Abstract
Blockade of the transient receptor potential channel vanilloid subfamily 1 (TRPV1) is suggested as a therapeutic approach to pain relief. However, TRPV1 is a widely expressed protein whose function might be critical in various non-neuronal physiological conditions. The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is overexpressed in many human epithelial cancers and is a potential target for anticancer drugs. Here we show that TRPV1 interacts with the EGFR leading to EGFR degradation. Notably, the absence of TRPV1 in mice results in a striking increase in skin carcinogenesis. The TRPV1 is the first membrane receptor shown to have a tumor-suppressing effect associated with the downregulation of another membrane receptor. The data suggest that even though a great deal of interest has focused on the TRPV1 as a target for pain relief, the chronic blockade of this pain receptor might increase the risk for cancer development.
INTRODUCTION
The transient receptor potential channel vanilloid subfamily 1 (TRPV1) belongs to a family of proteins comprised of non-selective cation channels (1). TRP proteins are involved in a variety of sensory signaling ranging from thermal and mechanical nociception to vision, taste, olfaction, touch and osmo-sensation (2). The TRPV1 is not only expressed in neuronal tissues, but is detected in epidermis, dermal blood vessels, normal human keratinocytes, mast cells, appendage epithelial structures, human cultured fibroblasts, human hair follicles, human lung BEAS-2B cells, and HaCaT cells (3–7), but the function of TRPV1 in non-neuronal cells and tissues is unclear. In contrast to other TRPs, TRPV1 is unique in its ability to interact with capsaicin, the pungent ingredient of hot peppers (8). The role of capsaicin in cancer development is controversial with some reports indicating that capsaicin inhibits phorbol ester-induced skin cancer (9) and NF-κB activation (10); whereas others observed a carcinogenic effect (11). Capsaicin activates and eventually destroys small diameter nociceptive sensory neurons and pain fibers that contain TRPV1, which has made TRPV1 an important target for the management of chronic pain (8). However, little is known about other physiologic effects that might occur from long-term blockade of this receptor.
The epidermal growth factor receptor (EGFR) is a widely expressed receptor tyrosine kinase that plays an important role in regulating the development of the epidermis and its appendages. This receptor is abundantly expressed in the basal layer of the epidermis and in the outer sheath of the hair follicles. The expression of EGFR decreases when keratinocytes differentiate and migrate to the suprabasal epidermal layers (12). The EGFR is overexpressed in human epithelial cancers, including lung, colon, ovary, bladder, and head and neck (13), and is pursued as a potential target for anticancer drugs (14).
Here we showed that TRPV1 interacts with the EGFR, which facilitated EGFR ubiquitylation by Cbl, an ubiquitin ligase, resulting in subsequent EGFR degradation through the lysosomal pathway. Additional experiments indicated that TRPV1 knockout (TRPV1−/−) mouse skin expressed strikingly higher levels of the EGFR compared to TRPV1 wildtype (TRPV1+/+) mouse skin. Notably, the absence of the TRPV1 in mice resulted in a marked increase in papilloma development. Overall, results indicated that TRPV1 is a membrane receptor exhibiting a tumor-suppressing effect that is associated with the down-regulation of another membrane receptor, the EGFR, which is known to be important in skin cancer development.
MATERIALS AND METHODS
Chemicals and antibodies
Tris, NaCl, SDS, cycloheximide, leupeptin, and lactacystin were from Sigma-Aldrich Corp. (St. Louis, MO). Restriction enzymes and some modifying enzymes were from Roche Diagnostics Corp. (Indianapolis, IN) and Taq DNA polymerase was from Qiagen, Inc. (Valencia, CA). The DNA ligation kit (v. 2.0) was purchased from TAKARA Bio, Inc. (Otsu, Shiga, Japan). Cell culture media and supplements were from Life Technologies, Inc. (Rockville, MD). Antibodies against EGFR (528), Cbl (A-9), or ubiquitin (P4D1) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and the anti-capsaicin receptor (Ab-1) was from Calbiochem (San Diego, CA). Antibodies for the detection of phosphorylated ERKs, MEK1/2, RSK, CREBs, and ATF1, and total and phosphorylated EGFR were purchased from Cell Signaling Technology, Inc. (Beverly, MA).
