Abstract
BACKGROUND
Understanding the excretion of 3,4-methylenedioxymethamphetamine (MDMA) and metabolites in sweat is vital for interpretation of sweat tests in drug treatment, criminal justice, and workplace programs.
METHODS
Placebo, low (1.0 mg/kg), and high (1.6 mg/kg) doses of oral MDMA were given double-blind in random order to healthy volunteers (n = 15) with histories of MDMA use. Participants resided on the closed clinical research unit for up to 7 days after each dose. Volunteers wore PharmChek® sweat patches (n = 640) before, during, and after controlled dosing. Patches were analyzed by solid phase extraction and GC-MS for MDMA, methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxyamphetamine (HMA), and 4-hydroxy-3-methoxymethamphetamine (HMMA). Limits of quantification (LOQ) were 2.5 ng/patch for MDMA and 5 ng/patch for HMA, HMMA, and MDA.
RESULTS
MDMA was the primary analyte detected in 382 patches (59.7%), with concentrations up to 3007 ng/patch. MDA was detected in 188 patches (29.4%) at <172 ng/patch, whereas no HMMA or HMA was detected; 224 patches (35.0%) and 60 patches (9.4%) were positive for MDMA and MDA, respectively, at the 25-ng/patch threshold proposed by the Substance Abuse and Mental Health Services Administration.
CONCLUSIONS
Sweat testing was shown to be an effective and reliable method for monitoring MDMA use in this controlled MDMA administration study. However, variability in sweat excretion suggests that results should be interpreted qualitatively rather than quantitatively. These data provide a scientific database for interpretation of MDMA sweat test results.
3,4-Methylenedioxymethamphetamine (MDMA)5 and other sympathomimetic amines including 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxyethylamphetamine (MDEA), methamphetamine, and amphetamine are popular recreational drugs in the US, Europe, and Australia (1-3). Hallucinogenic effects begin as early as 20 min after consumption and last 4−6 h (4-6). MDMA produces feelings of euphoria, intimacy, and diminished anxiety and fear. Adverse effects include dry mouth, tachycardia, depression, paranoia, involuntary teeth clenching, nausea, and tremors (4-13). In addition, no clear consensus has been reached on human neurotoxicity in relation to effects on the dopaminergic and serotonergic systems have yet to be fully resolved (14-16).
Drug monitoring is important in forensic toxicology for workplace drug testing, criminal justice, drug abuse treatment, and sport doping control programs. Recently, alternate biological matrices including sweat have been investigated to monitor illicit drug use (17-23). The sweat patch consists of an absorbent pad covered by a protective membrane similar to a bandage. Before application, the area is swabbed with isopropyl alcohol, and then the patch is affixed to the skin, usually on the upper arm, the abdomen, or the back, and typically worn for 1 week to collect drugs excreted in perspiration. Sweat testing has several advantages over blood and urine analyses, including less invasive and safer specimen collection, reduced potential for adulteration, facilitation of acquisition of a cumulative drug exposure record, and in some situations, longer detection windows than plasma or urine. A positive sweat test indicates drug use shortly before or during patch wear. Sweat testing disadvantages include a single extraction opportunity, low analyte concentrations, external contamination potential, and large variations in sweat production. Environmental factors such as temperature, exercise, and stress influence the amount of excreted sweat, and also drug concentrations in sweat. Sweat drug detection was proposed in the Substance Abuse Mental Health Services Administration's (SAMHSA) Mandatory Guidelines for Federal Workplace Drug Testing Program in 2004 (24). Proposed SAMHSA requirements for a positive sweat test include a positive amphetamines screen at ≥25 ng/patch and a positive confirmation of MDMA, MDA, MDEA, methamphetamine, and/or amphetamine at 25 ng/patch. Sweat testing for MDMA can serve as a deterrent to drug use in drug abuse treatment, law enforcement, military, and workplace drug testing programs.
Although sympathomimetic amines have been reported to be present in human perspiration (25), little is known about the time course of MDMA and metabolite disposition in sweat (26-28). In a controlled oral administration experiment, Samyn et al. (29) administered 75 mg of MDMA to 12 healthy recreational MDMA users. Pharmacokinetic data were obtained for traditional body fluids (blood and urine), and alternative matrices such as oral fluid and sweat wipes, collected during the first 5 h after administration. Mean MDMA concentrations for 5 h after ingestion did not exceed 25 ng/wipe.
Pichini et al. (28) administered a single oral dose of 100 mg MDMA to 9 individuals and detected parent drug in sweat patches after only 1.5 h, with peak concentrations at 24 h. There was large intersubject variability, with peak concentrations ranging from 3.2−1326 ng/patch. In addition, trace amounts of MDA were reported in the sweat of 7 of 9 individuals. Kintz and Samyn (27) also documented the presence of MDMA, MDA, and MDEA in sweat patches worn by 4 “techno ravers” for 28 h during a rave party. MDMA was always present in higher concentration (138−431 ng/patch) than MDA (13−56 ng/patch). MDEA detection suggests impurities in the ingested MDMA or polydrug use.
