Summary
Epidemiological studies report that a third of the cases of anaemia in older persons is unexplained. We compared erythropoietin (EPO), inflammatory markers and major comorbidities between older subjects with normal haemoglobin levels and those with different aetiologic forms of anaemia, including unexplained anaemia. Participants were a representative sample of 964 persons aged ≥65 years, with no evidence of bleeding, complete blood tests, and a complete blood count within 6 h of phlebotomy. Anaemia was defined as haemoglobin <130 g/l in men and 120 g/l in women, and classified as a result of chronic kidney disease, iron deficiency, chronic disease and B12/folate deficiency anaemia, or unexplained anaemia based on standard criteria. Of the 124 anaemic participants, 42 (36.8%) had unexplained anaemia. Participants with anaemia of chronic diseases had significantly higher interleukin-6 (IL-6) and C-reactive protein (CRP) levels, while those with unexplained anaemia had significantly lower CRP than non-anaemic controls. Iron deficiency anaemia was characterised by significantly higher EPO levels compared with other types of anaemia and normal haemoglobin, B12 and/or folate deficiency. Unexplained anaemia was characterised by unexpectedly low EPO and low lymphocyte count. Unexplained anaemia is associated with reduced kidney EPO response, low levels of pro-inflammatory markers and low lymphocyte counts.
Keywords: unexplained anaemia, ageing, erythropoietin, inflammation, Invecchiare in Chianti
Older age is an important risk factor for the development of anaemia (Balducci, 2003). Using data from a nationwide representative sample enrolled in the National Health and Nutrition Examination Survey (NHANES) III, Guralnik et al (2004) found that after the age of 50 years, the prevalence of anaemia rose rapidly, to a rate greater than 20% at age 85 years and older, and estimated that as many as 3 million Americans aged 65 years and older are anaemic. Iron and vitamin deficiencies, kidney diseases, and anaemia of chronic disease, alone or in combination, accounted for approximately 2/3 of anaemia cases, but no evident cause was found in 1/3 of the cases. In a study conducted amongst residents of a skilled nursing facility, Artz et al (2004) confirmed that the anaemia remains pathophysiologically ‘unexplained’ in approximately 1/3 of older persons with this condition. Researchers have proposed that the ‘unexplained’ anaemia of older persons may be caused by a chronic pro-inflammatory state, reduced renal function, inadequate erythropoietin (EPO) production, inadequate bone marrow response to EPO, intrinsic defects in bone marrow cell proliferation and/differentiation and reduced erythrocyte survival (Ershler, 2003; Balducci et al, 2005). However, none of these hypotheses have been confirmed in a representative sample of the general population.
To better understand the link between ageing and anaemia, we used data collected in a representative sample of older adults enrolled in the InCHIANTI (Invecchiare in Chianti, ageing in the Chianti area) study to test the hypothesis that the unexplained anaemia of ageing is associated with a marked pro-inflammatory state and low circulating EPO levels. Understanding the pathophysiology of old-age anaemia is important because anaemia in older persons is associated with poor physical functioning and is a strong risk factor for accelerated functional decline, excess healthcare resource utilisation, poor quality of life and reduced survival (Chaves et al, 2002, 2004, 2005; Cesari et al, 2004; Penninx et al, 2003, 2004, 2006; Longo, 2005; Onder et al, 2005).
Methods
Study population
InCHIANTI is a study conducted in the Tuscany Region of Italy (Ferrucci et al, 2000). In 1998, 1270 persons aged ≥65 years were randomly selected from the residents of Greve in Chianti and Bagno a Ripoli, two towns in the Chianti geographical area (Tuscany, Italy). Of the 1155 (90.1%) subjects who agreed to participate, 100 refused to donate a blood sample and 49 were excluded because they had a medical history of cancer, gastrointestinal disease or a medical condition potentially associated with bleeding. The final study population included 964 participants (413 men and 551 women) who had complete data for the analysis presented here. The Institutional Review Board of the Istituto Nazionale Riposo e Cura Anziani (INRCA) approved the study protocol. Participants provided written informed consent to take part and to have their blood samples analysed for scientific purposes. For those unable to fully consent because of cognitive or communication problems, surrogate consents were obtained from close relatives. Fasting blood samples and 24-h urine samples were obtained and aliquots of serum and urine were stored at −80°C and thawed just prior to analysis.
Anaemia related measures
Erythrocyte and differential white blood cell counts, erythrocyte mean cell volume (MCV) and haemoglobin were measured using the Coulter LH 750 (Beckman Coulter, Instrumentation Laboratory, Milan, Italy) within 6 h of phlebotomy. EPO was measured in duplicate using the Advantage EPO chemiluminescence immunoassay (Nichols Institute Diagnostic, San Clemente, CA), which has a sensitivity of 1.2 mU/ml, and a coefficient of variation (CV) <6%. Serum and 24-h urinary creatinine levels were measured using a modified Jaffe method and used to calculate creatinine clearance.
