Abstract
Objectives
To develop and validate mortality and hospitalization prognostic tools based upon information readily available to primary care physicians (PCPs).
Design
Population-based cohort study. Baseline predictors were patient demographics, a seven-item questionnaire on functional status and general health, use of five or more drugs, and previous hospitalization.
Setting
Community-based study.
Participants
Prognostic indexes were developed in 2,470 subjects and validated in 2,926 subjects, all community-dwelling, aged 65 and older, and randomly sampled from the rosters of 98 PCPs in Florence, Italy.
Measurements
Fifteen-month mortality and hospitalization.
Results
Two scores were derived from logistic regression models and used to stratify participants into four groups. With Model 1, based upon the seven-item questionnaire, mortality rate ranged from 0.8% in the lowest-risk group (0–1 point) to 9.4% in the highest risk group (≥3 points), and hospitalization rate ranged from 12.4% to 29.3%; area under the receiver operating characteristic curves (AUC) was 0.75 and 0.60, respectively. With Model 2, considering also drug use and previous hospitalization, mortality and hospitalization rates ranged from 0.3% to 8.2% and from 8.1% to 29.7%, for the lowest-risk to the highest-risk group; the AUC increased significantly only for hospitalization (0.67).
Conclusion
Prediction of death and hospitalization in older community-dwelling people can be easily obtained with two indexes using information promptly available to PCPs. These tools might be useful for guiding clinical care and targeting interventions to reduce the need for hospital care in older persons.
Keywords: elderly, mortality, hospitalization, screening
The aging of the population raises concerns of health professionals and policy makers around the world. Older persons in general require a large amount of medical attention and absorb a share of healthcare resources disproportionately greater than their prevalence in the population. In Italy, persons aged 65 and older represent more than 19% of the population, but they accounted for 35% of hospitalizations in 2000 and 40% of drug consumption in 2004.1,2 A small number of frail older persons who are vulnerable and usually affected by severe chronic comorbidities and functional limitations account for most of the excess healthcare spending.
Healthcare systems have developed different strategies in the attempt to provide the best possible care to older persons with chronic diseases and physical dependency in a situation of limited resources. In general, these innovative strategies are centered on the shift from traditional care, fragmented into episodic interventions in response to acute conditions, to an integrated and multidisciplinary approach toward chronicity, in which continuity of care is highly valued. Within this new paradigm of care, primary care physicians (PCPs) are frequently involved and assigned an important, proactive role, with the ultimate goals of postponing death and the onset of disability3–6 and minimizing the risk of unnecessary hospital admissions while containing costs.7 In Italy, because of their close, longstanding relationships with patients and their families, PCPs are usually knowledgeable about the clinical, personal, and environmental factors affecting the overall well-being of their patients, as well as about formal and informal supports that can be activated in case of need.8 Thus, PCPs are well positioned to identify frail older persons who are at risk for adverse medical outcomes and intense use of healthcare resources and who may benefit the most from integrated, continuous care interventions, planned and conducted in collaboration with geriatricians.9,10
Several indexes have been developed that allow the identification of high-risk older subjects in the community. These indexes usually integrate information from demographics, medical diagnoses, functional status, and laboratory values.11,12 The concurrent use of several prognosticators may increase the overall explanatory power of a screening tool, although this requires the availability of a greater amount of data, which might not be routinely available in primary care, and implies more complex calculations.13
This study used the results of the “Assistenza Socio-Sanitaria in Italia” (ASSI), an observational study of older community-dwelling people, to calculate and validate a prognostic index based on simple information easily available to PCPs. It had preliminarily been shown that the questionnaire used in ASSI, aimed at identifying the presence of general health problems, accurately predicted mortality.14 The prognostic index has now been further refined to estimate short-term mortality and hospitalization risk separately. Such tools might support clinicians' intuition and judgment when selecting appropriate therapeutic options or counseling patients and their families.
Methods
Participants and Study Protocol
ASSI is a prospective cohort study of persons aged 65 and older randomly abstracted from the rosters of 98 PCPs in Florence, Italy.15 At the January 2003 baseline, using a seven-item structured questionnaire, PCPs screened 5,445 (92%) of 5,927 eligible older community-dwelling people living in the local health unit of Florence, Italy, which covers the entire metropolitan area, for the presence of disability and other general health problems.
