Abstract
The hypoxic response is an ancient stress response triggered by low ambient oxygen (O2)1. It is controlled by hypoxia inducible transcription factor-1 (HIF-1), whose α subunit is rapidly degraded under normoxic conditions but stabilized when O2-dependent prolyl hydroxylases (PHDs) that target its O2-dependent degradation domain (ODD) are inhibited2–4. Thus the amount of HIF-1α, which controls many genes involved in energy metabolism and angiogenesis is regulated post-translationally. Another ancient stress response is the innate immune response, regulated by several transcription factors, among which NF-κB plays a central role5, 6. NF-κB activation is controlled by IκB kinases (IKK), mainly IKKβ, which are required for phosphorylation-induced degradation of IκB inhibitors in response to infection and inflammation6. Recently, IKKβ was found to be activated in hypoxic cell cultures when PHDs that suppress its activation are inhibited7. However, defining the relationship between NF-κB and HIF-1α has proven elusive. Using in vitro systems, it was reported that HIF-1α activates NF-κB8, that NF-κB controls HIF-1α transcription9 and that activation of HIF-1α may be concurrent to inhibition of NF-κB10. We used mice lacking IKKβ in different cell types to demonstrate that NF-κB is a critical transcriptional activator of HIF-1α in macrophages responding to bacterial infection and in liver and brain of hypoxic animals. IKKβ deficiency results in defective induction of various HIF-1α target genes including vascular endothelial growth factor (VEGF) and elevated astrogliosis in hypoxic mice. Hence, IKKβ provides an important physiological link between the hypoxic response and innate immunity/inflammation, two ancient stress response systems.
Hypoxia is characterized by reduced O2 pressure within a tissue and can occur under several pathophysiological situations including ischemia, cancer and inflammation11. During an ischemic event, flow of nutrients and O2 to damaged tissues is reduced and HIF-1α activation leads to induction of genes whose products restore blood supply, nutrients and energy production, thereby maintaining tissue integrity and homeostasis12, 13. The hypoxic response is important for proper function of tissue macrophages and infiltrating neutrophils that encounter low O2 pressure in infected tissues14. HIF-1α was also suggested to promote expression of inflammatory cytokines, known to be regulated by NF-κB15, in LPS-stimulated macropahges16 and mediate NF-κB activation in anoxic neutrophils8. However, it was also reported that hypoxia leads to activation of IKKβ by inhibiting PHDs that negatively modulate IKKβ activity7. We, therefore decided to critically explore the relationship between IKKβ, NF-κB and HIF-1α under in vivo conditions using IKKβ-deficient mice and primary macrophages.