Folic acid and vitamin B12 were measured by a (ICN Pharmaceuticals, SimulTRAC-SNB Radioassay, Orangeburg, NY. Sensitivity and CV: 1.36 nmol/l and 7.1% for folic acid; 18.5 pmol/l and 12.3% for vitamin B12). Vitamin B12 deficiency was defined as a concentration <150 pmol/l and folate deficiency as a concentration <5 nmol/l (Kratz & Lewandrowski, 1998).
Ferritin (Abbott Diagnostics, Abbott Park, IL. Sensitivity 11.2 pmol/l, CV 7%) and serum transferrin receptor (sTfr, Nichols Institute Diagnostics, San Clemente, CA. Sensitivity 0.1 nmol/l, CV 7%) were measured in duplicate using chemiluminescence methods. Iron was assessed by a colorimetric assay (Roche Diagnostics, GmbH, Mannheim, Germany. Sensitivity 0.9 μmol/l, CV < 3%). A serum sTfr/log(ferritin) ratio above 1.5 or a ferritin level <34 pmol/l were considered to indicate iron deficiency. In the absence of direct bone marrow observation, the sTfr/log(ferritin) ratio was considered the most reliable indicator of iron stores (Baillie et al, 2003).
Inflammatory markers
Serum interleukin 6 (IL-6) and tumour necrosis factor α (TNF-α) were measured in duplicate by high sensitivity enzyme-linked immuno-absorbent assays (ELISA; Biosource, Camarillo, CA). Sensitivity was 0.1 pg/ml for IL-6 and 0.09 pg/ml for TNF-α and the CV was <7% for both tests. C-reactive protein (CRP) was measured in duplicate using an ELISA and colorimetric competitive immunoassay (Sensitivity 0.03 mg/l and interassay CV < 5%).
Comorbid diseases
Diseases were ascertained by an experienced clinician according to pre-established criteria that combined information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations and blood tests. Diseases included in the current analysis were coronary heart disease (CHD, including angina and myocardial infarction), congestive heart failure (CHF), stroke, transient ischaemic attack, diabetes, hypertension, severe osteoarthritis (hip, knee or back with pain causing impairment most days of the week) and Parkinson disease (including Parkinsonian syndromes). Diagnostic algorithms were modified versions of those created for the Women’s Health and Ageing Study (Guralnik et al, 1995).
Statistical analysis
Anaemia and its pathophysiological forms were classified by applying sequentially the following criteria: (i) women with haemoglobin <120 g/l and men with haemoglobin <130 g/l were considered anaemic (Izaks et al, 1999); (ii) anaemic participants with creatinine clearance <0.5 ml/s were classified as anaemia of chronic kidney disease. In a previous study, we demonstrated that mild reduction of kidney function with creatinine clearance >0.5 ml/s was not a significant risk factor for anaemia (Ble et al, 2005); (iii) those with Tfr/log(ferritin) ratio >1.5 and/or ferritin <34 pmol/ml were classified as iron deficiency anaemia; (iv) those with low circulating iron (<10.7 μmol/l) and no evidence of iron deficiency (Tfr/log(ferritin) ≤1.5 and ferritin ≥34 pmol/ml) were classified as anaemia of chronic disease, a condition characterised by reduced intestinal absorption and decreased macrophage release of iron, despite adequate or increased total iron stores (Andrews, 2004); (v) anaemic participants with folate (<5 nmol/l) or vitamin B12 (<150 pmol/l) deficiency were classified as ‘B12/folate deficiency’ anaemia (Chaves et al, 2004) and (vi) in accordance with Guralnik et al (1995), participants with anaemia and normal renal function, normal circulating iron and no iron, folate or vitamin B12 deficiency were classified as ‘unexplained anaemia of ageing’.
Variables are reported as mean values ± standard deviations (SD), median and inter-quartile range (Q1–Q3) or percentages, and compared between participants with and without anaemia and across anaemia pathogenic groups by linear and logistic regression models. Variables with skewed distributions were rank-transformed before the analysis. Cytokine levels according to anaemic status and across different forms of anaemia were depicted as box-plots (see description in the figure legends). To obtain age-, sex- and haemoglobin-adjusted EPO values, which are directly interpretable and comparable between groups, log-transformed EPO values were first introduced into a regression analysis performed on the entire study population and included EPO as dependent variable and age and sex as independent variable. The residuals of this linear model were re-scaled and back-transformed to interpretable EPO values. Adjusted EPO values were used to create box-plots and scatterplots. It was important to use age- and haemoglobin-adjusted EPO values in these plots because age and anaemia severity, which are independent factors affecting EPO level, were significantly different between anaemia groups. Note that statistical comparison between groups were performed by fitting covariate-adjusted (including age, sex and haemoglobin) linear regression models on unadjusted, log transformed inflammatory markers and EPO values. All analyses were performed using the SAS statistical package, version 9.1 (SAS Institute Inc., Cary, NC). A P-value <0.05 was considered as a threshold for statistical significance.
