Abstract
Organotypic brain slice cultures are used for a variety of molecular, electrophysiological, and imaging studies. However, the existing culture methods are difficult or expensive to apply in studies requiring long-term recordings with multielectrode arrays (MEAs). In this work, a novel method to maintain organotypic cultures of rodent hippocampus for several weeks on standard MEAs in an unmodified tissue culture incubator is described. Polydimethylsiloxane (Sylgard) mini-wells were used to stabilize organotypic cultures on glass and MEA surfaces. Hippocampus slices were successfully maintained within PDMS mini-wells for multiple weeks, with preserved pyramidal layer organization, connectivity, and activity. MEAs were used to record the development of spontaneous activity in an organotypic cultures for four weeks. This method is compatible with integration of microchannels into the culture substrate. Microchannels were incorporated into the mini-wells and applied to the guidance of axons originating within the slice, paving the way for studies of axonal sprouting using organotypic slices.
Keywords: organotypic, hippocampus, multielectrode array, MEA, PDMS, soft lithography, microchannel, axon
1. Introduction
Organotypic brain slice cultures are used for a variety of molecular, electrophysiological, and imaging studies, and are usually prepared via the static culture on porous membranes (Yamamoto et al., 1989, Stoppini et al., 1991), or the roller-tube method (Gahwiler, 1981). Unfortunately, both of these methods have limitations on the types of culture substrates that can be used, due to the need to maintain a brain slice at the liquid-air interface to improve oxygen concentration in the tissue and avoid hypoxia and necrosis. In the method developed by Stoppini et al., this is accomplished by placing the slices on a nanoporous membrane with culture medium below; with only a thin sheath of medium covering the tissue and allowing ample oxygen flow to the cells. The roller-tube method calls for attaching a slice with either a blood clot or collagen gel to a glass cover slip, which is then placed in a roller-tube and rotated at slow speed to alternatively dip the slice into culture medium or bring it into the air.
Both of the existing methods are not directly compatible with the long-term use of organotypic cultures on substrates containing microfabricated structures such as substrate-integrated electrodes or microchannels for microenvironment control. Several groups have published methods for culturing organotypic cultures on multiple electrode arrays (MEAs), with MEAs fabricated on porous substrates to emulate the “Stoppini” method(Thiebaud et al., 1997, Kristensen et al., 2001), or with the MEAs rocked in the culture incubator to replicate the conditions of the roller-tube method(Egert et al., 1998, van Bergen et al., 2003). The first technique increases the difficulty of MEA fabrication, and consequently, its cost, while the latter methodology requires the use of specialized tissue culture equipment. An alternative is to culture the slices normally and transfer them to the MEA for the recording session(Bastrikova et al., 2008); however, the potential to use the MEA for chronic recordings is then lost.
In this work, a novel method to maintain organotypic cultures of rodent hippocampus for several weeks on stationary MEAs and glass surfaces is described. Polydimethylsiloxane (PDMS, sold as Sylgard 184, Dow Corning) was used to create mini-wells to stabilize the organotypic cultures on MEAs. PDMS is a material that is widely used for fabrication of micro-sized devices via a “soft lithography”(McDonald et al., 2000). In soft lithography, patterns with features as small as 1 μm or less are created on PDMS chips or films by pattern transfer from a pre-fabricated silicon wafer (see Figure 1, left, also Methods). This technique that holds the potential to significantly enhance the ability to create precisely controlled in vitro environments(El-Ali et al., 2006) for study of the neural circuit physiology. Soft lithography-defined stencils can be used to pattern and separate various cell types on the same culture substrate at micro-scale dimensions while allowing the investigator to define cell-to-cell interactions(Whitesides et al., 2001). In addition, this technology allows the delivery of fluids to cultured cells in a multiplexed and spatially defined manner(King et al., 2008). Soft lithography-based microfluidic technology has been increasingly applied to neuroscience(Pearce and Williams, 2007), with investigators focusing on generating molecular gradients to direct cell proliferation and differentiation(Chung et al., 2005), and axon growth(Joanne Wang et al., 2008, Li et al., 2008). Microchannels have also been used for separating neuronal soma into isolated “Campenot”-type(Campenot, 1977) compartments interconnected by microchannel-confined axons(Taylor et al., 2005) to study the relationship between cell and circuit morphology and neural activity, detected by substrate-integrated multiple electrode arrays(Claverol-Tinture et al., 2005, Morin et al., 2006, Ravula et al., 2006). While various cell types ranging from neural cell lines to primary neurons to neural stem cells have been placed in microfluidic devices, the microfluidic applications have, to date, been confined to dissociated cells.
