Summary
Mast cell mediator release represents a pivotal event in the initiation of inflammatory reactions associated with allergic disorders. These responses follow antigen-mediated aggregation of immunoglobulin E (IgE)-occupied high affinity receptors for IgE (FcεRI) on the mast cell surface,a response which can be further enhanced following stem cell factor-induced ligation of the mast cell growth factor receptor KIT. Activation of tyrosine kinases is central to the ability of both FcεRI and KIT to transmit downstream signaling events required for the regulation of mast cell activation. Whereas KIT possesses inherent tyrosine kinase activity, FcεRI requires the recruitment of Src family tyrosine kinases and Syk to control the early receptor-proximal signaling events. The signaling pathways propagated by these tyrosine kinases can be further upregulated by the Tec kinase Bruton's tyrosine kinase (Btk) and downregulated by the actions of the tyrosine Src homology 2 domain-containing phosphatase 1 (SHP1) and SHP2. In this review, we discuss the regulation and role of specific members of this tyrosine kinase network in KIT and FcεRI-mediated mast cell activation.
Keywords: mast cells, FcεRI, KIT, Btk, Lyn, Fyn
Introduction: mast cell activation
Mast cells are tissue-resident cells of hematopoietic lineage which are derived from CD13+CD34+KIT(CD117)+ bone marrow progenitors (1). Although mast cells play an important role in the innate immune response elicited as part of the body's host defense reaction to invading pathogens (2,3), these cells are also responsible for the detrimental exaggerated reactions to antigen observed in anaphylaxis, atopy, and rhinitis (4). These conditions are induced following the release of potent inflammatory mediators as a consequence of receptor-mediated mast cell activation (5). Such mediators include the following: (i) granule-associated mediators, including histamine, serotonin (5-hydroxytryptamine), and a variety of proteases and peptidases which are pre-synthesized and released following fusion of the secretory granules with the cytosolic membrane; (ii) eicosanoids such as prostaglandin D2 (PGD2) and leukotriene C4 (LTC4), which are generated and released following activation of cytosolic phospholipase A2 (PLA2), which catalyzes the hydrolysis of arachidonyl-containing phospholipids to yield the eicosanoid precursor, arachidonic acid; and (iii) cytokines including interleukin-2 (IL-2), IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor α (TNFα), and chemokines including CCL-2, CCL-3, CCL-5, and CXCL8, which are generated following transcriptional activation (6-9).
Although multiple receptors are capable of mediating or modifying mast cell activation (10,11), the major receptor responsible for the clinical manifestations of this response is FcεRI, the high affinity receptor for immunoglobulin E (IgE)(6,12,13). The FcεRI expressed on mast cells is a complex of four receptor subunits (Fig. 1): a single transmembrane-spanning α subunit that contains the IgE-binding site within its extracellular domain; the β chain that has four membrane spans and an immunoreceptor tyrosine-based activation motif (ITAM) within the C-terminal cytosolic domain; and two single transmembrane spanning γ chains that exist as a homodimer (6). As with the β chain, the γ chain COOH-terminal cytosolic domain also contains an ITAM.
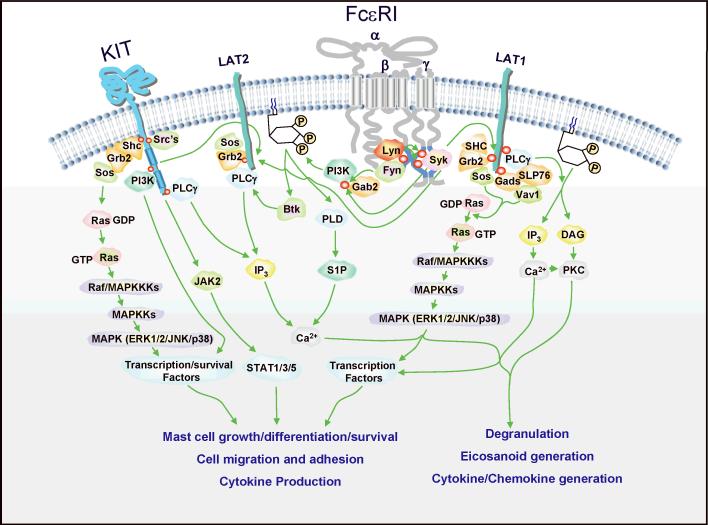
Fig. 1. Signaling pathways leading from activated KIT and aggregated FcεRI to mast cell responses.
Antigen-induced aggregation of IgE-occupied FcεRI induces activation of the Src family tyrosine kinase, Lyn, whereas SCF-induced KIT dimerization induces activation of its intrinsic KIT kinase activation. Phosphorylation of tyrosine residues within the receptor chains thus allows recruitment of SH2 domain-containing signaling molecules. In the case of FcεRI, Syk is recruited via ITAMs contained in the γ chain-cytoplasmic domains. Resulting activation of Syk, following its phosphorylation, leads to consequential phosphorylation of the transmembrane adapter molecules LAT1 and LAT2 (NTAL/LAB). Upon phosphorylation, these proteins serve as scaffolds for multimolecular signaling complexes comprising various cytosolic adaptor molecules such as Gads, Grb2, SLP76, and SHC, GTP exchangers including Sos and Vav1 and the signaling enzymes PLCγ1 and PLCγ2. PLCγ catalyzes the hydrolysis of PtdIns2 to yield diacylglycerol (DAG) and IP3, which, respectively, result in the activation of PKC and the liberation of intracellular calcium. Following depletion of the intracellular calcium stores, the calcium signal is maintained by store operated calcium entry (not depicted). These signals lead to mast cell degranulation and eicosanoid generation and also contribute to activation of transcription factors required for cytokine and chemokine production. In parallel to this pathway, PI3K is activated following binding to Gab2 upon the phosphorylation of this cytosolic adapter molecule by Fyn and/or Syk, phosphorylation of the p85α adapter subunit of PI3K, and activation of the catalytic subunit by small GTP-binding proteins. In the case of KIT, the p85α subunit directly binds to the phosphorylated molecule. The subsequent formation of membrane associated PtdInsP3 results in the recruitment of PH domain-containing signaling molecules such as Btk, PLD, and potentially others. PI3K-regulated pathways serve to enhance/maintain LAT/PLCγ1-regulated degranulation and, as depicted for KIT, regulate mast cell growth, differentiation, survival, migration, adhesion, and cytokine production. KIT- and FcεRI-mediated activation of the Ras-Raf-MAPK pathway following Sos- and Vav-regulated GDP-GTP exchange of Ras also contributes to these processes. The MAPK ERK1/2 also regulates PLA2 activation, which leads to the liberation of arachidonic acid for the generation of eicosanoids (not depicted). The role of LAT2 in mast cell activation is still enigmatic; however, it has been proposed to both upregulate and downregulate antigen-mediated responses. It does appear to be required for the ability of KIT to enhance FcεRI-dependent degranulation. Due to the complexity of the signaling cascades depicted, some of the intermediary steps involved in these processes could not be illustrated in this figure. For further details, readers are referred to other recent review articles (6,7,11).
Phosphorylation of these ITAMs at their canonical tyrosine residues creates novel sites for the binding of a variety of other signaling proteins, through the recognition of this modification by phospho-tyrosine binding regions (domains), such as the Src homology 2 (SH2) domain, found in many proteins (6,11,14,15). However, the β and γ ITAMs are functionally distinct. The γ ITAM provides the ability to initiate downstream signals, whereas the β ITAM functions to amplify these signals (12,16,17). This functional divergence is defined, at least in part, by the unique subsets of proteins that interact with the respective ITAMs (6,11,14,18,19). Thus, this multimeric receptor is capable of initiating and regulating IgE-dependent mast cell activation in a manner that elicits potent but controlled cellular responses.
In addition to the FcεRI, a number of other receptors that regulate or modify mast cell function are expressed on mast cells. The most critical of these receptors is KIT (Fig. 1), the receptor for stem cell factor (SCF), also termed steel factor or KIT ligand, which is responsible for mast cell development and homeostasis (9,20-24). As with specific G protein-coupled receptor (GPCR) ligands such as adenosine, PGE2 and C3a (7,10), SCF can also substantially potentiate antigen-mediated mast cell activation (25). Both KIT- and FcεRI-mediated signaling are intimately controlled by the highly regulated balance between the phosphorylated and non-phosphorylated states of component signaling proteins (Fig. 1). Whereas latent or intermediary signaling processes are primarily controlled by serine/threonine or lipid phosphorylation, immediate receptor-proximal events are primarily controlled by tyrosine phosphorylation as regulated by specific tyrosine kinases. These events modify critical signaling proteins, which either results in enhanced activation or allows inducible binding to other interacting signaling molecules. Hence, tyrosine kinases can be considered as the central driving force for critical downstream processes (Fig. 1) leading to mast cell activation. In this review, we thus discuss the receptor-mediated activation of specific classes of tyrosine kinases in mast cells and the roles that these kinases play in mast cell biology. However, multiple other downstream signaling processes are also involved in receptor-mediated mast cell activation. Readers are referred to a number of recent review articles for further discussion of these events (6, 7, 10, 11, 13, 26, 27).
Mast cell tyrosine kinases
Role of KIT in mast cell activation
KIT (CD117) is a protein of approximately 145 kDa whose expression is largely but not exclusively restricted to cells of hematopoietic lineage and melanocytes (28, 29) (Fig. 2). It is a type III tyrosine kinase and member of the transmembrane receptors with tyrosine kinase activity superfamily (28, 30-34). Dimerization of KIT, following ligation with SCF, induces activation of the inherent catalytic activity associated with the split tyrosine kinase domain contained within the cytosolic COOH-terminal region (28, 33). The major substrates for activated KIT are specific tyrosine residues contained within the cytosolic domain of KIT (Fig. 2). In human KIT, these include tyrosine (Y)568 and Y570 in the juxtamembrane region, Y703, Y721, Y730, and Y747 in the linker region between split catalytic domains, Y823 and Y900 in the COOH-terminal catalytic domain, and Y936 in the COOH-terminus (28). However, as discussed below, other signaling proteins are also directly phosphorylated by KIT (28, 30, 32). The phosphorylated residues on KIT provide docking sites for associating signaling molecules required for the biological responses elicited by KIT (Figs 1 and 2). These include phospholipase Cγ1 (PLCγ1)[phospho (p)Y936], the p85 subunit of phosphoinositide 3-kinase (PI3K) (pY721), the Src family tyrosine kinases Lyn and Fyn (pY568 / pY570), the cytosolic adapter molecules Grb2 (pY703 and pY936) and SHC (py568 / pY570), and the tyrosine SH2 domain-containing phosphatase 1 (SHP1) (pY568 and pY570) (28,30) (Fig. 2). As with the FcεRI, early events associated with activated KIT likely occur in specialized gycolipid-enriched membrane microdomains conceptually termed lipid rafts (35). Thus, the binding of critical signaling molecules to phosphorylated KIT, following auto-/trans-phosphorylation, allows recruitment of these proteins to the membrane-associated receptor signaling complex resident within these domains.
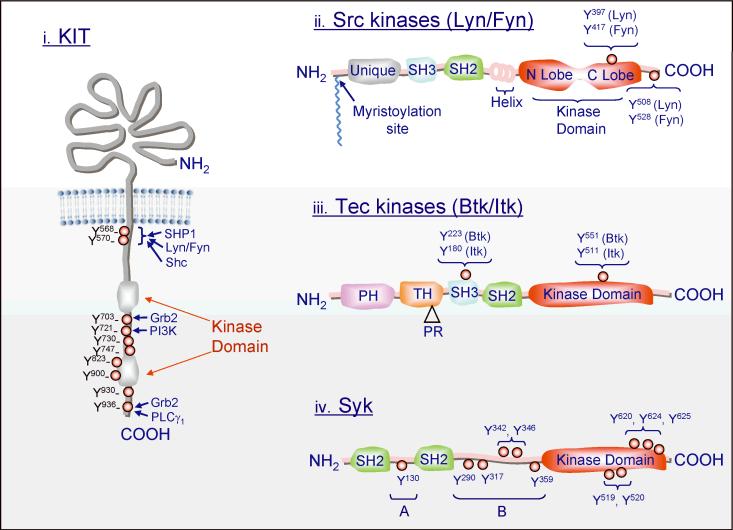
Fig. 2. Basic structures of the major tyrosine kinases involved in mast cell activation.
