Abstract
Drinking alcohol is associated with the disturbance of normal sleep rhythms, and insomnia is a major factor in alcoholic relapse. The thalamus is a brain structure that plays a pivotal role in sleep regulation and rhythmicity. A number of studies have implicated GABAA receptors (GABAA-Rs) in the anxiolytic, amnestic, sedative, and anesthetic effects of ethanol. In the present study, we examined the effects of ethanol on both synaptic and extrasynaptic GABAA-Rs of relay neurons in the thalamus. We found that ethanol (≥50 mM) elicits a sustained current in thalamocortical relay neurons from the mouse ventrobasal thalamus, and this current is associated with a decrease in neuronal excitability and firing rate in response to depolarization. The steady current induced by ethanol was totally abolished by gabazine and was absent in relay neurons from GABAA-R α4 subunit knockout mice, indicating that the effect of ethanol is to enhance tonic GABA-mediated inhibition. Ethanol (50 mM) enhanced the amplitude of tonic inhibition by nearly 50%. On the other hand, ethanol had no effect on spontaneous or evoked inhibitory postsynaptic currents (IPSCs) at 50 mM but did prolong IPSCs at 100 mM. Ethanol had no effect on the paired-pulse depression ratio, suggesting that the release of GABA from presynaptic terminals is insensitive to ethanol. We conclude that ethanol, at moderate (50 mM) but not low (10 mM) concentrations, can inhibit thalamocortical relay neurons and that this occurs mainly via the actions of ethanol at extrasynaptic GABAA-Rs containing GABAA-R α4 subunits.
Drinking alcohol can promote the onset of sleep, but it can also disrupt the normal sleep pattern, increase nocturnal awakenings, and reduce sleep quality (Drummond et al., 1998). Sleep disturbance caused by chronic alcohol can play a role in the progression of alcoholism, and poor sleep quality is often cited as a factor in alcoholic relapse (Brower et al., 1998; Brower, 2001). Inhibition in the thalamus plays an important role in the normal regulation of sleep cycles (Steriade, 2000; Huguenard and McCormick, 2007; Jia et al., 2007) and may, therefore, be involved in both the sedative effects of acute alcohol and in the development of alcoholism.
The inhibitory neurotransmitter GABA has long been implicated in the anxiolytic, amnestic, sedative, and anesthetic effects of alcohol. A large number of studies have investigated the interactions of alcohol with GABAA receptors (GABAA-Rs). The standard forms of recombinant GABAA-Rs that are found at GABAergic synapses (α1βγ2 and α2βγ2 subtypes) are modulated only by >60 mM ethanol (Sigel et al., 1993; Mihic et al., 1997). Most investigators have failed to observe direct postsynaptic actions of alcohol (<60 mM) on GABA-mediated inhibitory postsynaptic currents (IPSCs) in brain slices, except at high levels (Ariwodola and Weiner, 2004; Weiner and Valenzuela, 2006). In several brain areas, however, ethanol has been shown to facilitate synaptic inhibition by a presynaptic mechanism, for example, in the amygdala (Roberto et al., 2003, 2004; Roberto and Siggins, 2006; Zhu and Lovinger, 2006), cerebellum (Carta et al., 2004; Hanchar et al., 2005; Ming et al., 2006; Kelm et al., 2007), hippocampus (Ariwodola and Weiner, 2004; Sanna et al., 2004; Galindo et al., 2005), and nucleus accumbens (Nie et al., 2000; Crowder et al., 2002).
A novel form of “tonic inhibition” has also been described in the CNS, which is generated by the persistent activation of extrasynaptic GABAA-Rs (Semyanov et al., 2004; Farrant and Nusser, 2005; Mody, 2005). GABAergic tonic inhibition has been shown to regulate the excitability of individual neurons and the behavior of neural networks. Tonic inhibition is most often generated by activation of GABAA-Rs that contain δ subunits, which are normally located at extrasynaptic or perisynaptic sites (Nusser et al., 1998; Wei et al., 2003). Several laboratories have reported that extrasynaptic GABAA-Rs containing δ subunits are sensitive to low concentrations (≤30 mM) of alcohol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003, 2006; Wei et al., 2004; Hanchar et al., 2005, 2006; Santhakumar et al., 2006; Glykys et al., 2007); however, other laboratories have reported contradictory results (Carta et al., 2004; Borghese et al., 2006; Botta et al., 2007; Korpi et al., 2007).