The ethics committee of the academic hospital Azienda Ospedaliero-Universitaria Careggi approved the study protocol.
Data Collection and Measurement
The seven-item questionnaire answered by the PCPs provided summary information on complete inability and need for help in basic activities of daily living (BADLs: eating, toileting, bathing, dressing, transferring, and walking across the room) and instrumental activities of daily living (IADLs: grocery shopping, preparing meals, washing clothes, managing medications, and showering); poor vision (inability to read newspapers headings); poor hearing (inability to hold a conversation); recent unintentional weight loss (>3 kg in the previous year); use of homecare services (personal assistance, rehabilitation, or nursing services), which in Italy are usually prescribed by the PCP; and inadequacy of income. The PCPs answered this questionnaire based upon personal knowledge of their enrollees' status in 71.0% of cases and upon direct interview in the remaining cases.
In addition to the data gathered from the PCPs, information on the number of drug prescriptions and on hospitalizations in the 6 months before baseline assessment was obtained from registries of the regional health system of Tuscany, which contain data on pharmacy claims for out-of-hospital drug prescriptions, hospital discharges, and deaths. The same databases were used to identify the outcomes of interests: death or hospitalization within 15 months from baseline (through March 31, 2004).
Statistical Analysis: Development and Validation of the Predictive Models
Of the 5,445 baseline participants, 49 were lost follow-up, leaving 5,396 individuals available for analysis. The predictive indexes were developed in subjects from the northwestern area of Florence (N = 2,470), and validated using the population from the southeastern region (N = 2,926). The two subsamples were comparable in terms of demographics and social characteristics.
In the development cohort, the number of positive responses to the screening questionnaire, age (65–74, 75–84, and ≥85), sex, taking five or more prescription drugs, and having been hospitalized in the 6 months before the baseline assessment were tested in separate logistic regression models as predictors of death or hospitalization. Previous studies have reported that taking five or more drugs (usually referred to as polypharmacy) is an independent risk factor for death and other adverse health outcomes including hospitalizations.16,17 Because all of these variables were significantly associated with both of these outcomes, they were entered together into multivariable logistic models for the prediction of death and hospitalization.
In accordance with methods described in previous studies,11,12,18 each risk factor was assigned a score resulting from the ratio, rounded to the nearest integer, between the regression b coefficient for that variable and the lowest significant b coefficient in the corresponding logistic model, which resulted to be associated with being aged 75 to 84. A summary point score was calculated for each participant by adding the points for each risk factor present. The sample was then stratified on the basis of the individual risk scores. A sensitivity analysis was also performed to determine the stability of the results obtained by excluding from the main model the variables “taking five or more prescription drugs” and “having been hospitalized in the 6 months before baseline”.
The predictive accuracy of the point scoring systems for mortality and hospitalization was estimated in the development cohort and in the validation cohort by calculating the sensitivity and specificity for each risk stratum and the area under the receiver operating characteristic curves (AUCs) for the overall test. The AUC expresses the probability that, in all the possible pairs of participants of whom, for example, one lives and the other dies, a higher risk is assigned to the participants who died than to the ones who survived.19
Survival analysis across the risk strata was performed. Survival time was defined as the number of months between baseline interview and the date of mortality or hospitalization or, in absence of the mentioned outcomes, through March 31, 2004. Kaplan–Meier curves were used to examine the performance of the prognostic indexes over time. Statistical analyses were performed with Stata for Windows 8.0 (StataCorp., College Station, TX). Two-tailed P <.05 was considered statistically significant.
Results
Study Population
In the development cohort, mean age ± standard deviation was 75.3 ± 7.2; 56.2% were female, and 17.1% lived alone. Based on the questionnaire, 7.4% were dependent in one or more BADLs and 12.3% in one or more IADLs. At baseline, 22.2% participants were taking five or more prescription drugs, and 9.7% had been hospitalized in the previous 6 months. In the validation cohort, mean age was 75.1 ± 7.2, 58.9% were female, and 17.4% lived alone. A total of 7.5% participants were dependent in one or more BADLs and 13.9% in one or more IADLs. At baseline, 20.7% participants were taking five or more prescription drugs, and 11.7% had been hospitalized in the previous 6 months (Table 1). After 15 months of follow-up, 115 subjects from the development cohort (4.7%) and 114 from the validation cohort (3.9%) had died, whereas 445 (18.0%) and 504 (17.2%), respectively, had been hospitalized at least once.