Figure 1.

Left: fabrication sequence for creating PDMS mini-wells. Top right: schematic representation of the hippocampus slice in a PDMS mini-well. Bottom right, micrograph of an organotypic hippocampus slice in a mini-well, 7 DIV
To demonstrate the compatibility of the organotypic culture technique described here with “soft lithography”, microchannels were imprinted onto the PDMS mini-wells to provide physical guidance cues to the axons extended by the organotypic slice. The technique described here can serve a number of potential applications, in particular the selective fluid delivery to the organotypic slice or extended processes for studies of axonal sprouting, and long-term studies of the developing neural activity in the compartmented slices and extended axons with multiple electrode arrays.
2. Methods
2.1 Fabrication of Sylgard culture wells and microchannels
Cross-linked polydimethylsiloxane (PDMS, sold as Sylgard 184 by Dow Corning) was used to fabricate slice culture wells patterned with microchannels via modified “soft lithography” technique. As the first step in the fabrication sequence (Figure 1, left panel), silicon mold masters were prepared by defining a negative relief of microchannel pattern via SU-8 (5) photolithography (according to manufacturer supplied protocol, Microchem) on a 4″ diameter silicon wafer (Silicon Quest International, Inc). SU-8 patterns of 5 μm height, 50 to 100 μm width, and 100 to 200 μm center-to-center spacing (corresponding to microchannel depth, width, and spacing, respectively) were fabricated.
Liquid PDMS was spin-coated onto the silicon master at 500 rpm, and cured (cross-linked) at 70° C for at least 4 hours. The resulting flexible 150 μm Sylgard membrane bearing imprinted microchannels was removed from the silicon wafer. Mini-wells of 2 mm diameter for hippocampus slice placement, with a large channel for culture medium access were then cut from the membrane.
Glass coverslips (25 mm diameter circles, ESCO cover glass, Erie Scientific Company), to be used as culture substrates, were cleaned in 0.5 M NaOH for at least 1 hour, sterilized in hydrogen peroxide, washed in three changes of sterile-filtered deionized water, and dried. 200 μl of 1 mg/ml sterile solution of poly-D-lysine (Sigma) was deposited onto each coverslip and carefully spread to cover the entire surface. The cover slips were incubated in polylysine in humidified atmosphere at 37 °C overnight, washed in three changes of sterile deionized water, and dried. Some coverslips were prepared as above, but without the polylysine coating step, to act as controls.
Pre-cut Sylgard mini-wells were sterilized in 100% ethanol, dried, and sealed against a polylysine-coated glass coverslip. The watertight seal between Sylgard and glass forms spontaneously on contact, provided that both surfaces are thoroughly cleaned, and stays stable in aqueous solutions for several weeks. The mini-well bearing coverslips were then placed in a standard 6-well tissue culture plate, immersed in 2 ml/well of serum-free medium (see below), and incubated at 37 °C overnight to allow medium to fill the microchannels and remove any soluble impurities from PDMS.
2.2. Organotypic cultures
Hippocampal slices of 350 μm thickness were dissected from postnatal day 3 and 4 mouse and rat pups (FVB mice, Charles River Laboratories, and Spague-Dawley rats, Harlan) and placed into PDMS mini-wells on glass cover slips (Figure 1, top right panel) into a 6-well plate. The plate was then filled with just enough serum-containing (1:1:2 horse serum, Hanks' Balanced Salt Solution, and Basal Medium Eagle, supplemented with 0.5 mM glutamine and 30 μg/ml gentamicin, all from Invitrogen) medium to cover the bottom of the well, and incubated in a humidified 5% CO2 incubator. Serum-free medium (Neurobasal A/B27, with 0.5 mM glutamine and 30 μg/ml gentamicin, all from Invitrogen) was substituted in on the third day of organotypic culture, and was used in all subsequent medium changes (every 3 days).