Tonic inhibition also occurs in the relay neurons of the thalamus (Porcello et al., 2003; Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005, 2008b; Chandra et al., 2006; Bright et al., 2007; Peden et al., 2008), where the extrasynaptic GABAA-Rs contain α4, β2, and δ subunits (Belelli et al., 2005; Chandra et al., 2006). Thalamic extrasynaptic GABAA-Rs have distinct pharmacological properties that differentiate them from synaptic GABAA-Rs, consisting mainly of α1, β2, and γ2 subunits (Pirker et al., 2000; Browne et al., 2001; Jia et al., 2005). Several studies show that hypnotics and anesthetics are much more potent at thalamic extrasynaptic GABAA-Rs than at synaptic receptors (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005; Chandra et al., 2006). Investigating the effects of alcohol on both synaptic and extrasynaptic inhibition in the thalamus should enhance our understanding of the mechanisms underlying the interaction between alcohol and sleep. Therefore, we examined the actions of ethanol on the function of GABAA-Rs of thalamocortical relay neurons in the mouse ventrobasal (VB) thalamus.
Materials and Methods
Electrophysiological Recordings in Brain Slices
Experiments were performed in accordance with institutional and federal guidelines, using mice between 23 and 60 days old (C57BL/6, Gabra4+/+, and Gabra4−/−) by methods we have described previously (Jia et al., 2005). The knockout (Gabra4−/−) and wild-type (Gabra4+/+) littermates used were age-matched and on the same genetic background (129 × 1/S1 × C57BL/6J hybrid; F2–F4 generations) (Chandra et al., 2006). The experimenters were blind to genotype.
Animals were anesthetized with halothane, and brains were removed and placed in ice-cold slicing solution, which contained 2.5 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 220 mM sucrose, 11 mM glucose, 10 mM MgSO4, and 0.5 mM CaCl2, before horizontal slices (300 μm thick) were cut on a microslicer (VT 1000S; Leica, Wetzlar, Germany). Slices were perfused with carbogenated artificial cerebrospinal fluid, which contained 124 mM NaCl, 2.5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, and 10 mM glucose. Whole-cell patch-clamp recordings from visually identified thalamic neurons were performed at room temperature as described previously (Jia et al., 2005). Intracellular solution for most voltage-clamp recordings contained 140 mM CsCl, 4 mM NaCl, 1 mM MgCl2, 0.05 mM EGTA, 2 mM ATP-Mg2+, 0.3 mM GTP-Na+, and 10 mM HEPES, pH adjusted to 7.25 with CsOH. For voltage-clamp recordings involving acamprosate, intracellular solution contained 130 mM CsCH3SO3, 8.3 mM NaCH3SO3, 1.7 mM NaCl, 1 mM CaCl2, 10 mM EGTA, 2 mM ATP-Mg2+, 0.3 mM GTP-Na+, and 10 mM HEPES, pH adjusted to 7.2 with CsOH. Intracellular solution for current-clamp recordings contained 130 mM K+-gluconate, 5 mM NaCl, 2 mM MgCl2, 10 mM HEPES, 0.5 mM EGTA, 2 mM ATP-K+, and 0.3 mM GTP-Na+, pH adjusted to 7.25 with KOH.
Spontaneous inhibitory postsynaptic currents were recorded at −65 mV and isolated by bath application of 3 to 5 mM kynurenic acid (Jia et al., 2005), and evoked IPSCs were elicited by electrical stimulation (50 μs; 2 × threshold) with a bipolar metal electrode (FHC, Bowdoin, ME) located in the reticular thalamic nucleus. The interval between successive stimuli was long (>15 s) to prevent cumulative synaptic depression. Access resistance was monitored throughout the recording period and was less than 20 MΩ throughout.
Drugs and Data Analysis
Gabazine [4-[6-imino-3-(4-methoxyphenyl)pyridazin-1-yl]butanoic acid hydrobromide], kynurenic acid (4-oxo-1H-quinoline-2-carboxylic acid), baclofen [4-amino-3-(4-chlorophenyl)-butanoic acid], and ethanol were purchased from Sigma (St. Louis, MO). Acamprosate calcium (3-acetamidopropane-1-sulfonic acid calcium salt) was obtained from Toronto Research Chemicals Inc. (North York, ON, Canada). Off-line analysis was performed using MiniAnalysis 5.5 (Synaptosoft, Decatur, GA), SigmaPlot 6.0 (SPSS, Chicago, IL), and Excel 2000 (Microsoft, Redmond, WA), as described in our previous publications. Tonic currents were measured as illustrated in Fig. 2C. The holding current was calculated by averaging an IPSC-free 5-ms section from every 100 ms of record. The all-points histograms were fitted with a Gaussian curve. The difference between the peaks of these Gaussian curves in the presence and absence of drug were calculated to determine the change of holding current. Spontaneous IPSCs were detected and analyzed using MiniAnalysis as described previously (Jia et al., 2005). Numeric data are expressed as mean ± S.E.M., except where indicated. The statistical significance of results was assessed using Student's t test or one-way ANOVA, and a level of p < 0.05 was considered significant.