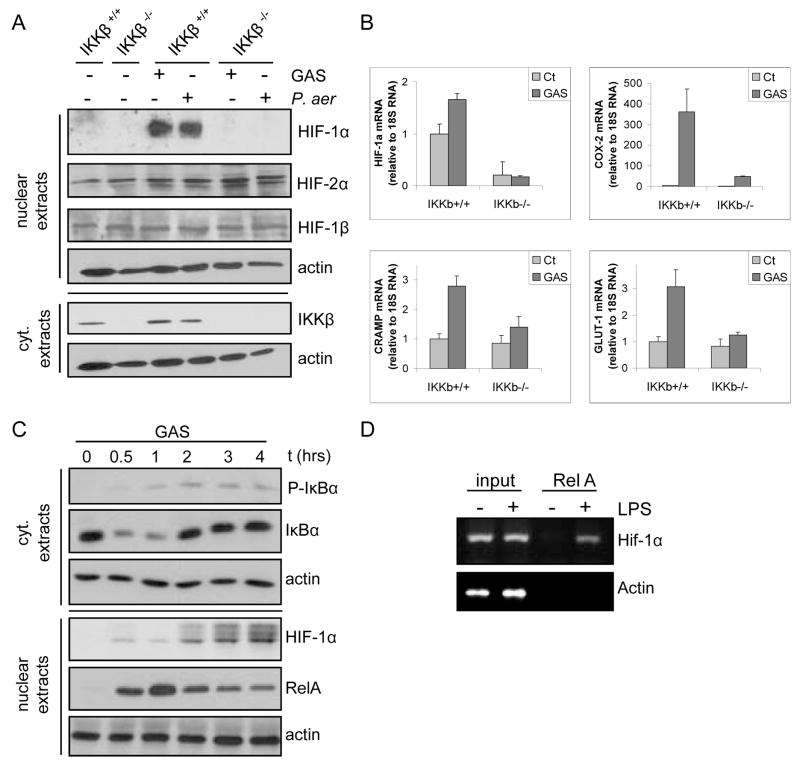
We first examined bone marrow-derived macrophages (BMDM) from either IkkβF/F or IkkβF/F/Mx1Cre mice challenged with poly(I:C), which induces interferon (IFN) and thereby drives CRE recombinase expression from the Mx1 promoter to delete Ikkβ in IFN-responsive cells of the resulting IkkβΔ mice17. BMDM were incubated with Gram positive (group A Streptococcus, GAS) and Gram negative (Pseudomonas aeruginosa) bacteria. Both species induced HIF-1α accumulation in an IKKβ-dependent manner (Fig. 1A). Induction of HIF-1 target genes involved in the hypoxic and innate immune responses was also dependent on IKKβ (Fig. 1B). These genes included Cox-2, which is directly regulated by NF-κB and HIF-1α, Cnlp, which encodes the murine antimicrobial peptide mCRAMP, whose expression is not directly responsive to NF-κB18, and Glut-1, a glucose transporter. Moreover, HIF-1α mRNA was dramatically downregulated in IKKβ-deficient cells even before infection, suggesting that IKKβ-dependent NF-κB may control HIF-1α gene transcription. We investigated this possibility by chromatin immunoprecipitation (ChIP) in LPS-stimulated macrophages and found that the RelA NF-κB subunit is recruited to the HIF-1α promoter, which contains a classical κB site at −197/−188 bp, conserved between mice and men (Fig. 1C).
Figure 1. IKKβ is required for microbial-induced HIF-1α expression in macrophages.
a) BMDM from either IkkβF/F (IKKβ+/+) or poly(IC)-injected IkkβF/F/Mx1-Cre (IkkβΔ; IKKβ−/−) mice were incubated with either with GAS or P. aeruginosa (MOI of 10 for 4 hrs). HIF-1α expression was analyzed by immunoblotting. b) RNA was extracted from BMDM incubated with GAS and gene expression was analyzed by quantitative (Q) RT-PCR. Results are averages of 3 separate experiments done in triplicate. Values were normalized relative to 18S rRNA. c) ChIP was performed with an anti-RelA antibody using fixed and sheared chromatin isolated from RAW264.7 mouse macrophages incubated with or without LPS. The HIF-1α promoter fragment, which contains a κB site at −197/−188 bp, was detected by PCR amplification.