The major tyrosine phosphorylation sites are represented by red circles. The tyrosine numbers designated for KIT, Lyn, Fyn, Btk, and Itk are based on the human sequence and for Syk on the rat sequence. The major signaling molecules recruited to specific phosphorylated tyrosines are depicted for KIT. PR in the Tec kinases structure represents a proline-rich region. A and B in the Syk structure shows the interdomain regions A and B of this kinase.
At least in human mast cells, auto/transphosphorylation of KIT is a very rapid but transient event following SCF challenge, with peak phosphorylation being observed within 1-2 min and then substantially but not completely decreasing by 5-10 min (36). KIT-dependent tyrosine phosphorylation, both directly and indirectly, of downstream signaling molecules such as linker for activated T cells 2 (LAT2), Bruton's tyrosine kinase (Btk), and PLCγ1 (Fig. 1) display similar kinetics (36, 37). However, secondary signaling events, following KIT activation, including the phosphorylation of AKT, components of the molecular target of rapamycin (mTOR) cascade, and the mitogen-associated protein kinases (MAPKs) extracellular signal-regulated kinases 1 and 2 (ERK1/2), p38, and c-Jun N-terminal kinase (JNK), are delayed with maximal phosphorylation being observed 5-10 min after SCF challenge (37-39). As with KIT phosphorylation, these responses are rapidly downregulated (37-39); thus, they likely become refractory to further SCF challenge. However, downstream signaling events (Fig. 1), especially those regulating transcription, must persist to prevent the mast cells from undergoing apoptosis. Certainly, mast cells deprived of SCF will display manifestations of apoptosis and start to die within 24-48 h (40). Thus mice defective in SCF (Sl/Sld) (41) or functional KIT (W/Wv and W/Wsh) (42, 43) are deficient in mast cells. This observation illustrates that SCF-induced KIT activation is an essential process for the expansion of mast cells from their progenitor cells and their subsequent maturation and survival in their resident tissues (24). In addition to its role in mast cell development and homeostasis, SCF-induced KIT activation may contribute to the homing of mast cells to their sites of residence in vivo, as suggested by its potent chemotactic properties for both mouse and human mast cells (38, 44) and its ability to promote adhesion of mast cells to extracellular matix proteins (38, 45).
A number of activating mutations have been described to be present in KIT that result in constitutively enhanced kinase activity associated with specific myeloproliferative disorders (9, 31). In particular, an activating aspartic acid to valine substitution at amino acid position 816 within the second catalytic domain of human KIT is linked to systemic mastocytosis (9, 22), whereas a valine to glycine substitution at amino acid position 560, which enhances the catalytic activity by preventing binding of and downregulation of KIT activity by SHP1, is associated with gastrointestinal stomal cell tumors (GIST).
SCF, at least under experimental conditions, can markedly enhance mast cell activation, although on its own, at physiological concentrations, SCF has little effect on mast cell degranulation or cytokine production (36). Of note is the ability of SCF to dramatically enhance degranulation at concentrations of antigen that produce little detectable degranulation in the absence of SCF (46). Should such a relationship exist in vivo, then it is quite conceivable that SCF may be as equally important as antigen in disorders such as asthma, anaphylaxis, and rhinitis resulting from exaggerated activation of mast cells. Although there is little evidence to support such a concept in vivo, the role of the SCF-KIT interaction in mast cell homeostasis implies that antigen-mediated mast cell activation in vivo would occur with ongoing KIT activation. Of particular interest is the reported incidence of anaphylaxis in approximately 50% of mastocytosis patients (47) and the corresponding observation that a subset of patients diagnosed with idiopathic anaphylaxis display markers of mastocytosis, including the D816V mutation in KIT (48). These observations would imply that the aberrant KIT activity associated with mastocytosis produces a hyperactive mast cell phenotype.
Our studies and those conducted in collaboration with the laboratory of Dr. Michael Beaven (NHLBI/NIH) have sought to determine the mechanisms regulating the synergistic enhancement of antigen-mediated mast cell degranulation and cytokine production by SCF. Initial studies conducted in human mast cells revealed that the inability of SCF to induce degranulation in the absence of SCF may be related to its similar inability to promote the activation of protein kinase C (PKC) (36), an essential signaling component for the aforementioned response (7). Conversely, the enhanced degranulation and potentially enhanced cytokine generation observed following concurrent challenge with SCF and antigen appeared to be linked to a similar enhancement of PLCγ1 activation leading to an exaggerated calcium signal (36). These studies were extended to investigate which receptor-proximal events regulated this response. By examining the early tyrosine phosphorylation events elicited by both receptors, we determined that both antigen and SCF induced the rapid phosphorylation of the transmembrane adapter molecule LAT2 (formerly termed non-T cell activation linker or linker for activated B cells) (Fig. 1), in both mouse and human mast cells (46). Furthermore, concurrent addition of antigen and SCF elicited a greater response than that induced by either agent alone (36, 46). In contrast to the antigen-mediated response, however, LAT2 phosphorylation in response to SCF was observed in the absence of LAT1 phosphorylation (46).
Although the FcεRI required the cytosolic tyrosine kinases Lyn and spleen tyrosine kinase (Syk) for antigen-induced LAT2 phosphorylation, SCF/KIT-induced LAT2 phosphorylation required neither Lyn or Syk nor other tyrosine kinases including Fyn and Btk (46, 49). These data, which suggested that KIT could directly phosphorylate LAT2, were confirmed in studies utilizing KIT and LAT2 constructs transduced in 293T cells (49). Further studies conducted in 293T cells, using mutant LAT2 constructs, surprisingly revealed that the suite of tyrosine residues phosphorylated by KIT, Lyn, and Syk were dramatically different (49). Whereas Syk primarily phosphorylated tyrosines (Y136, Y193 and Y233 of human LAT2) contained within putative binding sites (-YXN-) for the cytosolic adapter molecule Grb2, Lyn and KIT phosphorylated tyrosines (Y95, Y118 and Y136 for Lyn and Y118 for KIT) within the putative Grb2-binding sites and tyrosines (Y110 and Y119 for Lyn and Y110 for KIT) outside of the putative Grb2-binding sites (49). In pull down studies, we observed that PLCγ1 could bind to the two terminal tyrosines (Y193 and Y233) phosphorylated by Syk. However, as these are not recognized binding sites for PLCγ1, these interactions likely are indirect via Grb2 or similar cytosolic adapter molecule (49). Although we could not determine the signaling molecules recruited to LAT2 following its phosphorylation by Lyn or KIT, the flanking sequence around Y110, which is phosphorylated by Lyn and KIT, is highly homologous to the sequence containing Y568 and Y570, which is phosphorylated in KIT following its activation. This sequence in KIT has been proposed to bind SHP1, Lyn, and Fyn. Thus, it is possible that these molecules are also recruited to LAT2 in a similar KIT- or Lyn-dependent manner.
While the identity of the molecules recruited to LAT2 following phosphorylation by KIT have yet to be elucidated, gene knockdown studies have provided evidence to support a role for KIT-mediated LAT2 phosphorylation and indeed FcεRI-dependent LAT2 phosphorylation in mast cell activation. In this respect, human mast cells pre-incubated for 64 h with small interfering RNA (siRNA) oligonucleotides targeting LAT2 or human mast cells stably transfected with LAT2-targeted short hairpin (shRNA) display significantly reduced antigen-mediated and KIT-enhanced degranulation (46, 49). In addition, although LAT1 was not observed to be phosphorylated by KIT, knockdown of LAT1 also substantially reduced these responses (46). These and other observations led us to propose that whereas LAT1 may be the principle coordinator of signaling pathways regulating antigen-mediated degranulation through the eventual activation of PLCγ1, LAT2 may serve to coordinate an amplification pathway to further enhance or maintain PLCγ1 activity (7). By interacting with this latter pathway, assuming that LAT1 is concurrently phosphorylated following antigen challenge, KIT provides the necessary phosphorylation events required for the enhancement of PLCγ1 and subsequently elevation of the intracellular calcium concentration. As further discussed in the section describing the roles of Tec kinases in mast cell activation, this latter response may be mediated via a PI3K-Btk regulated pathway, downstream of LAT2 phosphorylation.
Although KIT possesses inherent tyrosine kinase activity, studies revealed that specific KIT-mediated mast cell responses are also dependent of the activation of the Src family tyrosine kinases Lyn and Fyn. Activated KIT both binds, as discussed above, and activates Lyn and Fyn. Mutation of the pY567 (mouse sequence) Src kinase-binding site in KIT abrogates SCF-induced calcium flux and chemotaxis (50). Similarly SCF-mediated mast cell proliferation and chemotaxis is substantially reduced in lyn-/- bone marrow-derived mast cells (BMMCs) (51-53), and SCF-mediated chemotaxis is reduced in fyn-/- BMMCs (51, 54). In lyn-/- BMMCs, this defect was associated with decreased KIT phosphorylation (pY823, pY703, pY719, pY730 and pY567/570, mouse sequence) in addition to a decrease in activation of JNK and decrease in STAT3 phosphorylation (51). In contrast to these observations, the defective chemotactic response observed in fyn-/- BMMCs was not associated with defective KIT phosphorylation but was associated with a decrease in KIT-mediated SHP-2 and p38 phosphorylation (51). Although it is unclear whether Fyn plays a role in the ability of SCF to enhance either antigen-mediated degranulation or cytokine production, as discussed later, Lyn is essential for this response. The roles of Lyn and Fyn in the context of FcεRI-mediated mast cell signaling are discussed in greater detail below.
Src family kinases in mast cell activation
The role of Src family protein tyrosine kinases (Src PTKs) in the FcεRI-mediated activation and function of mast cells is complex. The structure of these kinases is well defined (Fig. 2), and their mechanism of activation has been previously reviewed (55) (Fig. 3). These kinases are used by a variety of cell surface receptors on the mast cell (56), and accumulated evidence suggests that they act in both a positive and negative manner to control the activation and subsequent responses of the mast cell. In this section, we primarily discuss the recent advances in how the FcεRI utilizes these Src PTKs to initiate and regulate cellular signals and responses. The findings demonstrate an increasing complexity in how Src PTKs function and shed new light on their key regulatory role in controlling mast cell activation.
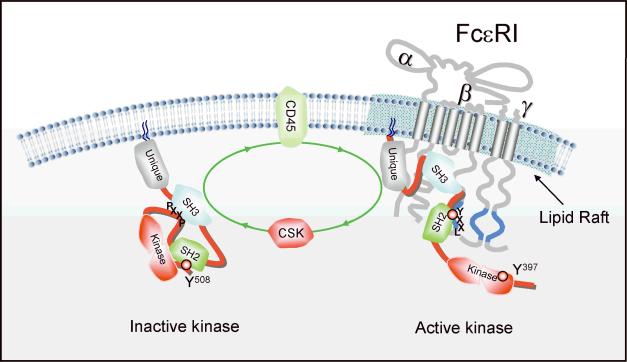
Fig. 3. Regulation of Src PTK recruitment to FcεRI.
The equilibrium of active and inactive Src PTKs, like Lyn, normally favors their inactive or closed conformation (as depicted on the left). This is mediated through the interaction a negative regulatory tyrosine (Y) at the COOH-terminus of Src PTKs (Y508 for Lyn) with its own SH2 domain. This balance is regulated by CD45 and Csk. In resting cells, Csk phosphorylates this site maintaining the closed conformation. FcεRI stimulation shifts the balance to CD45, which dephosphorylates Y508 allowing the SH2 domain to become available and bind this receptor. Once open, the phosphorylation of the activation loop tyrosine (Y397) increases, enhancing activity and stabilizing the open/active conformation. Fyn recruitment seems to function similarly although where this interaction might occur is not yet defined.