Fig. 2.
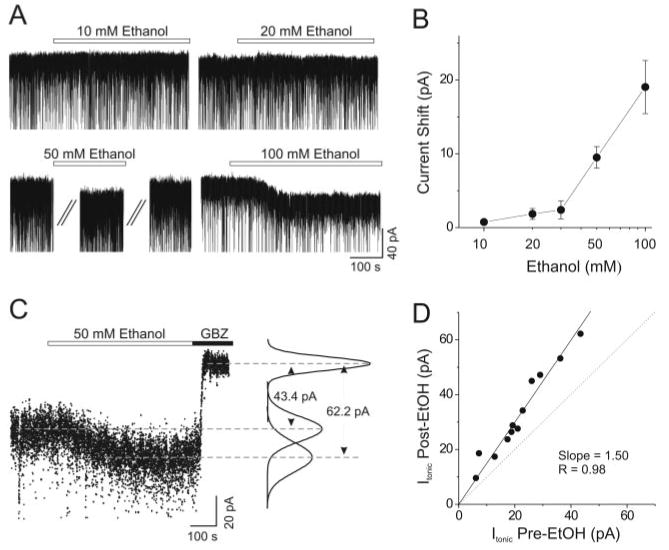
Ethanol (≥50 mM) enhances tonic currents mediated by extrasynaptic GABAA-Rs. A, typical voltage-clamp recordings of four VB neurons in response to the applications of different concentrations (10–100 mM) of ethanol. Ethanol (≥50 mM) induced substantial current-shifts. B, the averaged current-shifts elicited by ethanol are dose-dependent (10 mM: 0.8 ± 0.3 pA, n = 12; 20 mM: 1.8 ± 0.7 pA, n = 18; 30 mM: 2.4 ± 1.2 pA, n = 10; 50 mM: 9.5 ± 1.5 pA, n = 19; 100 mM: 19.0 ± 3.6 pA, n = 12). C, gabazine (20 μM) occluded the enhancement of tonic currents by 50 mM ethanol and revealed the background tonic current. The dotted trace and Gaussian fittings were made from the raw trace as described under Materials and Methods. Tonic currents before and after ethanol application in this case are 43.4 and 62.2 pA, respectively. D, each point corresponds to the tonic currents before (x-axis) and after (y-axis) ethanol application from individual experiment similar to the one shown in C. The points analyzed from twelve experiments were fitted by a straight line pretty well (r = 0.98). The slope of the fitted line is 1.50, well above the unitary line (y = x, the gray dashed line).
Results
Ethanol Decreases Excitability in Thalamic Relay Neurons via GABAA-Rs
We began by investigating whether ethanol modulates tonic firing of action potentials in depolarized thalamocortical relay neurons (Sherman, 2001). The membrane potentials of VB neurons were maintained at around −60 mV by constant current injection. At this membrane potential, VB neurons were generally silent but displayed sustained AP firing in response to depolarizing current steps. Ethanol (50 mM) decreased the excitability of VB relay neurons (Fig. 1A) and shifted the input-output curve to the right. This effect of alcohol to inhibit neuronal excitability is dependent on GABAA-Rs, because in the presence of gabazine (20 μM), a specific GABAA-R antagonist, ethanol had no effect on the input-output relationship (Fig. 1B).
Fig. 1.

Ethanol (50 mM) decreases the excitability of VB neurons via GABAA-Rs. A, representative current clamp traces demonstrate AP firing evoked by current steps (40–160 pA, duration 500 ms) in a VB neuron. After ethanol (50 mM) perfusion, AP firing decreased, and the input-output curve shifted rightwards. B, representative current clamp traces demonstrating AP firing evoked by current steps in the presence of gabazine (20 μM) in another VB neuron. Ethanol (50 mM) perfusion failed to change the input-output curves when GABAA-Rs were blocked. C, exemplar current trace demonstrating that 20 mM ethanol induced no change on firing rate evoked by depolarized current steps. On average, ethanol (20 mM) also makes no change on the numbers of APs (9.6 ± 1.5 versus 9.4 ± 1.6, p > 0.05, n = 5). N.S., not significant. D, pooled data show that 50 mM ethanol significantly reduced the number of evoked action potentials from 10.9 ± 1.4 to 8.5 ± 1.4 (**, p < 0.01, n = 6). E, when GABAA-Rs are blocked by gabazine, 50 mM ethanol fails to inhibit tonic action potential firing (12.5 ± 1.7 to 12.4 ± 1.9, p > 0.05, n = 5).
