Abstract
OBJECTIVES
To investigate the relationship between circulating uric acid (UA) levels and plasma antioxidants and whether antioxidant levels modulate the association between UA and physical function.
DESIGN
Cross-sectional.
SETTING
Community-based.
PARTICIPANTS
Nine hundred sixty-six elderly persons participating in the baseline assessment of the Invecchiare in Chianti Study.
MEASUREMENTS
UA, carotenoid, tocopherol, and selenium concentrations were assayed. Physical function was measured using the Short Physical Performance Battery (SPPB) and difficulties in instrumental activities of daily living (IADLs). Potential confounders were assessed using standardized methods.
RESULTS
Total carotenoids (P =.008), in particular α-carotene (P =.02), lutein (P<.001), zeaxanthin (P<.001), lycopene (P =.07), cryptoxanthin (P =.29), and selenium (P =.04) were inversely associated with UA levels. Total tocopherols (P =.06) and α-tocopherol (P =.10) had a positive trend across UA levels. SPPB (P =.01) and IADL disability (P =.002) were nonlinearly distributed across the UA quintiles. Participants within the middle UA quintile (4.8–5.3 mg/dL) were less disabled in IADLs and had better SPPB scores than those in the extreme UA quintiles. There was a significant interaction between UA and selenium in the model predicting SPPB score (P =.02).
CONCLUSION
UA levels are inversely associated with circulating carotenoids and selenium. Participants with intermediate UA levels had a higher prevalence of good physical functions, higher SPPB scores, and lower IADL disability. This study suggests that older subjects with intermediate UA levels may have an optimum balance between proinflammatory and antioxidant compounds that may contribute to better physical performance.
Keywords: uric acid, antioxidants, metabolism, inflammation, physical function, disability
Uric acid (UA), a constituent of the cell cytosol generated by nucleotide catabolism, reflects cell death and turnover and dietary intake composition. Based on biochemical evidence, UA is considered to be a reactive oxygen species (ROS) scavenger, and the genetic mutation that allows for higher UA circulating levels in humans than in all other species1,2 has been interpreted as an evolutionary strategy to counteract ROS-mediated damage, an unavoidable by-product of aerobic metabolism.3–5 Despite this interpretation, epidemiological studies have demonstrated that hyperuricemia is a strong predictor of mortality in women,6–8 older persons9,10 and those with preexisting cardiovascular disease.11–12 In subjects with chronic heart failure, UA levels have been positively associated with impaired oxidative metabolism, hyperinsulinemia, high levels of proinflammatory cytokine activation, and impaired endothelial function.13–14 Although an association between serum UA levels and various cardiovascular morbidity exists, most scientists believe that UA is a compensatory mechanism aimed at limiting oxidative stress, rather than having a causal role in the pathogenesis of these conditions.4
In partial contrast with this hypothesis, an association was recently demonstrated between UA and several proinflammatory parameters in humans.15 Consistently preclinical studies showed that UA can directly trigger an immunological response.16 In practice, the potential effects of UA on health status remain uncertain.
To gain insight into the relationship between UA levels and oxidative processes and its effect on general health status, it was proposed that the relationship between circulating UA and antioxidant levels, such as carotenoids, tocopherols, and selenium, and between UA and measures of physical function that in the geriatric literature are generally considered to be good indicators of global health status be studied.
This approach may help discover whether UA behaves as an antioxidant compound in humans and whether levels of circulating antioxidants modulate the effect of UA on health status, with the final aim being to provide insight into the pathophysiology of disability in older persons.
METHODS
Study Sample
The present analyses used data from participants in the Invecchiare in Chianti (Aging in the Chianti area, InCHIANTI) study, an epidemiological study performed in the Chianti, Italy, countryside. The rationale, design, and data collection procedures have been described elsewhere.17 The study protocol complies with the Declaration of Helsinki and was approved by the ethical committee of the Italian National Institute of Research and Care of Aging.
Briefly, in August 1998, 1,270 people aged 65 and older were randomly selected from the population registry of Greve in Chianti (population 11,709) and Bagno a Ripoli (population 4,704); of the 1,256 eligible subjects, 1,155 (90.1%) agreed to participate. Of the 1,155 participants, 1,055 (91.3%) persons aged 65 and older participated in the blood drawing from September 1998 to March 2000.
From the original sample of 1,055 subjects, 1,042 subjects had a serum UA determination. Six subjects with severe renal failure (estimated glomerular filtration rate (eGFR) <30 mL/min per 1.73 m2),18 six subjects taking vitamin or mineral supplements, and 64 subjects with cancer were excluded. Of the 966 subjects included in the final sample (534 women and 432 men), none had gout.
A detailed description of blood sampling procedures has been previously published.16 Blood samples were collected in the morning after overnight fasting and sitting for 15 minutes. The blood samples were centrifuged at 4°C to separate plasma. Plasma aliquots were protected from light with aluminum foil, stored at − 80°C, and not thawed until analyzed.
Uric Acid
UA (mg/dL) was measured using enzymatic-colorimetric methods (Roche Diagnostics, GmbH, Mannheim, Germany). The lower limits of detection were 0.2 mg/dL (range 0.2–25.0 mg/dL), intra-assay and interassay coefficients of variation (CVs) were 0.5% and 1.7%, respectively. According to values provided by the centralized laboratory at the SM Annunziata Hospital, Florence, Italy, hyperuricemia was defined as a serum urate concentration greater than 7.5 mg/dL (450 μmol/L) in men and greater than 6.2 mg/dL (372 μmol/L) in women. For statistical analysis, UA was divided into quintiles according to the following cutpoints: 4.0, 4.7, 5.3, and 6.1.