Coupling of Src kinase activity to FcεRI
The lack of intrinsic kinase activity of the multimeric FcεRI makes its association with a kinase a prerequisite for transducing signals that elicit mast cell responses (Fig. 3). The activity of the Src PTK Lyn is essential for phosphorylation of the tyrosine residues in the ITAMs through transphosphorylation (57). However, interaction of FcεRI with Lyn must precede the former's phosphorylation. Such interactions have been postulated to occur via the residence of Lyn in lipid rafts and the recruitment of FcεRI into these domains upon its aggregation by cell-bound IgE antibodies that encounter a specific antigen (58-60) (Fig. 3). Lipid rafts are not only enriched in cholesterol but also in sphingolipids and other saturated phospholipids. They also contain a variety of signaling proteins including Lyn kinase. Thus, aggregation of FcεRI may cause coalesence of receptors with Lyn based on the biophysical properties of the surrounding lipid milleu around both the FcεRI and Lyn. However, some studies argue that protein-protein interactions are required for FcεRI phosphorylation, based on the findings that a small fraction of Lyn can be found to weakly interact with the FcεRIβ subunit prior to engagement of this receptor and that exclusion of Lyn from lipid rafts only modestly affects FcεRI phosphorylation (61-63). It is known that the interaction of Lyn with FcεRI is greatly enhanced by stimulation of this receptor. This increased interaction is mediated by Lyn SH2 domain binding to the phosphorylated Y219 in the FcεRIβ ITAM (18,19). Mutation of Y219 of the FcεRIβ ITAM causes a marked reduction in FcεRI phosphorylation, suggesting that this interaction is key to maintain receptor phosphorylation. Obviously, FcεRI-Lyn interactions are likely to be greatly increased when both of these proteins are concentrated in lipid rafts. Thus, while there is still substantial uncertainty as to whether lipid rafts are necessary for initiating FcεRI phosphorylation, their contribution to the maintenance of receptor phosphorylation appears more certain (59, 62, 64).
The aforementioned requirements of protein-protein versus protein-lipid interactions, however, should not be viewed as mutually exclusive. Two-photon fluorescence lifetime imaging microscopy and fluorescence polarization anisotropy imaging studies have recently provided support for a mutually inclusive model. Studies in which the cholesterol-rich membrane domains are labeled with the lipid analog dil-C18 and FcεRI is labeled with Alexa 488-IgE demonstrated that aggregation of FcεRI causes the coalesence of dil-C18-labeled membrane domains and redistribution to membrane patches containing FcεRI (65). This results in an increased fluorescence lifetime of both dil-C18 and Alexa 488-IgE; an increase in dil-C18 fluoresence lifetime was previously shown to reflect increased lipid order. Furthermore, flouresence resonance energy transfer (FRET) occurs between the Alexa 488-labeled receptor and dil-C18-labeled domains (66) following FcεRI engagement, suggesting the reordering and close proximity of the cholesterol-enriched membrane and the aggregated FcεRI. These studies are consistent with prior work using high resolution electron microscopy (67, 68). Here it was found that compartmentation of FcεRI and Lyn occured in small electron-dense microdomains prior to FcεRI engagement. Consistent with the above studies, aggregation of FcεRI enhanced the size of these microdomains increasing the numbers of receptors and Lyn within.
Collectively, these studies define the cholesterol-rich membrane microdomains as a lipid environment that is dynamic, small, and likely to contain limited numbers of proteins prior to cell stimulation. A unifying hypothesis for the differing views on how Lyn couples to FcεRI phosphorylation emerges. One might postulate that a small fraction of FcεRI is found, at any given time, within these dynamic cholesterol-rich microdomains, an environment that may also contain Lyn kinase. In this model, aggregation of FcεRI would induce transphosphorylation with a neighboring receptor as it brings together receptors with or without Lyn kinase. The coalescence of the lipid domains would stabilize and/or increase FcεRI phosphorylation and cause assembly of a stable signaling complex. This model is also in agreement with the finding that FcεRI aggregation induces membrane changes that allow coalesence of proteins and lipids required for efficient propagation of signals (65).
The Src PTK Fyn (Fig. 2) has also been shown to be important for activation of mast cells upon FcεRI aggregation and to co-immunoprecipitate with this receptor (69). Functionally, the loss of Fyn in mast cells impairs mast cell degranulation and cytokine production (69,70). How Fyn might couple to FcεRI was until recently unknown. In unpublished work (Nora Fierro and Juan Rivera), Fyn was found to associate with the FcεRIβ ITAM and appears to be recruited, similarly to Lyn, via an SH2 domain-phosphotyrosine interaction (Fig. 3). However, unlike Lyn, Fyn does not appear to participate in the phosphorylation of FcεRI (70). Two striking features are revealed in the recent work (Nora Fierro and Juan Rivera, unpublished observations). First, chemical cross-linking studies, whereby Fyn or Lyn are covalently bound to the FcεRI, demonstrated that distinct populations of receptors associate with Fyn or Lyn. Second, FRET experiments show that Lyn can be found to associate with FcεRI in resting conditions, whereas Fyn association requires FcεRI aggregation. Moreover, Lyn association with FcεRI appears to be more transient than that of Fyn. These findings suggest that these interactions may be taking place in different compartments, a postulate that is currently under investigation. Nonetheless, the findings to date argue that the interaction of FcεRI with Fyn is likely to be spatiotemporally distinct from that with Lyn but is key in promoting the downstream signaling required for mast cell effector responses.
Src kinases: beyond FcεRI proximal interactions
Fyn kinase
Beyond its coupling to FcεRI, Fyn primarily functions to positively regulate mast cell responsiveness. Previous studies have demonstrated a role for this Src PTK in regulating the activation of PI3K (69, 71) and thus its product phosphatidylinositol-3,4,5-triphosphate (PIP3)(Fig. 1). Fyn-deficient mast cells show a substantial reduction in PIP3, which correlates with the reduced degranulation response of these cells. Moreover, considerable evidence has been accumulated for an essential role of PIP3 in mast cell responses. This has been demonstrated through varied approaches that encompass the use of PI3K inhibitors and through genetic manipulation of PI3K itself (72). PIP3 is a potent lipid second messenger that functions to assemble signalosomes at the cell membrane and coordinates the activity of multiple signaling proteins with calcium responses, the cytoskeleton, and the nucleus (73).
In an early study of Fyn function in mast cells, a more transient calcium response was noted relative to wildtype cells (69). Using more sensitive fluorometric methods, we found that the loss of Fyn showed little effect on the mobilization of calcium from intracellular stores but instead impaired calcium influx (Ryo Suzuki and Juan Rivera, unpublished observation). The mechanism for this effect is unclear; however, several possibilities exist. One possible mechanism is through Fyn-dependent phosphorylation of plasma membrane calcium channels. Members of the transient receptor potential channel (TRPC) family are known substrates for Src family kinase, like Fyn (74). Moreover, TRPC channels have been shown to contribute to calcium influx in a mast cell line (RBL-2H3) (75). A second scenario is that Fyn could contribute to calcium influx through its role in the activation of sphingosine kinase 2 (SphK2) and the generation of sphingosine-1-phosphate (S1P) (Fig. 1). S1P is a highly biologically active molecule that functions as an intracellular lipid second messenger as well as a ligand for a family of GPCRs expressed on the cell surface of many cell types. Fyn kinase is required for the activation of SphK2 (76). Much like the defective calcium influx that is seen upon loss of Fyn expression, the genetic deletion of SphK2 also caused a loss in calcium influx (77). However, whether Fyn's control of calcium influx is entirely mediated via its role in the production of S1P or is combined with its ability to phosphorylate calcium channels is not known. Obviously, the intracellular targets of Fyn and S1P must be identified to appropriately address this issue with certainty.
Various other functions of Fyn have been described in mast cells. Fyn has been shown to phosphorylate PLD2 in the RBL mast cell line (Fig. 1) (78), and it is thought that this may contribute to its role in promoting degranulation, as PLD2 activity is required for this event (79). Fyn has also been implicated in the organization of the cell cytoskeleton, in vesicle trafficking, and chemotaxis. Regulation of microtubule formation and its nucleation from the membrane in activated mast cells was shown to be dependent on complexes of γ-tubulin with Fyn and Syk kinases (80, 81). Microtubule function and the ability to translocate preformed mast cell granules to the plasma membrane also appears to depend on Fyn and is independent of calcium (82). Moreover, association of Fyn with cytoskeletal proteins like vimentin has been shown in mast cells, and such proteins are likely targets of Fyn activity (83). Thus, beyond its role in FcεRI proximal signaling, it appears that Fyn functions in many of the cellular processes required for mast cell activation and effector function.
Lyn kinase
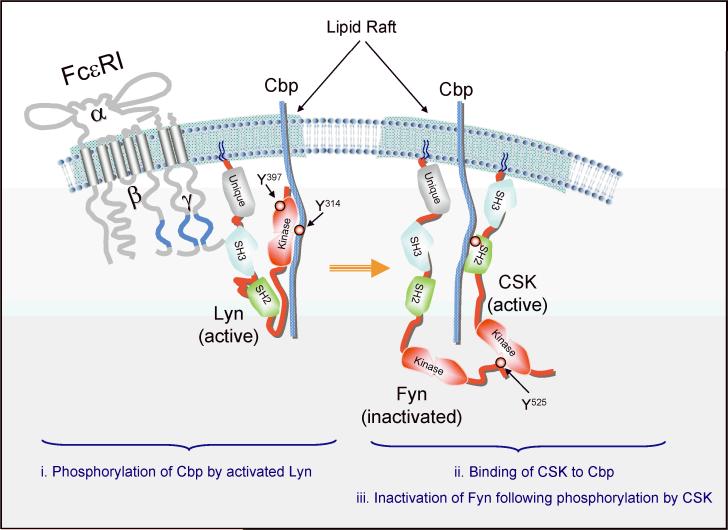
As mentioned above, Lyn kinase plays a positive role in activating mast cells through its phosphorylation of FcεRI (Figs 1 and 3). In addition, Lyn phosphorylation of other substrates such as Syk, Btk, the adapter LAT2, and the cytokine regulator Tom1L1 (reviewed in 6, 84), seemingly has a positive effect on mast cell functions. Unlike Fyn, however, Lyn also plays an important role as a negative regulator of mast cell effector responses (85-87). Lyn-deficient mice develop atopic-like allergic disease, and mast cells derived from these mice are hyperresponsive when stimulated via FcεRI (85). We now know that Lyn is required for phosphorylation of the lipid raft-localized C-terminal Src kinase (Csk)-binding protein (Cbp) and, thus, for membrane targeting of the regulatory kinase Csk, a kinase that negatively regulates Src PTKs through phosphorylation of a negative regulatory tyrosine in their COOH-termini (Figs 4 and 5). This causes Src PTKs to assume a `closed' conformation through intramolecular interaction of the phosphorylated C-terminal tyrosine with the kinases' own SH2 domain. This regulatory step is mediated by the Lyn kinase that is localized in lipid rafts, where Cbp is also found, and is required to downregulate Fyn kinase activity (63) (Fig. 4).
Fig. 4. Cbp/CSK-mediated regulation of Fyn activity.
In resting mast cells, active Lyn kinase phosphorylates the adapter Cbp at Y314 at low levels. Phosphorylation of this site causes the binding and membrane localization of CSK, which is normally localized in the cytoplasm. CSK can then target the COOH-terminal negative regulatory tyrosine (Y) residue found in Src PTKs. In Fyn, Y525 is a target for CSK activity leading to Fyn inactivation by inducing a closed conformation via intramolecular interaction with its own SH2 domain. The low level of Cbp phosphorylation maintains the equilibrium between active and inactive Src PTKs in resting cells. Upon FcεRI stimulation, the balance shifts towards increased activation of Src PTKs, likely through CD45. This new equilibrium is governed by a Lyn-dependent increase in Cbp phosphorylation and membrane CSK recruitment, which controls the activity of Fyn by inactivation as described above. Thus, the absence of Lyn leads to a loss in control of Fyn activity and mast cells are hyperresponsive (63, 85).