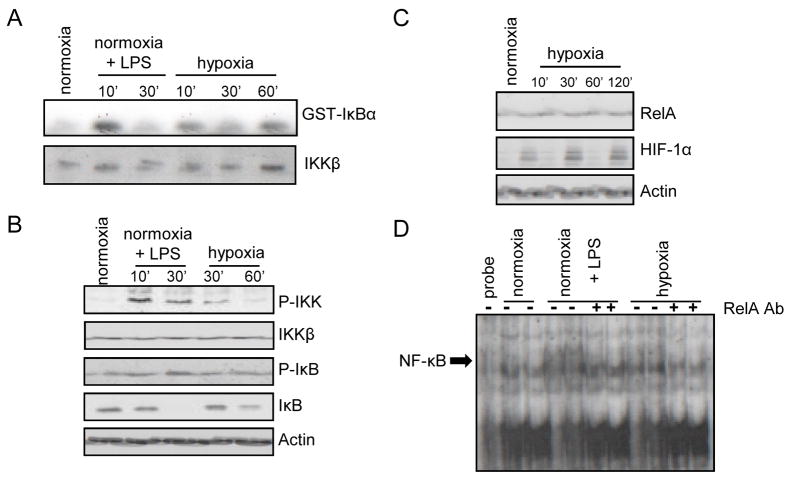
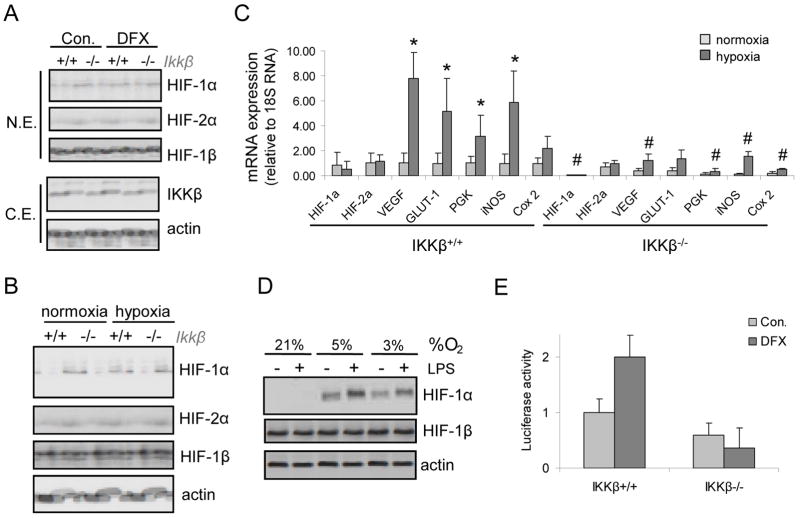
As found by Cummins et al.7, we observed that hypoxia activated IKK in macrophages (Fig. 2A), induced IKKα/β and IκBα phosphorylation and promoted IκBα degradation (Fig. 2B). NF-κB DNA binding to a canonical κB site was also induced by hypoxia (Fig. 2C). Given that IKKβ and NF-κB are activated by hypoxia we examined whether IKKβ was required for hypoxia-induced HIF-1α accumulation in macrophages, a response that is thought to be mainly dependent on inhibition of HIF-1α degradation3, 4. Remarkably, IKKβ was required for HIF-1α accumulation in BMDM incubated with the hypoxia mimetic desferrioxamine (DFX) as well as in response to actual hypoxia (Fig. 3A,B). The hypoxia-dependent induction of HIF-1 target genes, such as VEGF and Glut-1, was nearly abolished without IKKβ (Fig 3C). Expression of HIF-1α, but not HIF-2α, mRNA was also downregulated without IKKβ (Fig. 3C). Similar results were obtained in mouse embryonic fibroblasts (Supplementary Fig 1), where IKKβ was also required for activation of the HIF-1α promoter upon DFX treatment (Fig. 3D).
Figure 2. Hypoxia activates the NF-kB pathway in macrophages.
RAW264.7 mouse macrophages were incubated with or without LPS or cultured under hypoxia (O2 = 0.5 %). a) At the indicated time points of LPS stimulation or hypoxia, IKK activity was measured by an immunocomplex kinase assay using GST-IκBα as a substrate. b) Cell lysates were prepared and IKKβ and IκBα phosphorylation and abundance were analyzed by immunoblotting. c) Nuclear extracts were prepared at 2 hrs post-LPS or -hypoxia and NF-κB DNA binding activity was examined by a mobility shift assay. Antibody inhibition was performed using an anti-RelA antibody.