Controlled drug administration data are needed to accurately interpret sweat test results. In this randomized, within-subject, double-blind, placebo-controlled, multiple-dose MDMA study, we examined sweat from 15 individuals before, during, and after oral MDMA administration. Times of first detection, duration of excretion, and peak MDMA and metabolite concentrations were determined by GC-MS. Data were analyzed with the assay's limit of quantification and the proposed SAMHSA guidelines for sweat testing. This is the first study that evaluated MDMA detection in sweat after controlled administration of multiple doses over periods longer than 24 h.
Materials and Methods
STUDY POPULATION
Fifteen adult MDMA users (11 African American, 3 white, 1 Hispanic; 10 men, 5 women; mean (SD) age 21.1 (2.2) years, range 18−26 years) were recruited to participate in this MDMA administration study (6, 30). All study participants provided written informed consent to participate, and the study protocol was approved by the National Institute on Drug Abuse Institutional Review Board. Current good health of study participants was confirmed by a thorough medical and psychological evaluation. History of MDMA exposure was verified by a positive amphetamine urine test or MDMA hair test. Throughout the study (up to 23 days), participants resided on the secure clinical research unit under 24-h medical surveillance to ensure safety and to prevent additional drug use.
DRUG ADMINISTRATION
Placebo, low (1.0 mg/kg), and high (1.6 mg/kg) doses of oral MDMA were given double blind in random order while subjects resided on a closed clinical research unit for at least 7 days after each MDMA dose; 4 participants stayed on the unit for 23 consecutive days. For safety purposes, the maximum MDMA dose was limited to 150 mg. MDMA was a 50:50 racemic mixture of d,l-MDMA HCl, synthesized by Lipomed. Identical placebo capsules contained only lactose filler.
SWEAT COLLECTION
Sweat was collected with PharmChek® sweat patches generously provided by PharmChem (Haltom City, TX). The sweat patch consists of a rectangular, absorbent, cellulose pad attached to an adhesive polyurethane backing. The adhesive membrane allows oxygen, carbon dioxide, and water vapor to escape while non-volatile constituents in sweat accumulate in the absorbent pad. The skin was thoroughly cleaned with 70% isopropyl alcohol to remove external contamination and improve patch adherence. Patches were applied to the participant's back and/or abdomen and worn for intervals ranging from 2.5 h to 1 week. A maximum of 5 patches were worn at one time.
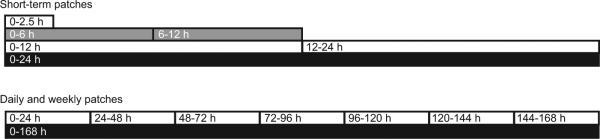
Four types of patches were used: washout patches, patches worn for 12 h or less (short-term patches), daily patches, and weekly patches. Washout patches were applied on admission and removed within 65 h of dosing to detect previously self-administered drugs. Short-term patches were applied before drug administration and worn ≤12 h (0−2.5, 0−6, 6−12, 0−12, or 12−24 h). These patches were applied to evaluate when drugs could first be detected, and for comparisons with patches worn for longer periods of time (Fig. 1). Daily patches consisted of 7 consecutive 24-h patches worn during and after dosing (0−24, 24−48, 48−72, 72−96, 96−120, 120−144, and 144−168 h). Duplicate weekly patches were applied before drug administration and removed 7 days later (0−168 h). Patches were stored at −20 °C until analysis. Sixteen (2.4%) of 674 sweat patches did not adhere throughout the wear period; 5 were weekly patches. An additional 18 patches (2.7%) were unavailable for analysis owing to clinical, administrative, or technical issues.
Fig. 1. The sweat patch application and removal schedule for short-term, daily, and weekly patches worn during MDMA administration.
Short-term patches were applied before drug administration and worn ≤12 h. Daily patches consisted of 7 consecutive 24 h patches worn during and after dosing, and weekly patches were applied before drug administration and removed 7 days later.
SWEAT PATCH ANALYSIS
Specimens were analyzed for MDMA, MDA, MDEA, 4-hydroxy-3-methoxyamphetamine (HMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) by solid-phase extraction and GC-MS procedures as described by DeMartinis et al. (19). Although MDEA was not an expected metabolite of MDMA, we tested washout patches for MDEA to monitor potential concentrations found following self-administered MDEA. Additionally, testing for MDEA after controlled MDMA administration would empirically document that MDEA was not produced from, or found in, pharmaceutical-grade MDMA and was not produced as an analytical artifact by the method.