To facilitate the comparison of firing rates, the amplitude of the current step (500 ms duration) was adjusted in each neuron to induce ∼10 APs, corresponding to a firing frequency of ∼20 Hz. We then compared the number of APs evoked by depolarizing current steps before and after ethanol application. Ethanol (20 mM) failed to decrease firing rate (9.6 ± 1.5 versus 9.4 ± 1.6; p > 0.05, n = 5; Fig. 1C). In contrast, 50 mM ethanol significantly reduced the number of evoked APs from 10.9 ± 1.4 to 8.5 ± 1.4 (p < 0.01, n = 6; Fig. 1D). Preapplication of gabazine completely prevented the inhibitory effects of 50 mM ethanol on firing (p > 0.05, n = 5; Fig. 1E). These results demonstrate that a sedative-hypnotic concentration (50 mM) of ethanol can reduce the excitability of depolarized VB relay neurons mainly through a GABAA-R mechanism.
Ethanol Enhances Tonic Inhibition in VB Neurons
We made whole-cell voltage-clamp recordings to explore the modulation by ethanol of synaptic and extrasynaptic GABAA-Rs. First, we examined whether ethanol induces any change of the tonic current mediated by extrasynaptic GABAA-Rs. At low concentrations (10–30 mM), ethanol induced no significant change of holding current (10 mM: 0.8 ± 0.3 pA, n = 12; 20 mM: 1.8 ± 0.7 pA, n = 18; 30 mM: 2.4 ± 1.2 pA, n = 10), but at higher concentrations (50–100 mM), ethanol elicited a steady and sustained current shift (50 mM: 9.5 ± 1.5 pA, n = 19; 100 mM: 19.0 ± 3.6 pA, n = 12; Fig. 2, A and B). In some experiments, we applied 20 μM gabazine following the application of 50 mM ethanol. Gabazine not only blocked the ethanol-induced current shift but also revealed the underlying tonic inhibition, indicating that the sustained currents induced by ethanol were due to enhancement of tonic inhibition mediated by GABAA-Rs. We measured the tonic currents before and after ethanol perfusion, as shown in Fig. 2C, and the pooled data from all experiments (n = 12) were well fitted by a straight line (r = 0.98), with a slope of 1.50, indicating that 50 mM ethanol enhanced tonic currents by 50%.
Ethanol-Evoked Currents Are Absent in VB Neurons from Gabra4−/− Mice
We have previously demonstrated that extrasynaptic GABAA-Rs are absent from the thalamus of Gabra4−/− mice (Chandra et al., 2006) and, therefore, investigated the actions of ethanol in VB neurons from Gabra4−/− mice and their wild-type littermates (Fig. 3). Ethanol (50 mM) failed to induce any shift in baseline current in VB neurons from α4 knockout mice (1.7 ± 0.9 pA, n = 10). In contrast, wild-type neurons showed measurable current shifts in response to 50 mM ethanol (8.6 ± 2.6 pA, n = 6), which were comparable to ethanol-induced currents recorded from C57BL/6 mice. This difference between the genotypes was highly significant (p < 0.01). These results are consistent with the idea that low concentrations of ethanol selectively enhance the activity of extrasynaptic GABAA-Rs in VB neurons that contain α4/δ subunits (Jia et al., 2005; Chandra et al., 2006).
Fig. 3.

Ethanol-induced current-shift is absent in VB neurons from mice lacking the GABAA-R α4 subunit. A, ethanol (50 mM) evoked a holding current shift (∼10 pA) in a VB neuron from a wild-type mouse. In contrast, ethanol produced no current shift in a VB neuron from a Gabra4−/− mouse. WT, wild-type; KO, knockout. B, bar graph demonstrates that ethanol (50 mM) induced current shifts in wild-type, but not α4 knockout, VB neurons (knockout: 1.7 ± 0.9 pA, n = 10; wild type: 8.6 ± 2.6 pA, n = 6; **, p < 0.01).