Plasma Antioxidants
Serum levels of α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene were determined using high-performance liquid chromatography.19 Total carotenoids were calculated as the sum of α-carotene, β-carotene, cryptoxanthin, lutein, zeaxanthin, and lycopene in μmol/L. Within- and between-assay CVs were 7.3% and 9.7% for α-carotene, 4.6% and 5.5% for β-carotene, 2.7% and 3.5% for cryptoxanthin, 2.6% and 7.1% for lutein, 6.2% and 6.8% for zeaxanthin, and 7.6% and 7.9% for lycopene, respectively.
Circulating concentrations of α- and γ-tocopherol were measured using reverse-phase high-performance liquid chromatography using a 3-m C18 reverse-phase column. A detailed description of the analytical method and procedures has been previously published.20 Total tocopherols was defined as the sum of α- and γ-tocopherols. Total cholesterol was measured using an automated enzymatic method, and the values were used to compute the tocopherol:cholesterol ratios.21 Serum selenium was measured using graphite furnace atomic absorption spectrometry with a Perkin Elmer AAnalyst 600 with Zeeman background correction. Samples were diluted 1:4 with a Triton-X correction and nitric acid solution (Fisher Scientific, Pittsburgh, PA); the matrix modifier was a palladium and magnesium nitrate solution (both Perkin Elmer, Norwalk, CT). The instrument was calibrated daily using known serum selenium standards (UTAK Laboratories, Inc., Valencia, CA). Within- and between-assay CVs for selenium were 5.8% and 4.8%, respectively. Selenium concentration was expressed in μmol/L. For statistical analysis, selenium was divided into quartiles according to the following cutpoints: 0.85, 0.95, 1.05, and 1.87.
Inflammatory Markers
Serum level of interleukin-6 (IL-6) was measured using enzyme linked immunosorbent assay (ELISA) using ultrasensitive commercial kits (Human Ultrasensitive, Biosource International Inc., Camarillo, CA). The minimum detectable concentration of IL-6 was 0.10 pg/mL, and the inter-assay CV was 7%. Serum C-reactive protein (CRP) was measured using a ELISA high-sensitivity test using purified protein and polyclonal anti-CRP antibodies with standardization according to the World Health Organization First International Reference Standard. The minimum detectable concentration was 0.03 mg/L, and the interassay CV was 5.0%. The average of two IL-6 and CRP measures for each sample was used in the analysis.
Physical Function
Physical function was assessed using standardized questionnaire and performance measures. Disability in instrumental activities of daily living (IADLs) was assessed by asking participants whether they needed help performing eight IADLs (shopping, doing light housework, preparing meals, managing money, using the telephone, taking medications, using transportation, doing laundry).22 The number of reported disabilities was used for the present study.
The Short Physical Performance Battery (SPPB) assessed walking speed, standing balance, and ability to rise from a chair. Walking speed was defined as the best performance (time in seconds) of two 4-m normal-pace walks along a corridor. For standing balance, participants were asked to stand in three progressively more-difficult positions for 10 seconds each: a position with the feet side by side, a semitandem position, and a full-tandem position. For the chair-stand test, participants were asked to stand up from and sit down in a chair five times as quickly as possible without using arms; the performance was timed. Each physical performance test was categorized into a five-level score, with 0 representing inability to do the test and 4 representing the highest level of performance, according to previously validated cutpoints.23 A summary performance measure ranging from 0 (poorest) to 12 (best) was developed by summing categorical scores of the individual performance tests.
Covariates
Standardized questionnaires were used to collect data on demographics, smoking, and medications. Smoking was measured as pack-years exposure, combining intensity and duration (packs smoked per day × years of smoking), based on self-report. Average energy intake (Kcal/d), average intake of fruits and vegetables (g/d), alcohol intake (<30 vs <30 g/d), and animal protein intake (g/d) were assessed by administering the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire.24 The information provided by the questionnaire was transformed into average daily intake of macro- and micro-nutrients using custom software that uses as a reference the table of food composition for Italian epidemiological studies, edited by the European Institute of Oncology.25
Physical activity in the previous year was based on responses to multiple questions and was rated as sedentary (inactive or light-intensity physical activity (walking, light housework) <1 h/wk), light physical activity (light-intensity physical activity 2–4 h/wk), moderate-high physical activity (light physical activity >4 h/wk or moderate physical activity (brisk walking, playing soccer, gardening) ≥1 h/wk). Renal function was assessed according to serum creatinine and creatinine-based eGFR, using the simplified Modification of Diet in Renal Disease equation.18
A trained geriatrician examined all participants. Diseases were ascertained according to preestablished criteria that combined information from physician diagnosis, medical records, clinical examination, and blood clinical tests.26 In the analysis presented here, cardiovascular disease is defined as the occurrence of angina pectoris, myocardial infarction, or congestive heart failure, and cerebrovascular disease includes stroke and transient ischemic attack. Metabolic syndrome is defined according to the criteria from the National Cholesterol Education Program’s Adult Treatment Panel III.27 All participants underwent carotid ultrasound and ankle-brachial index (ABI) estimation at the initial evaluation. Atherosclerosis was defined as bilateral carotid atherosclerotic plaques, any carotid stenosis more severe than 40%, or presence of peripheral arterial disease. Carotid atherosclerosis plaques and stenosis were estimated using Doppler flow ultrasonography. Peripheral artery disease is defined as ABI less than 0.90 using standardized measurement of the Winsor Index.28
Statistical Analyses
Variables with symmetric distribution were reported as means and standard deviations. Variables with asymmetric distribution were summarized as medians and interquartile ranges and were used log-transformed in regression analyses and back-transformed for data presentation. Differences across UA quintiles were estimated using analysis of variance and chi-square tests, as appropriate.
