Abstract
A multistudy analysis of positron emission tomography data identified three right prefrontal and two left prefrontal cortical sites, as well as a region in the anterior cingulate gyrus, where neuronal activity is correlated with the maintenance of episodic memory retrieval mode (REMO), a basic and necessary condition of remembering past experiences. The right prefrontal sites were near the frontal pole [Brodmann's area (BA) 10], frontal operculum (BA 47/45), and lateral dorsal area (BA 8/9). The two left prefrontal sites were homotopical with the right frontal pole and opercular sites. The same kinds of REMO sites were not observed in any other cerebral region. Many previous functional neuroimaging studies of episodic memory retrieval have reported activations near the frontal REMO sites identified here, although their function has not been clear. Many of these, too, probably have signaled their involvement in REMO. We propose that REMO activations largely if not entirely account for the frontal hemispheric asymmetry of retrieval as described by the original hemispheric encoding retrieval asymmetry model.
Remembering past events involves a large number of processes. Identification of these processes and their interactions continues as a primary goal of today's memory research. One major set of these processes concerns “ecphory” (recovery of stored information); another has to do with what is called episodic “retrieval mode” or REMO (1, 2). REMO refers to a neurocognitive set, or state, in which one mentally holds in the background of focal attention a segment of one's personal past, treats incoming and on-line information as “retrieval cues” for particular events in the past, refrains from task-irrelevant processing, and becomes consciously aware of the product of successful ecphory, should it occur, as a remembered event. In experimental studies of episodic retrieval, the cognitive set and the corresponding brain state are established by task instructions and maintained throughout the task as a task-related variable (3, 4). REMO guides item-related processes (3), such as ecphory, that operate on individual stimulus events within the task. Thus, REMO is a pivotal necessary condition for remembering past events.
The question under scrutiny here concerns the evidence for the brain state that underlies REMO. Specifically, can neuroanatomical regions whose activity is correlated with REMO be identified? Previous research with functional neuroimaging has already provided some positive evidence (2, 3, 5–11). The experimental logic used in these studies is simple (5). A brain region can be regarded as a neuroanatomical correlate of REMO if it (i) becomes differentially active during attempted retrieval of past events and (ii) does so independently of the level of ecphory. The implementation of this logic requires the comparison of regional neuronal activity in three kinds of cognitive task: (i) attempted retrieval combined with a high level of ecphory, (ii) attempted retrieval combined with a low level of ecphory, and (iii) a control condition that neither requires nor allows episodic retrieval but in other respects is similar to the two retrieval conditions. A brain region that shows activity that is equal in tasks i and ii and significantly higher than activity in task iii can be said to be a neuroanatomical correlate of REMO or a “REMO site.”
Previous studies of neuroanatomical correlates of REMO (2, 3, 5–11) have identified putative REMO sites in a number of neocortical locations, primarily in prefrontal cortex and especially in the right hemisphere. This pattern of localization is interesting, because it is similar to retrieval aspects of the hemispheric encoding retrieval asymmetry (HERA) model, according to which, episodic retrieval processes involve right frontal regions more than left (12–14). Such a correspondence in the localization of REMO and HERA suggests that at least some of the right frontal retrieval sites may represent regions directly involved in REMO processes.
The HERA model has been described in terms of the relations among three sets of concepts: anatomy (left vs. right hemisphere), memory systems (episodic vs. semantic), and memory processes (encoding vs. retrieval). Because it has allowed an interesting empirical regularity to emerge from a large collection of experiments, this description has been useful. Yet, it has been clear from the outset (12) that there are problems with HERA that require attention. One kind of problem is that the description of HERA is rather gross, lacking both conceptual and neuroanatomical specificity. Retrieval consists of a number of subprocesses, but nothing in the HERA model speaks to the issue of which of these subprocesses, or which combinations, are reflected in the prefrontal activity. Similarly, prefrontal cortex constitutes a large portion of the brain and comprises many anatomical and presumably functional subregions. It is unlikely that all of them are equally involved in retrieval processes, but HERA has been silent on the issue of topographic specificity. Finally, encoding and retrieval activations have been observed in brain regions other than prefrontal cortex (15, 16), further enhancing the uncertainty about neuroanatomical localization of subprocesses of retrieval.
The results of the previous functional neuroimaging studies of REMO, by differentiating between REMO and ecphory, have clarified HERA with respect to process specificity. But they have been less successful in identifying specific REMO sites in prefrontal regions. Although many previous relevant studies have reported the involvement of frontal pole regions [Brodmann's area (BA) 10] in REMO—bilaterally but with the right-hemisphere bias—other putative REMO sites have shown considerable variability in their frontal topography. The present project was undertaken to redress this shortcoming. Its primary purpose was to make use of the power of multistudy analyses of functional neuroimaging data (17–19) to obtain more sharply converging evidence about specific cortical sites that are involved in REMO. A secondary purpose was to relate the findings to HERA.