Results
The characteristics of the study population according to anaemia status and different forms of anaemia are reported in Table I. The overall prevalence of anaemia was 11% (124/964). Amongst participants with anaemia, nine (7.9%) were classified as chronic kidney disease anaemia, 19 (16.7%) as iron deficiency anaemia, 32 (28.1%) as anaemia of chronic disease, 12 (10.5%) as vitamin B12 and/or folate deficiency and 42 (36.8%) as unexplained anaemia (no evident cause for anaemia). Participants with anaemia were older than those without anaemia (P < 0.0001). Amongst the anaemic, those with anaemia of chronic disease were older than the others (P < 0.0001). Participants with chronic kidney disease anaemia and anaemia of chronic disease had a significantly lower body mass index (BMI) than those in the other groups.
Table I.
Characteristics of the study population, according to anaemia status and pathogenetic form of anaemia (n = 964).
| Non-anaemic (n = 850) |
CKD (n = 9) | IDA (n = 19) | ACD (n = 32) | B12/folate (n = 12) |
UAA (n = 42) | |
|---|---|---|---|---|---|---|
| Percentage of the whole study population (%) | 89 | 11 | ||||
| Percentage of participant with anaemia (%) | 7.9 | 16.7 | 28.1 | 10.5 | 36.8 | |
| Age (years, mean ± SD) | 75 ± 7 | 88 ± 6 | 80 ± 11 | 83 ± 8 | 83 ± 8 | 81 ± 8 |
| Sex (% males) | 42.9 | 44.4 | 68.4 | 53.1** | 33.3 | 23.8 |
| BMI (kg/m2, mean ± SD) | 27.7 ± 4.0 | 23.2 ± 2.6* | 27.0 ± 3.8 | 24.5 ± 3.1* | 26.7 ± 5.3 | 26.7 ± 4.3 |
| Waist circumference (cm, mean ± SD) | 92.9 ± 10.2 | 87.2 ± 8.2 | 90.4 ± 9.4 | 88.6 ± 8.4 | 95.3 ± 10.1 | 89.9 ± 10.9 |
| Creatinine Clearance (mL/s, mean ± SD) | 1.30 ± 0.41 | 0.32 ± 0.11** | 1.21 ± 0.44 | 1.32 ± 0.35 | 1.10 ± 0.34 | 1.18 ± 0.43 |
| Haemoglobin (g/l, mean ± SD) | 140 ± 11 | 102 ± 17** | 107 ± 13** | 111 ± 12** | 119 ± 8** | 116 ± 7** |
| Iron (μmol/l, mean ± SD) | 15.2 ± 4.3 | 9.4 ± 3.5* | 5.8 ± 2.5** | 7.7 ± 2.3** | 15.7 ± 3.4 | 15.3 ± 4.3 |
| Ferritin (pmol/l, median [Q1–Q3 range]) | 256 [133–448] | 242 [139–301] | 20.2 [15.7–22.5] | 256.8 [111.4–307.8] | 222.5 [155.0–465.1] | 267.4 [173.0–462.9] |
| Soluble transferrin receptor (mmol/l, mean ± SD) | 16.5 ± 4.8 | 22.5 ± 8.9** | 33.1 ± 19.9** | 23.0 ± 10.6** | 15.1 ± 3.5 | 16.8 ± 5.1 |
| sTfr/log(Ferritin) ratio (mean ± SD) | 0.29 ± 0.15 | 0.47 ± 0.34* | 1.30 ± 1.1** | 0.49 ± 0.30** | 0.25 ± 0.07 | 0.28 ± 0.11 |
| Iron deficiency (sTfr >1.5 or ferritin <34 pmol/l, %) | 2.9 | 11.1 | 100** | 0 | 0 | 0 |
| Vit B12 (pmol/l, median [Q1–Q3 range]) | 277 [204–374] | 251 [202–818] | 263 [173–402] | 257 [111–308) | 193 [134–285]** | 344 [228–495]* |
| Vit B12 deficiency (<150 pmol/l, %) | 4.8 | 0 | 10.5 | 18.8* | 25.0** | 0 |
| Folic acid (nmol/l, median [Q1–Q3 range]) | 7.3 [5.4–10.0] | 8.4 [6.3–13.6] | 6.6 [4.8–10.4] | 6.1 [4.8–9.1] | 4.1 [3.9–4.8]** | 8.4 [6.3–12.0]* |
| Folate deficiency (<5.0 nmol/l, %) | 18.2 | 11.1 | 26.3 | 28.1 | 91.7** | 0 |
| Coronary heart disease (%) | 7.1 | 22.2 | 5.3 | 15.6 | 8.3 | 4.8 |
| Congestive heart failure (%) | 4.8 | 33.3 | 5.3 | 12.5 | 8.3 | 2.4 |
| Stroke (%) | 3.9 | 22.2 | 10.5 | 15.6** | 0 | 3.9 |
| Diabetes (%) | 11.2 | 0 | 5.3 | 15.6 | 16.7 | 9.5 |
| Hypertension (%) | 60.9 | 55.6 | 63.2 | 68.8 | 58.3 | 71.4 |
| Severe osteoarthritis (%) | 15 | 22.2 | 15.8 | 10 | 16.6 | 50.0** |
| Parkinson disease or syndrome (%) | 0.8 | 0 | 5 | 6.3 | 8.3 | 33.3** |
P < 0.05 compared with non-anaemic, from age- and sex-adjusted linear and logistic regression models.