2.3. Electrophysiology
2.3.1 Glass electrode recordings
Individual slices were removed from PDMS mini-wells for electrophysiology on day 10 of culture, and transferred to the interface-type recording chamber perfused with modified artificial cerebrospinal fluid (120 mM NaCl, 3.3 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 1.3 mM CaCl2, 0.9 mM MgCl2, and 10 mM glucose) at 32-34°C and bubbled with 95%O2/5% CO2. Bipolar stimulating electrodes (twisted insulated tungsten wires) and 1 MΩ recording electrodes filled with the recording solution were used for testing the connectivity between dentate gyrus, CA1, and CA3 in the organotypic slices. For CA1 and CA3 responses, the recording electrode was placed in the pyramidal layer, and stimulating electrode in the granule layer of DG and pyramidal layer of CA1, respectively, aided by a stereo microscope (Olympus). Responses were evoked with single biphasic current pulses (200-400 μA) of 100 μs duration.
2.3.1 Multiple Electrode Array (MEA) long-term recording
MEAs were fabricated on glass substrates (Fisher Scientific), with SU-8 insulated gold microelectrodes of 30 μm diameter and 200 μm center-to-center spacing, similar to the commercially available electrode arrays(Oka et al., 1999). A 25 mm diameter polycarbonate well was used to insulate the connector pads from the center area of the array. The electrode array was then sterilized with hydrogen peroxide, washed in three changes of sterile deionized water, and allowed to dry. PDMS mini-well was centered with the electrode-containing area and sealed against the MEA surface. Slice culture was carried out as described above. For recording, the culture medium was replaced with modified artificial cerebrospinal fluid (120 mM NaCl, 3.3 mM KCl, 1.25 mM NaH2PO4, 10 mM HEPES, 1.3 mM CaCl2, 0.9 mM MgCl2, and 10 mM glucose), and the MEA, maintained at 32-34°C and atmospheric oxygen/CO2, was connected to an amplifier (EX4-400, Dagan Corporation) fitted with high-impedance preamplifier stage(4002, Dagan Corporation). Upon completion of a recording session, the MEA was re-filled with serum-free culture medium and returned to the tissue culture incubator.
2.4. Morphology
For histological analysis, organotypic slices were fixed in 4% paraformaldehyde for at least 1 hr, removed from the culture substrate, and sectioned with a cryostat at 20 μm. The resulting sections were Nissl-stained with Cresyl Violet (Sigma), cleared, and mounted for microscopy.
Viability staining of axon outgrowth was carried out with calcein indicator, per manufacturer's specifications (Live/Dead viability kit, Invitrogen), and visualized with an epifluorescent microscope (Carl Zeiss).
3. Results
3.1 Viability of organotypic slice cultures in PDMS mini-wells
Viable cultures could be maintained for at least four weeks in Sylgard mini-wells. The hydrophobicity of PDMS combined with the surface tension of the medium to limit the amount of liquid covering the brain slices to a very thin layer. This property permitted sufficient oxygen diffusion from the atmosphere into the slice, as evidenced by the lack of necrosis. The PDMS wells served to hold the slices in place for the duration of the culture period, while the nutrient supply to the slices was ensured by a small opening in the well that connects to the medium filling the culture dish outside the device. Hippocampal slices maintained under these conditions preserved their well-defined pyramidal and granule cell layers (Figure 2 A, results typical for 6 slices which were histologically analyzed), and displayed strong functional connectivity in the tri-synaptic pathway with preserved afferent connection from dentate gyrus to CA1 (Figure 2B, left) and CA1 to CA3 (Figure 2B, right), with 3 slices tested. These slices also exhibit spontaneous activity characteristic of hippocampal organotypic slices from postnatal day 4 rodents (Figure 2C,D)(Mohajerani and Cherubini, 2005).
Figure 2.
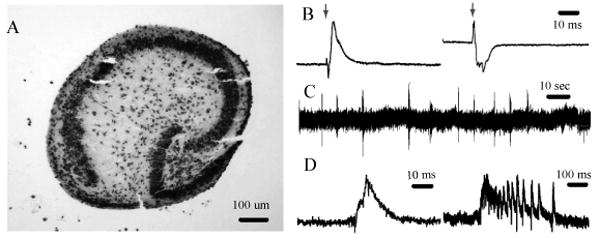
A: Nissl-stained 20 μm cryosection of DIV 7 hippocampus slice. B: Evoked activity, arrow shows the stimulus artifact, left: stimulus electrode in DG, recording electrode in CA3, right: stimulus electrode in CA3, recording electrode in CA1. C: spontaneous activity in CA1, D: short and long spontaneous bursts in CA1. Recordings in B-D were carried out with glass electrodes.