Materials and Methods
The logic of the identification of REMO sites described above was applied to the data from four different experiments conducted in our laboratory (2, 3, 5, 11). All experiments shared the defining features of the logic: (i) a task requiring attempted episodic retrieval, combined with a high level of ecphory, (ii) a task requiring attempted episodic retrieval, combined with a low level of ecphory, and (iii) a control condition that neither required nor allowed episodic retrieval but in other respects was comparable to the two retrieval conditions.
Each of our four constituent studies had been approved by the Human Subjects Use Committee of Baycrest Centre, and informed written consent had been obtained. The reader is directed to the published reports of the four constituent studies for a detailed account of the methodologies (see refs. 2, 3, 5, and 11). Subjects were right-handed healthy volunteers (16 females and 37 males; age range of 20–39). The retrieval tasks consisted of old–new recognition judgments about previously visually presented or nonpresented items (words, sentences, or landscapes), administered in a blocked-trial (60-s scans) design. The control tasks consisted of intentional study, silent reading, or semantic judgments of nonstudied items. Table 1 provides, for each study, a description of the three conditions of interest, the number of subjects, the type of materials used, and the density of old–new targets in the two recognition conditions. The data for our analyses were based on a total of 53 subjects and a total of 280 scans. Given such large samples, we had reasons to expect the results to be highly reliable (19, 20).
Table 1.
Summary table for the four component studies
| Study | n | Materials | Recognition
|
Control tasks | |
|---|---|---|---|---|---|
| New, percentage old | Old, percentage old | ||||
| Kapur et al. (5) | 19 | Words | 15 | 85 | Semantic encoding |
| Nyberg et al. (2) | 11 | Words | 0 | 100 | Silent reading |
| Düzel et al. (3) | 12 | Words | 15 | 85 | Semantic encoding |
| Nyberg et al. (11) | 11 | Sentences | 0 | 100 | study sentences |
| Landscapes | 0 | 100 | Study landscapes | ||
The primary data for the multistudy analysis were provided by unprocessed positron emission tomography (PET) scans of regional cerebral blood flow taken during a 60-s scanning window. The PET method used in each of the four studies followed that described by Nyberg et al. (21). For each PET study, images were realigned, normalized, and smoothed (10-mm filter) by using spm96 (Wellcome Department of Cognitive Neurology, London) implemented in matlab (Mathworks, Sherborn, MA) on a sun ultra1 workstation. spm96 for windows was used to perform the statistical analyses. The effects of the conditions (cognitive tasks) on the regional cerebral blood flow at each voxel were estimated with a general linear model wherein the changes in global counts were considered as a covariate. Two specific sets of contrasts were examined. First, a conjunction analysis (22) examined activations that were common to the recognition new control task and the recognition old control task comparisons. Two other contrasts were performed to examine differences between “old” and “new” recognition: old–new and new–old. Unless otherwise stated, the statistical threshold for intensity for all of these comparisons was set to P < 0.05, corrected for multiple comparisons.
Results
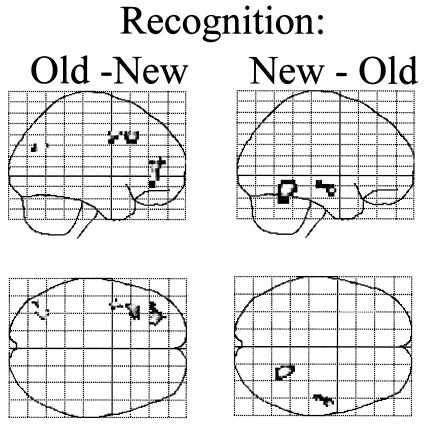
The conjunction analysis of the four-study data identified brain regions that showed greater activity during the recognition tasks (either new or old) compared with the control tasks. One large cluster of activations was observed in the right prefrontal cortex, and two small clusters were observed in the left hemisphere. Table 2 lists the peaks of activity and their statistical properties. The principal peaks of activity in the right hemisphere occurred at an anterior cingulate site, a dorsolateral cortex site, a frontal operculum site, and a frontal pole site (Fig. 1). The two small foci of activation in the left hemisphere consisted of homotopic regions to the right frontal operculum site and the right frontal polar site (Fig. 2). According to the rationale outlined above, we designate these prefrontal and anterior cingulate activations as REMO sites.
Table 2.
REMO activations as identified by the conjunction analysis of the recogition old control task and new control task contrasts
| Talairach coordinates
|
Z value | No. of voxels | Region of activation | BA | REMO site | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Left hemisphere | |||||||
| −26 | 56 | 8 | 4.64 | 135 | Frontal pole | 10 | 1 |
| −34 | 18 | 8 | 4.53 | 43 | Frontal operculum | 45/47 | 3 |
| Right hemisphere | |||||||
| 30 | 46 | 8 | 7.95 | 2,150* | Frontal pole | 10 | 2 |
| 32 | 24 | 0 | 6.52 | Frontal operculum | 45/47 | 4 | |
| 38 | 18 | 32 | 5.56 | Dorsal prefrontal cortex | 8/9 | 5 | |
| 2 | 22 | 40 | 7.78 | Anterior cingulate | 32 | 6 | |
This amount is the total number of voxels for all four activated regions.