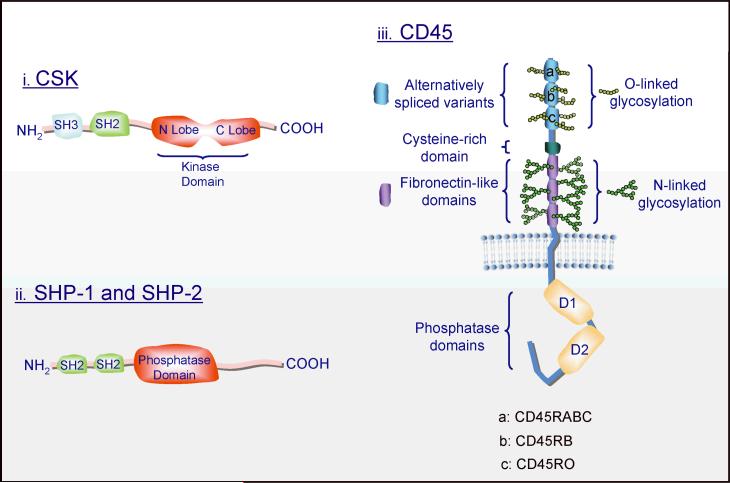
Fig. 5. Basic structures of the tyrosine kinase, CSK, and the tyrosine phosphatases SHP1, SHP2, and CD45.
Several splice variants of various lengths of CD45 have been described as depicted by A, B, and C in the figure.
Another dominant mechanism by which Lyn exerts negative control on mast cell effector responses is through the SH2 domain-containing inositol phosphatase-1 (SHIP-1) and SHIP-2. In the absence of Lyn, SHIP-1 activity is increased, causing an increase in the concentration of intracellular PIP3 (86). As mentioned above, PIP3 is important for mast cell degranulation and other responses. Unlike Fyn-deficient mast cells, Lyn-deficient mast cells have increased levels of intracellular PIP3, which is associated with their enhanced degranulation (85). Both SHIP-1- and SHIP-2-deficient mast cells also show an enhanced degranulation and cytokine response (88, 89). Furthermore, constitutive increases in intracellular PIP3 through downregulation of another inositol phosphatase, phosphatase and tensin homolog (PTEN), also results in enhanced degranulation and cytokine production (90). Thus, Lyn plays a key negative regulatory role in controlling the levels of PIP3 and thus suppressing the extent of a mast cells response.
One proposed mechanism by which Lyn kinase may play both a positive and negative role in mast cell activation has been revealed by studies of the effect of low or high strength stimulation on responses in wildtype and Lyn-deficient mast cells (87). This work shows that Lyn activity is required for mast cell degranulation and cytokine production when encountering a low strength stimulus. In contrast when encountering a high strength stimulus, Lyn activity is dispensable for the observed enhanced degranulation and cytokine production (87). These studies also conclude that negative regulation by Lyn is mediated through its interaction with the FcεRIβ. Taken together with the finding that Lyn's negative role is predominantly mediated by the pool of Lyn in lipid rafts (63), one might conclude that the interaction of Lyn with FcεRIβ is likely to occur upon coalesence of these domains. Therefore, this fits well with the concept that a strong stimulus, which leads to extensive coalesence of microdomains (65, 66), would serve to promote the negative role of Lyn kinase in order to control the extent of the inflammatory response.
Fgr, Hck, and Src kinases
The Src PTKs, Fgr, Hck, and Src are abundantly expressed in mast cells (13). However, much less is known about their role in mast cell activation and function. Early studies showed that Src co-immunoprecipitated with FcεRI (91), but more recent work suggests that these observations may have been due to the failure to solubilize lipid rafts with the detergent conditions used in these studies. In contrast, Src has been found to associate with PKCδ (92), a kinase that phosphorylates the FcεRIγ on threonine residues (93), but the role of Src in regulating this event is not known. Fgr, like Fyn, is able to phosphorylate PLD2 in mast cells (78). Thus, it also likely contributes to the activation of this lipase and to its role in promoting mast cell degranulation. In contrast, Fgr (and Lyn and Hck as well) does not appear to be required for mast cell spreading and lamellipodia formation in mast cells (54). Moreover, our own unpublished observations (Yasuko Furumoto, Santa Eglite, and Juan Rivera) rule out an appreciable direct role for Fgr, Hck, or Src in mast cell degranulation. Nonetheless, Hck appears to have an indirect role in promoting mast cell degranulation. This view is supported by studies on Hck-deficient mast cells (94), where the findings point to Hck-mediated negative regulatory control on Lyn activation. Thus, in the absence of Hck, Lyn activity is substantially increased suppressing degranulation and cytokine production when cells encounter a strong stimulus. The mechanism for this effect is unknown, but it does not appear to be mediated through loss of Cbp phosphorylation and, thus, the loss of Csk-mediated control of Lyn activity, since Cbp phosphorylation was enhanced. Moreover, Fyn activity was normal in the absence of Hck, suggesting normal Cbp/Csk-mediated regulation of Fyn (Fig. 4). One possible explanation comes from studies demonstrating that the lipid raft environment is essentially devoid of phosphatases that dephosphorylate the active site tyrosine of Lyn (58) as well as studies showing that the loss of some lipid raft proteins like LAT1 causes the increased presence of similar proteins, like LAT2, in these domains (95,96). Furthermore, we have found that the loss of Lyn expression causes more Fyn to localize in lipid rafts (Nora Fierro and Juan Rivera, unpublished observations). Thus, if the loss of Hck expression results in the increased localization of Lyn in lipid rafts, this would be consistent with increased Lyn activity and with the finding that the negative regulatory role of Lyn is localized to lipid raft-residing Lyn kinase. While this activity remains to be tested, it is clear that Hck appears to dampen the negative function of Lyn kinase. Collectively, the few studies to date on Fgr, Hck, and Src suggest that these kinases have contributory but not dominant roles in mast cell effector functions.
Syk kinase in mast cell activation and function
Syk and its closely related family member ζ-associated protein of 70 kDa (ZAP-70) have long been recognized as key components of signaling pathways in B cells and T cells, respectively (97, 98). The structure of Syk (Fig. 2) is well defined and is a topic that has been extensively reviewed (99-101). Beyond its catalytic function, key elements for Syk function include its NH2-terminal tandem SH2 domains, and the multiple phosphorylated tyrosine residues that form novel binding sites for interacting proteins of which the majority are found in the interdomain A and B regions of this kinase (Fig. 2). Syk activation occurs primarily through its binding of phosphorylated ITAMs on receptors that utilize its activity for eliciting cellular effector functions (reviewed in 99,101). However, recent reports suggest alternate methods of activation, such as by PKCδ's phosphorylation of Syk (102). An alternatively spliced form of Syk (SykB) has also been identified that differs from Syk by lacking 23 amino acids in the interdomain B region (reviewed in 99,101). While the catalytic activity of SykB is comparable to that of Syk, it seems less efficient in binding to phosphorylated ITAMs.
Syk is essential for the propagation of signals in mast cells (6), following binding to the phosphorylated ITAMs of FcεRIγ (103, 104) (Fig. 1). Syk binding to the phosphorylated γ chain ITAM is a necessary step for its activation in response to FcεRI stimulation. While weak interaction of Syk with FcεRIβ phospho-ITAM peptides can be observed in vitro, there is little evidence of this interaction in vivo. Once activated, Syk is essential in the amplification of mast cell signaling and in driving normal mast cell effector responses (105, 106). Syk function in mast cells and other immune cells has been well reviewed (99, 101, 107), so it will be covered very briefly herein. However, it is important to note that Syk deficiency results in a non-responsive mast cell that shows no calcium response, degranulation, or cytokine production following FcεRI stimulation (105,108). Thus, Syk is critical for calcium responses. Key to its amplification function and its ability to regulate calcium responses is its capacity to interact with multiple signaling proteins and to phosphorylate the key substrates required to assemble signaling networks, namely adapter molecules.
There are 10 tyrosine residues that can be modified by phosphorylation when Syk is activated. These phospho-Ys appear to mediate distinct functions. Y130 is localized in interdomain A and is thought to play a role in both mediating the activation of Syk and its release from interacting with an antigen receptor (109). Somewhat consistent with these results, in B cells the deletion of interdomain A resulted in the loss of interaction of Syk with the B-cell antigen receptor (BCR) (110). However, in this case, Syk became phosphorylated independent of BCR engagement and failed to induce calcium responses. There are five Y residues in interdomain B (Fig. 2), and Y290 lies within the 23 amino acid insert that is deleted in the alternatively spliced SykB isoform. As indicated above, SykB is less effective in interacting with ITAMs. However, upon FcεRI stimulation, the mutation of Y290 does not appear to affect the function of Syk (111). In contrast, Y317, Y342, and Y346 all appear to be important for Syk function. Mutation of Y317 has been shown to result in a gain of function in mast cells (112). The corresponding Y residue in ZAP-70 was shown to bind the negative regulator c-Cbl (113), which has been described to regulate the degradation of active Syk (114). Phosphorylation of Y342 of Syk is thought to be important for interaction and phosphorylation of Vav1 (115) (Fig. 1). However, in mast cells, mutation of Y342 did not alter the phosphorylation of Vav1 but instead showed decreased PLCγ phosphorylation and a decreased ability to interact with phosphorylated ITAMs (116). Importantly, this mutant form of Syk failed to reconstitute degranulation when expressed in Syk-deficient mast cells. Based on its analogous Y residue in ZAP-70, mutation of Y346 of Syk in mast cells might have been predicted to cause a loss of PLCγ interaction and function that would manifest in poor mast cell degranulation (reviewed in 99). However, mutation of this Y346 showed minimal effect on mast cell degranulation and probing of this site with a phospho-Y346-specific antibody suggests that this site is not heavily phosphorylated upon FcεRI stimulation of mast cells (116). At the moment there is no information on the function of Y358 of Syk. Y519 and Y520 lie in the activation loop of Syk. Mutation of either or both of these Y residues showed no loss in Syk kinase activity (106). Surprisingly, however, upon expression of these Syk mutants, at levels comparable to endogenous Syk in mast cells, there was a failure to transduce signals with no observable protein tyrosine phosphorylation or mast cell degranulation. Overexpression of these mutants could partially restore some signaling. Thus, these Y residues appear to be important in the propagation of signals by Syk through an as yet to be defined mechanism. This view is consistent with the finding that their phosphorylation mirrors the downstream propagation of signals required for mast cell degranulation (117). The function of the COOH-terminal cluster of Y624 and Y625 in mast cells is not known. However, in T cells, expression of a Syk chimera with mutations at these sites has demonstrated enhanced T-cell antigen receptor signaling, thus implying a negative regulatory role for these tyrosines (reviewed in 99).
Syk activation is central for mast cell effector function and the allergic response. Thus, its activity should be tightly regulated to avoid the injurious effects of spurious or sustained activation. Regulation of Syk activity occurs at multiple levels. Syk is maintained in an inactive state through intramolecular interactions of its SH2 domains with phosphorylated Y residues (Y130 and Y317 have been implicated in this binding) in its interdomains A and B (100, 118). What causes the loss of this intramolecular binding so that Syk can achieve the appropriate conformation to be active is not known, since phosphatase-mediated dephosphorylation of these sites has not been demonstrated. However, activation might simply be mediated through a shift in the equilibrium where the abundance of phosphorylated ITAMs upon FcεRI stimulation may provide a higher affinity binding site that stabilizes Syk in the open conformation leading to its increased activity. This view is supported by the finding that Syk can be activated by incubation with phosphorylated ITAM peptides (104, 119). This also suggests a potential negative regulatory step in its activation might be through dephosphorylation of the ITAMs (120), a topic further discussed in a following section. Once activated, Syk activity can be controlled by multiple mechanisms. In B cells, Syk binds to Cbl and is ubiquitylated and degraded (114). In mast cells, the loss of Cbl-b expression (but not that of c-Cbl) caused enhanced Syk phosphorylation and enhanced mast cell effector responses (121).