3.2 Axon extension in microchannels
The attachment of the slice to the glass surface, and the extension of axons outside the perimeter of the organotypic slice were found to be strongly dependent on the surface coating. For instance, the slices in right bottom panel of Figure 1, and in Figure 3A, representing typical results, were cultured on polylysine-free and polylysine-coated glass surfaces, respectively, in otherwise identical culture conditions. The slice cultured on uncoated glass exhibited very little neurite outgrowth by the end of first week in culture, whereas the slice cultured on polylysine coated coverslip has significant neurite extension from subiculum, CA1, and CA3 by the third day in vitro (3 DIV). Surface coating played a role in the development of slice morphology in vitro as well. On uncoated substrates, slices tended to contract after the first week in culture, and remain at least 150 μm thick. On polylysine-coated substates, slices spread out and thinned out to a depth of a 3 or 4 cell layers after the first week. Slices cultured on uncoated glass were easier to remove from the coverslip for electrophysiology and histology than slices cultured on polylysine-coated glass, signifying a stronger attachment of the slice to the coated surface. The coating did not have a noticeable effect on slice viability, however, as slices were successfully maintained on both surface types for at least four weeks.
Figure 3.
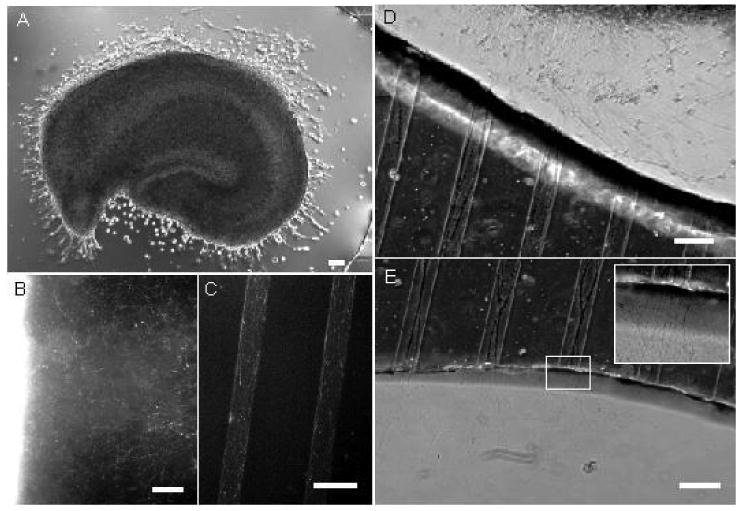
A: Hippocampus slice with neurite extension at 3 DIV, cultured on a polylysine-coated surface. B: calcein staining of neurite outgrowth from the slice (bright region on the left), 7 DIV, C: calcein staining of microchannel-guided axons, 7 DIV, D: axons extended by a hippocampus slice enter the microchannels, 10 DIV, E: axons span the length of the microchannels and emerge on the other side of the PDMS mini-well, 10 DIV.
By 7 DIV, slices placed on polylysine-coated substrate were surrounded by a “halo” of extended neurites, visualized in Figure 3B with a fluorescent viability dye. The neurites are likely to be both axons and dendrites, although the outgrowth that is longer than 1 mm is primarily composed of axons. When axons reach the border of the PDMS mini-well and encounter the opening of the microchannels, they align with the microchannel walls (Figure 3D). The microchannels exert a guidance effect on the axons (Figure 3C), similar to results published by groups working with dissociated neurons(Claverol-Tinture et al., 2005, Taylor et al., 2005), making it possible for axonal outgrowth to span the entire length of a microchannel (> 500 μm) and emerge on the other side of the PDMS compartment by DIV 7-10 (Figure 3E). The channel guidance effect was strongly affected by channel depth, with largely aligned axons in microchannels 5 μm deep, and more randomly oriented axons in 15 μm deep microchannels.
3.3 Long-term recording with substrate-integrated MEA
The PDMS mini-wells placed on the surface of the MEA imparted long-term stability to MEA-based recordings of spontaneous activity in the organotypic slice. The mini-wells prevented the mechanical disturbance of the slice cultured on MEA surface during the medium changes and recording sessions. Spontaneous bursts were recorded with the same electrode over the period of 28 DIV (Figure 4). Same activity pattern, but with smaller burst magnitude, could be detected with other electrodes located directly under the slice. It was found that perfusion was not necessary for the spontaneous activity to occur, provided that the recording medium level was kept low enough to maintain the slice at air-liquid interface.
Figure 4.