Figure 1.
Right hemisphere REMO activations. The numbers in the left upper corners denote the distance in millimeters to the midline in the Talairach system (30). The activation map is displayed onto sagittal sections of a magnetic resonance brain image that conforms to the dimension of the Talairach space.
Figure 2.
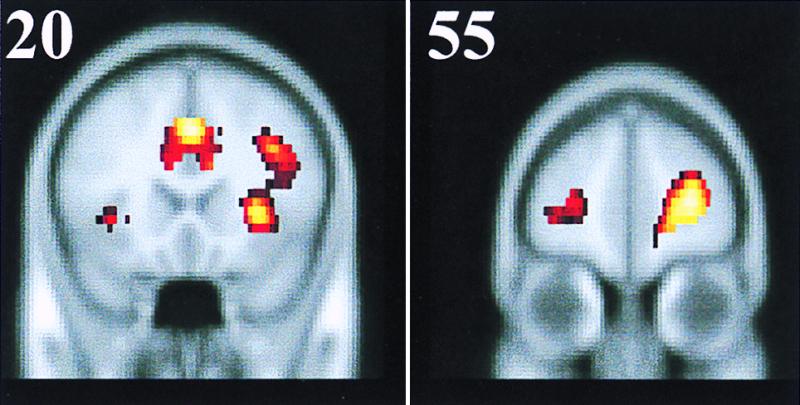
Coronal sections showing the homotopic left and right prefrontal activations for the frontal operculum region (Left) and for the frontal pole region (Right). Numbers in the upper left corners denote the distance in millimeters from the anterior commissure point in the Talairach system (30).
The old–new and new–old contrasts yielded no statistically significant differences in activation at the P < 0.05 threshold (corrected) anywhere in the brain. However, because some previous PET and functional MRI studies examining old–new contrasts have reported greater prefrontal activation during the recognition of old items (7, 9, 23) and because the present conjunction analysis associated several prefrontal regions with REMO, we lowered the height threshold to a Z value of 2.33 (P < 0.01, uncorrected) and the extent threshold to 30 continuous voxels to determine whether activity in any of the identified REMO sites or other prefrontal regions was related to the old–new manipulation.
This more lenient old–new contrast revealed left lateralized activation in three prefrontal regions and in a temporoparietal region. Differential activation in these brain areas in old–new comparisons is in keeping with previous data (24–26). Fig. 3 Left illustrates the location of the activations from the old–new comparison. Only the most anterior left prefrontal activation revealed by the old–new comparison overlapped spatially with any of the sites identified in the conjunction analysis. The new–old comparison revealed right lateralized activation in the middle temporal gyrus and fusiform gyrus, which have been previously reported in novelty detection (27, 28). Fig. 3 Right illustrates the location of these activations. It should be noted that none of the analyses with the liberal threshold revealed any differential activations in right prefrontal and anterior cingulate regions.
Figure 3.
Orthogonal projections of brain regions showing increased activity (P < 0.01, uncorrected) for the old–new (Right) and new–old comparisons (Left). For the old–new comparison, significant activations were observed in left frontal pole region (Z value = 3.35, xyz = −28 42 0), left middle frontal gyrus (Z value = 3.05, xyz = −44 22 36; Z value = 3.29, xyz = −44 6 40), and left temporoparietal region (Z value = 3.37, xyz = −40 −68 28). For the new–old comparison, significant activations were observed in right middle temporal gyrus (Z value = 3.46, xyz = 54 −6 −16) and right fusiform gyrus (Z value = 3.79, xyz = 24 −52 −16).
Retrieval Activations and REMO.
Before we discuss the results of our four-study analysis, we present another set of results from a survey of other previous episodic retrieval activations reported in the literature, because these results speak to the second purpose of our study—relating the identified REMO sites to HERA.
The idea is as follows. In functional neuroimaging studies of episodic retrieval, REMO and ecphory have almost always been confounded, which means that retrieval activations—activations yielded by contrasts between retrieval and nonretrieval (or less effective retrieval) conditions—that were observed in these studies may have reflected REMO, ecphory, or various interactions between these two sets of processes. It is reasonable—even if not strictly logical—to assume that those retrieval activations in previous studies that occurred in the close proximity of the REMO sites identified in the present study represented unidentified, and at the time unidentifiable, REMO sites. Therefore, we undertook a survey for the purpose of identification of these “candidate REMO” sites.
In conducting the survey, we relied on the expanded version of a database described elsewhere (29), as well as on a survey by Cabeza and Nyberg (16). We selected PET and functional MRI studies in which an episodic retrieval task was contrasted with a nonepisodic retrieval task and in which the data were reported in terms of the Talairach system (30). We excluded studies in which retrieval of different materials (e.g., refs. 33 and 34) or two episodic retrieval tasks (e.g., refs. 23, 31, and 32) were compared, because in these studies, REMO is present in both the target and the reference conditions and its anatomic correlates are thereby largely “cancelled out.” We also excluded studies that examined only a single region of interest (e.g., ref. 35). Finally, for studies reporting overlapping data (e.g., refs. 36 and 37) only one article was kept. The 40 PET and functional MRI studies that met these criteria constituted the database for the exercise.