The association between circulating antioxidants and UA levels was examined using generalized linear models in which covariates were progressively added. In Model 1, the association was adjusted for age; sex; and total energy, protein, and vitamin intake. Confounders from Model 1 and alcohol consumption, smoking, physical activity, and eGFR were tested in Model 2, and relevant clinical conditions, such as hypertension, cardiovascular and cerebrovascular diseases, metabolic syndrome, diabetes mellitus, atherosclerosis, and arthritis were added in Model 3. In addition, each antioxidant was regressed on UA and a propensity score, which summarized the confounding effect of all variables in the model.29 The propensity score was estimated by regression of all covariates on UA. Homogeneous distribution of the propensity score across the UA quintiles was achieved.
Generalized linear models were used to examine the association between IADL disability, SPPB score, and UA levels, as a linear and quadratic effect, independent of age, sex, total energy and protein intake, body mass index (BMI), and smoking (Table 3, Model 1), and these initial covariates plus carotenoids, tocopherols, and selenium (Table 3, Model 2). Finally, relevant clinical conditions such as hypertension, cardiovascular and cerebrovascular diseases, diabetes mellitus, metabolic syndrome, arthritis, and eGFR were included as potential confounders in Model 3. Interaction terms between UA and each antioxidant were also tested in the fully saturated models.
Table 3.
Linear Regression Models Describing the Relationship Between Uric Acid (UA), Circulating Antioxidants, and Physical Functioning Assessed According to Instrumental Activities of Daily Living (IADLs) and Short Physical Performance Battery (SPPB) Score
| IADL Disability |
SPPB Score |
|||
|---|---|---|---|---|
| Adjustment Covariate | β (SE) | P-Value | β (SE) | P-Value |
| Model 1 | ||||
| UA | −0.252 (0.125) | .049 | 0.045 (0.036) | .23 |
| UA2 | 0.029 (0.009) | .003 | −0.006 (0.002) | .04 |
| Model 2 | ||||
| UA | −0.233 (0.126) | .07 | 0.048 (0.036) | .18 |
| UA2 | 0.029 (0.009) | .002 | −0.006 (0.002) | .02 |
| Model 3 | ||||
| UA | −0.298 (0.125) | .01 | 0.075 (0.037) | .03 |
| UA2 | 0.031 (0.009) | .001 | −0.008 (0.002) | .003 |
| UA × selenium | 0.021 (0.215) | .92 | 0.144 (0.065) | .017 |
Note: β-coefficient, standard error (SE), and P-test are estimated in Models 1 through 3.
Model 1 includes age, sex, body mass index, total energy and protein intake, smoking.
Model 2 includes all variables in Model 1 plus carotenoid, tocopherol, and selenium concentrations.
Model 3 includes all variables in Model 2 plus estimated glomerular filtration rate, hypertension, arthritis, cardiovascular diseases, cerebrovascular diseases, and metabolic syndrome.
=estimated coefficient from regression models; SE =standard error.
The linearity of the relationship between UA and antioxidants and between UA and physical functioning were explored using scatterplots and by examining the average antioxidant values and IADL or SPPB scores according to UA quintiles. In addition, the assumption of constant variance was explored using residual plots. All analyses were performed using the SAS statistical package, version 8.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Characteristics of the Study Sample
Demographic and health-related characteristics of study participants overall and across UA quintiles are displayed in Table 1. From the lowest to the highest UA quintile, participants were older, consumed more alcohol, and had lower eGFR and higher BMI. In participants from the lowest to the highest UA quintile, total carotenoid and selenium plasma levels tended to decrease, whereas tocopherols increased slightly. The prevalence of participants with hypertension, cardiovascular disease, cerebrovascular diseases, metabolic syndrome, atherosclerosis, and knee and hip arthritis increased with increasing UA quintile. In parallel, participants were more likely to have IADL disabilities and a lower SPPB score. Participants in the middle UA quintile reported higher physical activity in the previous year, were less likely to have one or more IADL disabilities and had better lower extremity physical performance than those in the other groups.
Table 1.
Baseline Characteristics of the Entire Sample
| Uric Acid (Quintiles) |
||||||
|---|---|---|---|---|---|---|
| Characteristic | Entire Sample (N =966) | 1 (n =203) | 2 (n =189) | 3 (n =200) | 4 (n =181) | 5 (n =193) |
| Variable, mean ± SD | ||||||
| Age, yr | 74.8 ± 7.2 | 75.0 ± 6.5 | 74.3 ± 7.2 | 75.1 ± 7.2 | 74.3 ± 6.7 | 76.4 ± 7.8* |