P < 0.01 compared with non-anaemic, from age- and sex-adjusted linear and logistic regression models.
CKD, anaemia due to chronic kidney disease; IDA, iron-deficiency anaemia; ACD, anaemia of chronic disease; UAA, unexplained anaemia; B12/folate, vitamin B12/folate-deficiency anaemia.
Anaemia from multiple causes was frequent. For example, participants with chronic kidney disease anaemia had also lower iron levels and higher prevalence of iron deficiency while participants with anaemia of chronic disease were more likely to have B12 and folate deficiency than non-anaemic controls. The prevalence of CHD, CHF, stroke and diabetes was higher in the anaemia of chronic disease group than in any other group, although after adjusting for age and sex, the difference was statistically significant only for stroke. Of note, the prevalence of severe osteoarthritis of the hip, knee or spine and the prevalence of Parkinson disease or Parkinsonian syndrome was substantially higher in participants affected by unexplained anaemia (33%) than in the rest of the study population (1.4%).
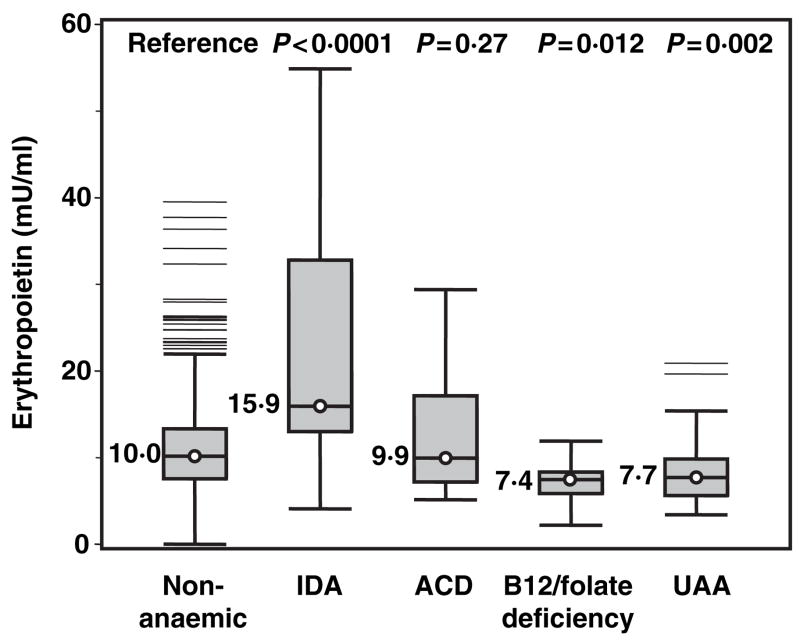
Figure 1 shows box-plots depicting the distribution of IL-6, TNF-alpha and CRP according to anaemia status. The nine participants with chronic kidney disease anaemia had extremely high values of inflammatory markers, severely compromised health status and very low EPO levels and were excluded from this analysis. Participants with anaemia of chronic disease had higher levels of IL-6, TNF-α and CRP than non-anaemic controls, although the difference was not statistically significant for TNF-α. Interestingly, participants with unexplained anaemia had significantly lower CRP than non-anaemic controls, and lower IL-6, TNF-α and CRP values than any other type of anaemia. Figure 2 shows box-plots for the EPO distribution, according to anaemia status. Age-, sex- and haemoglobin-adjusted EPO values are reported to enable direct comparison between groups. Only participants with iron deficiency anaemia showed clearly elevated EPO levels compared with non-anaemic controls. Interestingly, in spite of the anaemia, participants with B12 and/or folate deficiency and those with unexplained anaemia had adjusted EPO levels that were significantly lower than the non-anaemic controls.
Fig 1.