We computed the three-dimensional vector distance, a measure of spatial proximity (38), between each REMO site identified in our four-study analysis and the peak of every single retrieval activation in the database. Activations in the database whose vector distance from a given REMO site was less than 10 mm were classified as a REMO “matches,” and those with vector distances greater than 10 mm but smaller than 16 mm were classified as “near matches.” Of the total of 40 studies in the database, 32 yielded at least one REMO match, and another set of 5 studies yielded at least one near match.
The studies in the database yielding matches and near matches with REMO sites are listed in Table 3, organized by individual sites, the first author of the study, year of the publication, and the identification number of its reference. Table 3 shows at a glance that a large number of previous studies have indeed reported retrieval activations in close proximity to the REMO sites. Moreover, the number of matches and near matches is higher for the right prefrontal and the anterior cingulate sites than for the sites in left prefrontal cortex. The only three studies that did not yield any REMO matches or near matches are those of Kapur et al. (39), Schacter et al. (40), and Bäckman et al. (41).
Table 3.
Listing of PET and functional MRI studies that have produced matches (vector distance 10 mm or less) and near matches (vector distance between 10 and 16 mm) with the six REMO sites described in Table 2
| Site | Talairach coordinates
|
Matches | Near matches | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1, left polar frontal | −26 | 56 | 8 | Blaxton 1996 (49) | Rugg 1998 (9) |
| Buckner 1995 (46) | |||||
| Dalla Barba 1998 (54) | |||||
| Ghaem 1997 (67) | |||||
| Krause 1999 (61) | |||||
| Schacter 1996 (6) | |||||
| Schacter 1997 (68) | |||||
| 2, right polar frontal | 30 | 46 | 8 | Andreasen 1995 (52) | Blaxton 1996 (49) |
| Buckner 1995 (46) | Buckner 1998 (7) | ||||
| Buckner 1996 (47) | Cabeza 1997 (48) | ||||
| Dalla Barba 1998 (54) | Jernigan 1998 (70) | ||||
| Haxby 1996 (43) | Nyberg 1996 (21) | ||||
| Rugg 1997 (69) | Roland 1995 (71) | ||||
| Rugg 1998 (9) | Schacter 1995 (72) | ||||
| Schacter 1996 (6) | Schacter 1997 (68) | ||||
| Wagner 1998 (10) | |||||
| 3, left frontal operculum | −34 | 18 | 8 | Buckner 1995 (46) | Petrides 1995 (74) |
| Buckner 1998 (7) | |||||
| Fujii 1997 (73) | |||||
| Henson 1999 (58) | |||||
| Jernigan 1998 (70) | |||||
| Schacter 1997 (68) | |||||
| Wagner 1998 (10) | |||||
| 4, right frontal operculum | 32 | 24 | 0 | Andreasen 1995 (52) | Blaxton 1996 (49) |
| Andreasen 1996 (42) | Fletcher 1998 (57) | ||||
| Buckner 1995 (46) | Fujii 1997 (73) | ||||
| Buckner 1996 (47) | Jernigan 1998 (70) | ||||
| Buckner 1998 (7) | Roland 1995 (71) | ||||
| Cabeza 1997 (48) | |||||
| Fink 1996 (75) | |||||
| Henson 1999 (58) | |||||
| Johnsrude 1999 (76) | |||||
| McDermott, (77) | |||||
| Nyberg 1996 (21) | |||||
| Owen 1996 (45) | |||||
| Schacter 1997 (68) | |||||
| Shallice 1994 (36) | |||||
| Wagner 1998 (10) | |||||
| 5, right dorsal frontal | 38 | 18 | 32 | Andreasen 1995 (52) | Andreasen 1996 (42) |
| Blaxton 1996 (49) | Haxby 1996 (43) | ||||
| Buckner 1995 (46) | Heckers 1998 (80) | ||||
| Buckner 1996 (47) | Henson 1999 (58) | ||||
| Buckner 1998 (7) | Jernigan 1998 (70) | ||||
| Henke 1997 (78) | Köhler 1998 (53) | ||||
| Johnsrude 1999 (76) | Krause 1999 (61) | ||||
| Moscovitch 1995 (44) | McDermott, (77) | ||||
| Owen 1996 (45) | Petrides 1995 (74) | ||||
| Roland 1995 (71) | Schacter 1995 (72) | ||||
| Wagner 1998 (10) | Taylor 1998 (81) | ||||
| Wiggs 1999 (79) | |||||
| 6, medial anterior cingulate | 2 | 22 | 40 | Andreasen 1995 (52) | Blaxton 1996 (49) |
| Andreasen 1996 (42) | Buckner 1995 (46) | ||||
| Buckner 1996 (47) | Buckner 1998 (7) | ||||
| Cabeza 1997 (48) | Ghaem 1997 (67) | ||||
| Dalla Barba 1998 (54) | Jernigan 1998 (70) | ||||
| Haxby 1996 (43) | Krause 1999 (61) | ||||
| Kim 1999 (82) | Schacter 1997 (83) | ||||
| Köhler 1998 (53) | Taylor 1998 (81) | ||||
| Petrides 1995 (74) | |||||
| Schacter 1997 (68) | |||||
| Shallice 1994 (36) | |||||
| Wagner 1998 (10) | |||||
Studies are identified by the name of the first author, year of publication, and the identifying number in the list of references.