Tec kinases in mast cell activation
Btk
Tec kinases are a group of structurally conserved cytosolic tyrosine kinases which comprise five family members: Btk, Etk (BMX), Itk, Rlk, and Tec (122,123). Although mast cells express four of these members, Btk, Itk, Rlk, and Tec, only Btk has been documented to directly play a major role in mast cell responses. A lack of Btk is linked to immunodeficiency in both the mouse [X-linked immunodeficiency (XID)] and human (X-linked agammaglobulinemia) as a consequence of defective B-cell signaling (124). As with other members of the Tec kinase family, Btk contains an N-terminal pleckstin homology (PH) domain, a Tec homology (TH) domain, an SH2 domain, an SH3 domain, and a C-terminal catalytic domain (122) (Fig. 2). Btk is activated following phosphorylation of critical tyrosines as a consequence of receptor aggregation. The initial Src kinase-dependent phosphorylation of Y551 within the activation loop (125) results in an increase in catalytic activity leading to autophosphorylation at Y223 within the SH3 domain (126), thereby further enhancing its activation status. As assessed by immunoprecipitation studies, in vitro kinase assays, and by the use of phospho-specific antibodies (recognizing pY223 and pY551), Btk is rapidly activated upon tyrosine phosphorylation in mast cells following challenge with either antigen or SCF, where maximal phosphorylation is observed within 60 s of cell activation (37). Although not so apparent at early time points (< I min), at later time points (>5 min) a synergistic enhancement of Btk phosphorylation is observed when both agents are added concurrently. These events are largely dependent on Lyn, as evidenced by the markedly attenuated Btk phosphorylation at position Y233 in lyn-/- BMMCs challenged with either SCF or antigen (37). However, as Btk phosphorylation has been reported to also be dependent on Syk (127), it is possible that such control is indirect via Syk activation.
In addition to being phosphorylated, Btk is also translocated from the cytosol to the membrane in response to antigen (125). This is necessary for Btk to interact with the receptor-signaling complex (Fig. 1) and to access its substrate(s). As in B cells, this interaction likely involves binding of the PH domain of Btk to membrane-associated PIP3 following its generation as a consequence of the activation of PI3K (128, 129). Although additional molecular interactions for Btk and other Tec family members have been documented in other cell types including B and T cells (122, 123), these interactions in mast cells are relatively unknown. Certainly, Btk does not appear to bind to the receptor subunits (130). Nevertheless, in addition to PIP3, under experimental conditions, the PH domain of Btk can interact with PKC family members including PKCα, PKCβ,I and PKCβII, PKCε, and PKCδ (127,131). In activated mast cells however, Btk primarily associates with PKCβI (131). PKC phosphorylates Btk at serine (S)223, thereby inhibiting its catalytic activity (127). Conversely, the phosphorylation of PKCβ1 and its translocation to the membrane is, in part, Btk dependent (Fig. 1), implying that PKCβ1 may function as a regulator of an inhibitory feedback loop for the regulation of Btk activity (132).
Evidence for a role for Btk in mast cell function has primarily come from studies conducted in mast cells derived from the bone marrow of XID and btk-/- mice and supported by studies utilizing gene knockdown approaches and phamacological inhibitors of Btk activity. In both XID (133-135) and btk-/- BMMCs (37,133-136), antigen-mediated degranulation is significantly reduced, although this defect is more marked in btk-/- BMMCs (~50% reduction) compared to XID BMMCs (~25%) (133). Similarly, XID mice, primed with anti-DNP-IgE then triggered with antigen, displayed a slightly reduced early phase and markedly reduced late phase anaphylactic response (135). Inhibition of antigen-mediated degranulation has also been observed in both human and mouse mast cells treated with pharmacological compounds, including terreic acid (137), desatinib (138, 139), and hypothemycin (140), that block Btk activity, and in RBL 3H3 cells treated with Btk-targeted siRNA oligonucleotides (141). The release of cytokines including TNF-α, IL-6, IL-13 from btk-/- BMMCs (37, 127, 135, 136, 140) and IL-2 is also reduced to various extents in cultures of btk-/- BMMCs with the release of IL-2 being almost completely attenuated (136, 142). The residual degranulation and cytokine production observed in response to antigen are virtually abolished in btk-/-lyn-/- BMMCs (136). As Btk activation is dependent on Lyn (37), this implies that Btk and Lyn may be components of both linear and parallel pathways regulating mast cell activation.
In contrast to the partial inhibition of antigen-mediated degranulation observed in the btk-/- BMMCs, we have observed that the ability of SCF to enhance the residual antigen-mediated degranulation is essentially absent in the btk-/- BMMCs and indeed in the lyn-/- BMMCs (37), suggesting that the SCF-mediated response is more dependent on the Lyn-dependent activation of Btk than is the antigen-mediated response. We previously demonstrated that ability of SCF to enhance antigen-mediated degranulation was similarly dependent on PI3K (72). However, we have not consistently observed synergistic enhancement of Akt (also known as PKB) phosphorylation, a surrogate marker for PI3K activation, following antigen and SCF challenge in mast cells. Indeed, if anything, the phosphorylation of Akt, at least in mouse BMMCs, appears to be lower when the cells are triggered with SCF in the presence of antigen than with SCF alone (37). This latter response is not observed in lyn-/- BMMCs, suggesting that Lyn may regulate a negative regulatory pathway for PI3K activation in mast cells. Furthermore, these data suggest that although PI3K is a critical component of the signaling pathway leading to the enhancement of antigen-mediated degranulation by SCF, it is not responsible for the synergy per se. An explanation for this apparent dichotomy may be that PI3K is required to recruit Btk to the membrane, whereas the synergistic activation is mediated by enhanced Btk activation.
The attenuated antigen-mediated degranulation observed in the btk-/- BMMCs can be explained by a defect in regulatory pathways leading to calcium mobilization in these cells. Both PLCγ1 and PLCγ2 phosphorylation is significantly reduced in btk-/- BMMCs and virtually abolished in btk-/-lyn-/- cells (37, 136). By extension from studies on Tec kinases conducted in T cells and B cells (122, 143), it is likely that PLCγ1 and PLCγ2 are direct substrates of Btk. Downstream inositol-1,4,5,-triphosphate (IP3) generation and the consequential increase in intracellular calcium concentrations observed in response to antigen are decreased in a similar manner in btk-/- BMMCs (136). Furthermore, the SCF-dependent enhancement of antigen-induced PLCγ1 phosphorylation and calcium flux is almost entirely abrogated in the btk-/- BMMCs (37). These observations thus support our conclusion that the PI3K-dependent, Lyn-mediated activation of Btk may be part of a signaling pathway, downstream of LAT2, which controls the amplification and/or maintenance of the PLCγ1-dependent calcium signal initially dependent upon interactions of PLCγ1 with LAT and SLP-76.
The defective calcium signal observed in btk-/- BMMCs may in part explain the reduced cytokine production observed in these cells. In this respect, we observed that the activation-state phosphorylation of S54 on the calcium-dependent transcription factor NFAT, in responses to both antigen and SCF, was diminished in btk-/- BMMCs (37). It is likely, however, that other transcriptional pathways also contribute to the Btk-regulated cytokine generation in mast cells. For example, we have observed that phophorylation of the transcription factor NFκB in response to both antigen and SCF is also decreased in btk-/- BMMCs (37). Antigen-dependent phosphorylation of the MAPKs p38 and JNK but not ERK is also reduced in btk-/- BMMCs, a defect that is more marked in btk-/-lyn-/- BMMCs (37,136). However, these responses appear to more obvious at earlier (10 min) than later (30 min) time points. Nevertheless, these and other data (136, 142, 144) have provided evidence that at least IL-2 generation may be controlled by JNK-dependent AP1 transcriptional regulation downstream of Btk.
In addition to mast cell degranulation, Btk may also be involved in other mast cell responses. For example, it has been observed that btk-/- BMMCs grown in the presence of IL-3 show greater expansion in culture than do wildtype BMMCs grown under the same conditions (145). This may be explained by reduced apoptosis due to inhibition of the JNK pro-apoptotic signal rather than enhanced division. Furthermore, studies using the btk-/- BMMCs suggest that Btk contributes to the signaling pathays mediating mast cell spreading in response to highly cytokinergic IgE (146) but not in response to SCF (133).
Itk
Itk (EMT) has also been proposed to play a role in mast cell activation, although the evidence to support this role is conflicting. As with Btk, Itk activity is regulated by the Src kinase-dependent phosphorylation of a critical tyrosine (Y511) in the activation loop followed by autophosphorylation of a regulatory tyrosine (y180) in the SH3 domain (147). Itk is tyrosine phosphorylated in mouse BMMCs following antigen challenge (148), suggesting a role for this response in mast cell activation. Indeed, it has been reported that antigen-induced degranulation of airway and cultured itk-/- mast cells in response to compound 48/80 is reduced (149). In vivo, whereas there were limited changes in mast cell-dependent allergic responses in itk-/- mice, there was a dramatic attenuation of both acute (plasma extravasation) and late phase inflammatory allergic responses (infiltration of eosinophils and lymphocytes and T-helper 2 cytokine production in the lungs). In this study, no differences were observed in levels of circulating levels of IgE or IgG. Similarly, mice lacking Itk were reported to have dramatically reduced lung inflammation and mucous production and a reduced T-cell influx into the lung (150). However, in this study, it was reported that circulating levels of IgE were elevated in the itk-/- mice. Furthermore, itk-/- mice have reported reduced airway responses to methacholine and diminished hyperresponsiveness to antigen accompanied by reduced T-helper 2 cytokine production (151).
In contrast to these reports, a recent study has reported that mouse BMMCs lacking itk have no discernable defects in antigen-mediated degranulation or in signaling events regulating this response (152). However, the release of cytokines, including IL-13 and TNF-α, in response to antigen was increased, and this increase appeared to be linked to elevated levels of nuclear NFAT. Furthermore, in this study, the reduced airway responsiveness to dinitrophenol-human serum albumin (DNP-HSA) following passive sensitization with anti-DNP IgE in vivo was associated with elevated circulating IgE levels. In addition, transfer of itk-/- BMMCs to W/Wv mast cell-deficient mice fully rescued histamine release in vivo, suggesting that the defective allergic responses observed in itk-/- was not due to lack of expression of the kinase in mast cells (152). Thus, the authors concluded that the observed defects in the allergic responses the itk-/- mice in vivo was due to the increased circulating IgE levels competing for the anti-DNP IgE rather than a defect in the mast cell signaling process (152).
Regulation of the tyrosine kinase network by dephosphorylation
A number of regulatory steps that control the activity of the tyrosine kinases expressed in mast cells were discussed in the preceding sections. Here, we focus on the converse key regulatory step that controls the tyrosine phosphorylation status of signaling proteins, namely dephosphorylation. Tyrosine dephosphorylation is mediated by protein tyrosine phosphatases (PTPs), which catalyze the removal of the phosphate group from Y residues in a selective manner. Mast cells express a variety of PTPs (153), and, while some progress has been made in understanding their function, our knowledge of their role is still quite limited. Here we will focus on those PTPs where there is evidence for a role in regulating mast cell signaling or function.
A role for CD45 in mast cell activation
The PTP CD45 increases the activity of Src PTKs by dephosphorylation of the COOH-terminal tyrosine that inactivates these kinases (Figs 3 and 5). As briefly mentioned above, phosphorylation of the Y residue at the COOH-terminus of the Src PTK promotes an intramolecular interaction with its own SH2 domain and suppresses catalytic activity by inducing a closed conformation of the kinase (6,107). Dephosphorylation of the COOH-terminal Y residue by CD45 causes an open conformation of the kinase allowing accessibility to the ATP-binding site in the catalytic domain and promoting the phosphorylation of an activation loop Y residue, which increases Src PTK activity.