Briefly, sweat patches (fortified calibrators, quality control (QC) samples, or clinical specimens) were folded, placed into screw-top vials with 3 mL of 0.2 mol/L sodium acetate buffer (pH 5.0), deuterated internal standards added, and tubes shaken for 30 min. Then 1 mL of buffered extract was applied to preconditioned SPEC MP1 (10 mL/70 mg) columns, obtained from Varian. The columns were washed sequentially with 500 μL of distilled water, 250 μL 0.1 mol/L acetic acid, and 400 μL of methanol and then dried under full vacuum for 5 min. Analytes of interest were eluted into clean 5-mL disposable glass centrifuge tubes with two 1-mL aliquots of freshly prepared elution solvent (ethyl acetate/methanol:ammonium hydroxide 78:20:2, vol/vol/vol); 15 μL of 1% hydrochloric acid in methanol (vol/vol) was added before vortex mixing and evaporating under nitrogen. Dried extracts were reconstituted with 100 μL 0.10 mol/L triethylamine in heptane and 10 μL heptafluorobutyric acid anhydride. The tubes were capped, vortex-mixed, and incubated at 60 °C for 20 min. After cooling, 200 μL 0.05 mol/L Tris buffer (pH 7.4) was added, the tubes were vortex mixed for 2 min, and centrifuged at room temperature. The organic layers were then transferred for GC-MS analysis on an Agilent 6890 gas chromatograph interfaced with an Agilent 5973 mass-selective detector. Intraand interassay imprecision was <7.2%, and mean percent recovery from sweat patches was between 85% and 112%. Quantification was performed in the selectedion monitoring mode by monitoring 3 ions for each analyte and 2 ions for each internal standard (quantitative ions in parentheses): MDA-d0 135, (162), 375; MDA-d5 (167), 380; HMA-d0 163, (240), 360; MDMA-d0 162, 210, (254); MDMA-d5 213, (258); HMMA-d0 210, (254), 360; MDEA-d0 162, 240, (268) and MDEA-d6 244, (274).
CALIBRATORS AND QC
Working calibration solutions of MDMA, MDA, MDEA, HMMA, and HMA at 100, 10, 1, and 0.1 mg/L were prepared and stored at −20 °C. Blank sweat patches were premoistened with artificial sweat (31) and fortified with calibrator solutions to create daily calibration curves from 2.5−10000 ng/patch. QC solutions at the same concentrations were prepared in methanol with different ampules of reference standards than were used for preparing calibration standards. QC sweat patches were prepared at 7.5, 75, 300, 750, 3000, and 6000 ng/patch.
Deuterated MDMA, MDA, and MDEA were combined and diluted in methanol to produce a working internal standard solution at 1 mg/L. In the absence of commercially available stable isotopes for HMA and HMMA, MDA-d5 and MDMA-d5 were employed as internal standards for HMA and HMMA, respectively.
DATA ANALYSIS
For each analytical batch, 2 calibration curves were constructed for each analyte. Drugs were quantified by linear regression with a 1/x weighting factor. Low-concentration calibration curves were constructed with 2.5−500 ng/patch for MDMA and MDEA, 5−500 ng/patch for MDA, and 5−100 ng/patch for HMA and HMMA. High concentration curves at 500−10 000 ng/patch for MDA, MDMA, and MDEA and a smaller dynamic range of 100−2500 ng/patch were prepared for HMA and HMMA. Coefficients of determination (R2) for all curves were >0.990. Analytes were identified by comparing retention times (±0.15 min) and qualifier ion ratios (±20%) to corresponding mean values of calibrators assayed in the same batch. Peak abundance ratios of analytes to corresponding internal standards were calculated for each concentration. Calibrator concentrations calculated against the full calibration curve were required to be within 20% of target.
Results
OVERALL SWEAT-PATCH RESULTS
Of 559 sweat patches collected during and after controlled oral MDMA administration, 370 contained 1 or more MDMA analytes (Table 1). Parent MDMA was the primary analyte, with no patches positive for HMMA or HMA. At the method limit of quantification (LOQ) of 2.5 ng/patch, 64.4% of patches were positive for MDMA, whereas 38.8% were positive at the proposed SAMHSA cutoff of 25 ng/patch. For MDA, 31.1% were positive at 2.5 ng/patch and 10.4% at 25 ng/patch. In addition, 10 patches (1.8%) contained MDA ≥LOQ without concurrent MDMA. However, all of these patches were collected from individuals (n = 3) who received placebo as the first dose; and 3 of these were weekly patches.
Table 1.
Detection rates and percentage of patches positive for MDMA and MDA in sweat patches during and after MDMA administration and in washout sweat patches at the limits of quantification and proposed SAMHSA cutoffs (25 ng/patch). LOQs were 2.5 ng/patch for MDMA and 5 ng/patch for MDA.
| Total patches (N = 559) | MDMA | MDA | MDMA only | MDA only | MDMA & MDA | Total |
|---|---|---|---|---|---|---|
| LOQ (ng/patch) | 2.5 | 5 | ||||
| ≥LOQ | 360 | 174 | 196 | 10 | 164 | 370 |
| Positive, % | 64.4 | 31.1 | 35.1 | 1.8 | 29.3 | 66.2 |
| Range, ng/patch | 2.5−3007.7 | 5.0−171.2 | 2.5−346.6 | 5.8−41.1 | ||
| ≥SAMHSA cutoff (25 ng/patch) | 217 | 58 | 162 | 3 | 55 | 220 |
| SAMHSA positive, % | 38.8 | 10.4 | 29 | 0.5 | 89.8 | 39.4 |
| Washout patches (n = 81) | MDMA | MDA | MDMA only | MDA only | MDMA & MDA | Total |
| ≥LOQ | 22 | 14 | 13 | 5 | 9 | 27 |
| % Positive, % | 27.2 | 17.3 | 16 | 9.8 | 11.1 | 33.3 |
| Range, ng/patch | 2.6−287.2 | 6.4−31.7 | 2.6−25.7 | 9.2−20.4 | ||
| ≥SAMHSA cutoff (25 ng/patch) | 7 | 2 | 1 | 6 | 1 | 8 |
| SAMHSA positive, % | 8.6 | 2.5 | 1.2 | 7.4 | 1.2 | 9.9 |
Based on the proposed SAMHSA cutoff (≥25 ng/patch), >90% of weekly patches were positive after a single low or high oral MDMA dose. Maximum concentrations of up to 3007 (MDMA) and 171 (MDA) ng/patch were observed in weekly patches after a single high dose. Peak MDMA concentrations in short-term and daily patches for all individuals (n = 15) ranged from 9.9−894.0 and 51.9−2777.2 ng/patch for the low and high doses, respectively. MDA concentrations for the low and high doses ranged from 5.1−77.0 and 5.0−92.2 ng/patch, respectively.