We performed two additional analyses for the sake of completing the overall picture. In the first, we searched for matches and near matches with REMO sites among the encoding activations of the database. With a single exception, very few such matches were observed, suggesting that most of our REMO sites do not seem to be differentially involved in memory encoding. The exception was REMO Site 3, in the left frontal operculum, which was matched by a larger proportion of encoding (36/610) than retrieval (19/824) activations.
The second additional analysis concerned our four constituent studies that provided the data for the multistudy analysis. When we considered them individually, analogously with the studies in the survey of previous retrieval studies, we found that they produced only a total of seven matches or near matches within a 16-mm vector distance. One of the four studies (3) produced no matches, and another one (11) produced one match.
Discussion
The purpose of our study was to identify cerebral regions that are selectively activated in REMO. We used the combined data from four previous experiments whose designs included the requisite conditions. The findings pointed to six specific cortical regions, noted in Table 2, at which recognition testing of old items showed as much differential activation as did testing of new items. One of these sites was in the anterior cingulate (BA 32). The other five were in prefrontal cortex and included two homotopic sites situated bilaterally and symmetrically: one in BA 10 and the other near BA 47/45. The fifth one was a right dorsal prefrontal site near BA 8/9. We conclude that these six REMO sites, in addition to whatever other cognitive functions they may serve, are involved in the establishment and maintenance of a brain state that subserves the cognitive set of episodic memory REMO.
The claims that there are REMO sites and that their brain coordinates are as indicated need to be tempered by several caveats. First, the materials used in the four constituent studies on which our analysis was based were almost exclusively verbal. Therefore, it is not known whether the identified REMO sites represent REMO in general or REMO for verbal materials. It is worth noting, however, that some of the studies reported in Table 3 used nonverbal materials (e.g., refs. 42–45). Thus, it is conceivable that the location of REMO sites has some generality across different materials. Second, the retrieval tasks in all four studies consisted of old–new recognition. Therefore, it is not known whether the sites reflect REMO in general or only that limited to the recognition task. Importantly, though, several of the studies that provided matches in Table 3 included tests of episodic retrieval other than yes/no recognition (44, 46–49). Third, in subtraction analyses of PET data, the location of activated region is always jointly determined by both the target and reference tasks (50). Therefore, it is possible that the localization of the REMO sites in our four-study analysis was specific to the combined control tasks used in our constituent studies. Fourth, as discussed above, REMO comprises different mental activities and corresponding neurocognitive processes. However, the data presented here are silent on the relation between individual REMO sites and the different subprocesses of REMO. The activity detected at any given REMO site may be associated with a single REMO subprocess or some combination of them. The possibilities are many, but because of the lack of relevant data, speculations on this issue would be premature.
Previous PET studies have established the involvement of a number of brain regions other than frontal lobes in episodic memory retrieval (15, 16, 51), thereby encouraging ideas about specific but widely distributed episodic retrieval networks (11, 52–56). Individual components of these networks are assumed to be specialized for specific aspects of retrieval, whereas the connections among them allow for interactions that assure the integrity of the whole process of retrieval. Thus, although the neural activity at REMO sites is correlated with aspects of REMO, the non-REMO retrieval activations in dorsolateral (BA 46/9) portions of prefrontal cortex (57, 58), in medial temporal lobes (29, 59), medial parietal and posterior cingulate regions (36, 60, 61), and other portions of the cerebrum are involved in other, yet to be identified subprocesses of retrieval. Future research undoubtedly will allow a more precise characterization of functional neuroanatomy of REMO as well as non-REMO retrieval processes.
Relation to HERA.
Two REMO sites appeared in the left hemisphere. As Table 2 shows, their Z values were lower, and their spatial extents were considerably smaller than those of the homotopic sites on the right. Such an asymmetry raises the possibility that the left REMO sites do not quite serve the same function as the homotopic right sites. The data currently available, however, do not provide any obvious hints. Additionally, right frontal Site 5 was not duplicated on the left. Consequently, left/right distribution of the “candidate REMO” sites mimics the left/right distribution of retrieval activations as described by HERA. This pattern can therefore be seen as suggesting that the previously reported retrieval asymmetry of HERA could be largely if not wholly accounted for in terms of the distribution and activity of REMO sites. That is, if REMO is held constant, there may be no HERA-like frontal hemispheric asymmetry remaining. If so, whatever empirical regularities retrieval activations have may have to be interpreted in terms other than the simple contrast between encoding and retrieval (12–15, 62).