Immunoprecipitation
For protein binding, an EGFR (528) antibody was used for immunoprecipitation with membrane fractions from A431 (150 µg), HEK293, HaCaT cells (400 µg), or cytosolic protein (150 µg) fractions. Antibody binding was carried out at 4 °C overnight and proteins visualized by Western blotting using specific antibodies against TRPV1, EGFR, ubiquitin, or Cbl.
Expression of GST-TRPV1-NT and GST-TRPV1-CT
To identify the domain of TRPV1 that binds with EGFR, we amplified cDNA fragments spanning the cytoplasmic N-terminal domain (1–423 aa; sense, 5’-GCAGGATCCAGATGGAACAACGGGCTAGCTTAGAC-3’; antisense, 5’-GCTCTAGAGCGCTTGACAAATCTGTCCCACTT-3’) and the C-terminal domain (680–838 aa; sense, 5’-CGGAATTCGCTCTCATGGGTGAGACCGTC AAC-3; antisense, 5’ATAGTTTAGCGGCCGCTAATTTCTCCCCTGGGACCATGGA-3’). The N-terminal DNA fragment was introduced into BamHI/XbaI of pGEX5XC (pGST-TRPV1-NT) and the C-terminal DNA fragment was recombined into EcoRI/NotI of pGEX5X1 (pGST-TRPV1-CT) and confirmed by DNA sequencing. Plasmids were transformed into BL21 cells and induced with 500 µM IPTG at 25 °C for 5 h. Soluble GST-fusion proteins were purified using Sepharose 4B beads and 10 mM reduced glutathione in 50 mM Tris-HCl (pH 8.0) buffer.
GST-pull down
GST-TRPV1-NT or -CT proteins bound to Sepharose 4B beads were mixed with A431 cell lysate (200 µg) and incubated overnight at 4 °C with suspension buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100). The beads were washed 4X with suspension buffer at 4 °C, then denatured by adding SDS sample buffer and visualized by Western blotting using appropriate antibodies.
Extraction of membrane and cytosolic proteins
Cells were harvested, disrupted with lysis buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl) and freezing and thawing. The supernatant fraction, including cytosolic and nuclear soluble proteins, was recovered by centrifugation and the pellet was resuspended in buffer. The cell suspension was stirred on ice 1 h, then centrifuged and membrane proteins were recovered in the supernatant fraction. To prepare membrane fractions from mouse skin, 0.5 g of tissue was homogenized with 10 volumes of buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl) and centrifuged at 10,000g for 10 min at 4 °C. Supernatant fractions were subjected to ultracentrifugation at 160,000g for 2 h at 4 °C and the pellet was collected and used as a whole membrane fraction. The membrane proteins were solubilized with membrane suspension buffer (20 mM Tris-HCl, pH 8.0, 1% Triton-X-100) and protein concentration was measured using the DC protein assay kit (Bio-Rad, Hercules, CA). The proteins were visualized by Western blotting.
Analysis of protein degradation pathway
A431 cells (1×106 cells) were seeded in 60-mm cell culture dishes and transfected with a mock or TRPV1 plasmid. At 48 h after transfection, cells were treated for 6 h with cycloheximide (CHX) in 10% FBS/DMEM to block protein synthesis, and lactacystin to inhibit the proteosomal degradation pathway; or with CHX and leupeptin to inhibit the lysosomal degradation pathway. Cells were harvested, separated into membrane and cytosolic protein fractions, and EGFR protein level was visualized by Western blotting.
Genomic PCR and RT-PCR analysis
To verify the TRPV1 knockout, we conducted genomic PCR analysis. Genomic DNA (50 ng) extracted from the tail of TRPV1 wildtype and knockout mice was used for genomic PCR with primers for wildtype (sense: 5’-CGA GGA TGG GAA GAA TAA CTC ACT G-3’ and antisense; 5’-GGA TGA TGA AGA CAG CCT TGA AGT C-3’) and for knockout (sense: 5’-TTT GTC AAG ACC GAC CTG TCC-3’ and antisense: 5’-CCC TCA GAA GAA CTC GTC AAG AAG-3’). To examine whether the TRPV1 is expressed in skin, total RNA (1 µg) from the dorsal skin of wildtype and TRPV1 knockout mice was used for reverse transcription (RT) with superscript II reverse transcriptase (Invitrogen Corp, Carlsbad, CA). The TRPV1 cDNA was amplified with Hotstart Taq DNA polymerase (Qiagen, Inc., Valencia, CA) using specific primers (sense: 5’-TGT GGC TTC CAT GGT GTT CTC-3’ and antisense: 5’-AAG GCC TTC CTC ATG CAC TTC-3’). The bands were visualized by agarose gel electrophoresis and ethidium bromide staining.