A role for CD45 in IgE-mediated degranulation was first reported in basophils with the finding that a monoclonal antibody directed to CD45 inhibited histamine release from human basophils (154). Later studies on the role of CD45 in RBL variants expressing high or low levels of CD45 demonstrated a relationship between the levels of CD45 expression and the degranulation response (155). Low levels of CD45 caused poor degranulation, most notably when IgE-loaded cells were stimulated with suboptimal doses of antigen. FcεRI-associated PTK activity was also reduced in cells expressing low levels of CD45 as was the phosphorylation of this receptors β and γ subunits, suggesting a loss of Lyn activity. In contrast, high expression of CD45 resulted in strong phosphorylation of the FcεRI β and γ subunits and facilitated mast cell degranulation even at low doses of antigen. Co-immunoprecipitation of CD45 with FcεRI was also found, suggesting that CD45 is a key component in FcεRI-mediated mast cell activation. Beyond its role in FcεRI-mediated mast cell activation, CD45 was also demonstrated to play a role in IL-3-mediated mast cell activation and responses. BMMCs from cd45-/- mice stimulated with IL-3 showed increased activation of the Janus kinase 2 (JAK2) and enhanced proliferative responses (156). This effect appeared to be independent of Src PTKs and was attributed to negative regulation of JAK activity by CD45, since in vitro experiments demonstrated the interaction of CD45 with JAKs and the dephosphorylation of the latter.
CD45 (Fig. 5) thus appears to negatively regulate mast cell activation and function. However, many questions remain to be answered. For example, the mechanism by which CD45 mediates its activating role in mast cell activation is not known. Moreover, whether Lyn is the target (or the sole target) of CD45 activity is not completely clear. Given the recent evidence that multiple Src PTKs play a role in mast cell activation and function, revisiting what targets of CD45 activity may be key to mast cell activation seems warranted. Regardless, it is of particular interest to determine if CD45 plays a role in the sensitivity of the response of an allergic individual upon encountering an allergen.
SH2 domain-containing nonreceptor PTPs: SHP-1 and SHP-2
SHP-1 is expressed mostly in hematopoietic cells, whereas SHP-2 is more ubiquitously expressed. Both SHP-1 and SHP-2 contain two adjacent NH2-terminal SH2 domains, and several alternatively spliced isoforms of SHP-1 exclude one of these SH2 domains (Fig. 5). SHP-2 also contains a proline-rich sequence at its COOH-terminus that appears suited to interact with proteins containing SH3 domains. Deficiency in either SHP-1 or SHP-2 manifests in severe disease, a topic that has been recently reviewed (157).
These phosphatases function to negatively regulate the activation of mast cells. While their mode of action is not completely defined, these PTPs appear to function upon FcεRI engagement or regulate FcεRI signals through inhibitory receptors that recruit these PTPs and downregulate FcεRI-mediated mast cell activation. This latter topic of immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptors and their function has also been well reviewed (158) and will not be covered herein. Instead, herein we focus on how these PTPs may regulate FcεRI-mediated mast cell signaling and function.
Co-immunoprecipitation of SHP-1 and SHP-2 with FcεRI has been shown (103, 104). SHP-1 is found to be constitutively associated with FcεRI, whereas SHP-2 is recruited upon antigen stimulation of this receptor. In vitro phospho-ITAM peptide pulldown experiments revealed an association of SHP-2 with the FcεRIβ ITAM but no association of SHP-1 with either the FcεRIβ or γ ITAM was observed, suggesting that the observed association in co-immunoprecipitation experiments was likely to be indirect (104, 159). SHP-1 appears to exert opposing roles in FcεRI-mediated mast cell signaling. Overexpression experiments in the RBL mast cell line demonstrated that SHP-1 caused the decreased phosphorylation of FcεRI and Syk but, in contrast, an enhanced phosphorylation of JNK and increased the production of TNF was observed (160). This finding suggests that while SHP-1 may play a negative role proximal to FcεRI, its downstream function may be required to activate pathways needed for gene transcription or translation. Curiously, these experiments found no role for SHP-1 in degranulation (as measured by histamine release), even though Syk phosphorylation was considerably reduced. Beyond its ability to bind to multiple signaling proteins like Grb2, Dos/Gab2 (71), and FcεRI (161), surprisingly little is known about SHP-2 function in regulating mast cell activation. Thus, while SHP-1 and SHP-2 appear to be strong candidates in the regulation of FcεRI phosphorylation, more direct evidence of this role is required.
A recent study (120) on the regulation of Syk activation in mast cells, however, suggest a key role for PTPs in regulating Syk activation and demonstrates the potential role of SHP-1 and SHP-2 in the dephosphorylation of the FcεRIγ ITAM Y residues. Using mass spectrometry, it was noted that phosphorylation of Y58 (C-terminal ITAM Y) of the FcεRIγ appears to be less abundant than that at Y47 (N-terminal ITAM Y). In addition, expression of a mutant FcεRIγ (Y47F) in mast cells derived from FcεRIγ-null mice revealed that, upon FcεRI stimulation, there was minimal phosphorylation at the Y58 site. Since these Y residues are known targets of Lyn kinase, the ability of Lyn to phosphorylate both sites was tested and shown to be similar. Moreover, the inhibition of PTP activity with pervanadate demonstrated that both Y residues could be efficiently phosphorylated in cells. Mutation of either Y residue inhibited mast cell responses. However, the meager phosphorylation at Y58 alone was able to induce modest Syk activation and weak calcium responses. Furthermore, Syk was found to bind to a mutant FcεRIγ where only Y58 could be phosphorylated but not to one where only Y47 could be phosphorylated. Collectively, the findings suggested that, once phosphorylated, the Y58 site FcεRIγ is likely a target of preferential dephosphorylation. A test of this postulate revealed that Y58 is more susceptible to dephosphorylation by SHP-1 and SHP-2 than Y47 (120). Thus, one might surmise that control of Y58 phosphorylation is essential in regulating the activation of Syk and the extent of mast cell response.
Other PTPs in mast cells
Multiple other PTPs appear to be expressed in mast cells (reviewed in 153). These include HePTP, PTP20, PRL1, PRL2, PTP-MEG1, and PTP-MEG2. However, beyond establishing their presence in primarily the RBL mast cell line, there is little known on their role in mast cell activation and function. HePTP is preferentially expressed in hematopoietic cells, and this PTP is tyrosine phosphorylated upon FcεRI stimulation of RBL cells (162). However, this phosphatase does not co-immunoprecipitate with FcεRI, suggesting that it is not associated with this receptor and may function in controlling downstream signaling. Consistent with this view was the finding that tyrosine phosphorylation of HePTP can be stimulated by the calcium ionophore A23187 and that the absence of calcium in the extracellular medium causes a dramatic reduction in its phosphorylation when cells are stimulated via FcεRI. As the influx of calcium is also required for mast cell degranulation and calcium ionophores bypass the early signaling steps in FcεRI-mediated mast cell activation, it is suggested that HePTP is likely to function at a distal step to early signaling events in mast cells. However, it is not known what role tyrosine phosphorylation of HePTP plays in its activity in mast cells. Thus, much work is left to do in evaluating the role of this PTP in mast cell function.
PTP-MEG2 also appears to have a role in mast cell function. This PTP is expressed in RBL cells and is localized on secretory vesicles (163). Interestingly, the overexpression of this PTP caused a dramatic enlargement of secretory vesicles suggestive of fusion. This response may reflect a role for this PTP in controlling late steps in the secretory apparatus of mast cells that may be key to the degranulation process, since fusion of granules takes place during this process. Obviously, the target(s) of PTPMEG2 activity may be of considerable therapeutic interest.
As might be garnered from this section, there is still much to be learned about the role of PTPs in controlling the tyrosine kinase network involved in mast cell activation. Nonethless, it is clear that PTPs also play both positive and negative roles in mast cell activation and function. Moreover, the PTP network is equally complex and equally critical to the tyrosine kinase network in regulating mast cell function. The coming efforts are likely to provide discoveries that challenge the existing paradigm of PTPs as regulators rather than initiators of signaling pathways.
Conclusions and perspectives
It is clear from the discussions presented in this review that the tyrosine phosphorylation status of key regulatory proteins is critical for the ability of antigen to induce mast cell activation and for SCF to modify this response. Protein tyrosine phosphorylation is a highly regulated process depending on the equilibrium of phosphorylation, induced by tyrosine kinases, and dephosphorylation induced by tyrosine phosphatases.
Because of its central role in controlling calcium and in propagating signals required for FcεRI-mediated mast cell degranulation, Syk is considered a potential therapeutic target in allergic disease (164). This topic has been extensively reviewed (165, 166) and is beyond focus of this review. However, strategies that target the catalytic function of Syk (167) as well as those that target its interactions (168,169) have shown efficacy in inhibiting mast cell effector functions. These studies reflect the central role of Syk, as a kinase whose activity and interactions are important in driving mast cell signaling and function. Likewise, Src kinases play a central role in FcεRI-mediated mast cell activation and the ability of KIT to enhance this response. However, the recognition that Lyn may negatively regulate mast cell activation and that the absence of Lyn in mice can lead to an increased anaphylactic response questions the utility of agents targeting this kinase activity in the treatment of anaphylaxis and atopic disease. At the moment, our knowledge of whether Fyn kinase might serve as a valuable therapeutic target is limited. However, given that it is expressed in many cells, it is unlikely that the generalized approach of inhibiting its kinase activity would not result in some undesirable effects. Nonetheless, as we gain knowledge of its role in mast cell signaling and function, new avenues with therapeutic potential may be identified.
The Tec kinase Btk appears to be a component of an amplification pathway contributing to FcεRI-mediated degranulation and cytokine production and regulating the KIT-mediated enhancement of this response. Although compounds inhibiting Btk activity have proved to effectively inhibit mast cell-mediated responses in vivo, as with Src kinase and Syk inhibitors, a potential detrimental issue for the treatment of allergic disease in humans would be immunodeficiency, due to targeting of these critical kinases in B cells. The ability of KIT to enhance FcεRI-mediated mast cell activation has led to the suggestion that targeting KIT kinase activity may be a possible adjuvant approach for the treatment of mast cell disorders (170, 171). Certainly, they would appear to have potential utility for the treatment of mastocytosis (170,171). A compound co-targeting KIT and FcεRI-mediated signaling was also demonstrated to effectively inhibit an anaphylactic response in the mouse (140).
The approach of targeting both regulatory and initiating receptors has also shown efficacy when co-crosslinking inhibitory and activatory receptors on mast cells (172, 173). In some cases, this is mediated by the targeting of a tyrosine phosphatase associated with the inhibitory receptor (like paired Ig-like receptor-B) to cause dephosphorylation of components normally phosphorylated by engagement of the activating receptor, like FcεRI (174). This strategy holds promise for therapeutic intervention, as it increases target specificity, since FcεRI is expressed primarily on mast cells and basophils. Regardless, it is clear that increasing our knowledge on how tyrosine kinase networks function in mast cell activation will likely inform us on the suitability of potential therapeutic targets and continue to define new areas of therapeutic interest.
Acknowledgements
Research in the authors' laboratory is supported by the NIAMS (J.R.) NIAID (A.M.G.) Intramural Programs within the National Institutes of Health, USA.