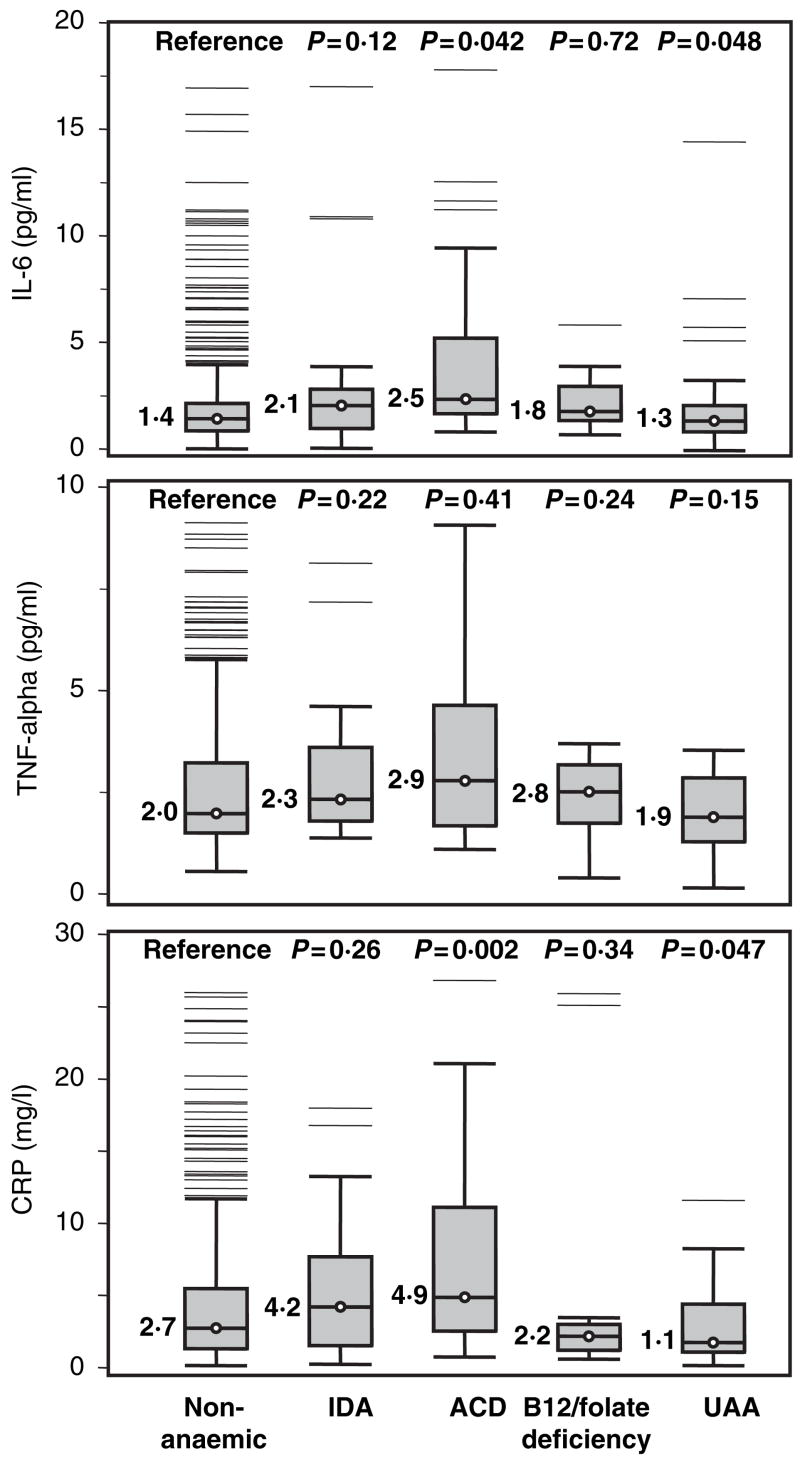
Box-plots depicting interleukin-6 (IL-6), tumour necrosis factor α (TNF-α) and C-reactive protein (CRP) distributions in non-anaemic controls and participants with different clinical forms of anaemia. P-values were calculated from age- and sex-adjusted linear regression models. The central line within each box indicates the median value of the distribution and the top and bottom box limits are the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme points located within 1.5 times the interquartile distance below and above the box. Points outside this range are singularly plotted as outliers. IDA, iron deficiency anaemia; ACD, anaemia of chronic disease; UAA, unexplained anaemia.
Fig 2.
Box-plots depicting age-, sex- and haemoglobin-adjusted erythropoietin (EPO) levels according to anaemia status in non-anaemic controls and in participants with different clinical forms of anaemia. P-values were calculated from age-, sex- and haemoglobin-adjusted linear regression models. Description of the box-plots and abbreviations are given in Fig 1.