Table 1. Baseline Characteristics of the Study Participants in the Development and Validation Cohort.
| Characteristic | Development (N=2,470) |
Validation (N=2,926) |
|---|---|---|
| Demographic | ||
| Female, n (%) | 1,387 (56.2) | 1,722 (58.9) |
| Age, mean ± standard deviation | 75.3 ± 7.2 | 75.1 ± 7.2 |
| Age, n (%) | ||
| 65–74 | 1,305 (52.8) | 1,618 (55.3) |
| 75–84 | 891 (36.1) | 998 (34.1) |
| ≥85 | 274 (11.1) | 310 (10.6) |
| Living alone, n (%) | 422 (17.1) | 510 (17.4) |
| Health services utilization, n (%) | ||
| Hospitalized in the prior 6 months | 238 (9.7) | 342 (11.7) |
| Taking ≥5 prescribed drugs | 549 (22.2) | 605 (20.7) |
| Responses to the screening instrument, n (%) | ||
| Need help performing basic activities of daily living | 182 (7.4) | 218 (7.5) |
| Need help performing instrumental activities of daily living | 304 (12.3) | 406 (13.9) |
| Poor vision | 123 (5.0) | 135 (4.6) |
| Poor hearing | 86 (3.5) | 127 (4.3) |
| Self-perceived inadequacy of income | 105 (4.3) | 127 (4.3) |
| Absence of home care services | 2,401 (97.2) | 2,856 (97.6) |
| Weight loss >3 kg in previous year | 122 (4.9) | 230 (7.9) |
Multivariable Results
Logistic regression was used to identify factors that predicted death or hospitalization during follow-up. As indicated in Table 2, the same variables were consistently identified as significant predictors of both outcomes yet with different risk estimates. In the development cohort, the risk of death increased dramatically with the number of problems identified in the screening questionnaire (being almost five times as high with four to six positive answers as with no positive answers) and with advancing age (point estimate of more than four associated with age ≥85), and it was twice as high in men as in women. Previous hospitalization and use of five or more prescription drugs were also strong predictors; in particular, they were major predictors of hospital admission, obtaining the highest point estimates across the entire set of independent variables (Table 2).
Table 2. Risk Factors Associated with Mortality and Hospitalization in the Development Cohort in Multi-variate Analyses.
| Mortality | Hospitalization | |||||
|---|---|---|---|---|---|---|
| Variables | Adjusted OR (95% CI) |
P-Value | Points | Adjusted OR (95% CI) |
P-Value | Points |
| Positive responses to the screening test | ||||||
| 0–1 | 1.0 | — | 0 | 1.0 | — | 0 |
| 2–3 | 2.53 (1.54–4.14) | <.001 | 2 | 1.67 (1.25–2.22) | <.001 | 2 |
| 4–6 | 5.56 (3.03–10.2) | <.001 | 4 | 1.53 (1.03–2.48) | .03 | 1 |
| Age | ||||||
| 65–74 | 1.0 | — | 0 | 1.0 | — | 0 |
| 75–84 | 1.57 (1.01–2.62) | .046 | 1 | 1.38 (1.08–1.75) | .008 | 1 |
| ≥85 | 4.89 (2.74–8.75) | <.001 | 4 | 1.53 (1.06–2.20) | .02 | 1 |
| Sex | ||||||
| Female | 1.0 | — | 0 | 1.0 | — | 0 |
| Male | 2.30 (1.52–3.50) | <.001 | 2 | 1.62 (1.30–2.01) | <.001 | 1 |
| Hospitalization in the previous 6 months | ||||||
| No | 1.0 | – | 0 | 1.0 | – | 0 |
| Yes | 2.77 (1.70–4.52) | <.001 | 2 | 3.60 (2.66–4.87) | <.001 | 4 |
| ≥5 prescriptions | ||||||
| No | 1.0 | – | 0 | 1.0 | – | 0 |
| Yes | 1.98 (1.30–3.08) | .001 | 2 | 2.24 (1.77–2.84) | <.001 | 3 |
OR = odds ratio; CI = confidence interval.