Acamprosate Enhances Tonic Inhibition in VB Neurons Only at High Concentrations
Our recent work has shown that taurine is also a potent agonist for thalamic extrasynaptic GABAA-Rs (Jia et al., 2008b). Acamprosate (3-acetamidopropane-1-sulfonic acid) is a taurine analog that has been used to treat alcohol abuse and alcoholism (De Witte et al., 2005; Gupta et al., 2005), although its mechanism of action is not yet fully understood. Given that neurons become hyperexcitable during alcohol withdrawal, we wondered whether acamprosate might reduce excitability and inhibit the neurons via extrasynaptic GABAA-Rs in a way similar to taurine. Our recordings showed that 1 μM acamprosate was unable to induce any change in the tonic current in VB neurons (0.1 ± 1.6 pA, n = 6). Tonic currents were also insensitive to 10 and 100 μM acamprosate (0.3 ± 1.8 pA, n = 5, and 2.8 ± 2.0 pA, n = 6, respectively). At higher concentrations, 200 and 500 μM acamprosate did elicit modest currents (14.7 ± 3.8 pA, p < 0.05, n = 6, and 42.5 ± 6.8 pA, p < 0.01, n = 6, respectively; Fig. 4). These results are consistent with a recent report (Reilly et al., 2008) that shows that acamprosate at low, clinically relevant concentrations has no effect on α4β3δ GABAA-Rs expressed in Xenopus laevis oocytes.
Fig. 4.

The tonic currents evoked by acamprosate at high concentrations. A, a typical recording of a VB neuron in response to 1 μM acamprosate. B, pooled data show that 200 and 500 μM, but not 1 to 100 μM, acamprosate elicits significant current-shift in VB neurons.
High Concentrations of Ethanol Prolong IPSC Decay Time
We investigated next whether ethanol changes the properties of IPSCs. Spontaneous inhibitory synaptic currents are readily observed in VB neurons and can be blocked by gabazine (data not shown), indicating that they are mediated by synaptic GABAA-Rs. The averaged data from this set of experiments (Fig. 5C) show that, at all of the three concentrations that we tested (20, 50, and 100 mM), ethanol induced no significant change in the frequency (percentage change: 20 mM: −3.2 ± 3.6%, n = 9; 50 mM: 3.8 ± 3.1%, n = 16; 100 mM: 7.0 ± 6.6%, n = 8) or the amplitude of spontaneous IPSCs (percentage change: 20 mM: −1.0 ± 2.3%, n = 9; 50 mM: −1.2 ± 2.4%, n = 16; 100 mM: 1.5 ± 4.5%, n = 8). At 100 mM, ethanol did significantly increase the decay time of spontaneous IPSCs by 8.5 ± 2.0% (p < 0.01, n = 8).
Fig. 5.

The effects of ethanol on spontaneous IPSCs. A, a typical recording of spontaneous IPSCs in a VB neuron in the absence and presence of 50 mM ethanol. Averaged spontaneous IPSC traces before (black) and after (gray) ethanol application are superimposed to illustrate the similarity in amplitude and decay time. B, a representative experiment shows that the frequency, amplitude, and decay time of spontaneous IPSCs did not change during the ethanol application for more than 20 min. C, pooled data demonstrate that spontaneous IPSCs are largely insensitive to ethanol. Only 100 mM ethanol significantly increases the decay time of spontaneous IPSCs (**, p < 0.01).
We also compared evoked inhibitory synaptic currents before and after ethanol applications. Gabazine completely blocked evoked IPSCs (data not shown), suggesting that they were mediated by GABAA-Rs, and ethanol (20–100 mM) did not enhance the amplitude of evoked IPSCs (percentage change: 20 mM: 1.7 ± 1.4%, n = 7; 50 mM: 2.3 ± 3.1%, n = 8; 100 mM: 0.3 ± 2.5%, n = 13); only 100 mM ethanol increased the decay time of evoked IPSCs (percentage change: 20 mM: −0.5 ± 1.5%, n = 7; 50 mM: 5.8 ± 2.6%, n = 8; 100 mM: 13.6 ± 2.5%, p < 0.001, n = 13; Fig. 6, A and B). Ethanol (≤50 mM) does not appear to modulate the function of synaptic GABAA-Rs in VB neurons.
Fig. 6.

The effects of ethanol on evoked IPSCs and paired-pulse depression. A, exemplar evoked IPSC traces demonstrating that 100 mM ethanol increase the decay time, but not the amplitude of evoked IPSCs. B, average data show that evoked IPSCs are insensitive to ethanol less than 100 mM. Only 100 mM ethanol increased the decay time of evoked IPSCs significantly (***, p < 0.001). C, sample traces showing paired-pulse responses before (in dark) and after 100 mM ethanol (in gray) application. The superimposed traces clearly show the similar degree of paired-pulse depression. D, bar graph demonstrates that paired-pulse ratio is insensitive to ethanol (20–100 mM), which indicates that the presynaptic GABA release probability is not modified by ethanol.