| Body mass index, kg/m2 | 27.4 ± 4.0 | 25.7 ± 3.7 | 26.7 ± 3.8 | 28.0 ± 4.0 | 28.5 ± 4.1 | 28.3 ± 4.0ρ |
| Uric acid, mg/dL | 5.2 ± 1.4 | 3.5 ± 0.4 | 4.4 ± 0.2 | 5.0 ± 0.2 | 5.7 ± 0.2 | 7.3 ± 1.3ρ |
| Creatinine, mg/dL | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.2ρ |
| Estimated glomerular filtration rate, mL/min per 1.73m2 | 77.0 ± 16.3 | 79.5 ± 15.9 | 77.9 ± 15.4 | 77.1 ± 17.0 | 77.2 ± 17.6 | 69.3 ± 17.4ρ |
| Circulating antioxidant | ||||||
| Total carotenoids, μmol/L, median (IR) | 1.7 (0.8) | 1.8 (0.9) | 1.8 (0.8) | 1.9 (0.7) | 1.7 (0.5) | 1.6 (0.8)ρ |
| α-tocopherol, μmol/L, median (IR) | 28.9 (10.6) | 28.3 (8.7) | 29.5 (11.5) | 28.9 (7.29) | 30.3 (8.4) | 29.1 (11.6)* |
| γ-tocopherol, μmol/L, median (IR)† | 1.7 (0.3) | 1.6 (0.2) | 1.7 (0.2) | 1.6 (0.2) | 1.7 (0.3) | 1.7 (0.3)* |
| Total tocopherols, μmol/L, median (IR) | 30.2 (11.1) | 29.8 (9.1) | 30.5 (12.0) | 29.6 (11.2) | 31.8 (10.9) | 30.9 (11.7) |
| Selenium, μmol/L, mean ± SD | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.1 |
| Behavior-related variables | ||||||
| Never smokers, n (%) | 573 (59.3) | 148 (25.8) | 122 (21.3) | 106 (18.5) | 103 (18.0) | 94 (16.4)ρ |
| Current smokers, n (%) | 134 (13.9) | 22 (16.4) | 33 (24.6) | 31 (23.1) | 24 (17.9) | 24 (17.9) |
| Smoking, packs/year, median (IR) | 12.5 (21.1) | 0.0 (1.3) | 0.0 (14.3) | 0.0 (27.9) | 0.0 (27.4) | 0.6 (32.8)ρ |
| Energy intake, Kcal/d, mean ± SD | 1,931 ± 565 | 1,823 ± 477 | 1,894 ± 594 | 1,931 ± 608 | 2,063 ± 604 | 1,908 ± 524* |
| Carotenoids intake, mg/d, mean ± SD | 2.1 ± 1.1 | 2.1 ± 1.2 | 2.2 ± 1.2 | 2.1 ± 1.0 | 2.2 ± 1.2 | 2.1 ± 1.0 |
| Vitamin E intake, mg/d, mean ± SD | 6.2 ± 2.0 | 5.89 ± 1.7 | 6.3 ± 2.2 | 6.2 ± 2.0 | 6.5 ± 2.0 | 6.2 ± 1.9* |
| Total protein intake, g/d, mean ± SD | 75.2 ± 20.8 | 69.8 ± 18.4 | 74.6 ± 20.8 | 75.5 ± 21.7 | 79.1 ± 22.5 | 74.1 ± 20.1 |
| Alcohol intake, g/d, median (IR) | 14.6 (19.8) | 5.7 (13.6) | 3.8 (16.6) | 7.9 (26.8) | 13.3 (27.1) | 13.4 (26.6)ρ |
| Physical activity, (h/wk), mean ± SD | 3.2 ± 1.1 | 3.1 ± 0.9 | 3.2 ± 1.1 | 3.3 ± 1.1 | 3.3 ± 1.2 | 3.0 ± 1.1* |
| Physical function | ||||||
| Instrumental activity of daily living disability, median (IR) | 0.0 (1.0) | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (1.0)* |
| Subjects with disability, n (%) | 257 (25.6) | 47 (23.2) | 48 (25.4) | 43 (21.5) | 45 (24.89) | 64 (33.2)* |
| Short Physical Performance Battery score | ||||||
| Median (IR) | 11.0 (3.0) | 11.0 (3.0) | 11.0 (2.0) | 11.0 (3.0) | 12.0 (3.0) | 11.0 (4.0) |
| Mean ± SD‡ | 2.2 ± 0.6 | 2.2 ± 0.3 | 2.2 ± 0.6 | 2.3 ± 0.5 | 2.2 ± 0.6 | 2.1 ± 0.7 |
| Medical conditions, n (%) | ||||||
| Hypertension | 632 (66.8) | 126 (62.1) | 121 (64.0) | 130 (65.0) | 119 (65.8) | 155 (80.3)° |
| Cardiovascular diseases | 264 (28.0) | 49 (24.1) | 50 (26.5) | 58 (29.0) | 48 (26.5) | 74 (38.3)° |
| Cerebrovascular diseases | 66 (7.2) | 13 (6.4) | 11 (6.1) | 11 (5.6) | 14 (7.8) | 22 (11.9)* |
| Diabetes | 171 (18.2) | 41 (20.2) | 34 (18.0) | 34 (17.0) | 28 (15.5) | 48 (24.9) |
| Metabolic syndrome | 203 (21.5) | 35 (17.2) | 25 (13.2) | 43 (21.5) | 35 (17.2) | 65 (33.7)ρ |
| Atherosclerosis | 177 (20.9) | 32 (17.9) | 27 (15.9) | 32 (17.8) | 45 (24.9) | 46 (27.2)° |
| Chronic renal failure | 243 (27.2) | 57 (29.7) | 46 (26.4) | 51 (26.3) | 46 (28.4) | 59 (33.3)* |
| Knee and hip arthritis | 99 (10.6) | 7 (3.5) | 14 (7.4) | 12 (6.0) | 15 (8.3) | 50 (25.9)ρ |
| Use of diuretics | 90 (9.5) | 24 (11.8) | 16 (8.5) | 28 (14.0) | 15 (8.3) | 22 (11.4) |
Values are expressed as tocopherol/cholesterol ratios.
Reported as continuous variable.
SD =standard deviation; IR =interquartile range.
Statistical comparisons are from analysis of variance:
P<.05,.01,.001.
Relationship Between Circulating Levels of UA and Antioxidants
Independent of age, sex, and total energy, protein, and vitamin intake, circulating UA levels were inversely associated with total carotenoids, in particular, α-carotene, lycopene, lutein, and zeaxanthin, and with selenium (Table 2, Model 1). The association between circulating UA and antioxidant levels remained statistically significant after adjustment for lifestyle factors such as total energy, protein, and vitamin intake, alcohol consumption, smoking, and physical activity and for kidney function using eGFR (Table 2, Model 2). The associations between total carotenoids, α-carotene, lycopene, lutein, zeaxanthin, selenium, and UA circulating levels were virtually unchanged after further adjustment for comorbid conditions (Table 2, Model 3). A similar but nonsignificant trend was observed for β-carotene and β-cryptoxanthin (P =.96 and P =.29, respectively).