Risk Scoring System
Based on the results of the logistic models, points were assigned to each of the final risk factors, as listed in Table 2. The mean risk scores were 2.8 (range 0–14) for mortality and 2.3 (range 0–11) for hospitalization.
When participants were stratified according to risk scores, mortality ranged from 0.2% to 9.6% in the development cohort and 0.3% to 8.2% in the validation cohort, whereas hospitalization ranged from 6.1% to 30.5% in the development cohort and 8.1% to 29.7% in the validation cohort (Table 3). Overall, the point scoring system predicted death better than hospitalization (development cohort AUC = 0.68; validation cohort AUC = 0.67) and to the same extent in the two cohorts (AUC = 0.75). When the risk strata were compared, the optimal cutoff point was at a score of 3, which offered the best compromise between sensitivity and specificity to predict mortality (sensitivity 91.2%, specificity 58.6%) and hospitalization (sensitivity 61.3%, specificity 68.9%).
Table 3. Performance of Prognostic Indexes for Mortality and Hospitalization in the Development and Validation Cohorts According to Risk Stratum.
| Threshold for Being at High Risk* |
Mortality | Hospitalization | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Who Died/N at Risk |
%† (95% CI) |
Sensitivity | Specificity | AUC (95% CI) |
N Hospitalized/ N at Risk |
%† (95% CI) |
Sensitivity | Specificity | AUC (95% CI) |
|
| Development cohort‡ | ||||||||||
| 0 | 1/508 | 0.2 (0.04–1.1) | 100.0 | 0.0 | 0.75 (0.72–0.78) | 31/508 | 6.1 (4.1–8.5) | 100.0 | 0.0 | 0.68 (0.66–0.71) |
| 1 | 4/278 | 1.4 (0.4–3.6) | 99.1 | 21.5 | 89/789 | 11.2 (9.1–13.6) | 93.0 | 23.5 | ||
| 2 | 7/612 | 1.1 (0.4–2.3) | 95.6 | 33.1 | 59/303 | 19.4 (15.1–24.3) | 73.0 | 58.1 | ||
| ≥3 | 103/1,072 | 9.6 (7.9–11.5) | 89.6 | 58.8 | 266/870 | 30.5 (27.5–33.7) | 59.8 | 70.1 | ||
| Validation cohort‡ | ||||||||||
| ≥0 | 2/625 | 0.3 (0.03–1.1) | 100.0 | 0.0 | 0.75 (0.73–0.78) | 51/625 | 8.1 (6.1–10.5) | 100.0 | 0.0 | 0.67 (0.65–0.70) |
| 1 | 3/328 | 0.9 (0.1–2.1) | 98.2 | 22.6 | 92/907 | 10.1 (8.2–12.2) | 89.8 | 23.7 | ||
| 2 | 5/706 | 0.7 (0.2–1.1) | 95.6 | 33.7 | 52/355 | 14.6 (11.1–18.7) | 71.6 | 57.3 | ||
| ≥3 | 104/1,267 | 8.2 (6.7–9.8) | 91.2 | 58.6 | 309/1,039 | 29.7 (26.9–32.6) | 61.3 | 68.9 | ||
Threshold above which persons might be detected as being at high risk for mortality or hospitalization.
Frequency of mortality and hospitalization over total population at risk.
Risk scores are calculated according to the scoring system reported in Table 2.
The area under the receiver operating characteristic curve (AUC) is reported for the overall scores.
CI = confidence interval.
Additional AUC statistics were calculated in subgroups identified using demographic characteristics. In the validation cohort, the accuracy of the point scoring system in predicting mortality was higher in men (0.79) than in women (0.68) and in participants younger than 75 (0.79) than in those aged 75 and older (0.61). Conversely, participant demographics did not affect the accuracy of the scoring system in predicting hospitalization.
Sensitivity analysis was performed with models in which the scores attributed to previous hospitalization and to taking five or more prescription drugs were excluded, because these two conditions may vary across regions only because of contextual factors. The results indicate similar accuracy for the prediction of death (AUC = 0.75) and substantial poorer accuracy for the prediction of hospitalization (AUC = 0.60).