Figure 3. IKKβ regulates hypoxia-induced HIF-1α and target genes in mouse macrophages.
a) BMDM from IkkβF/F (IKKβ+/+) or IkkβΔ (IKKβ−/−) mice were incubated with desferrioxamine (DFX) for 4 hrs. HIF-1α, HIF-1β and IKKβ expression were analyzed by immunoblotting. b) BMDM were obtained as above and cultured under hypoxia (O2 = 0.5% for 4 hrs). HIF-1α expression was analyzed by immunoblotting. c) BMDM were treated as above and mRNA expression was analyzed by Q-RT-PCR. Results are averages of three separate experiments done in triplicates. p<0.05: *, vs normoxic Ikkβ+/+ cells; #, vs hypoxic Ikkβ+/+ cells. d) MEF from either Ikkβ+/+ or Ikkβ−/− embryos were transfected with a luciferase reporter gene driven by the HIF-1α promoter. After 36 hrs the cells were incubated for 3 hrs with DFX. p<0.05: *, vs normoxic Ikkβ+/+ cells; #, vs hypoxic Ikkβ+/+ cells.
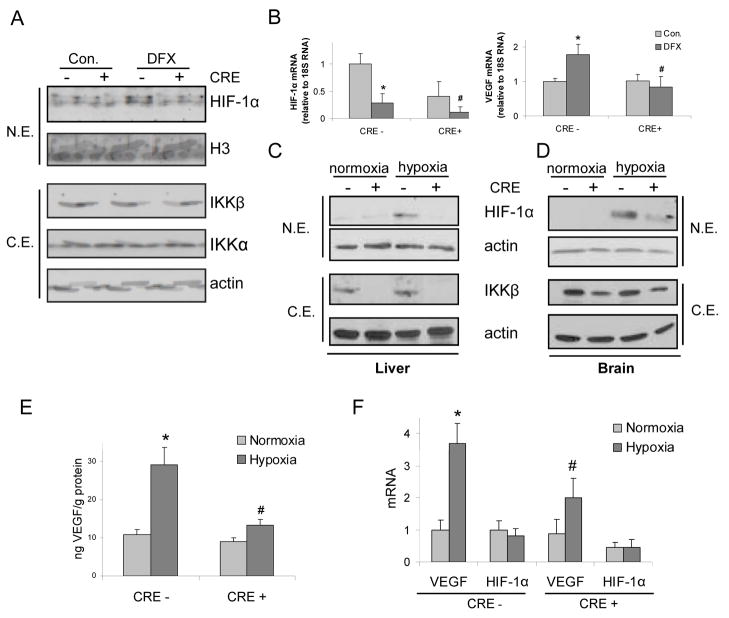
Having established the role of IKKβ in HIF-1 activation in macrophages, we examined its role in HIF-1 activation in intact mice. DFX administration induced HIF-1α expression in liver of IkkβF/F mice but not in IkkβΔ mice (Fig. 4A), which lack Ikkβ in both hepatocytes and Kupffer cells19. IkkβΔ mice also contained less HIF-1α and VEGF mRNA in their livers (Fig 4B). Next, we examined the role of IKKβ in the response to actual hypoxia. Mice were placed in a chamber with ambient O2 concentration of 8% (thus mimicking an altitude of 7000 m20). Under these conditions, we observed hypoxia-induced HIF-1α accumulation in liver (Fig 4C) and brain (Fig 4D) and in both cases HIF-1α induction was dependent on IKKβ in IFN-responsive cells. Furthermore, hypoxia-dependent induction of VEGF protein (Fig 4E) and mRNA (Fig 4F) in the brain also depended on IKKβ in IFN-responsive cells, which include brain endothelial cells and microglia21, 22. Surprisingly, IkkβΔ mice exhibited a profound increase in cerebellar astrocyte activation, marked by glial fibriliary acidic protein (GFAP), relative to IkkβF/F mice (Fig. 5). This may be due to defective production of VEGF, a cytokine with anti-inflammatory properties, shown to promote tissue repair23. Microglia produce VEGF24 and astrocytes express VEGF receptors under ischemic conditions25. VEGF is also a potent neuroprotective factor26, whose decreased production may potentiate hypoxia-induced neuronal damage and thereby augment astrocyte activation. This situation maybe akin to the loss of IKKβ in intestinal epithelial cells, previously found to exacerbate ischemic damage to the intestinal mucosa27. These results suggest that IKKβ inhibitors may not be useful in treatment of neuro-inflammatory disorders and that individuals treated with IKKβ or NF-κB inhibitors should not be exposed to hypoxic conditions such as those associated with high altitude mountain climbing.