Ethanol Has No Presynaptic Effect on IPSCs in VB Neurons
In many parts of the CNS, alcohol alters synaptic inhibition via a presynaptic mechanism, often by an increase in frequency. These presynaptic effects have been reported in the amygdala and cerebellum, for example (Siggins et al., 2005; Breese et al., 2006; Roberto et al., 2006; Weiner and Valenzuela, 2006). As mentioned above, we recorded evoked IPSCs of VB neurons and used the well described paired-pulse stimulation protocol (Zalutsky and Nicoll, 1990), which is widely used to detect a change in transmitter release from presynaptic terminals. In response to paired stimuli (separated by 150 ms), IPSCs in VB relay neurons show substantial paired-pulse depression. At all of the concentrations we tested (20–100 mM), ethanol had no effect on the paired-pulse ratio. In contrast, baclofen (5 μM), a GABAB receptor agonist that acts by decreasing GABA release from the presynaptic terminal, significantly increased the paired-pulse ratio from 0.59 ± 0.04 to 0.97 ± 0.17 (p < 0.05, n = 7). These results suggest that ethanol does not modulate synaptic GABA release in the thalamus.
Discussion
Alcohol is one of the most widely abused drugs. Blood alcohol levels between 5 and 20 mM reduce anxiety and produce mild sedation; these levels are commonly associated with light to moderate intoxication associated with social drinking. Blood ethanol (20–50 mM) typically elicits profound sedation, cognitive impairment, amnesia, and loss of motor coordination. Higher concentrations of ethanol (100 mM) in normal individuals cause general anesthesia, decreased ventilation, and risk of death (Deitrich and Harris, 1996; Little, 1999). All of these effects are less pronounced in chronic alcoholics, who routinely tolerate extraordinary high levels of the drug. Many of the pharmacological properties of ethanol are shared by drugs, such as the benzodiazepines and barbiturates, that have long been known to achieve their effects via regulation of the GABAA-Rs, and a large body of evidence implicates GABAA-R as an important target for ethanol in the CNS (Martz et al., 1983; Grobin et al., 1998). However, the mechanisms by which ethanol enhances GABAergic transmission are unclear and may vary substantially among brain regions (Weiner and Valenzuela, 2006).
The main findings of this study are as follows. i) Ethanol (50 mM), but not 20 mM ethanol, reduces firing rate of depolarized VB neurons via GABAA-Rs; ii) ethanol (≥50 mM) enhances tonic inhibition mediated by extrasynaptic GABAA-Rs; iii) ethanol (100 mM), but not 20 to 50 mM ethanol, exerts a postsynaptic action to prolong IPSCs on VB neurons; iv) enhancement of tonic currents by ethanol is absent in VB relay neurons from α4 subunit knockout mice; and v) ethanol has no presynaptic action at inhibitory synapses made by reticular thalamic nucleus neurons on to VB neurons.
In the present study, we demonstrate that ethanol (≤50 mM) does not change the amplitude or decay time of spontaneous IPSCs or evoked IPSCs in VB relay neurons. In contrast, 100 mM ethanol significantly prolongs both spontaneous IPSCs and evoked IPSCs. This finding is consistent with most previous studies on recombinant “synaptic” GABAA-Rs (α1βγ2 and α2βγ2 subtypes) heterologously expressed in cultured cells (Sigel et al., 1993; Mihic et al., 1997) and native synaptic GABAA-Rs in slices of different brain regions (Weiner and Valenzuela, 2006), which suggests that synaptic GABAA-Rs may not act as the direct target of ethanol at subanesthetic concentrations (≤50 mM).
An indirect action of ethanol on GABAA-Rs via presynaptic sites has been observed in many brain regions, including the amygdala (Roberto et al., 2003, 2004; Roberto and Siggins, 2006; Zhu and Lovinger, 2006), the cerebellum (Carta et al., 2004; Hanchar et al., 2005; Ming et al., 2006; Kelm et al., 2007), the hippocampus (Ariwodola and Weiner, 2004; Sanna et al., 2004; Galindo et al., 2005), and the nucleus accumbens (Nie et al., 2000; Crowder et al., 2002). However, we were unable to detect any change of spontaneous IPSC frequency or paired-pulse ratio of evoked IPSCs by ethanol (20–100 mM) in VB relay neurons, which suggests that presynaptic GABA release in the thalamus is insensitive to ethanol.