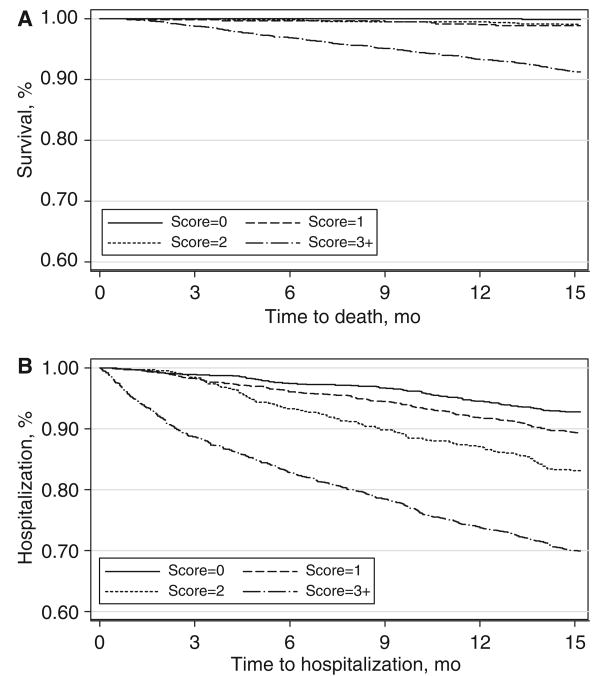
The findings reported above were confirmed using Kaplan–Meier survival curves, which demonstrated that mortality trajectories were comparable for scores from 0 to 2 and diverged only in participants who scored 3 or higher (Figure 1A). The survival-without-hospitalization curves showed good discrimination for a threshold score of 3 or higher (Figure 1B).
Figure 1.
Kaplan–Meier survival curves of the risk of mortality (A) and hospitalization (B) stratified according to risk scores in the validation cohort. mo = months.
Discussion
In this study, two prognostic indexes were developed and validated, based on a simple point scoring system, that office-based physicians can use to effectively classify older community-dwelling people into groups at variable risk of mortality and hospitalization. Items from an ad hoc questionnaire exploring general health status were the basis of the prognostic tools, because they had proven accurate in the preliminary analyses of mortality prediction.14 With the inclusion of a few other elements of information, the indexes showed good discrimination, with AUCs of 0.75 for mortality and 0.67 for hospitalization in the validation cohort.
Overall, this performance compares favorably with that of definitively more-complex prognostic indexes, generally developed in hospital settings, that require the availability of medical diagnoses and biochemical markers. One screening instrument based on sex, functional status, diagnosis of congestive heart failure and cancer, and high serum creatinine and low plasma albumin levels predicted 1-year mortality with a c statistic (analogous to an AUC) of 0.80.12 The High-Risk Diagnoses for the Elderly Scale,20 which retrieves information from administrative data, achieved a c statistic of 0.68 when 10 different comorbidities were included into the model. The Community Assessment Risk Screening Instrument,21 which includes three main predictors, such as having two or more associated illnesses, taking five or more prescriptions, and having been hospitalized over the previous year, predicted the risk of 1-year hospitalization with a c statistic of 0.67. Finally, the Probability of Repeated Admission questionnaire, based on eight items (age, sex, self-perceived health, number of hospital admissions in previous year, number of physician visits in previous year, presence of diabetes mellitus, presence of coronary heart disease, and availability of a caregiver), predicted the 1-year risk of hospital admission with a c statistic of 0.64.22
In the present study, survival and AUC analyses suggested that a score of 3 or higher, identifying the group at highest risk, represented the most convenient threshold to distinguish between high- and low-risk elderly subjects, because it correctly classified 91.2% of persons who died and 61.3% of those who would require hospitalization in the following 15 months (sensitivity), with corresponding specificity figures of 58.6% and 68.9%, respectively. In addition, the use of such scoring system allowed a large (more than 50% of the sample) group of robust elderly people scoring 2 or less, whose mortality and hospitalization rates were low (<1% and 10%, respectively), to be identified.
The ultimate usefulness of identifying frail older persons at risk for poor outcomes is debated. Some believe that, because of limited chances of recovery, the frailest individuals should not be the target of community intervention programs, although others disagree.23 Balancing the available evidence, one study underscored the importance of risk stratification in old age, suggesting that a simple health risk appraisal be conducted in low-risk individuals aged 60 to 75 and preventive home visits in those aged 75 and older who live independently and that higher-intensity services be restricted to the frailest subjects.24 Along this line of thought, the findings reported in this study should be of interest to policy makers, who need to allocate public health resources appropriately, because, in these days of constant pressure for cost containment, effective interventions need to be targeted to the appropriate patient subgroups.