Figure 4. IKKβ regulates HIF-1α expression in hypoxic mice.
IkkβF/F (CRE-) or IkkβΔ (CRE+) mice were treated with DFX (600 mg/Kg). After 15 hrs, livers were removed for protein (a) and RNA (b) analysis. a) HIF-1α and IKKβ expression was analyzed by immunoblotting. b) Expression of HIF-1α and VEGF mRNA was examined by Q-RT- PCR (n=3). p<0.05: *, vs normoxic CRE- mice; #, vs DFX-treated CRE- mice. c,d) IkkβF/F and IkkβΔ mice were kept under normoxia or hypoxia (O2 = 8%) for 24 hrs and HIF-1α expression was analyzed by immunoblotting of liver (c) or brain (d) extracts. e) VEGF expression in brain of mice from above experiment was analyzed by ELISA. p<0.05: *, vs normoxic CRE- mice; #, vs hypoxic CRE- mice. f) VEGF and HIF-1α mRNA expression was analyzed by Q-RT-PCR of total brain RNA. p<0.05: *, vs normoxic CRE- mice; #, vs hypoxic CRE- mice (n=3).
Figure 5. IKKβ deficiency results in increased astrogliosis in brains of hypoxic mice.
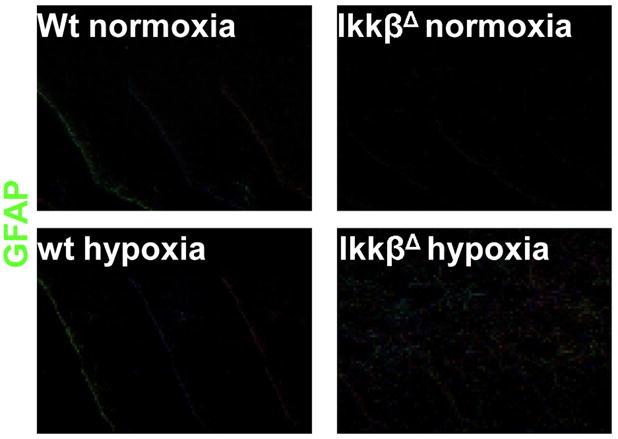
Mice of the indicated genotypes were kept under normoxia or hypoxia (O2 = 8%) for 24 hrs. After this period the mice were perfused with a fixative and the brain was collected and frozen. Brain sections at the cerebellar region (10 μm) were stained with an antibody against GFAP (an astrocyte marker). Magnification x20.
Although early studies had demonstrated induction of HIF-1α mRNA in experimental animals during development and hypoxia28, 29, numerous in vitro studies led to the current model that HIF-1α accumulation is regulated predominantly at the post-translational level via inhibition of O2-dependent PHDs that drive HIF-1α degradation in normoxic cells3, 4. Our results clearly demonstrate that transcriptional activation of the HIF-1α gene by IKKβ-responsive NF-κB is of critical importance under pathophysiologically relevant conditions ex vivo and in vivo. Both macrophages infected with bacteria and mice subjected to hypoxia reveal a pronounced HIF-1α induction defect upon loss of IKKβ. These results, together with the previous finding that IKKβ catalytic activity is controlled by O2 sensitive PHDs7 establish NF-κB as a hypoxia-regulated transcription factor that controls HIF-1α expression and thereby, serves as an important regulator of the hypoxic response. Previous findings identified a connection between HIF-1α and innate immunity/inflammation but it was not clear how microbial infection or inflammation led to HIF-1α activation under normoxic conditions14, 18. The current findings have far-reaching physiological significance as they indicate the existence of a tight coupling between two evolutionary ancient stress responses: innate immunity and the hypoxic response. By controlling HIF-1α activation in macrophages during microbial infections, that may lower local O2 tension, NF-κB can enhance glycolytic energy metabolism and production of angiogenic factors, in addition to its well established role in expression of proinflammatory cytokines, chemokines and anti-microbial peptides. Thus the ability of NF-κB to enhance HIF-1α expression expands its regulatory potential, leading to more effective execution of the host-defense response. In turn, the ability of NF-κB to promote HIF-1α activation during hypoxia expands its prosurvival function, since the HIF-1-dependent hypoxic response is critical for providing cells and tissues undergoing ischemia with sufficient energy supplies and allows them to resist cell death.