WASHOUT PATCHES
Washout patches (n = 81) were worn before dosing to detect previously self-administered drugs. Patches were worn an average of 15.2 h (10.5−65.0 h). MDEA could have been present in the washout patches from previously self-administered drug, but none was detected in any patch. Results for washout sweat patches at the LOQ and SAMHSA cutoffs are shown in Table 1. Twenty-seven patches worn before dosing contained MDMA and/or MDA above the method LOQ and 8 above the proposed SAMHSA cutoff of 25 ng/patch.
Six participants received placebo as the first dose, allowing an extended detection window for self-administered drug. Three of these participants were negative for all analytes in washout patches worn for up to 33.8 h before their first dose. Two of these had no detectable analyte in any sweat patch for an additional week after their first (placebo) dose. One participant had only small amounts of MDA (5.6 and 10.5 ng/patch) in both weekly patches collected 168 h after placebo dosing. In 2 study participants with positive wash-out patches, MDMA and MDA were still present after the week of abstinence (following placebo dosing) at concentrations high enough to satisfy SAMHSA criteria for a positive sweat patch.
ONSET OF DRUG DETECTION
Onset of drug detection was defined by the first positive specimen ≥LOQ in patches worn less than 12 h (0−2.5, 0−6, 6−12, 12−24 h). MDMA was first detected in 40.0% and 53.3% of 0−2.5 h patches after low and high doses, respectively, and usually within the first 6 h. After the low dose, no MDMA was detected in short-term patches from one study participant, but appeared in the first daily patch, worn from 0−24 h. MDA was usually first detectable within6hof drug administration. Short-term patches from 4 study participants had no detectable MDA after the low dose and in 2 study participants after the higher dose.
The highest short-term patch MDMA and MDA concentrations were in 0−12 or 12−24 h patches. MDMA maximum short-term patch concentrations were 794.6 and 2777.2 ng/patch after low and high doses, respectively, with 52.5% and 66.7% exceeding the SAMHSA cutoff. Positive MDMA detection rates for combined low- and high-dose patches (n = 30) were 46.7%, 73.3%, 80.0%, and 96.7% for patches worn 0−2.5, 0−6, 6−12, and 12−24 h, respectively. Positive rates increased with wear duration, but MDMA concentrations did not correlate significantly with time worn or dose administered. Similar MDA results were obtained.
Maximum daily patch concentrations occurred in 0−24 h patches for MDMA (90%) and MDA (75%); peak concentrations always occurred within 72 h of dosing.
DURATION OF DRUG DETECTION
Three study participants who remained on the clinical unit throughout the study received placebo between low and high doses, allowing evaluation of MDMA detection duration for 2 weeks after dosing. One study participant had no positive sweat patches during the placebo session. Six of 7 consecutive daily patches from the second study participant were positive for MDMA (range 2.6−4.4 ng/patch), and both weekly patches contained MDMA (2.6 and 7.0 ng/patch) the week after a high dose. Similarly, 3 of 7 daily patches (range 5.4−9.8 ng/patch) and both weekly patches from the third study participant contained MDMA concentrations (25.8 and 31.9 ng/patch) exceeding the SAMHSA confirmation threshold 14 days after a high dose.
DRUG ACCUMULATION
To evaluate drug accumulation in the first 24 h after dosing (n = 15), concentrations in 2 consecutive 12-h patches (0−12, 12−24 h) and 3 consecutive short-term patches (0−6, 6−12, 12−24 h) were compared to the concentration in the first daily patch (0−24 h). As expected, patches worn for the longer time (0−24 h) usually contained the highest analyte concentration. However, after the low dose, only 5 of 15 first daily patches exceeded the total MDMA concentrations in 2 or 3 short-term patches. After the high dose, only 4 of 15 first daily patches were higher. The single daily patch contained more MDA than cumulative short-term patches in 50% of patches after low and high doses.
Cumulative MDMA and MDA excretion in weekly sweat patches was compared to total analyte concentrations in 7 consecutive daily patches (Table 2). There were 9 and 12 complete sets of 7 daily patches, with at least 1 weekly patch available for comparison after low and high doses, respectively. When available, the mean of 2 duplicate weekly patches was compared to the sum of daily patches. There was large variability in duplicate patch concentrations, with differences ranging from 0.2−1156.0 ng/patch. Weekly MDMA concentrations were less than the sum of 7 daily MDMA concentrations in all but 1 study participant after the low (n = 9) and high (n = 12) doses, respectively. The sum of daily MDA patch concentrations always exceeded weekly patch concentrations in which MDA was detected.
