenosis in DES showed incomplete neointimal healing as late as two years after implantation. 18 PES showed more pronounced signs of delayed healing than SES. Since this was observed in atherectomy specimens endothelialisation could not be assessed, but delayed healing was demonstrated by other features such as neointimal organisation, cellular content and extracellular matrix build up. In addition, it should be noted that this was observed in patients without signs of DES thrombosis. Therefore, it seems clear that DES do affect the vasculature in more ways than only endothelial presence and coronary endothelial function may be important in the long-term clinical outcomes of patients after DES implantation.
DES-induced endothelial dysfunction
Acetylcholine acts as a potent vasodilator in normal coronary vessels by promoting the release of nitric oxide (NO) by the endothelium.19 However, acetylcholine can also cause vasoconstriction in vessel segments where the endothelial NO release is impaired or insufficient. This method has been accepted for evaluation of coronary endothelial function.20 Hofma and colleagues showed abnormal coronary vasoconstrictive responses to acetylcholine after SES implantation but not BMS at six months follow-up.21 Togni and colleagues evaluated coronary vasomotion with biplane quantitative coronary angiography at rest and during supine bicycle exercise in patients with coronary artery disease who were treated with PES, SES and BMS.22,23 Both DES were associated with paradoxic exercise-induced vasoconstriction of the adjacent vessel segments. Shin and colleagues also showed that SES and PES implantation were associated with endothelial dysfunction at six to nine months, predominantly in the distal segment of the treated vessel.24 However, the BMS group did not demonstrate a significantly different response. Data from animal studies also suggest that DES are associated with pronounced endothelial dysfunction as shown for instance by a decrease in eNOS expression in PES as compared with BMS.25 Lack of eNOS expression may also reduce the anticoagulant properties of the endothelium. A study by Li and colleagues studied vasomotor function of coronary segments distal to SES in a porcine coronary artery model at one month after implantation.26 Distal conduit arteries devoid of anydirect mechanical injury showed vasomotor dysfunction distal to SES, but not BMS.
Preclinical studies on endothelialisation
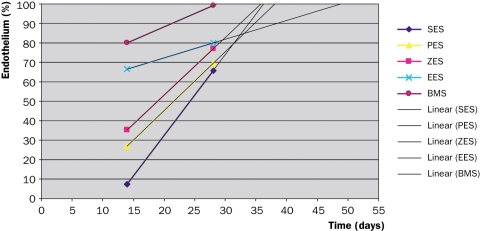
Porcine coronary arteries and rabbit iliac arteries are the most commonly used models to assess the vascular response to stent placement. DES has often yielded similar endothelialisation rates to BMS.27 Suzuki and colleagues reported similar endothelialisation rates between DES and BMS at 28 days in porcine coronary arteries.28 Also in a study by Klugherz using rabbit iliac arteries no evidence of delayed endothelialisation was noted with 28-day SES as compared with polymer only-coated stent or BMS.29 However, DES did reveal delayed endothelialisation and healing at segments where stents overlapped compared with BMS.30 Presumably this was the effect of high local drug concentrations. Importantly, overlap stenting has not been identified as a predictor of clinical LST, again putting the hypothesis of delayed endothelialisation up for discussion. In a recent paper, the same group of investigators compared endothelial coverage after implantation of four different DES (SES, PES, everolimus-eluting stent (EES) and ZES) for 14 and 28 days along with a BMS control in the rabbit iliac artery.31 While the endothelialisation ratio decreased in this study compared with overlap stenting, they now reported a significant disparity in endothelialisation at 14 days as compared with 28 days. EES and BMS demonstrated a greater endothelial coverage above struts, but remained poorest in SES, followed by PES and ZES. At 28 days, however, significant differences were no longer observed. Actually, SES showed the largest increase in endothelial regrowth as compared with all the others and especially everolimus. A forward projection of their data to predict the time point of complete endothelialisation (figure 2) shows that everolimus takes the longest (48 days) while zotarolimus, sirolimus and paclitaxel are actually all the same (36, 37 and 38 days, respectively). Considering the clinical evidence suggesting that PES is worse than SES, these data do not indicate that delayed endothelialisation is the culprit for LST. Alternatively, arterial healing seems to be multifactorial by combining both presence and function of cellular elements, which are also affected by stent design and drugrelease patterns.
Figure 2 .
Endothelialisation of drug-eluting stent (DES) in a rabbit iliac model of stenting. A forward projection of 14- and 28-day data suggests that both for bare metal stent (BMS) and most DES endothelialisation will be complete between 28 and 38 days (data derived from reference 29). ES=eluting stent, S=sirolimus, P=paclitaxel, Z=zotarolimus, E=everolimus
Conclusion
Despite the current concept about delayed endothelialisation after DES implantation, data from preclinical studies suggest that DES do not necessarily show differences in re-endothelialisation. Absence of thin layers of neointima and endothelial coverage cannot be ascertained by angioscopy. Arterial healing is multifactorial; endothelial dysfunction, inflammation, polymer coatings and drug release kinetics should also be considered in LST. Delayed endothelialisation could well be responsible for a pro-thrombogenic state in some but certainly not all patients.