In summary, our results show that IKKβ is a key regulator of the hypoxic response in vivo, in particular providing an important homeostatic function to the brain, an organ that is extremely sensitive to oxygen and glucose deprivation30.
Methods
A detailed methods section is available in Supplementary Information. To delete Ikkβ in IkkβF/F/Mx1Cre mice, 250 μg poly(I:C) (Sigma) was injected i.p. 3 weeks prior to hypoxia exposure or isolation of myeloid cells17. To induce hypoxia in vivo, mice were placed in a special chamber where N2 and O2 were injected to achieve an O2 concentration of 8±0.1%. This was controlled by the Oxycycler hydraulic system (Model A44x0, BioSpherix, Redfield, NY, USA) and ANA-Win2 Software (Version 2.4.17, Watlow Anafaze, Watsonville, CA, USA). Control mice were kept in the same room but under normal atmospheric O2 and were exposed to the same level of noise and light during the duration of each experiment. After 24 hrs of exposure to normoxia or hypoxia, mice were sacrificed and their livers and brains were rapidly removed and frozen in liquid N2 or OCT using a dry-ice/isobutanol bath.
Supplementary Material
Acknowledgments
J.R. was supported by a postdoctoral fellowship from the Spanish Ministry of Education and Science. Work in M.K., R.J., K.A., V.N. and G.H. laboratories was supported by grants from the NIH. We thank Dr. Ebbinghaus for HIF-1α-luciferase plasmid.
References
- 1.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxwell PH, MSW, GWC, SCC, ECV, MEC, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 3.Semenza G. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleu. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 4.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylase. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;357:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 7.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, Kietzmann T, Gorlach A. Hypoxia Up-Regulates HIF-1{alpha} Transcription by Involving PI-3 Kinase and NF{kappa}B in Pulmonary Artery Smooth Muscle Cells. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-04-0391. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, E A. RSUME, a Small RWD-Containing Protein, Enhances SUMO Conjugation and Stabilizes HIF-1alpha during Hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Paul SA, Simons JW, Mabjeesh NJ. HIF at the crossroads between ischemia and carcinogenesis. Cell Physiol. 2004;200:20–30. doi: 10.1002/jcp.10479. [DOI] [PubMed] [Google Scholar]
- 12.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, R J, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 16.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 17.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125:2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- 21.Schneider A, Zhang Y, Guan Y, Davis LS, Breyer MD. Differential, inducible gene targeting in renal epithelia, vascular endothelium, and viscera of Mx1Cre mice. Am J Physiol Renal Physiol. 2003;284:F411–F417. doi: 10.1152/ajprenal.00235.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ, Lee JD. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failur. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–2792. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- 24.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–445. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 25.Choi JS, Kim HY, Cha JH, Choi JY, Chun MH, Lee MY. Upregulation of vascular endothelial growth factor receptors Flt-1 and Flk-1 in rat hippocampus after transient forebrain ischemia. J Neurotrauma. 2007;24:521–531. doi: 10.1089/neu.2006.0139. [DOI] [PubMed] [Google Scholar]
- 26.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 27.Chen LW, Egan L, Li ZW, FRG, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 28.Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73:117–23. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 29.Elson DA, Ryan HE, Snow JW, Johnson RS, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189-61–85. [PubMed] [Google Scholar]
- 30.Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. BMJ. 1998;317:1370–1373. doi: 10.1136/bmj.317.7169.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.