The instruments proposed here offer a definite advantage over previous tools, because they achieve comparable, or better, prognostic accuracy and represent a simpler and low-cost way to identify older community-dwelling people at risk for poor outcomes who require close monitoring and might benefit from targeted interventions. Because all information used to build the prognostic indexes are in general already available to PCPs and in most cases stored in an electronic form, the most appropriate setting for the application of such instrument could be the PCP office. For patients who screen positive, the PCP would be prompted to review current therapies, plan frequent follow-up visits, communicate with the family, and take other actions directed at improving the management of high-risk older patients. To enhance widespread use of these tools, they might be incorporated into computerized medical records integrated with already available systems.25,26 With the investment of just a few minutes of time, PCPs can gain worthwhile clinical and prognostic information about their patients by adding the assessment of the functional and social status included into the seven-item questionnaire.
Information on drug treatment and previous hospitalizations affected the assessment of the risk of dying less than that of being hospitalized. This finding is in agreement with previous studies, demonstrating that measures of functional status, along with age and sex, are per se powerful predictors of the risk of 1-year mortality, even beyond the predictive power of medical diagnoses or physiologic measures.27,28 One study12 found that, after adjustment for functional status, of all the medical diagnoses included in the Charlson Comorbidity Index, only congestive heart failure and cancer remained independently associated with mortality. The strong prognostic value of functional status, resulting from the current study as well as from other studies, can be easily interpreted, considering that physical performance represents the common final pathway through which most illnesses and psychosocial factors exert their burden.29
Conversely, information on use of five or more prescribed drugs and previous hospitalizations substantially improved the prediction of hospitalization to a greater extent than functional status. Nevertheless, it should be emphasized that, even with a wide array of explanatory variables, the prediction of hospital admission remained substantially less satisfactory than the prediction of death. This is not an unexpected finding, because it has been consistently reported in several previous studies that highlighted the role of prior hospitalization and of drug treatment–related measures in predicting the subsequent use of healthcare resources.21,22,30,31 Several authors have also argued that population measures of deprivation or social factors such as living alone, rather that strict clinical indicators, might explain the variability in hospital admission rates. Moreover, lower admission rates have been found to be associated with some markers of quality in primary care, such as the provision of specialist clinics, thus suggesting that organizations focusing on better integration between primary and secondary care and on patient self-management cost less and result in fewer hospitalizations.32–35 Thus, it appears that, in addition to physiological and clinical variables, us of healthcare resources in late life depends heavily—or, at least, more than mortality—on broad societal factors and, more specifically, on the architecture of healthcare services for the elderly.
Limits of the study should be recognized. Functional status of participants whose PCPs had indicated that they were impaired was subsequently verified using appropriate instruments,15 yet no representative sample of participants who had screened negative was evaluated. Thus, whereas the proportion of subjects who screened positive and were eventually confirmed as disabled from direct interview was high, some false negative subjects might have been missed. However, in a previous study, the screening instrument used in the current study proved to have excellent concurrent validity.14 Second, the current study did not consider cognitive impairment and behavioral symptoms, which are known to be reliable predictors of poor survival36–38 and hospitalization,39,40 even beyond somatic comorbidity, functional impairment, and living situation. Third, data on hospitalization and medication use were derived from claims databases, whereas in possible future applications, it is likely that direct input of these data would be required; it is unknown whether this different methodological approach to data retrieval might influence the performance of the prognostic index. Fourth, the prediction of major health outcomes could not be calibrated to a shorter time-frame, as would be useful in frail older persons, because only 43 deaths were observed in 6 months. Finally, the indexes were developed using information from home care settings, and additional validation studies might be needed before using them in other settings, such as residential care or long-term hospitals, or in other populations.41
In conclusion, our indexes provide potentially useful diagnostic tools to identify older, community-dwelling subjects at risk of death and hospitalization. They are calculated with only 11 variables, all easily available to PCPs, and a simple additive point system. Because of these characteristics, these indexes might be useful in identifying frail older individuals at greater risk of death and to target interventions aiming at the prevention of potentially avoidable hospital admissions.