References
- 1.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773–80. [DOI] [PubMed] [Google Scholar]
- 2.Park SJ, Shim WH, Ho DS, Raizner AE, Park SW, Hong MK, et al. A paclitaxel-eluting stent for the prevention of coronary stenosis. N Engl J Med 2003;348:1537–45. [DOI] [PubMed] [Google Scholar]
- 3.Collected_DES papers. Sections “perspectives”, “original articles” and “editorial”. N Engl J Med 2007;356:981–1039,1059–60.17296826 [Google Scholar]
- 4.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 2007;115:2435–41. [DOI] [PubMed] [Google Scholar]
- 5.Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006;47:2108–11. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins, KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007;356:998–1008. [DOI] [PubMed] [Google Scholar]
- 7.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 2007;370:937–48. [DOI] [PubMed] [Google Scholar]
- 8.Garg P, Cohen DJ, Gaziano T, Mauri L. Balancing the risks of restenosis and stent thrombosis in bare-metal versus drug-eluting stents: results of a decision analytic model. J Am Coll Cardiol 2008;51:1844–53. [DOI] [PubMed] [Google Scholar]
- 9.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large twoinstitutional cohort study. Lancet 2007;369:667–78. [DOI] [PubMed] [Google Scholar]
- 10.Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Jüni P, Vaina S, et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. J Am Coll Cardiol 2008;52:1134–40. [DOI] [PubMed] [Google Scholar]
- 11.Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007;356:1020–9. [DOI] [PubMed] [Google Scholar]
- 12.Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, et al. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation 2007;116:910–6. [DOI] [PubMed] [Google Scholar]
- 13.Awata M, Nanto S, Uematsu M, Morozumi T, Watanabe T, Onishi T, et al. Angioscopic comparison of neointimal coverage between zotarolimus and sirolimus-eluting stents. J Am Coll Cardiol 2008;52:787–92. [DOI] [PubMed] [Google Scholar]
- 14.van Beusekom HM, Whelan DM, Hofma SH, Krabbendam SC, van Hinsbergh VW, Verdouw PD, et al. Long-term endothelial dysfunction is more pronounced after stenting than after balloon angioplasty in porcine coronary arteries. J Am Coll Cardiol 1998;32:1109–17. [DOI] [PubMed] [Google Scholar]
- 15.Whelan DM, van der Giessen WJ, Krabbendam SC, van Vliet EA, Verdouw PD, Serruys PW, et al. Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart 2000;83:338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 2003;108: 1701–6. [DOI] [PubMed] [Google Scholar]
- 17.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 18.van Beusekom HM, Saia F, Zindler JD, Lemos PA, Swager-Ten Hoor SL, van Leeuwen MA, et al. Drug-eluting stents show delayed healing: paclitaxel more pronounced than sirolimus. Eur Heart J 2007;28:974–9. [DOI] [PubMed] [Google Scholar]
- 19.Takase B, Hamabe A, Satomura K, Akima T, Uehata A, Matsui T, et al. Comparable prognostic value of vasodilator response to acetylcholine in brachial and coronary arteries for predicting long-term cardiovascular events in suspected coronary artery disease. Circ J 2006;70:49–56. [DOI] [PubMed] [Google Scholar]
- 20.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–54. [DOI] [PubMed] [Google Scholar]
- 21.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J 2006;27:166–70. [DOI] [PubMed] [Google Scholar]
- 22.Togni M, Windecker S, Cocchia R, Wenaweser P, Cook S, Billinger M, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol 2005;46:231–6. [DOI] [PubMed] [Google Scholar]
- 23.Togni M, Räber L, Cocchia R, Wenaweser P, Cook S, Windecker S, et al. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int J Cardiol 2007;120:212–20. [DOI] [PubMed] [Google Scholar]
- 24.Shin DI, Kim PJ, Seung KB, Kim DB, Kim MJ, Chang K, et al. Drugeluting stent implantation could be associated with long-term coronary endothelial dysfunction: comparison between sirolimus-eluting stent and paclitaxel-eluting stent. Int Heart J 2007;48:553–67. [DOI] [PubMed] [Google Scholar]
- 25.Sorop O, Batenburg WW, Koopmans S-J, Dekker R, Duncker D, Danser A, et al. Taxus but not Cypher drug eluting stents induce endothelial dysfunction in the distal coronary microvasculature. Circulation 2007;116:II_293 [Google Scholar]
- 26.Li J, Jabara R, Pendyala L, Otsuka Y, Shinke T, Hou D, et al. Abnormal vasomotor function of porcine coronary arteries distal to sirolimus-eluting stents. J Am Coll Cardiol Intv 2008;1:279–85. [DOI] [PubMed] [Google Scholar]
- 27.Onuma Y, van Beusekom HM, Sorop O, van der Giessen WJ. The paradigm of endothelium and stent thrombosis in DES. EuroIntervention 2008;4: 17–21. [PubMed] [Google Scholar]
- 28.Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, et al. Stentbased delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 2001;104:1188–93. [DOI] [PubMed] [Google Scholar]
- 29.Klugherz BD, Llanos G, Lieuallen W, Kopia GA, Papandreou G, Narayan P et al. Twenty-eight-day efficacy and phamacokinetics of the sirolimuseluting stent. Coron Artery Dis 2002;13:183–8. [DOI] [PubMed] [Google Scholar]
- 30.Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005;112:270–8. [DOI] [PubMed] [Google Scholar]
- 31.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 2008;52:333–42. [DOI] [PubMed] [Google Scholar]