Table 2.
Individual and mean concentrations of MDMA and MDA in duplicate weekly sweat patches and cumulative amounts in 7 consecutive daily sweat patches worn for 24 h after dosing with 1.0 mg/kg (low dose) or 1.6 mg/kg (high dose) MDMA.
|
MDMA, ng/patch |
MDA, ng/patch |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study participant | Patch 1 | Patch 2 | Mean | 7 Daily patch totals | Patch 1 | Patch 2 | Mean | 7 Daily patch totals | |
| 1.0 mg/kg | 1 | 5.8 | —a | — | 9.1 | 0 | —a | — | 0 |
| 2 | 517.8 | —a | — | 447 | 37.6 | —a | — | 39.5 | |
| 3 | 270.9 | —a | — | 338.4 | 21.4 | —a | — | 24.9 | |
| 5 | 94.7 | 186.6 | 140.7 | 490.3 | 8.7 | 17.6 | 13.1 | 23.8 | |
| 7 | 389.3 | 63.5 | 226.4 | 1185.6 | 25.6 | 12.7 | 19.2 | 66 | |
| 8 | 445.9 | 442.3 | 444.1 | 2724.1 | 22.8 | 21.3 | 22.1 | 107 | |
| 10 | 59 | 47 | 53 | 75.5 | 5.7 | 5.3 | 5.5 | 6.4 | |
| 11 | 180.4 | 466.1 | 323.3 | 535.9 | 11.2 | 25.5 | 18.3 | 32.5 | |
| 12 | 61.9 | 84.1 | 73 | 95.4 | 0 | 5.5 | 2.7 | 5.4 | |
| 1.6 mg/kg | 1 | 58.7 | —a | — | 93.2 | 6 | —a | — | 7.2 |
| 2 | 1032.5 | —a | — | 870.9 | 73 | —a | — | 94 | |
| 3 | 634.6 | —a | — | 824.3 | 43.2 | —a | — | 59 | |
| 5 | 860 | 257.2 | 558.6 | 903 | 68.9 | 19.1 | 44 | 64.3 | |
| 7 | 3007.7 | 1851.7 | 2429.7 | 2753.3 | 171.2 | 91.2 | 131.2 | 175 | |
| 8 | 2638.2 | 2566.4 | 2602.3 | 3053 | 91.7 | 93.2 | 92.4 | 113.4 | |
| 9 | 816.4 | 367.6 | 592 | 957 | 71.7 | 35.8 | 53.7 | 81.4 | |
| 10 | 14.7 | 64.7 | 39.7 | 275.5 | 0 | 0 | 0 | 26.2 | |
| 11 | 376.6 | 422.6 | 399.6 | 1069 | 33.5 | 33.7 | 33.6 | 99.2 | |
| 12 | —a | 22 | — | 270.8 | —a | 0 | — | 17.8 | |
| 13 | 28.5 | 239.6 | 134 | 1237.9 | 0 | 5.5 | 2.7 | 27.2 | |
| 14 | 85.8 | 465.5 | 275.65 | 389.2 | 5.8 | 26.8 | 16.3 | 18.8 | |
Missing sweat patch.
DRUG EXCRETION
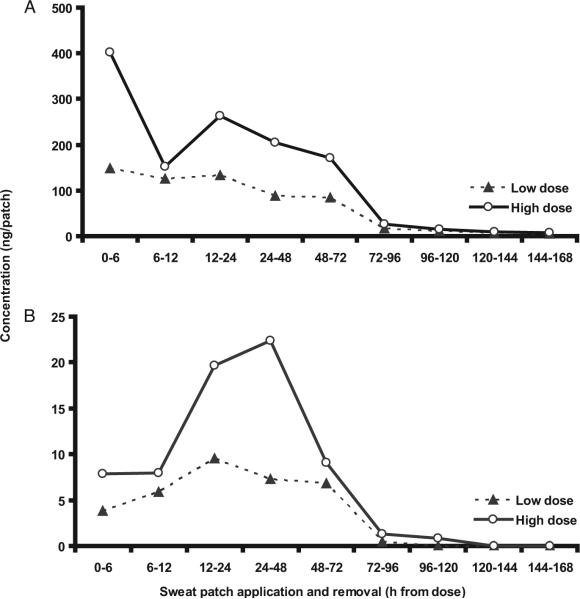
Approximately 64% of total MDMA was excreted on the first day, falling to 23% and 6% on successive days (Fig. 2A). Similar results were obtained for MDA excretion (Fig. 2B). For both analytes, large intra- and interindividual variability was noted.
Fig. 2.
(A), Mean (SE) MDMA sweat patch concentrations for 15 study participants after low (1.0 mg/kg) and high (1.6 mg/kg) doses in short-term and daily sweat patches.
(B), Mean (SE) MDA sweat patch concentrations for 15 study participants after low (1.0 mg/kg) and high (1.6 mg/kg) doses in short-term and daily sweat patches.