Table 2.
Linear Regression Models on the Relationship Between Circulating Antioxidants and Uric Acid
| Overall Population |
α-Carotene (μmol/L)* |
β-Carotene (μmol/L)* |
Lycopene (μmol/L)* |
Lutein (μmol/L)* |
Zeaxanthin (μmol/L)* |
Cryptoxantin (μmol/L)* |
Carotenoids (μmol/L)† |
α-tocopherol (μmol/L)* † |
Total Tocopherols (μmol/L)* † |
Selenium (μmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||||
| β | −0.04 | −0.02 | −0.03 | −0.04 | − 0.04 | −0.01 | −0.03 | 0.02 | 0.02 | −0.44 |
| SE | 0.01 | 0.01 | 0.013 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.28 |
| P | .005 | .15 | .04 | <.001 | <.001 | .62 | .001 | <.001 | .003 | .12 |
| Model 2 | ||||||||||
| β | −0.05 | −0.01 | −0.02 | −0.04 | − 0.05 | −0.13 | −0.02 | 0.01 | 0.01 | −0.79 |
| SE | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.30 |
| P | .002 | .74 | .13 | <.001 | <.001 | .43 | .005 | .01 | .005 | .007 |
| Model 3 | ||||||||||
| β | −0.04 | −0.01 | −0.03 | −0.04 | − 0.06 | −0.0212 | −0.0281 | 0.0106 | 0.0115 | −0.72 |
| SE | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.006 | 0.006 | 0.35 |
| P | .02 | .96 | .07 | <.001 | <.001 | .29 | .008 | .10 | .06 | .04 |
| Model 4 | ||||||||||
| β | −0.04 | −0.01 | −0.03 | −0.04 | − 0.06 | −0.02 | −0.03 | 0.01 | 0.01 | −0.62 |
| SE | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.36 |
| P | .02 | .87 | .06 | <.001 | <.001 | .25 | .007 | .11 | .08 | .09 |
Note: β-coefficient, standard error (SE), and P-test are estimated in Models 1 through 4.
Variable values log-transformed for analysis.
Values are reported as tocopherol/cholesterol ratios.
Model 1 adjusting for age, sex, total energy intake, protein intake, and vitamin intake.
Model 2 adjusting for for all variables present in Model 2 plus alcohol consumption, smoking, physical activity, and estimated glomerular filtration rate.
Model 3 adjusting for all variables present in Model 2 plus hypertension, cardiovascular diseases, cerebrovascular diseases, diabetes mellitus, metabolic syndrome, atherosclerosis, and arthritis.
Model 4 estimates are from antioxidants regressed on uric acid and propensity score (see Methods).
= estimated coefficient from regression models; SE =standard error.
Independent of demographic and lifestyle factors, total tocopherol and α-tocopherol were positively associated with UA levels (Table 2, Model 1 and 2), but the association was no longer significant after adjustment for comorbid conditions (Table 2, Model 3). The findings did not change when tocopherol:cholesterol level ratios or nonstandardized tocopherol levels were used in the analysis. The magnitude, direction, and statistical significance of the association between UA and antioxidants were substantially unchanged after adjustment for a propensity score that summarized the confounding effect of all covariates (Table 2, Model 4). The magnitude of the associations between UA and total carotenoids was greater in women than men, whereas no difference was observed between UA and tocopherols or selenium between the sexes.
The antioxidants independently and significantly associated with UA levels are reported in Panels 1 and 2 of Figure 1, according to UA quintiles. In Panel 3 of Figure 1, the distribution of IL-6 and CRP are reported to allow a direct comparison. All values in Figure 1 were expressed as the number of standard deviations from the population mean, to make them directly comparable. Total carotenoids and selenium were progressively and significantly lower across the UA quintiles. Total tocopherols and α-tocopherol had a positive trend, whereas IL-6 and CRP levels were progressively and significantly higher across UA quintiles (Figure 1).
Figure 1.
Mean values of antioxidants and inflammatory markers (expressed as number of standard deviations from the population mean), according to uric acid quintiles.
*SD =standard deviation; statistical comparisons are from age-and sex-adjusted test for trend.
IL-6 =Interleukin-6; CRP =C-reactive protein.
In the fully adjusted models fitted on 912 subjects within the normal UA range, a significant and negative association was confirmed between total carotenoids, α-carotene, lycopene, lutein, zeaxanthin, selenium, and UA levels, whereas a nonsignificant trend was observed for tocopherols (data not shown).
Relationship Between Physical Function and UA Circulating Levels
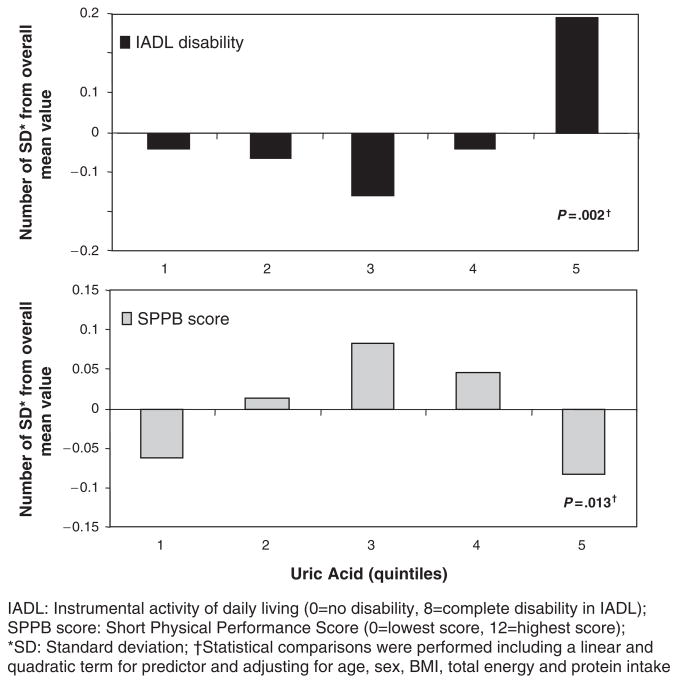
The prevalence of IADL disability, the average number of IADL disabilities, and average SPPB scores were nonlinearly distributed across the UA quintiles. The lowest IADL disabilities and the highest SPPB scores were found in participants in the middle UA quintile (third quintile) (Figure 2). IADL disability and SPPB score were significantly associated with UA levels as linear and quadratic terms, independent of age, sex, BMI, total energy, and protein intake (Table 3, Model 1). Both associations remained statistically significant after adjustment for circulating carotenoids, tocopherols, and selenium (Table 3, Model 2) and for comorbid conditions (Table 3, Model 3). The magnitude and direction of the association between UA and IADL disability or SPPB physical performance were similar between the sexes. In the fully adjusted model, predicting lower extremity performance, but not in the model predicting disability, a significant, positive interaction was found between UA and selenium. The interaction terms carotenoids by UA (P =.66) and tocopherols by UA (P =.71) were also tested but were not statistically significant for either functional outcome.