References
- 1.Kirshenbaum AS, et al. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 2.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 6.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 7.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:2318–2330. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 8.Tkaczyk C, Jensen BM, Iwaki S, Gilfillan AM. Adaptive and innate immune reactions regulating mast cell activation: from receptor-mediated signaling to responses. Immunol Allergy Clin North Am. 2006;26:427–450. doi: 10.1016/j.iac.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 12.Nadler MJ, Matthews SA, Turner H, Kinet JP. Signal transduction by the high-affinity immunoglobulin E receptor FcεRI: coupling form to function. Adv Immunol. 2000;76:325–355. doi: 10.1016/s0065-2776(01)76022-1. [DOI] [PubMed] [Google Scholar]
- 13.Blank U, Rivera J. The Ins and Outs of IgE-dependent mast cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Furumoto Y, et al. Rethinking the role of Src family protein tyrosine kinases in the allergic response: New insights on the functional coupling of the high affinity IgE receptor. Immunol Res. 2004;30:241–254. doi: 10.1385/ir:30:2:241. [DOI] [PubMed] [Google Scholar]
- 15.Rivera J. Molecular adapters in FcεRI signaling and the allergic response. Curr Opin Immunol. 2002;14:688–693. doi: 10.1016/s0952-7915(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 16.Dombrowicz D, et al. Allergy-associated FcRβ is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–529. doi: 10.1016/s1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 17.Kraft S, Rana S, Jouvin MH, Kinet JP. The role of the FcεRI β-chain in allergic diseases. Int Arch Allergy Immunol. 2004;135:62–72. doi: 10.1159/000080231. [DOI] [PubMed] [Google Scholar]
- 18.On M, Billingsley JM, Jouvin MH, Kinet JP. Molecular dissection of the FcRβ signaling amplifier. J Biol Chem. 2004;279:45782–45790. doi: 10.1074/jbc.M404890200. [DOI] [PubMed] [Google Scholar]
- 19.Furumoto Y, Nunomura S, Terada T, Rivera J, Ra C. The FcεRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IκB kinase phosphorylation and mast cell cytokine production. J Biol Chem. 2004;279:49177–49187. doi: 10.1074/jbc.M404730200. [DOI] [PubMed] [Google Scholar]
- 20.Tsai M, et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006;26:387–405. doi: 10.1016/j.iac.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura Y, Tsujimura T, Jippo T, Kasugai T, Kanakura Y. Regulation of development, survival and neoplastic growth of mast cells through the c-kit receptor. Int Arch Allergy Immunol. 1995;107:54–56. doi: 10.1159/000236929. [DOI] [PubMed] [Google Scholar]
- 23.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 24.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bischoff SC, Dahinden CA. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MS, Radinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera J, Fierro NA, Olivera A, Suzuki R. Chapter 3. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broudy VC, et al. Human umbilical vein endothelial cells display high-affinity c-kit receptors and produce a soluble form of the c-kit receptor. Blood. 1994;83:2145–2152. [PubMed] [Google Scholar]
- 30.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 31.Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 32.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 33.Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 34.Mol CD, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278:31461–31464. doi: 10.1074/jbc.C300186200. [DOI] [PubMed] [Google Scholar]
- 35.Jahn T, Leifheit E, Gooch S, Sindhu S, Weinberg K. Lipid rafts are required for Kit survival and proliferation signals. Blood. 2007;110:1739–1747. doi: 10.1182/blood-2006-05-020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hundley TR, et al. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 37.Iwaki S, et al. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 40.Metcalfe DD, Mekori JA, Rottem M. Mast cell ontogeny and apoptosis. Exp Dermatol. 1995;4:227–230. doi: 10.1111/j.1600-0625.1995.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura Y, Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979;53:492–497. [PubMed] [Google Scholar]
- 42.Tono T, et al. c-kit Gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal ckit coding region. Blood. 1992;80:1448–1453. [PubMed] [Google Scholar]
- 43.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 44.Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- 45.Lorentz A, Schuppan D, Gebert A, Manns MP, Bischoff SC. Regulatory effects of stem cell factor and interleukin-4 on adhesion of human mast cells to extracellular matrix proteins. Blood. 2002;99:966–972. doi: 10.1182/blood.v99.3.966. [DOI] [PubMed] [Google Scholar]
- 46.Tkaczyk C, et al. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following KIT activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 47.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 48.Akin C, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110:2331–2333. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwaki S, et al. Kit- and FcεRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell Signal. 2008;20:195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda S, et al. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood. 2002;99:3342–3349. doi: 10.1182/blood.v99.9.3342. [DOI] [PubMed] [Google Scholar]
- 51.Samayawardhena LA, Hu J, Stein PL, Craig AW. Fyn kinase acts upstream of Shp2 and p38 mitogen-activated protein kinase to promote chemotaxis of mast cells towards stem cell factor. Cell Signal. 2006;18:1447–1454. doi: 10.1016/j.cellsig.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Shivakrupa R, Linnekin D. Lyn contributes to regulation of multiple Kit-dependent signaling pathways in murine bone marrow mast cells. Cell Signal. 2005;17:103–109. doi: 10.1016/j.cellsig.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 53.O'Laughlin-Bunner B, et al. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–350. doi: 10.1182/blood.v98.2.343. [DOI] [PubMed] [Google Scholar]
- 54.Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007;109:3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 56.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 57.Pribluda VS, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young RM, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- 59.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcεRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- 61.Vonakis BM, Haleem-Smith H, Benjamin P, Metzger H. Interaction between the unphosphorylated receptor with high affinity for IgE and Lyn kinase. J Biol Chem. 2001;276:1041–1050. doi: 10.1074/jbc.M003397200. [DOI] [PubMed] [Google Scholar]
- 62.Kovarova M, et al. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcε receptor I aggregation. Mol Cell Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovarova M, et al. Cholesterol-deficiency in a murine model of Smith-Lemli-Opitz Syndrome reveals increased mast cell responsiveness. J Exp Med. 2006;203:1161–1171. doi: 10.1084/jem.20051701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera J, Gonzalez-Espinosa C, Kovarova M, Parravicini V. The architecture of IgE-dependent mast cell signaling. A complex story. Allergy Clin Immunol Int. 2002;14:25–36. [Google Scholar]
- 65.Davey AM, Walvick RP, Liu Y, Heikal AA, Sheets ED. Membrane order and molecular dynamics associated with IgE receptor cross-linking in mast cells. Biophys J. 2007;92:343–355. doi: 10.1529/biophysj.106.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davey AM, Krise KM, Sheets ED, Heikal AA. Molecular perspective of antigen-mediated mast cell signaling. J Biol Chem. 2008;283:7117–7127. doi: 10.1074/jbc.M708879200. [DOI] [PubMed] [Google Scholar]
- 67.Wilson BS, Pfeiffer JR, Surviladze Z, Gaudet EA, Oliver JM. High resolution mapping of mast cell membranes reveals primary and secondary domains of FcεRI and LAT. J Cell Biol. 2001;154:645–658. doi: 10.1083/jcb.200104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson BS, Pfeiffer JR, Oliver JM. Observing FcεRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parravicini V, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 70.Gomez G, et al. Impaired FcεRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn-deficiency in mast cells. J Immunol. 2005;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 71.Gu H, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 72.Ali K, et al. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 73.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 74.Hisatsune C, et al. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- 75.Ma HT, et al. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olivera A, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 77.Olivera A, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Choi WS, et al. Activation of RBL-2H3 mast cells is dependent on tyrosine phosphorylation of phospholipase D by Fyn and Fgr. Mol Cell Biol. 2004;24:6980–6992. doi: 10.1128/MCB.24.16.6980-6992.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi WS, Kim YM, Combs C, Frohman MA, Beaven MA. Phospholipases D1 and D2 regulate different phases of exocytosis in mast cells. J Immunol. 2002;168:5682–5689. doi: 10.4049/jimmunol.168.11.5682. [DOI] [PubMed] [Google Scholar]
- 80.Macurek L, et al. Regulation of microtubule nucleation from membranes by complexes of membrane-bound gamma-tubulin with Fyn kinase and phosphoinositide 3-kinase. Biochem J. 2008 doi: 10.1042/BJ20080909. doi:10.1042/BJ20080909. [DOI] [PubMed] [Google Scholar]
- 81.Sulimenko V, et al. Regulation of microtubule formation in activated mast cells by complexes of gamma-tubulin with Fyn and Syk kinases. J Immunol. 2006;176:7243–7253. doi: 10.4049/jimmunol.176.12.7243. [DOI] [PubMed] [Google Scholar]
- 82.Nishida K, et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nahm DH, et al. Identification of Fyn-binding proteins in MC/9 mast cells using mass spectrometry. Biochem Biophys Res Commun. 2003;310:202–208. doi: 10.1016/j.bbrc.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, Suzuki K, Hitomi T, Siraganian RP. TOM1L1 is a Lyn substrate involved in FcεRI signaling in mast cells. J Biol Chem. 2007;282:37669–37677. doi: 10.1074/jbc.M705168200. [DOI] [PubMed] [Google Scholar]
- 85.Odom S, et al. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hernandez-Hansen V, et al. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 87.Xiao W, et al. Positive and negative regulation of mast cell activation by Lyn via the Fc∊RI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huber M, et al. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leung WH, Bolland S. The inositol 5'-phosphatase SHIP-2 negatively regulates IgE-induced mast cell degranulation and cytokine production. J Immunol. 2007;179:95–102. doi: 10.4049/jimmunol.179.1.95. [DOI] [PubMed] [Google Scholar]
- 90.Furumoto Y, et al. Cutting edge: lentiviral shRNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 91.Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 92.Song JS, et al. Tyrosine phosphorylation-dependent and -independent associations of protein kinase C-δ with Src family kinases in the RBL-2H3 mast cell line: regulation of Src family kinase activity by protein kinase C-δ. Oncogene. 1998;16:3357–3368. doi: 10.1038/sj.onc.1201886. [DOI] [PubMed] [Google Scholar]
- 93.Germano P, Gomez J, Kazanietz MG, Blumberg PM, Rivera J. Phosphorylation of the γ chain of the high affinity receptor for immunoglobulin E by receptor-associated protein kinase C-δ. J Biol Chem. 1994;269:23102–23107. [PubMed] [Google Scholar]
- 94.Hong H, et al. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu M, Liu Y, Koonpaew S, Ganillo O, Zhang W. Positive and negative regulation of Fcε-mediated signaling by the adaptor protein LAB/NTAL. J Exp Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rivera J. NTAL/LAB and LAT: A balancing act in mast cell activation and function. Trends Immunol. 2005;26:119–122. doi: 10.1016/j.it.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Zoller KE, MacNeil IA, Brugge JS. Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]
- 98.Bu JY, Shaw AS, Chan AC. Analysis of the interaction of ZAP-70 and syk protein-tyrosine kinases with the T-cell antigen receptor by plasmon resonance. Proc Natl Acad Sci USA. 1995;92:5106–5110. doi: 10.1073/pnas.92.11.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sada K, Takano Y, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 100.Arias-Palomo E, Recuero-Checa MA, Bustelo XR, Llorca O. 3D structure of Syk kinase determined by single-particle electron microscopy. Biochem Biophys Acta. 2007;1774:1493–1499. doi: 10.1016/j.bbapap.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol Immunol. 2002;38:1229–1233. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 102.Bijli KM, Fazal F, Minhajuddin M, Rahman A. Activation of Syk by protein kinase C-δ regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via tyrosine phosphorylation of RelA/p65. J Biol Chem. 2008;283:14674–14684. doi: 10.1074/jbc.M802094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benhamou M, Ryba NJP, Kihara H, Nishikata H, Siraganian RP. Protein-tyrosine kinase p72syk in high affinity receptor signaling. Identification as a component of pp72 and associaition with the receptor γ chain after receptor aggregation. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- 104.Kihara H, Siraganian RP. Src homology 2 domains of Syk and Lyn bind to tyrosinephosphorylated subunits of the high affinity IgE receptor. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- 105.Costello PS, et al. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- 106.Zhang J, Kimura T, Siraganian RP. Mutations in the activation loop tyrosines of protein tyrosine kinase Syk abrogate intracellular signaling but not kinase activity. J Immunol. 1998;161:4366–4374. [PubMed] [Google Scholar]