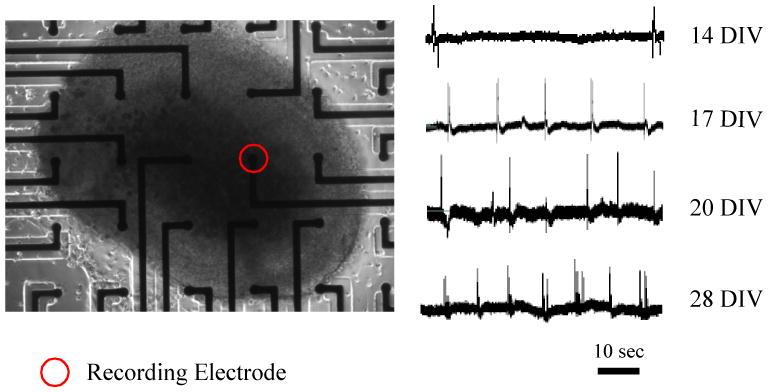
Left: hippocampus organotypic slice (20 DIV) on the MEA, Right: spontaneous population bursts recorded at different time points with the same recording electrode.
4. Discussion
The organotypic culture platform described here is modular, consisting of three components: PDMS mini-well for slice culture on stationary, solid substrate, microchannels that are integrated into the PDMS well for axon guidance, and the multiple electrode array integrated into the culture substrate. Different combinations of modules can be used for a variety of experiments; for example, PDMS mini-well can be used on a glass coverslip to replace the interface method of organotypic slice culture in experiments where improved visualization of live slices with an inverted microscope is desired. For studies of axonal sprouting out of the slice, microchannels of different geometries can be fabricated into the PDMS mini-well to provide axon guidance or to allow the delivery of precisely controlled gradients of growth factors. Several mini-wells can be interconnected by microchannels to create “Campenot”-type(Campenot, 1977) compartmented co-cultures of two or more slices, where individual slice environments (ionic composition of medium, presence of growth factors) can be independently controlled. Finally, a commercially available MEA can be used instead of the glass cover slip as the substrate for the PDMS mini-well for long-term electrophysiology experiments on compartmented slice cultures.
The PDMS mini-well is critical in order to enable the use of soft-lithography based microchannels and microfluidics with the organotypic slice cultures. In order to provide the optimal conditions for the organotypic slice survival, the geometry of the PDMS mini-well was designed to both position the slice at the medium/air interface and immobilize the slice on the culture substrate. The substrate coating, while sufficient to provide good adhesion for the layer of cells on the slice surface, is incapable of permanently anchoring the slice to the substrate. In early experiments, it was found that when the slice was submerged in the culture medium, which frequently happened during medium changes, it would detach from the substrate surface, leaving a layer of superficial cells and extracellular matrix behind. The shear force of the fluid moving across the slice proved to be greater than the force between adhesion molecules and the neural cell layers. Therefore, it was necessary to design the culture substrate to prevent fluid movement around the slice at any point during tissue culture. The PDMS mini-well almost completely surrounds the organotypic slice, shielding it from the medium motion and fluctuations in the larger well, while allowing the diffusion of necessary nutrients through the access channel. This property proved vital for enabling stable, long-term recording of neural activity in the slice with the MEA, and for allowing the axons to emerge from the borders of the slice and attach to the substrate without mechanical disturbance.
Neurite outgrowth beyond the borders of the organotypic slice was previously reported on a nanoporous membrane coated with collagen or laminin(Molnar and Blakemore, 1999). In the course of this study, it was found that the axon extension outside the borders of the hippocampal slice readily occurs in serum-free medium on a substrate that is coated with positively charged polyelectrolyte such as polylysine. Therefore, it can be inferred that the axons are extended spontaneously by neurons within the slice, likely as a response to the elimination of a portion of their axonal projection during dissection, but the appearance of axons outside of the borders of the slice depends on the presence of axonal growth-permissive surface.
The axons in microchannels aligned along the direction of the channel (Figure 3), suggesting that the channel walls exerted a guidance effect on the growth cone. The effect was more pronounced for shallower channel geometries. The mechanism by which channel depth affects axons was not immediately apparent, but probably stems from increased surface effects in more confined geometries.
5. Conclusions
The culture method described here allowed the first use of microchannels with organotypic slices. Hippocampus slices were successfully maintained within PDMS mini-wells for multiple weeks, with preserved pyramidal layer organization, connectivity, and activity. Mini-wells were imprinted with a microfluidic channel network, demonstrating how microfluidic devices can be integrated into the organotypic culture platform. Microchannels were used for guidance of axons originating within the slice, paving the way for the use of microdevices for studies of axonal sprouting from slices. MEAs were used to record from slices cultured in PDMS mini-wells to show how the development of neural activity in compartmented organotypic cultures can be monitored over several weeks.