The finding in our four-study analysis that the old–new contrast, even if only with the liberal threshold, showed activated regions in three left and no right prefrontal regions neatly illustrates what can happen when REMO is held constant. Similar findings, which hitherto have been regarded as “exceptions to HERA” (14), have been reported in other studies in which REMO has been controlled or held constant (24–26).
In sum, the findings of our study point to the necessity for a revision of the retrieval-related aspects of the HERA model, along the following lines: (i) the typically observed left/right asymmetry of retrieval activations seems to be attributable to REMO, either wholly or at least to a considerable extent; (ii) there need not be any frontal hemispheric asymmetry of activations associated with retrieval processes other than REMO; and (iii) REMO sites are located bilaterally with a prominent right hemisphere bias.
This reformulation of the HERA model does not touch the encoding side of HERA. Against the backdrop of some sparse exceptions (e.g., refs. 38 and 63), the hemispheric asymmetry with respect to encoding processes is still clearly present—left prefrontal regions are more involved than right ones. (For recent analyses of encoding processes in relation to HERA, see refs. 64–66.)
We conclude by noting that, although progress is being made in the understanding of what at the beginning was a puzzling hemispheric encoding/retrieval asymmetry, the new story of HERA leaves many previous puzzles standing and has created a number of new ones. We think of such a state of affairs as normal science.
Acknowledgments
We thank R. L. Buckner, S. Kapur, S. Köhler, K. B. McDermott, M. D. Rugg, and A. D. Wagner for comments. This research was supported by an endowment by Anne and Max Tanenbaum in support of research in cognitive neuroscience and by Natural Sciences and Engineering Research Council of Canada Grant A8632 (to E.T.). M.L. is supported by a Tanenbaum postdoctoral fellowship. L.N. is supported by Swedish Council for Research in the Humanistic and Social Sciences.
Abbreviations
- BA
Brodmann's area
- HERA
hemispheric encoding retrieval asymmetry
- PET
positron emission tomography
- REMO
retrieval mode
References
- 1.Tulving E. Elements of Episodic Memory. New York: Oxford Univ. Press; 1983. [Google Scholar]
- 2.Nyberg L, Tulving E, Habib R, Nilsson L, Kapur S, Houle S, Cabeza R, McIntosh A R. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- 3.Düzel E, Cabeza R, Picton T W, Yonelinas A P, Scheich H, Heinze H, Tulving E. Proc Natl Acad Sci USA. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson D I, Buckner R L. Neuron. 1999;22:412–414. doi: 10.1016/s0896-6273(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Craik F I M, Jones C, Brown G M, Houle S, Tulving E. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 6.Schacter D L, Alpert N M, Savage C R, Rauch S L, Albert M S. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner R L, Koutstaal W, Schacter D L, Wagner A D, Rosen B R. Neuroimage. 1998;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- 8.Buckner R L, Koutstaal W, Schacter D L, Dale A M, Rotte M, Rosen B R. Neuroimage. 1998;7:163–175. doi: 10.1006/nimg.1998.0328. [DOI] [PubMed] [Google Scholar]
- 9.Rugg M D, Fletcher P C, Allan K, Frith C D, Frackowiak R S J, Dolan R J. Neuroimage. 1998;8:262–273. doi: 10.1006/nimg.1998.0363. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A D, Desmond J E, Glover G H, Gabrieli J D E. Brain. 1998;121:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- 11.Nyberg, L., Persson, J., Habib, R., Tulving, E., McIntosh, A. R., Cabeza, R. & Houle, S. (2000) J. Cognit. Neurosci12, in press. [DOI] [PubMed]
- 12.Tulving E, Kapur S, Craik F I M, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckner R L. Psychon Bull Rev. 1996;3:149–158. doi: 10.3758/BF03212413. [DOI] [PubMed] [Google Scholar]
- 14.Nyberg L, Cabeza R, Tulving E. Psychon Bull Rev. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 15.Desgranges B, Baron J, Eustache F. Neuroimage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- 16.Cabeza, R. & Nyberg, L. (2000) J. Cognit. Neurosci.12, in press. [DOI] [PubMed]
- 17.Poline J B, Vandenberghe R, Holmes A P, Friston K J, Frackowiak R S. Neuroimage. 1996;4:34–54. doi: 10.1006/nimg.1996.0027. [DOI] [PubMed] [Google Scholar]
- 18.Tulving E, Markowitsch H J. Curr Opin Neurobiol. 1997;7:209–216. doi: 10.1016/s0959-4388(97)80009-8. [DOI] [PubMed] [Google Scholar]