- 107.Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keshvara LM, Isaacson C, Harrison ML, Geahlen RL. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J Biol Chem. 1997;272:10377–10381. doi: 10.1074/jbc.272.16.10377. [DOI] [PubMed] [Google Scholar]
- 110.Adachi T, Wienands J, Tsubata T, Kurosaki T. Interdomain A is crucial for ITAM-dependent and -independent regulation of Syk. Biochem Biophys Res Comm. 2007;364:111–117. doi: 10.1016/j.bbrc.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 111.Latour S, Zhang J, Siraganian RP, Veillette A. A unique insert in the linker domain of Syk is necessary for its function in immunoreceptor signalling. EMBO J. 1998;17:2584–2595. doi: 10.1093/emboj/17.9.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sada K, Zhang J, Siraganian RP. Point mutation of a tyrosine in the linker region of Syk results in a gain of function. J Immunol. 2000;164:338–344. doi: 10.4049/jimmunol.164.1.338. [DOI] [PubMed] [Google Scholar]
- 113.Meng W, Sawasdikosol S, Burakoff SJ, Eck MJ. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84–90. doi: 10.1038/18050. [DOI] [PubMed] [Google Scholar]
- 114.Rao N, et al. The non-receptor tyrosine kinase Syk is a target of Cbl-mediated ubiquitylation upon B-cell receptor stimulation. EMBO J. 2001;20:7085–7095. doi: 10.1093/emboj/20.24.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Berenstein E, Siraganian RP. Phosphorylation of Tyr342 in the linker region of Syk is critical for FcεRI signaling in mast cells. Mol Cell Biol. 2002;22:8144–8154. doi: 10.1128/MCB.22.23.8144-8154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J, Billingsley ML, Kincaid RL, Siraganian RP. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J Biol Chem. 2000;275:35442–35447. doi: 10.1074/jbc.M004549200. [DOI] [PubMed] [Google Scholar]
- 118.Sada K, Zhang J, Siraganian RP. SH2 domain-mediated targeting, but not localization, of Syk in the plasma membrane is critical for FcεRI signaling. Blood. 2001;97:1352–1359. doi: 10.1182/blood.v97.5.1352. [DOI] [PubMed] [Google Scholar]
- 119.Minoguchi K, et al. Activation of protein tyrosine kinase p72syk by FcεRI aggregation in rat basophilic leukemia cells. p72syk is a minor component but the major protein tyrosine kinase of pp72. J Biol Chem. 1994;269:16902–16908. [PubMed] [Google Scholar]
- 120.Yamashita T, et al. Differential dephosphorylation of the FcRγ ITAM tyrosines with dissimilar potential for activating Syk. J Biol Chem. 2008 doi: 10.1074/jbc.M802679200. doi/10.1074/jbc.M802679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang J, Chiang YJ, Hodes RJ, Siraganian RP. Inactivation of c-Cbl or Cbl-b differentially affects signaling from the high affinity IgE receptor. J Immunol. 2004;173:1811–1818. doi: 10.4049/jimmunol.173.3.1811. [DOI] [PubMed] [Google Scholar]
- 122.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat Rev Immunol. 2005;5:284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 123.Felices M, Falk M, Kosaka Y, Berg LJ. Tec kinases in T cell and mast cell signaling. Adv Immunol. 2007;93:145–184. doi: 10.1016/S0065-2776(06)93004-1. [DOI] [PubMed] [Google Scholar]
- 124.Lindvall JM, et al. Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev. 2005;203:200–215. doi: 10.1111/j.0105-2896.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 125.Rawlings DJ, et al. Activation of BTK by a phosphorylation mechanism initiated by Src family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 126.Park H, et al. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 127.Kawakami Y, et al. Regulation of protein kinase CβI by two protein-tyrosine kinases, Btk and Syk. Proc Natl Acad Sci USA. 2000;97:7423–7428. doi: 10.1073/pnas.120175097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawakami Y, Yao LB, Han W, Kawakami T. Tec family protein-tyrosine kinases and pleckstrin homology domains in mast cells. Immunol Lett. 1996;54:113–117. doi: 10.1016/s0165-2478(96)02659-4. [DOI] [PubMed] [Google Scholar]
- 129.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J Biol Chem. 2001;276:16201–16206. doi: 10.1074/jbc.M100873200. [DOI] [PubMed] [Google Scholar]
- 130.Kawakami Y, et al. Tyrosine phosphorylation and activation of bruton tyrosine kinase upon FcεRI cross-linking. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yao L, Kawakami Y, Kawakami T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kang SW, et al. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 2001;20:5692–5702. doi: 10.1093/emboj/20.20.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Setoguchi R, Kinashi T, Sagara H, Hirosawa K, Takatsu K. Defective degranulation and calcium mobilization of bone-marrow derived mast cells from Xid and Btk-deficient mice. Immunol Lett. 1998;64:109–118. doi: 10.1016/s0165-2478(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 134.Kawakami Y, Kitaura J, Hata D, Yao L, Kawakami T. Functions of Bruton's tyrosine kinase in mast and B cells. J Leukoc Biol. 1999;65:286–290. doi: 10.1002/jlb.65.3.286. [DOI] [PubMed] [Google Scholar]
- 135.Hata D, et al. Involvement of Bruton's tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kawakami Y, et al. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 137.Kawakami Y, et al. Terreic acid, a quinone epoxide inhibitor of Bruton's tyrosine kinase. Proc Natl Acad Sci USA. 1999;96:2227–2232. doi: 10.1073/pnas.96.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hantschel O, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci USA. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kneidinger M, et al. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood. 2008;111:3097–3107. doi: 10.1182/blood-2007-08-104372. [DOI] [PubMed] [Google Scholar]
- 140.Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcεRI-mediated signaling: coordinated suppression of mast cell activation. J Pharmacol Exp Ther. 2008;324:128–138. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heinonen JE, Smith CI, Nore BF. Silencing of Bruton's tyrosine kinase (Btk) using short interfering RNA duplexes (siRNA) FEBS Lett. 2002;527:274–278. doi: 10.1016/s0014-5793(02)03206-4. [DOI] [PubMed] [Google Scholar]
- 142.Hata D, et al. Bruton's tyrosine kinase-mediated interleukin-2 gene activation in mast cells. Dependence on the c-Jun N-terminal kinase activation pathway. J Biol Chem. 1998;273:10979–10987. doi: 10.1074/jbc.273.18.10979. [DOI] [PubMed] [Google Scholar]
- 143.Fluckiger AC, et al. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kawakami Y, Hartman SE, Holland PM, Cooper JA, Kawakami T. Multiple signaling pathways for the activation of JNK in mast cells: involvement of Bruton's tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J Immunol. 1998;161:1795–1802. [PubMed] [Google Scholar]
- 145.Kawakami Y, et al. Bruton's tyrosine kinase regulates apoptosis and JNK/SAPK kinase activity. Proc Natl Acad Sci USA. 1997;94:3938–3942. doi: 10.1073/pnas.94.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kitaura J, et al. Regulation of highly cytokinergic IgE-induced mast cell adhesion by Src, Syk, Tec, and protein kinase C family kinases. J Immunol. 2005;174:4495–4504. doi: 10.4049/jimmunol.174.8.4495. [DOI] [PubMed] [Google Scholar]
- 147.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem. 2003;278:37112–37121. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 148.Kawakami Y, et al. Activation and interaction with protein kinase C of a cytoplasmic tyrosine kinase, Itk/Tsk/Emt, on FcεRI cross-linking on mast cells. J Immunol. 1995;155:3556–3562. [PubMed] [Google Scholar]
- 149.Forssell J, et al. Interleukin-2-inducible T cell kinase regulates mast cell degranulation and acute allergic responses. Am J Respir Cell Mol Biol. 2005;32:511–520. doi: 10.1165/rcmb.2004-0348OC. [DOI] [PubMed] [Google Scholar]
- 150.Mueller C, August A. Attenuation of immunological symptoms of allergic asthma in mice lacking the tyrosine kinase ITK. J Immunol. 2003;170:5056–5063. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 151.Ferrara TJ, Mueller C, Sahu N, Ben-Jebria A, August A. Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase. J Allergy Clin Immunol. 2006;117:780–786. doi: 10.1016/j.jaci.2005.12.1330. [DOI] [PubMed] [Google Scholar]
- 152.Iyer AS, August A. The Tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses. J Immunol. 2008;180:7869–7877. doi: 10.4049/jimmunol.180.12.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Heneberg P, Draber P. Nonreceptor protein tyrosine and lipid phosphatases in type I Fcε receptor-mediated activation of mast cells and basophils. Int Arch Allergy Immunol. 2002;128:253–263. doi: 10.1159/000063864. [DOI] [PubMed] [Google Scholar]
- 154.Hook WA, Berenstein EH, Zinsser FU, Fischler C, Siraganian RP. Monoclonal antibodies to the leukocyte common antigen (CD45) inhibit IgE-mediated histamine release from human basophils. J Immunol. 1991;147:2670–2676. [PubMed] [Google Scholar]
- 155.Murakami K, Sato S, Nagasawa S, Yamashita T. Regulation of mast cell signaling through high-affinity IgE receptor by CD45 protein tyrosine phosphatase. Int Immunol. 2000;12:169–176. doi: 10.1093/intimm/12.2.169. [DOI] [PubMed] [Google Scholar]
- 156.Irie-Sasaki J, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 157.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 159.Kimura T, et al. Syk-independent tyrosine phosphorylation and association of the protein tyrosine phosphatases SHP-1 and SHP-2 with the high affinity IgE receptor. J Immunol. 1997;159:4426–4434. [PubMed] [Google Scholar]
- 160.Xie ZH, Zhang J, Siraganian RP. Positive regulation of c-Jun N-terminal kinase and TNF-α production but not histamine release by SHP-1 in RBL-2H3 mast cells. J Immunol. 2000;164:1521–1528. doi: 10.4049/jimmunol.164.3.1521. [DOI] [PubMed] [Google Scholar]
- 161.Kimura T, et al. Downstream signaling molecules bind to different phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) peptides of the high affinity IgE receptor. J Biol Chem. 1996;271:27962–27968. doi: 10.1074/jbc.271.44.27962. [DOI] [PubMed] [Google Scholar]
- 162.Swieter M, Berenstein EH, Swaim WD, Siraganian RP. Aggregation of IgE receptors in rat basophilic leukemia 2H3 cells induces tyrosine phosphorylation of the cytosolic protein-tyrosine phosphatase HePTP. J Biol Chem. 1995;270:21902–21906. doi: 10.1074/jbc.270.37.21902. [DOI] [PubMed] [Google Scholar]
- 163.Wang X, et al. Enlargement of secretory vesicles by protein tyrosine phosphatase PTP-MEG2 in rat basophilic leukemia mast cells and Jurkat T cells. J Immunol. 2002;168:4612–4619. doi: 10.4049/jimmunol.168.9.4612. [DOI] [PubMed] [Google Scholar]
- 164.Kovarova M, Rivera J. A molecular understanding of mast cell activation and the promise of anti-allergic therapeutics. Curr Med Chem. 2004;11:2083–2091. doi: 10.2174/0929867043364801. [DOI] [PubMed] [Google Scholar]
- 165.Wong BR, Grossbard EB, Payan DG, Masuda ES. Targeting Syk as a treatment for allergic and autoimmune disorders. Expert Opin Investig Drugs. 2004;13:743–762. doi: 10.1517/13543784.13.7.743. [DOI] [PubMed] [Google Scholar]
- 166.Masuda ES, Schmitz J. Syk inhibitors as treatment for allergic rhinitis. Pulm Pharmacol Ther. 2008;21:461–467. doi: 10.1016/j.pupt.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 167.Braselmann S, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 168.Moriya K, et al. ER-27319, a novel acridone-related compound, inhibits release of antigen-induced allergic mediators from mast cells by selective inhibition of FcεRI-mediated activation of Syk. Proc Natl Acad Sci USA. 1997;94:12539–12544. doi: 10.1073/pnas.94.23.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mazuc E, et al. A novel druglike spleen tyrosine kinase binder prevents anaphylactic shock when administered orally. J Allergy Clin Immunol. 2008;122:188–194. doi: 10.1016/j.jaci.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 170.Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jensen BM, Metcalfe DD, Gilfillan AM. Targeting kit activation: a potential therapeutic approach in the treatment of allergic inflammation. Inflamm Allergy Drug Targets. 2007;6:57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- 172.Bruhns P, Fremont S, Daeron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17:662–669. doi: 10.1016/j.coi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 173.Saxon A, Kepley C, Zhang K. “Accentuate the negative, eliminate the positive”: engineering allergy therapeutics to block allergic reactivity through negative signaling. J Allergy Clin Immunol. 2008;121:320–325. doi: 10.1016/j.jaci.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 174.Chen CC, Kong DW, Cooper MD, Kubagawa H. Mast cell regulation via paired immunoglobulin-like receptor PIR-B. Immunol Res. 2002;26:191–197. doi: 10.1385/ir:26:1-3:191. [DOI] [PubMed] [Google Scholar]