Immunostaining
A human skin cancer tissue array (5-micron, Cat#SK801, U.S. Biomax, Inc., Rockville, MD) and mouse dorsal skin samples (100-micron) were processed and immunostained. For the human skin cancer tissue array, we used anti-TRPV1 (Cat#GP14100, Neuromics) and anti-EGFR (Cat#4405, Cell Signaling) and Alexa Fluor 488 and 647 conjugated secondary antibodies. Mouse skin samples were immunostained with antibodies: a) 1:100 anti-TRPV1 made in guinea pig and 1:200 donkey anti-guinea pig IgG conjugated to Cy2 (Jackson ImmunoResearch, West Grove, PA) and b) anti-EGFR made in rabbit (Santa Cruz Biotechnology) and donkey anti-rabbit IgG conjugated to Cy5. Image stacks were captured at room temperature using laser scanning confocal microscopy (NIKON C1si Confocal Spectral Imaging System; CFI Plan Fluor 20× objective for tissue array and MRC 1000 Bio-Rad for skin samples).
In vivo mouse studies
B6, 129S4-Trpv1 (TRPV1−/−) mice and cDNA plasmids were kindly provided by Dr. David Julius (USCSB, Santa Barbara, CA) and TRPV1 wildtype (TRPV1+/+) mice were purchased from Jackson Laboratories (Bar Harbor, ME). For the two-stage carcinogenesis studies, age- and gender-matched TRPV1 WT and TRPV1 KO mice were divided into 2 groups each and all were initiated by topical application of 200 nmol of DMBA. Two weeks later, topical application of TPA (17 nmol) was begun and continued twice a week for a total of 21 weeks. Groups 1 (WT; n = 17) and 2 (TRPV1 KO; n = 16) were treated with vehicle (acetone) only; groups 3 (WT; n = 16) and 4 (TRPV1 KO; n =16) were treated with 17 nmol TPA only. Mice were weighed and tumors counted and measured once a week beginning when first measurable tumors (1 mm3) were observed (week 12). All animal experiments were carried out according to protocols approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC).
RESULTS
EGFR and TRPV1 interact in vitro and ex vivo
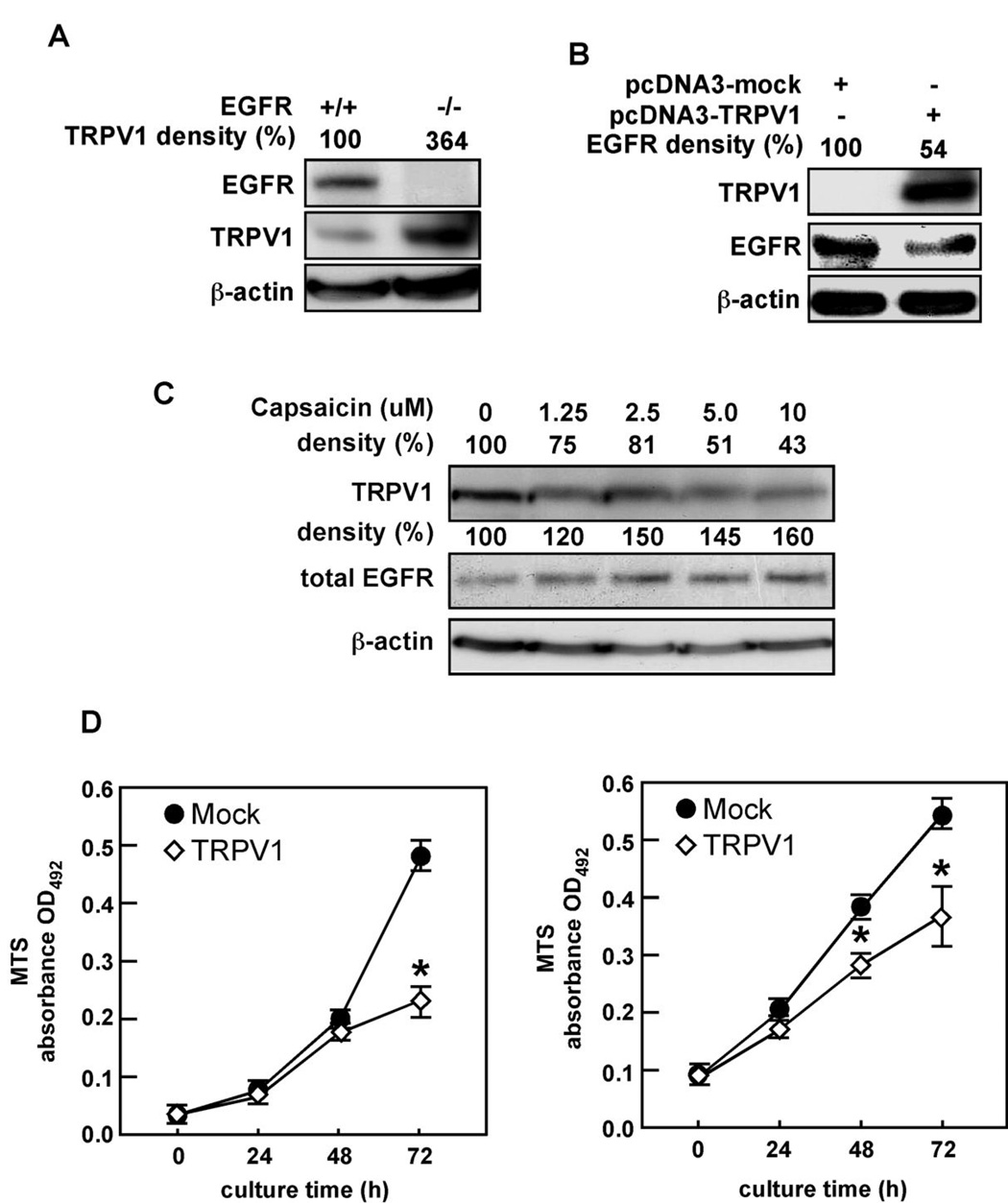