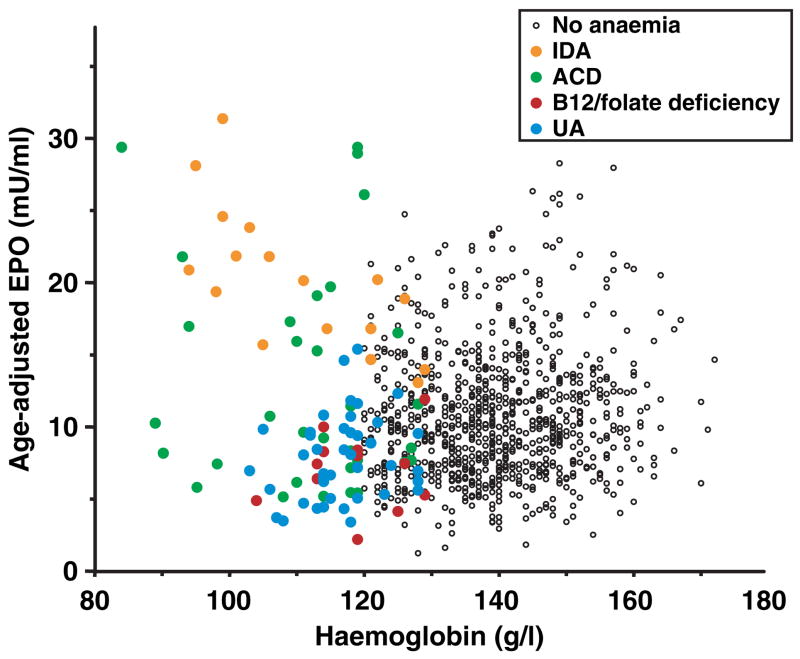
The severity of anaemia and the magnitude of the EPO compensatory response to anaemia clearly identified different pathophysiological forms of anaemia (Fig 3). Severe anaemia (haemoglobin <100 g/l) was mostly accounted for by iron deficiency and chronic disease anaemia, but while iron deficiency anaemia was associated with high compensatory EPO level, in chronic disease anaemia the EPO response was bimodal (low in some persons and high in others). B12 and/or folate deficiency and unexplained anaemia tended to be mild in severity and showed little EPO compensatory response. Indeed, EPO levels in vitamin B12 deficiency and unexplained anaemia were even lower than in non-anaemic controls.
Fig 3.
Scatterplot of the relationship between haemoglobin and erythropoietin (EPO) levels. EPO values are age and sex adjusted to enable comparison across the different forms of anaemia, which are indicated by circles of different colours (see legend). Abbreviations are given in Fig 1.
Some of the haematological characteristics of the different forms of anaemia compared with the non-anaemic controls are summarised in Table II. After adjusting for age and sex, when compared with non-anaemic controls, iron deficiency anaemia and anaemia of chronic disease were associated with lower MCV, and B12 and/or folate deficiency anaemia with higher MCV. Of note, unexplained anaemia was associated with low leucocyte count, which was mostly accounted for by a low lymphocyte count. Participants with iron deficiency anaemia had significantly more platelets than non-anaemic controls, as would be expected.
Table II.
Complete blood count parameters according to anaemia status and pathogenetic form of anaemia (n = 964).
| Non-anaemic (n = 850) | CKD (n = 9) | IDA (n = 19) | ACD (n = 32) | B12/folate (n = 12) | UAA (n = 42) | |
|---|---|---|---|---|---|---|
| Erythrocytes (1012/l; mean ± SD) | 4.56 ± 0.37 | 3.48 ± 0.54** | 3.94 ± 0.94** | 4.0 ± 0.45** | 3.86 ± 0.43** | 3.93 ± 0.40** |
| Leukocytes (109/l; mean ± SD) | 6.2 ± 1.6 | 7.4 ± 3.5 | 6.5 ± 1.8 | 5.7 ± 1.0* | 5.6 ± 0.7 | 5.5 ± 1.5* |
| Neutrophils (109/l; mean ± SD) | 3.88 ± 3.00 | 4.44 ± 2.84 | 4.22 ± 1.44 | 3.67 ± 0.92 | 3.51 ± 0.68 | 3.29 ± 106 |
| Lymphocytes (109/l; mean ± SD) | 1.95 ± 1.42 | 2.01 ± 1.02 | 1.65 ± 0.47 | 1.52 ± 0.44 | 1.52 ± 0.47 | 1.26 ± 0.45** |
| Mean corpuscular volume (fl; mean ± SD) | 90.8 ± 4.5 | 91.5 ± 6.9 | 84.7 ± 7.6** | 86.2 ± 6.8** | 95.6 ± 6.6** | 90.5 ± 6.7 |
| Platelets (109/l; mean ± SD) | 223 ± 60 | 219 ± 78 | 291 ± 106** | 219 ± 59 | 218 ± 76 | 239 ± 84 |
| Mean platelet volume (fl) | 11.1 ± 1.0 | 10.9 ± 1.2 | 10.6 ± 0.7* | 10.7 ± 0.2* | 11.0 ± 1.2 | 10.8 ± 1.0 |
P < 0.05 compared to non-anaemic, from age- and sex-adjusted linear regression models.
P < 0.01 compared to non-anaemic, from age- and sex-adjusted linear regression models.
CKD, anaemia due to chronic kidney disease; IDA, iron-deficiency anaemia; ACD, anaemia of chronic disease; UAA, unexplained anaemia; B12/folate, vitamin B12/folate-deficiency anaemia.