Footnotes
Author Contributions: G. Mazzaglia and M. Di Bari originated the idea for this study, performed the data analysis, and took primary responsibility for writing the manuscript. L. Ferrucci and N. Marchionni designed the ASSI project and supervised the data collection. A. Colombini refined the data analysis and interpretation and assisted in the composition of the manuscript, tables, and figures. L. Roti and G. Corsini were responsible for data collection and handling. G. Maciocco and E. Buiatti expanded the discussion of public health implications and provided useful references. All authors contributed to the writing and editing of the manuscript.
Sponsor's Role: The sponsor had no role in the design, recruitment, data collection, analysis, or preparation of the manuscript.
Conflict of Interest: The authors have no conflict of interest to disclose related to this article. The study was funded by the Agency for Regional Healthcare Services, Department of Health, Rome, Italy. M. Di Bari had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Istituto Nazionale di Statistica. La cura ed il ricorso ai servizi sanitari, Indagine Multiscopo sulle famiglie “Condizioni di salute e ricorso ai servizi sanitari” 1999–2000. Rome: Dec, 2001. [Google Scholar]
- 2.The Italian National Drug Use Monitoring Center (OsMed) Drug Use in Italy, Year 2004. Rome: Jun, 2005. [Google Scholar]
- 3.Kane RL. Managed care as a vehicle for delivering more effective chronic care for older persons. J Am Geriatr Soc. 1998;46:1034–1039. doi: 10.1111/j.1532-5415.1998.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 4.Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: Systematic review and meta-regression analysis. JAMA. 2002;287:1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 5.Stuck AE, Aronow HU, Steiner A, et al. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med. 1995;333:1184–1189. doi: 10.1056/NEJM199511023331805. [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt JB, Toseland RW, O'Donnell JC, et al. The effectiveness and efficiency of outpatient geriatric evaluation and management. J Am Geriatr Soc. 1996;44:847–856. doi: 10.1111/j.1532-5415.1996.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernabei R, Landi F, Gambassi G, et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. BMJ. 1998;316:1348–1351. doi: 10.1136/bmj.316.7141.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwan RT, Davison N, Forster DP, et al. Screening elderly people in primary care: A randomized controlled trial. Br J Gen Pract. 1990;40:90–97. [PMC free article] [PubMed] [Google Scholar]
- 9.Besdine R, Boult C, Brangman S, et al. American Geriatrics Society Task Force on the Future of Geriatric Medicine. Caring for older Americans: The future of geriatric medicine. J Am Geriatr Soc. 2005;53(6 Suppl):S245–S256. doi: 10.1111/j.1532-5415.2005.53350.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin JC, Avant RF, Bowman MA, et al. Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: A collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3–S32. doi: 10.1370/afm.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey EC, Walter LC, Lindquist K, et al. Development and validation of a functional morbidity index to predict mortality in community—dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 13.Coleman EA, Wagner EH, Grothaus LC, et al. Predicting hospitalization and functional decline in older health plan enrollees: Are administrative data as accurate as self-report? J Am Geriatr Soc. 1998;46:419–425. doi: 10.1111/j.1532-5415.1998.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 14.Roti L, Corsini G, Colombini A, et al. A screening instrument to identify older community-dwellers at risk for death and hospitalization in Tuscany, Italy. The “Assistenza Socio-Sanitaria in Italia” project. J Am Geriatr Soc. 2006;54(Suppl S):S90–S91. doi: 10.1111/j.1532-5415.2007.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Bari M, Pecchioli A, Mazzaglia G, et al. Care available to severely disabled older persons living at home in Florence, Italy. Aging Clin Exp Res. 2007 doi: 10.1007/bf03324745. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Bari M, Virgillo A, Matteuzzi D, et al. Predictive validity of measures of comorbidity in older community-dwellers: The Insufficienza Cardiaca negli Anziani Residenti a Dicomano study. J Am Geriatr Soc. 2006;54:210–216. doi: 10.1111/j.1532-5415.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: Results from the His-panic EPESE study. J Gerontol. 2006;61:170–175. doi: 10.1093/gerona/61.2.170. [DOI] [PubMed] [Google Scholar]