- 19.Fox P T, Parsons L M, Lancaster J L. Curr Opin Neurobiol. 1998;8:178–187. doi: 10.1016/s0959-4388(98)80138-4. [DOI] [PubMed] [Google Scholar]
- 20.Gold S, Arndt S, Johnson D, O'Leary D S, Andreasen N C. Neuroimage. 1997;5:280–291. doi: 10.1006/nimg.1997.0268. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg L, McIntosh A R, Cabeza R, Habib R, Houle S, Tulving E. Proc Natl Acad Sci USA. 1996;93:11280–11285. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price C J, Friston K J. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 23.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S J, Dolan R J. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 24.Düzel E, Yonelinas A P, Mangun G R, Heinze H J, Tulving E. Proc Natl Acad Sci USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugg M D, Walla P, Schloerscheidt A M, Fletcher P C, Frith C D, Dolan R J. Exp Brain Res. 1998;123:18–23. doi: 10.1007/s002210050540. [DOI] [PubMed] [Google Scholar]
- 26.Henson R N A, Rugg M D, Shallice T, Josephs O, Dolan R J. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulving E, Markowitsch H J, Craik F I M, Habib R, Houle S. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 28.Kopelman M D, Stevens T G, Foli S, Grasby P. Brain. 1998;121:875–887. doi: 10.1093/brain/121.5.875. [DOI] [PubMed] [Google Scholar]
- 29.Lepage M, Habib R, Tulving E. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 31.Tulving E, Kapur S, Markowitsch H J, Craik F I M, Habib R, Houle S. Proc Natl Acad Sci USA. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulving E, Markowitsch H J, Kapur S, Habib R, Houle S. NeuroReport. 1994;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Cabeza R, Mangels J A, Nyberg L, Habib R, Houle S, McIntosh A R, Tulving E. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 34.Wagner A D, Poldrack R U, Eldridge L L, Desmond J E, Glover G H, Gabrieli J D E. NeuroReport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- 35.Petersson K M, Elfgren C, Ingvar M. Neuroimage. 1997;6:1–11. doi: 10.1006/nimg.1997.0276. [DOI] [PubMed] [Google Scholar]
- 36.Shallice T, Fletcher P, Frith C D, Grasby P, Frackowiak R S J, Dolan R J. Nature (London) 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher P C, Frith C D, Grasby P M, Shallice T, Frackowiak R S J, Dolan R J. Brain. 1995;118:401–416. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- 38.MacLeod A K, Buckner R L, Miezin F M, Petersen S E, Raichle M E. Neuroimage. 1998;7:41–48. doi: 10.1006/nimg.1997.0308. [DOI] [PubMed] [Google Scholar]
- 39.Kapur N, Friston K J, Young A, Frith C D, Frackowiak R S J. Cortex. 1995;31:99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- 40.Schacter D L, Reiman E, Curran T, Yun L S, Bandy D, McDermott K B, Roediger H L., III Neuron. 1996;17:267–274. doi: 10.1016/s0896-6273(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 41.Bäckman L, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, Langström B. J Cognit Neurosci. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- 42.Andreasen N C, O'Leary D S, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins G L, Ponto L B, Hichwa R D. J Neuropsychiatry Clin Neurosci. 1996;8:139–146. doi: 10.1176/jnp.8.2.139. [DOI] [PubMed] [Google Scholar]
- 43.Haxby J V, Ungerleider L G, Horwitz B, Maisog J M, Rapoport S I, Grady C L. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moscovitch M, Kapur S, Köhler S, Houle S. Proc Natl Acad Sci USA. 1995;92:3721–3725. doi: 10.1073/pnas.92.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen A M, Milner B, Petrides M, Evans A C. J Cognit Neurosci. 1996;8:588–602. doi: 10.1162/jocn.1996.8.6.588. [DOI] [PubMed] [Google Scholar]
- 46.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckner R L, Raichle M E, Miezin F M, Petersen S E. J Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabeza R, Kapur S, Craik F I M, McIntosh A R, Houle S, Tulving E. J Cognit Neurosci. 1997;9:254–265. doi: 10.1162/jocn.1997.9.2.254. [DOI] [PubMed] [Google Scholar]
- 49.Blaxton T A, Bookheimer S Y, Zeffiro T A, Figlozzi C M, Gaillard W D, Theodore W H. Can J Exp Psychol. 1996;50:42–56. doi: 10.1037/1196-1961.50.1.42. [DOI] [PubMed] [Google Scholar]
- 50.Buckner R L, Tulving E. In: Handbook of Neuropsychology. Johnson R J, Baron J C, editors. Vol. 10. Amsterdam: Elsevier; 1995. pp. 439–466. [Google Scholar]
- 51.Cabeza R, Nyberg L. J Cognit Neurosci. 1997;9:1–26. doi: 10.1162/jocn.1997.9.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Andreasen N C, O'Leary D S, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins G L, Boles Ponto L L, Hichwa R D. Proc Natl Acad Sci USA. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Köhler S, Moscovitch M, Winocur G, Houle S, McIntosh A R. Neuropsychologia. 1998;36:129–142. doi: 10.1016/s0028-3932(97)00098-5. [DOI] [PubMed] [Google Scholar]