Discussion
To gain insight into the pathophysiology of the unexplained anaemia of ageing, we compared levels of EPO and inflammatory markers between older persons without anaemia and those with different pathophysiological forms of anaemia, including anaemia that remained unexplained. Unexplained anaemia was found to be characterised by low EPO level, low levels of pro-inflammatory markers and low lymphocyte counts. These findings support the hypothesis already proposed in the literature (Guralnik et al, 2005), that the unexplained anaemia of ageing is associated with low EPO levels and clearly indicates that the reduced EPO response to anaemia is not attributable to a pro-inflammatory state. Although the cause of the insufficient EPO response could not be defined, our study provided clues that may help generate preliminary hypotheses concerning the mechanism leading to unexplained anaemia in older persons.
The prevalence of severe osteoarthritis and Parkinson disease were, respectively, over three times (50% vs. 15.4%) and 20 times (33.3% vs. 1.4%) higher in participants with unexplained anaemia than in the rest of the study population. It can also be hypothesised that the same underlying mechanism that causes arthritis and Parkinson disease may also cause anaemia. For example, excessive and unopposed oxidative stress may cause anaemia (Dallalio & Means, 2003; Lang et al, 2005, 2006), is associated with cartilage damage, and is the main cause of neuronal death in the substantia nigra in Parkinson disease. Thus, it is possible that an inadequate dietary intake of antioxidants, often found in older adults, possibly associated with excessive oxidative stress, may be the main cause of unexplained anaemia in older persons (Rodriguez et al, 2001). This hypothesis is consistent with studies showing that chronic under nutrition in older persons is associated with a relative downregulated immune response and low lymphocyte counts (Lesourd, 2004), which were the two main correlates of unexplained anaemia in our study.
The finding that vitamin B12 or folate deficiency anaemia were characterised by low EPO levels confirms a previous observation (Remacha et al, 1997). Interestingly, rather than a diminished EPO production, low EPO level may reflect an increased consumption secondary to the intense bone marrow erythroid hyperplasia and ineffective erythropoiesis (failure of delivery of red cells to the peripheral blood), which are characteristic features of B12 and folate deficiency anaemia (Koury & Ponka, 2004).
The most important limitation of this study is the relatively limited sample size, which is especially problematic for the comparison of different pathophysiological forms of anaemia. However, since the prevalences of the different pathophysiological forms of anaemia, particularly the prevalence of unexplained anaemia, are remarkably similar to those reported by other studies (Artz et al, 2004; Guralnik et al, 2004), we are confident that our findings have strong external validity. In fact, not only was our study performed on a representative sample of the older population in the community, but also the different forms of anaemia were classified using measures and criteria that, with the exception of bone marrow inspection, are considered state of the art in clinical practice. It is conceivable that the availability of a bone marrow examination and other tests would have revealed that some of the cases of unexplained anaemia were due to relatively rare causes, such as myelodysplasia and hypothyroidism. However, it is unlikely that these additional diagnoses would account for 30% of the cases of anaemia in this population.
By characterising the unexplained anaemia of ageing as a form of mild anaemia associated with reduced EPO response, low circulating levels of pro-inflammatory markers, low lymphocyte counts and frequent association with severe osteoarthritis and Parkinsonian symptoms, this study represents a step forward in the comprehension of the pathophysiology of this highly prevalent and important syndrome (Rozzini et al, 2005). Whether the anaemia of ageing is associated with excessive oxidative stress and/or poor dietary intake should be verified in future studies. In the meantime, in patients with anaemia of ageing that remains unexplained in spite of comprehensive clinical testing, the administration of EPO and nutritional supplementation should be considered as treatment options.
Acknowledgments
The InCHIANTI study was supported as a ‘targeted project’ (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336), an unrestricted grant from Ortho Biotech, and by the US National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336). None of the sponsoring Institutions played a role in the collection, analysis, presentation and interpretation of the data reported in this article.
L. Ferrucci was the primary writer of this article. L. Ferrucci, J.M. Guralnik and DL Longo designed the study and supervised the statistical analysis. S Bandinelli, F. Lauretani and A.M. Corsi collected the data, discussed the analytical findings and extracted the clinical data from the charts. Carmelinda Ruggiero and William B. Ershler revised the first draft of the manuscript and provided feedback that was essential for the discussion. All authors have critically reviewed the manuscript and provided comments several times. Luigi Ferrucci, Jack M Guralnik, William B. Ershler, Carmelinda Ruggiero and Dan L Longo were employees of the National Institute on Aging, Intramural Research Program when this manuscript was prepared and submitted.