Recently, GABAA-Rs have been shown to be present at extrasynaptic sites as well as subsynaptic sites (Farrant and Nusser, 2005; Mody, 2005). Several groups have begun to look at the potential for alcohol action at extrasynaptic GABAA-Rs. First of all, two groups reported that recombinant α4β2δ (Sundstrom-Poromaa et al., 2002) and α4/6β3δ (Wallner et al., 2003) GABAA-Rs are extremely sensitive to alcohol, with enhancement of function noted at alcohol concentrations as low as 3 mM. However, the data in these two studies are inconsistent in terms of the dose-dependent ethanol response at α4β2δ subtype, and two other studies failed to observe the low concentration ethanol effects at recombinant α4βδ GABAA-Rs (Borghese et al., 2006; Yamashita et al., 2006). Enhancement of tonic currents mediated presumably by α6βδ, α4βδ, or α1βδ GABAA-Rs has also been observed in hippocampal or cerebellar brain slices (Wei et al., 2004; Hanchar et al., 2005; Glykys et al., 2007). These alcohol effects on α6βδ GABAA-Rs are further exaggerated by a mutation (R100Q) in the α6 subunit of the GABAA-Rs (Hanchar et al., 2005). Contrasting observations on native extrasynaptic GABAA-Rs have been reported by other groups (Carta et al., 2004; Valenzuela et al., 2005; Borghese et al., 2006; Botta et al., 2007; Korpi et al., 2007). The reasons for these discrepancies are still elusive.
In thalamocortical relay neurons and dentate granule cells, α4β2δ GABAA-Rs have been shown to be located at extrasynaptic sites and to mediate tonic inhibition (Belelli et al., 2005; Jia et al., 2005; Chandra et al., 2006; Herd et al., 2008). Small, but measurable, tonic currents have been recorded from β2 knockout mice (Belelli et al., 2005; Herd et al., 2008). The residual tonic currents suggest the possible contribution of the α4β3δ subtype or an unknown subunit compensation induced by gene knockout. Thalamic extrasynaptic GABAA-Rs have also been implicated in the action of hypnotics and anesthetics (Belelli et al., 2005; Cope et al., 2005; Chandra et al., 2006; Jia et al., 2008a).
In this study, we found that tonic currents are not significantly enhanced by ethanol at low concentrations (10–30 mM) associated with social alcohol drinking. It seems that extrasynaptic GABAA-Rs (mainly α4β2δ subtype) expressed in VB relay neurons are not as sensitive to ethanol as recombinant GABAA-Rs expressed in X laevis oocytes (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003) or the extrasynaptic GABAA-Rs of dentate granule cells (Wei et al., 2004). However, VB relay neurons are sensitive to sedative or anesthetic concentrations of ethanol (≥50 mM). At 50 mM, ethanol enhances tonic currents by approximately 50% and decreases the excitability of the relay neurons. The reasons for the discrepancies in this literature are still elusive. They may arise from methodological issues, for example, the differences in expression systems, tissue preparation, or recording temperatures, and one must also consider the possibility that ethanol might indirectly modulate the activity of extrasynaptic GABAA-Rs. For example, ethanol might enhance tonic inhibition by stimulating the nonvesicular release of taurine (Olive, 2002), a potent agonist of these receptors (Jia et al., 2008b).
We have shown previously that gabazine, an antagonist of GABAA-Rs, has little effect on the holding current in VB relay neurons from Gabra4−/− mice (Chandra et al., 2006), which is consistent with a loss of these extrasynaptic receptors. Likewise, 50 mM ethanol failed to evoke any significant current shift in VB neurons from Gabra4−/− mice. At this concentration, ethanol does not change the properties of IPSCs from wild-type and Gabra4−/− mice either. Therefore, ethanol (∼50 mM) selectively enhances the activity of extrasynaptic GABAA-Rs containing α4 subunits. Similar ablation of ethanol-induced tonic currents has also been shown in dentate gyrus neurons from Gabra4−/− mice (Liang et al., 2008).
Tonic inhibition mediated by extrasynaptic GABAA-Rs plays a crucial role in regulating excitability at the level of individual neurons and within neuronal networks (Semyanov et al., 2004). In the present study, we show that the tonic firing in VB relay neurons was decreased by 50 mM ethanol but not by 20 mM. In addition, preapplied gabazine occluded the inhibition of tonic firing by 50 mM ethanol, which indicates that GABAA-Rs are critical for the inhibitory effects of ethanol in the thalamus.