Figure 2.
Mean values of disability index assessed according to instrumental activity of daily living (IADL) and physical performance score assessed according to Short Physical Performance Battery (SPPB) score (expressed as number of standard deviations from the population mean), according to UA quintiles.
The relationship between UA levels and functional outcomes across selenium quartiles was examined and is reported in Figure 3. UA levels were negatively associated with selenium (P =.04), whereas SPPB score was positively associated with selenium (P<.001). These relationships remained significant after adjustment for age, sex, BMI, total energy and protein intake, and circulating carotenoids and tocopherols. The relationship between UA levels and IADL or SPPB across the selenium quartiles is summarized in Table 4. The association between UA levels and IADL disability was higher in the lowest selenium quartile and became progressively weaker with increasing selenium levels. Similarly, the association between UA levels and SPPB scores was lower in the lowest selenium quartile and increased progressively in the higher quartiles (Table 4).
Figure 3.
Mean values of uric acid levels and physical performance score assessed according to Short Physical Performance Battery score (expressed as number of standard deviations from the population mean), according to selenium quartiles.
Table 4.
Relationship Between Uric Acid Levels and Physical Function Across Selenium Quartiles
| Instrumental Activity of Daily Living Disability |
Short Physical Performance Battery Score |
||||
|---|---|---|---|---|---|
| Selenium, Quartile | Uric Acid, mg/dL, mean (SE) | β (SE) | P-Value | β (SE) | P-Value |
| 1 | 5.37 (1.67) | 0.23 (0.023) | .02 | −0.08 (0.02) | .003 |
| 2 | 5.22 (1.46) | 0.15 (0.09) | .02 | −0.03 (0.01) | .03 |
| 3 | 5.02 (1.34) | 0.16 (0.06) | .02 | −0.02 (0.01) | .29 |
| 4 | 5.06 (1.26) | −0.07 (0.06) | .23 | 0.02 (0.02) | .31 |
SE =standard error.
DISCUSSION
In community-dwelling older persons, serum carotenoid and selenium concentrations were inversely associated with circulating UA concentrations. Participants from the lowest to the highest UA quintiles had significantly lower levels of α-carotene, lycopene, lutein, and zeaxanthin but not β-carotene and cryptoxanthin. Serum total tocopherols and α-tocopherol tended to increase across UA quintiles. This is the first study that has addressed the relationship between circulating UA and antioxidant levels and found an inverse association between UA and several antioxidants in humans.
Circulating carotenoid levels depend mainly on vegetable and fruit consumption and exert their antioxidant properties within the lipid bilayers of cell membranes, quenching and reducing ROS to nonradical compounds.30–33 Selenium is a dietary trace element that regulates the intracellular redox status, thyroid metabolism, and inflammatory response.34,35 In particular, selenoproteins inhibit the binding of nuclear factor–kappa B to deoxyribonucleic acid (DNA) and modulates cell apoptosis via inhibition of the activity of the mitogen-activated protein kinase.36 Tocopherols are essential fat-soluble, ubiquitous components of biological membranes that protect the polyunsaturated fatty acids of membranes against ROS-mediated damage.37,38
Historically, researchers have hypothesized that different antioxidants act jointly and possibly synergistically to protect cell membranes, intracellular proteins, and DNA from oxidative-mediated damage.38 Recent studies suggest that antioxidants also exert powerful antiinflammatory effects, mostly by preventing the ROS-mediated activation of intracellular redox-sensitive signaling pathways that induce cytokine gene transcription.40,41 In particular, β-carotene levels were inversely related to CRP and leukocytes,42 lutein and lycopene levels were inversely related to soluble inter-cellular adhesion molecule-1,43 selenium levels were inversely related to urinary F2 isoprostanes,44,45 and cyclooxygenase-mediated inflammatory markers.44 In in vitro studies, carotenoids and tocopherols downregulated the redox-mediated activation of inflammatory transcription factors,39,45,46 the nuclear factor–kappa B pathway, and the production of IL-6, and TNF-α cytokines45,47,48 α-tocopherol and selenium reduced ROS, TNF-α, IL-1β, and IL-6 synthesis.49–51
The findings of an inverse association between circulating carotenoids, selenium, and UA levels and previous evidence of a positive association between proinflammatory markers and UA levels add further evidence about the potential antiinflammatory properties of antioxidants but also suggest that UA behaves more as a proinflammatory than an antioxidant compound.