References
- Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. The Journal of Clinical Investigation. 2004;113:1251–1253. doi: 10.1172/JCI21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz AS, Fergusson D, Drinka PJ, Gerald M, Bidenbender R, Lechich A, Silverstone F, McCamish MA, Dai J, Keller E, Ershler WB. Mechanisms of unexplained anemia in the nursing home. Journal of the American Geriatrics Society. 2004;52:423–427. doi: 10.1111/j.1532-5415.2004.52116.x. [DOI] [PubMed] [Google Scholar]
- Baillie FJ, Morrison AE, Fergus I. Soluble transferrin receptor: a discriminating assay for iron deficiency. Clinical and Laboratory Haematology. 2003;25:353–357. doi: 10.1046/j.0141-9854.2003.00548.x. [DOI] [PubMed] [Google Scholar]
- Balducci L. Epidemiology of anemia in the elderly: information on diagnostic evaluation. Journal of the American Geriatrics Society. 2003;51:S2–S9. doi: 10.1046/j.1532-5415.51.3s.4.x. [DOI] [PubMed] [Google Scholar]
- Balducci L, Hardy CL, Lyman GH. Hemopoiesis and aging. Cancer Treatment and Research. 2005;124:109–134. doi: 10.1007/0-387-23962-6_6. [DOI] [PubMed] [Google Scholar]
- Ble A, Fink JC, Woodman RC, Klausner MA, Windham BG, Guralnik JM, Ferrucci L. Renal function, erythropoietin, and anemia of older persons: the InCHIANTI study. Archives of Internal Medicine. 2005;165:2222–2227. doi: 10.1001/archinte.165.19.2222. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Lauretani F, Russo CR, Carter C, Bandinelli S, Atkinson H, Onder G, Pahor M, Ferrucci L. Hemoglobin levels and skeletal muscle: results from the In-CHIANTI study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2004;59:249–254. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? Journal of the American Geriatrics Society. 2002;50:1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? Journal of the American Geriatrics Society. 2004;52:1811–1816. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- Dallalio G, Means RT. Effects of oxidative stress on human erythroid colony formation: modulation by gamma-interferon. The Journal of Laboratory and Clinical Medicine. 2003;141:395–400. doi: 10.1016/S0022-2143(03)00041-6. [DOI] [PubMed] [Google Scholar]
- Ershler WB. Biological interactions of aging and anemia: a focus on cytokines. Journal of the American Geriatrics Society. 2003;51:S18–S21. doi: 10.1046/j.1532-5415.51.3s.2.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of the American Geriatrics Society. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty MD. The Women’s Health and Aging Study. Health and social characteristics of older women with disability. National Institute of Aging; Bethesda, MD: 1995. [Google Scholar]
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ershler WB, Schrier SL, Picozzi VJ. Anemia in the elderly: a public health crisis in hematology. Hematology. American Society of Hematology. Education Program. 2005;2005:528–532. doi: 10.1182/asheducation-2005.1.528. [DOI] [PubMed] [Google Scholar]
- Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annual Review of Nutrition. 2004;24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- Kratz A, Lewandrowski KB. Case records of the Massa-chusetts General Hospital. Weekly clinicopathological exercises. Normal reference laboratory values. The New England Journal of Medicine. 1998;339:1063–1072. doi: 10.1056/NEJM199810083391508. [DOI] [PubMed] [Google Scholar]
- Lang KS, Lang PA, Bauer C, Duranton C, Wieder T, Huber SM, Lang F. Mechanisms of suicidal erythrocyte death. Cellular Physiology and Biochemistry. 2005;15:195–202. doi: 10.1159/000086406. [DOI] [PubMed] [Google Scholar]
- Lang F, Lang KS, Lang PA, Huber SM, Wieder T. Mechanisms and significance of eryptosis. Antioxidants & Redox Signaling. 2006;8:1183–1192. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- Lesourd B. Nutrition: a major factor influencing immunity in the elderly. The Journal of Nutrition, Health & Aging. 2004;8:28–37. [PubMed] [Google Scholar]
- Longo DL. Closing in on a killer: anemia in elderly people. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60:727–728. doi: 10.1093/gerona/60.6.727. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Cesari M, Bandinelli S, Lauretani F, Bartali B, Gori AM, Pahor M, Ferrucci L. Anemia is associated with depression in older adults: results from the In-CHIANTI study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60:1168–1172. doi: 10.1093/gerona/60.9.1168. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. The American Journal of Medicine. 2003;115:104–110. doi: 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, Guralnik JM, Ferrucci L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. Journal of the American Geriatrics Society. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2006;61:474–479. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- Remacha AF, Bellido M, Garcia-Die F, Marco N, Ubeda J, Gimferrer E. Serum erythropoietin and erythroid activity in vitamin B12 deficiency. Haematologica. 1997;82:67–68. [PubMed] [Google Scholar]
- Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. Journal of Critical Care. 2001;16:36–41. doi: 10.1053/jcrc.2001.21795. [DOI] [PubMed] [Google Scholar]
- Rozzini R, Sabatini T, Cassinadri A, Boffelli S, Ferri M, Barbisoni P, Frisoni GB, Trabucchi M. Relationship between functional loss before hospital admission and mortality in elderly persons with medical illness. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60:1180–1183. doi: 10.1093/gerona/60.9.1180. [DOI] [PubMed] [Google Scholar]