Alcohol not only has sedative effects that can promote onset of sleep but also causes abnormal sleep patterns (Kubota et al., 2002). After drinking alcohol, the time spent in deep (slow-wave) sleep is increased, whereas the time spent in the dreaming state (rapid eye movement sleep) is decreased. There can be little argument concerning the pivotal role of the thalamus in controlling the sleep-wake transition and in sensory transmission. The thalamocortical circuit represents an important potential target for alcohol, as reflected in the electroencephalogram changes that occur during drinking. A previous in vivo study demonstrates that 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol, a hypnotic, works through extrasynaptic GABAA-Rs that contain the δ subunits (Winsky-Sommerer et al., 2007). Our results indicate that extrasynaptic GABAA-Rs may also play a role in the action of sedative concentrations of ethanol (∼50 mM) in the thalamus. Given that sleep disturbances have been suggested to play a reciprocal role in the progression of alcoholism (Brower et al., 1998; Brower, 2001), the extrasynaptic GABAA-Rs in the thalamus may be a potential therapeutic target for the treatment of alcoholism.
Acknowledgments
We thank Carolyn Ferguson for expert assistance. We thank Dr. Angelo Keramidas and Lindsay Tannenholz for the critical reading for the manuscript. We also thank Felix Wolf [Research Animal Resource Center (RARC), Weill Medical College, Cornell University], and the RARC staff for their assistance.
The work was supported by National Institutes of Health Grants AA 16393 (to N.L.H.) and AA 13004 and GM 47818 (to G.E.H.).
Abbreviations
- GABAA-R(s)
GABAA receptor(s)
- IPSC
inhibitory postsynaptic current
- AP
action potential
- CNS
central nervous system
- VB
ventrobasal thalamic nucleus
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF. Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not increased by a single amino acid change (R100Q) in the α6 GABAA receptor subunit. J Pharmacol Exp Ther. 2007;323:684–691. doi: 10.1124/jpet.107.127894. [DOI] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, et al. Basis of the GABAmimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABAA receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Browne SH, Kang J, Akk G, Chiang LW, Schulman H, Huguenard JR, Prince DA. Kinetic and pharmacological properties of GABAA receptors in single thalamic neurons and GABAA subunit expression. J Neurophysiol. 2001;86:2312–2322. doi: 10.1152/jn.2001.86.5.2312. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, et al. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder TL, Ariwodola OJ, Weiner JL. Ethanol antagonizes kainate receptor-mediated inhibition of evoked GABAA inhibitory postsynaptic currents in the rat hippocampal CA1 region. J Pharmacol Exp Ther. 2002;303:937–944. doi: 10.1124/jpet.102.038471. [DOI] [PubMed] [Google Scholar]
- De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Win T, Bittner S. Taurine analogues; a new class of therapeutics: retrospect and prospects. Curr Med Chem. 2005;12:2021–2039. doi: 10.2174/0929867054546582. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15–4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Harrison NL. GABAA receptors in the thalamus: α4 subunit expression and alcohol sensitivity. Alcohol. 2007;41:177–185. doi: 10.1016/j.alcohol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008a;324:1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABAA receptors in the thalamus. J Neurosci. 2008b;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, Rabe H, Bohme I, Aller MI, Wisden W, et al. Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Little HJ. The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol Ther. 1999;84:333–353. doi: 10.1016/s0163-7258(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of γ-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances γ-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2000;293:654–661. [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Interactions between taurine and ethanol in the central nervous system. Amino Acids. 2002;23:345–357. doi: 10.1007/s00726-002-0203-1. [DOI] [PubMed] [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586:965–987. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Lobo IA, McCracken LM, Borghese CM, Gong D, Horishita T, Harris RA. Effects of acamprosate on neuronal receptors and ion channels expressed in Xenopus oocytes. Alcohol Clin Exp Res. 2008;32:188–196. doi: 10.1111/j.1530-0277.2007.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, Valenzuela F, Zhu PJ, Lovinger D, Zhang TA, et al. Actions of acute and chronic ethanol on presynaptic terminals. Alcohol Clin Exp Res. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAA receptor α6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the α6 gene. J Neurosci. 2006;26:3357–3364. doi: 10.1523/JNEUROSCI.4799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relay functions. Prog Brain Res. 2001;134:51–69. doi: 10.1016/s0079-6123(01)34005-0. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P. Recombinant GABAA receptor function and ethanol. FEBS Lett. 1993;324:140–142. doi: 10.1016/0014-5793(93)81380-i. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated a4b2d GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Mameli M, Carta M. Single-amino-acid difference in the sequence of α6 subunit dramatically increases the ethanol sensitivity of recombinant GABAA receptors. Alcohol Clin Exp Res. 2005;29:1356–1357. doi: 10.1097/01.alc.0000171926.66397.94. author reply 1358. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci U S A. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABAA δ-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABAA receptors. J Pharmacol Exp Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]