Concentrations of duplicate weekly sweat patches applied and removed together were compared to determine MDMA excretion variability (Table 2). In patches worn during dosing (0−168 h), there were 6 paired patches after the low and 8 after the high dose. MDMA differences between these positive duplicate patches ranged from 3.7−325.8 ng/patch (relative percentage differences of up to 143.9%) after the low and 46.1−1156.0 ng/patch (relative percentage differences up to 157.5%) after the high doses. MDA differences were similar. All weekly patches (n = 35) worn during dosing were positive for MDMA ≥LOQ. After the low dose, 14 of 15 weekly patches (93.3%) exceeded the proposed SAMHSA confirmation cutoff (25 ng/patch), compared to 18 of 20 weekly patches (90.0%) following a single high dose.
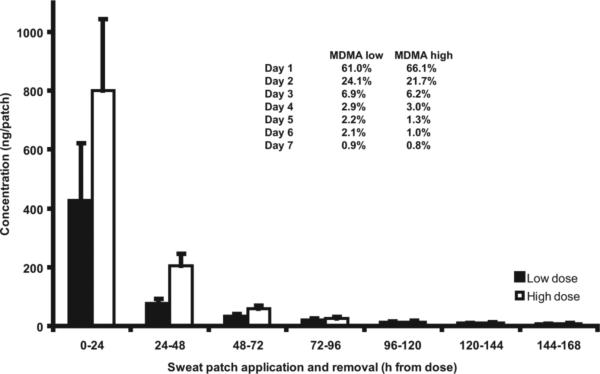
Mean daily MDMA excretion profiles were determined in 11 study participants after low and high doses (Fig. 3). Analyte concentrations were higher in daily patches worn close to the time of dosing. Mean sweat patch concentrations rapidly decreased the second and third days, with residual excretion continuing throughout the week. Similar results were observed with mean excretion rates (ng/h) (Table 3). The majority of the drug was excreted in the first 24 h, and the rate of excretion was highest in short-term patches.
Fig. 3. Mean (SE) daily MDMA excretion in 11 study participants.
Inset table shows mean percentages of total analyte excreted in consecutive daily patches.
Table 3.
Mean excretion rates (ng/h) of MDMA and MDA in sweat (n = 15) with median values and ranges.
| Time point | MDMA low (SE) | Median (range) | MDMA high (SE) | Median (range) | MDA low (SE) | Median (range) | MDA high (SE) | Median (range) |
|---|---|---|---|---|---|---|---|---|
| 0−6 h | 23.2 (11.0) | 3.5 (0−132.4) | 67.1 (32.8) | 12.1 (0−462.9) | 0.6 (0.3) | 0.0 (0−3.5) | 1.3 (0.6) | 0.0 (0−7.1) |
| 6−12 h | 20.8 (7.1) | 5.9 (0−75.2) | 25.4 (8.9) | 16.6 (0−136.0) | 1.0 (0.4) | 0.0 (0−3.7) | 1.3 (0.5) | 0.8 (0−7.6) |
| 12−24 h | 11.1 (3.0) | 7.3 (0−35.8) | 21.9 (3.4) | 18.4 (4.3−40.0) | 0.8 (0.3) | 0.5 (0−3.1) | 1.6 (0.3) | 1.5 (0−4.0) |
| 24−48 h | 3.7 (1.2) | 2.1 (0.1−18.0) | 8.5 (1.3) | 8.5 (0.9−17.5) | 0.3 (0.1) | 0.2 (0−1.7) | 0.9 (0.2) | 0.8 (0−2.8) |
| 48−72 h | 3.5 (2.4) | 0.9 (0−37.3) | 6.6 (4.5) | 2.5 (0−67.4) | 0.3 (0.2) | 0.0 (0−3.2) | 0.4 (0.2) | 0.3 (0−2.2) |
| 72−96 h | 0.5 (0.3) | 0.2 (0−3.5) | 0.9 (0.2) | 0.8 (0−2.6) | 0.0 (0.0) | 0.0 (0−0.2) | 0.0 (0.0) | 0.0 (0−0.4) |
| 96−120 h | 0.3 (0.2) | 0.2 (0−2.1) | 0.5 (0.1) | 0.4 (0−1.6) | 0.0 (0.0) | 0.0 (0) | 0.0 (0.0) | 0.0 (0−0.2) |
| 120−144 h | 0.2 (0.1) | 0.2 (0−1.7) | 0.3 (0.1) | 0.3 (0−1.1) | 0.0 (0.0) | 0.0 (0) | 0.0 (0.0) | 0.0 (0) |
| 144−168 h | 0.2 (0.1) | 0.0 (0−1.1) | 0.2 (0.1) | 0.2 (0−0.9) | 0.0 (0.0) | 0.0 (0) | 0.0 (0.0) | 0.0 (0) |
| Maximum excretion (ng/h) | 132.4 | 462.9 | 3.7 | 7.6 | ||||
| Time point | 0−6 h | 0−6 h | 0−6 h | 0−6 h | ||||
The mean percentage of total analyte concentration excreted in 7 consecutive daily patches (n = 11) is presented in Fig. 3. Consistent excretion patterns across doses for both MDMA and MDA were demonstrated. It appears that most drug is excreted in the first daily patch, and almost 90% within the first 3 days.