- 54.Dalla Barba G, Parlato V, Jobert A, Samson Y, Pappata S. Cortex. 1998;34:547–561. doi: 10.1016/s0010-9452(08)70513-6. [DOI] [PubMed] [Google Scholar]
- 55.Bäckman L, Andersson J L R, Nyberg L, Winblad B, Nordberg A, Almkvist O. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- 56.McIntosh A R. In: Memory, Consciousness, and the Brain: The Tallinn Conference. Tulving E, editor. Philadelphia: Psychology; 2000. , in press. [Google Scholar]
- 57.Fletcher P C, Shallice T, Frith C D, Frackowiak R, Dolan R J. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- 58.Henson R N A, Shallice T, Dolan R J. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- 59.Schacter D L, Wagner A D. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 60.Maguire E A, Frith C D, Morris R G. Brain. 1999;122:1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- 61.Krause B J, Schmidt D, Mottaghy F M, Taylor J, Halsband U, Herzog H, Tellmann L, Müller-Gärtner H. Brain. 1999;122:255–263. doi: 10.1093/brain/122.2.255. [DOI] [PubMed] [Google Scholar]
- 62.Nolde S F, Johnson M K, Raye C L. Trends Cognit Sci. 1998;2:399–406. doi: 10.1016/s1364-6613(98)01233-9. [DOI] [PubMed] [Google Scholar]
- 63.Kelley W M, Miezin F M, McDermott K B, Buckner R L, Raichle M E, Cohen N J, Ollinger J M, Akbudak E, Conturo T E, Snyder A Z, et al. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 64.Nyberg L, Cabeza R, Tulving E. Trends Cognit Sci. 1998;2:419–410. doi: 10.1016/s1364-6613(98)01242-x. [DOI] [PubMed] [Google Scholar]
- 65.Kelley W M, Buckner R L, Petersen S E. Trends Cognit Sci. 1998;2:421. doi: 10.1016/s1364-6613(98)01234-0. [DOI] [PubMed] [Google Scholar]
- 66.Buckner R L, Kelley W M, Petersen S E. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 67.Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M. NeuroReport. 1997;8:739–744. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- 68.Schacter D L, Buckner R L, Koutstaal W, Dale A M, Rosen B R. Neuroimage. 1997;6:259–269. doi: 10.1006/nimg.1997.0305. [DOI] [PubMed] [Google Scholar]
- 69.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S J, Dolan R J. NeuroReport. 1997;8:1283–1287. doi: 10.1097/00001756-199703240-00045. [DOI] [PubMed] [Google Scholar]
- 70.Jernigan T L, Ostergaard A L, Law J, Svarer C, Gerlach C, Paulson O B. Neuroimage. 1998;8:93–105. doi: 10.1006/nimg.1998.0350. [DOI] [PubMed] [Google Scholar]
- 71.Roland P E, Gulyás B. Cereb Cortex. 1995;5:79–93. doi: 10.1093/cercor/5.1.79. [DOI] [PubMed] [Google Scholar]
- 72.Schacter D L, Reiman E, Uecker A, Polster M R, Yun L S, Cooper L A. Nature (London) 1995;376:587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]
- 73.Fujii T, Okuda J, Kawashima R, Yamadori A, Fukatsu R, Suzuki K, Ito M, Goto R, Fukuda H. NeuroReport. 1997;8:1113–1117. doi: 10.1097/00001756-199703240-00010. [DOI] [PubMed] [Google Scholar]
- 74.Petrides M, Alivisatos B, Evans A C. Proc Natl Acad Sci USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fink G R, Markowitsch H J, Reinkemeier M, Bruckbauer T, Kessler J, Heiss W. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnsrude I S, Owen A M, Crane J, Milner B, Evans A C. Neuropsychologia. 1999;37:829–841. doi: 10.1016/s0028-3932(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 77.McDermott K B, Ojemann J G, Petersen S E, Ollinger J M, Snyder A Z, Akbudak E, Conturo T E, Raichle M E. Memory. 1999;7:661–678. doi: 10.1080/096582199387797. [DOI] [PubMed] [Google Scholar]
- 78.Henke K, Buck A, Weber B, Wieser H G. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 79.Wiggs C L, Weisberg J, Martin A. Neuropsychologia. 1999;37:103–118. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 80.Heckers S, Rauch S L, Goff D, Savage C R, Schacter D L, Fischman A J, Alpert N M. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 81.Taylor S F, Liberzon I, Fig L M, Decker L R, Minoshima S, Koeppe R A. Neuroimage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- 82.Kim J J, Andreasen N C, O'Leary D S, Wiser A K, Boles Ponto L L, Watkins G L, Hichwa R D. Brain. 1999;122:1069–1083. doi: 10.1093/brain/122.6.1069. [DOI] [PubMed] [Google Scholar]
- 83.Schacter D L, Uecker A, Reiman E, Yun L S, Bandy D, Chen K, Cooper L A, Curran T. NeuroReport. 1997;8:3993–3998. doi: 10.1097/00001756-199712220-00028. [DOI] [PubMed] [Google Scholar]