To better understand the effect of the inverse association between antioxidant and UA concentrations on general health status, the distribution of physical function measures across UA quintiles and the effect of adjusting for antioxidant levels in this relationship, including possible UA by antioxidant interactions, were explored. Measures of IADL disability and lower extremity physical performance showed nonlinear trajectory across UA levels, which remained virtually unchanged after adjustment for circulating carotenoids, selenium, and tocopherols. Participants in the middle UA quintile had higher physical performance scores and more independence in IADLs than those with higher and lower UA levels.
This is the first report of a nonlinear trend between physical function and UA levels within the normal range. Although difficult to fully explain, it suggests that UA levels may mark or participate in pathological processes that are detrimental to health status. It was hypothesized that participants in the middle UA quintile have a level of oxidative stress that allows appropriate functioning of ROS-dependent signaling pathway without excessive oxidative stress damage and a proinflammatory state. Above this threshold, excessive oxidative stress, revealed by low levels of antioxidants and high inflammatory markers and UA levels, may have a deleterious effect on health and functional status. The significant interaction between selenium and UA levels support the idea of a detrimental effect of UA on physical performance and IADL disability, showing that circulating antioxidants may counteract or buffer the effect of UA levels on physical function, although more research is needed to verify whether an optimal balance exists between circulating UA and antioxidants levels causally involved in the disability processes.
Although the tendency toward the positive association between tocopherols and higher UA levels and inflammatory markers is somewhat puzzling, care must be taken about the interpretation of this finding. Because dietary requirements for tocopherols are dependent upon the level of polyunsaturated fatty acids in the diet52,53 and in cell membranes,54–57 the measure of tocopherols used in this study, which does not consider fractionated fatty acids, may imply that the effect of inadequate consumption because of oxidative stress and, in turn, of the tocopherol proactive concentrations may not have been captured.
The main limitations of this study are the cross-sectional design, which does not allow for study of the direction of the causal pathway between UA and antioxidants, and the absence of direct measurements of oxidative damage, which does not allow for quantifying the ROS-mediated damage associated with poorer physical function. Points of strength of this study are that circulating UA and antioxidant levels were systematically measured in all participants enrolled in the InCHIANTI Study, together with detailed information about major confounders and dietary intake of antioxidants. In addition, physical function was assessed using subjective (IADL) and objective (SPPB) measures, and the use of these two different approaches measuring physical function provided consistent results.
In conclusion, this study demonstrated that circulating carotenoids and selenium are inversely associated with UA levels, whereas IADL disability and lower extremity performance are nonlinearly distributed across circulating UA levels. Participants with UA levels between 4.8 and 5.3 mg/ dL tended to have less IADL disability and higher physical performance than those with higher or lower UA levels.
Acknowledgments
Financial Disclosure: The InCHIANTI Study was supported as a ‘‘targeted project’’ (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336) and by the Intramural Research Program of the U.S. National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336). This research was also partially supported by an unrestricted grant by BRACCO imaging SpA, Italy.
Footnotes
Author Contributions: Ruggiero and Ferrucci had full access to all of the data in the study, analyzed the data, and prepared the manuscript. Cherubini, Guralnik, Semba, Lauretani, Maggio, and Bandinelli participated in the design of the study and acquisition and analysis of the data. Maggio, Guralnik, Semba, Ling, Lauretani, and Senin provided intellectual and critical support. All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
Sponsor’s Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
References
- 1.Wu XW, Muzny DM, Lee CC, et al. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 2.Hediger MA, Johnson RJ, Miyazaki H, et al. Molecular physiology of urate transport. Physiology. 2005;20:125–133. doi: 10.1152/physiol.00039.2004. [DOI] [PubMed] [Google Scholar]
- 3.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto FJ, Iribarren C, Gross MD, et al. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis. 2000;148:131–139. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 5.Glantzounis GK, Tsimoyiannis EC, Kappas AM, et al. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Williamson DF, Gunter EW, et al. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I epidemiologic follow-up study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 7.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National health and nutrition examination survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 8.Levine W, Dyer AR, Shekelle RB, et al. Serum uric acid and 11.5 year mortality of middle-aged women: Findings of the Chicago Heart Association Detection Project in industry. J Clin Epidemiol. 1989;42:257–267. doi: 10.1016/0895-4356(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RJ, Burchfiel CM, Benfante R, et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch Intern Med. 1995;155:686–694. [PubMed] [Google Scholar]
- 10.Franse LV, Pahor M, Di Bari M, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP) J Hypertens. 2000;18:1149–1154. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- 11.Alderman MH. Uric acid and cardiovascular risk. Curr Opin Pharmacol. 2002;2:126–130. doi: 10.1016/s1471-4892(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 12.Langford HG, Blaufox MD, Borhani NO, et al. Is thiazide-produced uric acid elevation harmful? Analysis of data from the hypertension detection and follow-up program. Arch Intern Med. 1987;147:645–649. doi: 10.1001/archinte.147.4.645. [DOI] [PubMed] [Google Scholar]