- 18.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariate models. Ann Intern Med. 1993;130:515–524. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. The meaning and use of the area under a receiving operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 20.Desai MM, Bogardus ST, Jr, Williams CS, et al. Development and validation of a risk-adjustment index for older patients: The high-risk diagnoses for the elderly scale. J Am Geriatr Soc. 2002;50:474–481. doi: 10.1046/j.1532-5415.2002.50113.x. [DOI] [PubMed] [Google Scholar]
- 21.Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): Identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925–933. [PubMed] [Google Scholar]
- 22.Wagner JT, Bachmann LM, Boult C, et al. Predicting the risk of hospital admission in older persons – validation of a brief self-administered questionnaire in three European countries. J Am Geriatr Soc. 2006;54:1271–1276. doi: 10.1111/j.1532-5415.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 23.Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: Systematic review and meta-analysis. BMJ. 2001;323:719–725. doi: 10.1136/bmj.323.7315.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuck AE, Beck JC, Egger M. Preventing disability in elderly people. Lancet. 2004;364:1641–1642. doi: 10.1016/S0140-6736(04)17365-0. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell E, Sullivan F, Watt G, et al. Using electronic patient records to inform strategic decision making in primary care. Medinfo. 2004;11(Part 2):1157–1161. [PubMed] [Google Scholar]
- 26.Filippi A, Sabatini A, Badioli L, et al. Effects of an automated electronic reminder in changing the antiplatelet drug-prescribing behavior among Italian general practitioners in diabetic patients: an intervention trial. Diabetes Care. 2003;26:1497–1500. doi: 10.2337/diacare.26.5.1497. [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 28.Covinsky KE, Justice AC, Rosenthal GE, et al. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997;12:203–208. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried LP, Guralnik JM. Disability in older adults: Evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 30.Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811–817. doi: 10.1111/j.1532-5415.1993.tb06175.x. [DOI] [PubMed] [Google Scholar]
- 31.Billings J, Dixon J, Mijanovich T, et al. Case finding for patients at risk of readmission to hospital: Development of algorithm to identify high risk patients. BMJ. 2006;333:327. doi: 10.1136/bmj.38870.657917.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landi F, Onder G, Cesari M, et al. Comorbidity and social factors predicted hospitalization in frail elderly patients. J Clin Epidemiol. 2004;57:832–836. doi: 10.1016/j.jclinepi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Saxena S, George J, Barber J, et al. Association of population and practice factors with potentially avoidable admission rates for chronic diseases in London: Cross sectional analysis. J R Soc Med. 2006;99:81–89. doi: 10.1258/jrsm.99.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blustein J, Hanson K, Shea S. Preventable hospitalizations and socioeconomic status. Health Aff (Millwood) 1998;17:177–189. doi: 10.1377/hlthaff.17.2.177. [DOI] [PubMed] [Google Scholar]
- 35.Feachem RGA, Sekhri NK, White KL, et al. Getting more for their dollars: A comparison of NHS with California's Kaiser Permanente. BMJ. 2002;324:135–143. doi: 10.1136/bmj.324.7330.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portin R, Muuriaisniemi ML, Joukamaa M, et al. impairment and the 10-year survival probability of a normal 62-year-old population. Scand J Psychol. 2001;42:359–366. doi: 10.1111/1467-9450.00247. [DOI] [PubMed] [Google Scholar]
- 37.Desmond DW, Moroney JT, Sano M, et al. Mortality in patients with dementia after ischemic stroke. Neurology. 2002;59:537–543. doi: 10.1212/wnl.59.4.537. [DOI] [PubMed] [Google Scholar]
- 38.Covinsky KE, Kahana E, Chin MH, et al. Depressive symptoms and 3-year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130:563–569. doi: 10.7326/0003-4819-130-7-199904060-00004. [DOI] [PubMed] [Google Scholar]
- 39.Kales HC, Blow FC, Copeland LA, et al. Health care utilization by older patients with coexisting dementia and depression. Am J Psychiatry. 1999;156:550–556. doi: 10.1176/ajp.156.4.550. [DOI] [PubMed] [Google Scholar]
- 40.Bula CJ, Wietlisbach V, Burnand B, et al. Depressive symptoms as a predictor of 6-month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161:2609–2615. doi: 10.1001/archinte.161.21.2609. [DOI] [PubMed] [Google Scholar]
- 41.Grundy E, Glaser K. Trends in, transition to, institutional residence among older people in England and Wales. J Epidemiol Community Health. 1997;51:531. doi: 10.1136/jech.51.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]