Discussion
This study is the first to evaluate disposition of MDMA and metabolites in human sweat after controlled oral administration of low and high doses, with samples taken up to 2 weeks after drug administration. We found that single oral MDMA recreational doses frequently produced positive weekly sweat patches at the LOQ of the analytical method, but also at the proposed SAMHSA confirmation cutoffs for federally-mandated drug testing. The onset of MDMA detection in sweat was rapid, generally within 6 h, and duration of drug detection from a single dose extended at least into the week after dosing in some cases. Drug excretion rate was highest on the dosing day, with substantial drug excretion on days 2 and 3, and low residual excretion throughout the week. There was high variability in excreted drug concentration in duplicate weekly patches. These data indicate that a positive MDMA sweat patch could indicate ecstasy use during the week of patch wear or possibly 1 to 2 weeks before patch application. Positive rates increased with wear duration, but MDMA and MDA concentrations did not correlate significantly with time worn or dose administered.
This variability in MDMA sweat excretion suggests that sweat patch tests should be interpreted qualitatively rather than quantitatively. We observed high within-subject variability of MDMA concentrations in duplicate weekly sweat patches, with percentage differences of up to 157.5% and correlation coefficients of 0.41−0.92.
Samyn et al (29) reported mean sweat concentrations <25 ng/wipe the first 5 h after 75 mg MDMA. However, mean MDMA concentrations in our short-term sweat patches (0−6 h) were higher, and more similar to those reported by Pichini et al (28). After our low (1.0 mg/kg, median 75 mg, range 43.2−105.7 mg) and high (1.6 mg/kg, median 120 mg, range 69.1−150 mg) MDMA doses, mean MDMA concentrations in 0−6 h patches were 148.9 and 625.2 ng/patch, respectively, compared with 229.3 ng/patch after 100 mg MDMA (28).
MDMA, MDA, and MDEA sweat concentrations were 138−431, 13−41, and 171−281 ng/patch, respectively, in 4 “techno ravers” who wore sweat patches for 28 h during a dance weekend (27). These concentrations were much lower than those detected in our first daily patch (0−24 h) after the low (up to 2220 ng/patch) or high (up to 2502 ng/patch) doses. This result may be due to the notoriously poor purity of recreational MDMA tablets, which often contain little or no MDMA (32-34). However, MDA concentrations were similar to those in the current study, 5.2−92.2 ng/patch.
There are few limitations to these data, which included sensitive and specific sweat patch analysis, controlled administration of 2 MDMA recreational doses, short-term, daily and weekly sweat patches to characterize the onset of drug excretion, time course, and daily rates of MDMA and metabolite excretion and drug accumulation. Although the study design provided an opportunity to evaluate duration of drug excretion and documented excretion beyond the 1-week sweat patch wear period, these data do not definitively delineate whether or not sweat patches worn 2 weeks after dosing would be positive. Participants resided on the closed research unit for up to 23 days; a longer residential period would be necessary to fully characterize the duration of MDMA detection in sweat after single oral MDMA doses. These data suggest that sweat testing is a good alternative matrix for monitoring MDMA use and that, for the highest detection rate, both MDMA and MDA should be assayed. The results should assist clinicians and other professionals in monitoring individuals in drug treatment and interpreting sweat test results in workplace drug testing programs.
Acknowledgments
The authors thank participants and NIDA IRP clinical research staff and PharmChem™ Inc. for generously providing sweat patches.
Research Funding: This research was funded by the Intramural Research Program, National Institutes of Health, National Institute on Drug Abuse. B.S. De Martinis, CAPES (Brazilian Coordination for the Improvement of Higher Education Personnel).
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: MDMA, 3,4-methylenedioxymethamphetamine; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxyethylamphetamine; SAMHSA, Substance Abuse Mental Health Services Administration; HMA, 4-hydroxy-3-methoxyamphetamine; HMMA, 4-hydroxy-3-methoxymethamphetamine; LOQ, limit of quantitation.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16:557–77. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- 3.Schifano F. A bitter pill. Overview of ecstasy (MDMA, MDA) related fatalities. Psychopharmacology (Berl) 2004;173:242–8. doi: 10.1007/s00213-003-1730-5. [DOI] [PubMed] [Google Scholar]
- 4.de Almeida SP, Silva MT. Ecstasy (MDMA): effects and patterns of use reported by users in Sao Paulo. Rev Bras Psiquiatr. 2003;25:11–7. doi: 10.1590/s1516-44462003000100004. [DOI] [PubMed] [Google Scholar]
- 5.de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, et al. Pharmacology of MDMA in humans. Ann NY Acad Sci. 2000;914:225–37. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 6.Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol. 2008;28:432–40. doi: 10.1097/JCP.0b013e31817ef470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–44. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology. 1998;19:241–51. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- 9.Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, et al. Human pharmacology of 3,4-methylenedioxymethamphetamine (“ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–66. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Downing J. The psychological and physiological effects of MDMA on normal volunteers. J Psychoactive Drugs. 1986;18:335–40. doi: 10.1080/02791072.1986.10472366. [DOI] [PubMed] [Google Scholar]