- 13.Anker SD, Doehner W, Rauchhaus M, et al. Uric acid and survival in chronic heart failure: Validation and application in metabolic, functional, and hemo-dynamic staging. Circulation. 2003;22(107):1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 14.Doehner W, Rauchhaus M, Florea VG, et al. Uric acid in cachectic and noncachectic patients with chronic heart failure: Relationship to leg vascular resistance. Am Heart J. 2001;141:792–799. doi: 10.1067/mhj.2001.114367. [DOI] [PubMed] [Google Scholar]
- 15.Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. Eur Heart J. 2006;27:1174–1181. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Walston J, Xue Q, Semba RD, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 20.Cherubini A, Martin A, Andres-Lacueva C, et al. Vitamin E levels, cognitive impairment and dementia in older persons: The InCHIANTI Study. Neurobiol Aging. 2005;26:987–994. doi: 10.1016/j.neurobiolaging.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- 22.Penninx BW, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 23.Penninx BW, Ferrucci L, Leveille SG, et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55A:691–697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 24.Pisani P, Faggiano F, Krogh V, et al. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:152–160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 25.Salvini S. A food composition database for epidemiological studies in Italy. Cancer. 1997;114:299–300. doi: 10.1016/s0304-3835(97)04686-7. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute of Aging; 1995. [Google Scholar]
- 27.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Final report Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 28.McDermott MM, Guralnik JM, Albay M, et al. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI study. J Am Geriatr Soc. 2004;52:405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 30.Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal S, Rao AV. Tomato lycopene and low density lipoprotein oxidation: A human dietary intervention study. Lipids. 1998;33:981–984. doi: 10.1007/s11745-998-0295-6. [DOI] [PubMed] [Google Scholar]
- 32.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. [PubMed] [Google Scholar]
- 33.McEligot AJ, Yang S, Meyskens FL., Jr Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Annu Rev Nutr. 2005;25:261–295. doi: 10.1146/annurev.nutr.25.050304.092633. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Whitfield ML, Xu T, et al. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell. 2004;15:506–519. doi: 10.1091/mbc.E03-07-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y, Zhang H, Hawthorn L, et al. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003;63:52–59. [PubMed] [Google Scholar]
- 36.Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 38.Erin AN, Spirin MM, Tabidze LV, et al. Formation of alpha-tocopherol complexes with fatty acids. A hypothetical mechanism of stabilization of biomembranes by vitamin E Biochim Biophys Acta. 1984;774:96–102. doi: 10.1016/0005-2736(84)90279-7. [DOI] [PubMed] [Google Scholar]
- 39.Bray TM. Dietary antioxidants and assessment of oxidative stress. Nutrition. 2000;16:578–581. doi: 10.1016/s0899-9007(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 40.Kheir-Eldin AA, Motawi TK, Gad MZ, et al. Protective effect of vitamin E, beta-carotene and N-acetylcysteine from the brain oxidative stress induced in rats by lipopolysaccharide. Int J Biochem Cell Biol. 2001;33:475–482. doi: 10.1016/s1357-2725(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–2432. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- 42.Erlinger TP, Guallar E, Miller ER, 3rd, et al. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med. 2001;161:1903–1908. doi: 10.1001/archinte.161.15.1903. [DOI] [PubMed] [Google Scholar]
- 43.van Herpen-Broekmans WM, Klopping-Ketelaars IA, Bots ML, et al. Serum carotenoids and vitamins in relation to markers of endothelial function and inflammation. Eur J Epidemiol. 2004;19:915–921. doi: 10.1007/s10654-004-5760-z. [DOI] [PubMed] [Google Scholar]
- 44.Helmersson J, Arnlov J, Vessby B, et al. Serum selenium predicts levels of F2-isoprostanes and prostaglandin F2alpha in a 27 year follow-up study of Swedish men. Free Radic Res. 2005;39:763–770. doi: 10.1080/10715760500108513. [DOI] [PubMed] [Google Scholar]
- 45.Alissa EM, Bahijri SM, Ferns GA. The controversy surrounding selenium and cardiovascular disease: A review of the evidence. Med Sci Monit. 2003;9:9–18. [PubMed] [Google Scholar]
- 46.Seo JY, Kim H, Seo JT, et al. Oxidative stress induced cytokine production in isolated rat pancreatic acinar cells: Effects of small-molecule antioxidants. Pharmacology. 2002;64:63–70. doi: 10.1159/000056152. [DOI] [PubMed] [Google Scholar]
- 47.Sharoni Y, Danilenko M, Dubi N, et al. Carotenoids and transcription. Arch Biochem Biophys. 2004;430:89–96. doi: 10.1016/j.abb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Qin F, Shite J, Liang CS. Antioxidants attenuate myocyte apoptosis and improve cardiac function in CHF: Association with changes in MAPK pathways. Am J Physiol Heart Circ Physiol. 2003;285:822–832. doi: 10.1152/ajpheart.00015.2003. [DOI] [PubMed] [Google Scholar]
- 49.Jialal I, Traber M, Devaraj S. Is there a vitamin E paradox? Curr Opin Lipidol. 2001;12:49–53. doi: 10.1097/00041433-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Bellezzo JM, Leingang KA, Bulla GA, et al. Modulation of lipopolysaccharide-mediated activation in rat Kupffer cells by antioxidants. J Lab Clin Med. 1998;131:36–44. doi: 10.1016/s0022-2143(98)90075-0. [DOI] [PubMed] [Google Scholar]
- 51.Haddad JJ. Science review: Redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: Role for nuclear factor-kappaB. Crit Care. 2002;6:481–490. doi: 10.1186/cc1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godbout JP, Berg BM, Kelley KW, et al. Alpha-tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol. 2004;149:101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 53.Bieri JG, Corash L, Hubbard VS. Medical uses of vitamin E. N Engl J Med. 1983;308:1063–1071. doi: 10.1056/NEJM198305053081805. [DOI] [PubMed] [Google Scholar]
- 54.Piers LS, Walker KZ, Stoney RM, et al. Substitution of saturated with mono-unsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90:717–727. doi: 10.1079/bjn2003948. [DOI] [PubMed] [Google Scholar]
- 55.Poppitt SD, Kilmartin P, Butler P, et al. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 2005;5:30. doi: 10.1186/1476-511X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aro A, Pietinen P, Valsta LM, et al. Effects of reduced-fat diets with different fatty acid compositions on serum lipoprotein lipids and apolipoproteins. Public Health Nutr. 1998;1:109–116. doi: 10.1079/phn19980017. [DOI] [PubMed] [Google Scholar]
- 57.Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr. 2006;136:565–569. doi: 10.1093/jn/136.3.565. [DOI] [PubMed] [Google Scholar]