Acknowledgments
This work was supported in part by NIH P41EB002503. Y.B. was supported by NIH F32 MH079662.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- Claverol-Tinture E, Ghirardi M, Fiumara F, Rosell X, Cabestany J. Multielectrode arrays with elastomeric microstructured overlays for extracellular recordings from patterned neurons. J Neural Eng. 2005;2:L1–7. doi: 10.1088/1741-2560/2/2/L01. [DOI] [PubMed] [Google Scholar]
- Egert U, Schlosshauer B, Fennrich S, Nisch W, Fejtl M, Knott T, Muller T, Hammerle H. A novel organotypic long-term culture of the rat hippocampus on substrate-integrated multielectrode arrays. Brain Res Brain Res Protoc. 1998;2:229–242. doi: 10.1016/s1385-299x(98)00013-0. [DOI] [PubMed] [Google Scholar]
- El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Thompson E, Audinat E, Robertson RT. Organotypic slice cultures of neural tissue. In: Banker G, Goslin K, editors. Culturing Nerve Cells. MIT Press; Cambridge: 1998. pp. 379–411. [Google Scholar]
- Joanne Wang C, Li X, Lin B, Shim S, Ming GL, Levchenko A. A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound ECM molecules and diffusible guidance cues. Lab Chip. 2008;8:227–237. doi: 10.1039/b713945d. [DOI] [PubMed] [Google Scholar]
- King KR, Wang S, Jayaraman A, Yarmush ML, Toner M. Microfluidic flow-encoded switching for parallel control of dynamic cellular microenvironments. Lab Chip. 2008;8:107–116. doi: 10.1039/b716962k. [DOI] [PubMed] [Google Scholar]
- Kristensen BW, Noraberg J, Thiebaud P, Koudelka-Hep M, Zimmer J. Biocompatibility of silicon-based arrays of electrodes coupled to organotypic hippocampal brain slice cultures. Brain Res. 2001;896:1–17. doi: 10.1016/s0006-8993(00)03304-7. [DOI] [PubMed] [Google Scholar]
- Li GN, Liu J, Hoffman-Kim D. Multi-molecular gradients of permissive and inhibitory cues direct neurite outgrowth. Ann Biomed Eng. 2008;36:889–904. doi: 10.1007/s10439-008-9486-z. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mohajerani MH, Cherubini E. Spontaneous recurrent network activity in organotypic rat hippocampal slices. Eur J Neurosci. 2005;22:107–118. doi: 10.1111/j.1460-9568.2005.04198.x. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. Development of signals influencing the growth and termination of thalamocortical axons in organotypic culture. Exp Neurol. 1999;156:363–393. doi: 10.1006/exnr.1999.7032. [DOI] [PubMed] [Google Scholar]
- Morin F, Nishimura N, Griscom L, Lepioufle B, Fujita H, Takamura Y, Tamiya E. Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: a step towards neuron-based functional chips. Biosens Bioelectron. 2006;21:1093–1100. doi: 10.1016/j.bios.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Oka H, Shimono K, Ogawa R, Sugihara H, Taketani M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods. 1999;93:61–67. doi: 10.1016/s0165-0270(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Pearce TM, Williams JC. Microtechnology: meet neurobiology. Lab Chip. 2007;7:30–40. doi: 10.1039/b612856b. [DOI] [PubMed] [Google Scholar]
- Ravula SK, McClain MA, Wang MS, Glass JD, Frazier AB. A multielectrode microcompartment culture platform for studying signal transduction in the nervous system. Lab Chip. 2006;6:1530–1536. doi: 10.1039/b612684g. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud P, de Rooij NF, Koudelka-Hep M, Stoppini L. Microelectrode arrays for electrophysiological monitoring of hippocampal organotypic slice cultures. IEEE Trans Biomed Eng. 1997;44:1159–1163. doi: 10.1109/10.641344. [DOI] [PubMed] [Google Scholar]
- van Bergen A, Papanikolaou T, Schuker A, Moller A, Schlosshauer B. Long-term stimulation of mouse hippocampal slice culture on microelectrode array. Brain Res Brain Res Protoc. 2003;11:123–133. doi: 10.1016/s1385-299x(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989;245:192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]