- 11.Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs. 1986;18:319–27. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RK. MDMA: nonmedical use and intoxication. J Psychoactive Drugs. 1986;18:349–54. doi: 10.1080/02791072.1986.10472368. [DOI] [PubMed] [Google Scholar]
- 13.Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001;154:161–8. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 14.Kraner JC, McCoy DJ, Evans MA, Evans LE, Sweeney BJ. Fatalities caused by the MDMA-related drug paramethoxyamphetamine (PMA). J Anal Toxicol. 2001;25:645–8. doi: 10.1093/jat/25.7.645. [DOI] [PubMed] [Google Scholar]
- 15.Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature (Lond) 1999;398:567–70. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 16.Kish SJ. How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol Biochem Behav. 2002;71:845–55. doi: 10.1016/s0091-3057(01)00708-0. [DOI] [PubMed] [Google Scholar]
- 17.Caplan YH, Goldberger BA. Alternative specimens for workplace drug testing. J Anal Toxicol. 2001;25:396–9. doi: 10.1093/jat/25.5.396. [DOI] [PubMed] [Google Scholar]
- 18.Barnes AJ, Smith ML, Kacinko SL, Schwilke EW, Cone EJ, Moolchan ET, Huestis MA. Excretion of methamphetamine and amphetamine in human sweat following controlled oral methamphetamine administration. Clin Chem. 2008;54:172–80. doi: 10.1373/clinchem.2007.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Martinis BS, Barnes AJ, Scheidweiler KB, Huestis MA. Development and validation of a disk solid phase extraction and gas chromatography-mass spectrometry method for MDMA, MDA, HMMA, HMA, MDEA, methamphetamine and amphetamine in sweat. J Chromatogr B. 2007;852:450–8. doi: 10.1016/j.jchromb.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huestis MA, Scheidweiler KB, Saito T, Fortner N, Abraham T, Gustafson RA, Smith ML. Excretion of Delta9-tetrahydrocannabinol in sweat. Forensic Sci Int. 2008;174:173–7. doi: 10.1016/j.forsciint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kacinko SL, Barnes AJ, Schwilke EW, Cone EJ, Moolchan ET, Huestis MA. Disposition of cocaine and its metabolites in human sweat after controlled cocaine administration. Clin Chem. 2005;51:2085–94. doi: 10.1373/clinchem.2005.054338. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Wtsadik AT, Scheidweiler KB, Fortner N, Takeichi S, Huestis MA. Validated gas chromatographic-negative ion chemical ionization mass spectrometric method for delta-9-tetrahydrocannabinol in sweat patches. Clin Chem. 2004;50:2083–90. doi: 10.1373/clinchem.2004.034868. [DOI] [PubMed] [Google Scholar]
- 23.Schwilke EW, Barnes AJ, Kacinko SL, Cone EJ, Moolchan ET, Huestis MA. Opioid disposition in human sweat after controlled oral codeine administration. Clin Chem. 2006;52:1539–45. doi: 10.1373/clinchem.2006.067983. [DOI] [PubMed] [Google Scholar]
- 24.DHHS Proposed revisions to mandatory guidelines for federal workplace drug testing programs. Federal Register. 2004;69:19673–732. [Google Scholar]
- 25.Vree TB, Muskens AT, Van Rossum JM. Excretion of amphetamines in human sweat. Arch Internationales de Pharmacodynamie et de Therapie. 1972;199:311–7. [PubMed] [Google Scholar]
- 26.Kintz P. Drug testing in addicts: a comparison between urine, sweat, and hair. Ther Drug Monit. 1996;18:450–5. doi: 10.1097/00007691-199608000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Kintz P, Samyn N. Determination of “ecstasy” components in alternative biological specimens. J Chromatogr B. 1999;733:137–43. doi: 10.1016/s0378-4347(98)00521-0. [DOI] [PubMed] [Google Scholar]
- 28.Pichini S, Navarro M, Pacifici R, Zuccaro P, Ortuno J, Farre M, et al. Usefulness of sweat testing for the detection of MDMA after a single-dose administration. J Anal Toxicol. 2003;27:294–303. doi: 10.1093/jat/27.5.294. [DOI] [PubMed] [Google Scholar]
- 29.Samyn N, De Boeck G, Wood M, Lamers CTJ, De Waard D, Brookhuis KA, et al. Plasma, oral fluid and sweat wipe ecstasy concentrations in controlled and real life conditions. Forensic Sci Int. 2002;128:90–7. doi: 10.1016/s0379-0738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 30.Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit. 2008;30:320–32. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skoop G, Potsch L, Moeller MR. On cosmetically treated hair: aspects and pitfalls of interpretation. Forensic Sci Int. 1997;84:43–52. doi: 10.1016/s0379-0738(96)02047-6. [DOI] [PubMed] [Google Scholar]
- 32.Pham JV, Puzantian T. Ecstasy: dangers and controversies. Pharmacotherapy. 2001;21:1561–5. doi: 10.1592/phco.21.20.1561.34474. [DOI] [PubMed] [Google Scholar]
- 33.Baggott M, Heifets B, Jones RT, Mendelson J, Sferios E, Zehnder J. Chem analysis of ecstasy pills. JAMA. 2000;284:2190. doi: 10.1001/jama.284.17.2190. [DOI] [PubMed] [Google Scholar]
- 34.Palhol F, Boyer S, Naulet N, Chabrillat M. Impurity profiling of seized MDMA tablets by capillary gas chromatography. Anal Bioanal Chem. 2002;374:274–81. doi: 10.1007/s00216-002-1477-6. [DOI] [PubMed] [Google Scholar]