Endogenous TRPV1 was barely detectable in murine embryonic fibroblasts (MEFs) that highly express wildtype EGFR (EGFR+/+, Fig. 1A). In contrast, EGFR-deficient (EGFR−/−) MEFs showed strikingly high levels of TRPV1 (Fig. 1A). Further examination revealed that ectopic expression of TRPV1 in HEK293 cells was associated with a substantial decrease in total EGFR protein expression (Fig. 1B). Further, capsaicin down-regulated TRPV1 dose-dependently (Fig. 1C, top panel), which supported other observations that capsaicin down-regulates or desensitizes TRPV1 (15). In contrast, we found that EGFR was increased in response to capsaicin treatment (Fig. 1C, middle panel). Furthermore, ectopic expression of TRPV1 decreased proliferation of NIH3T3 (Fig. 1D, left) or A431 (Fig. 1D, right) cells compared to mock transfected cells. These findings suggested that the EGFR and TRPV1 membrane receptors associate and possibly regulate each other in opposition.
Figure 1.
TRPV1 and EGFR are expressed in opposition to each other. (A) EGFR-deficient (EGFR−/−) MEFs express high levels of endogenous TRPV1 compared to wildtype (EGFR+/+) cells. (B) Ectopic expression of the TRPV1 suppresses the endogenous level of the EGFR protein. (C) Capsaicin treatment down-regulates the TRPV1 protein level but enhances total EGFR expression. A431 human skin cells were treated for 24 h with increasing doses of capsaicin as indicated. The cells were harvested, membrane proteins extracted, and TRPV1 and EGFR protein levels were analyzed by immunoblotting with specific antibodies. (D) Ectopic expression of TRPV1 decreases proliferation of NIH3T3 (left) or A431 (right) cells. Cells were transfected with pcDNA3-TRPV1 or pcDNA3-mock and cultured an additional 24 h and then cell proliferation was measured over time as indicated using the MTS assay (Promega). For A–C, β-actin was used to verify equal protein loading and for D, the asterisk (*) indicates a significance decrease in proliferation of TRPV1-transfected cells compared to mock-transfected cells (*, p < 0.01).
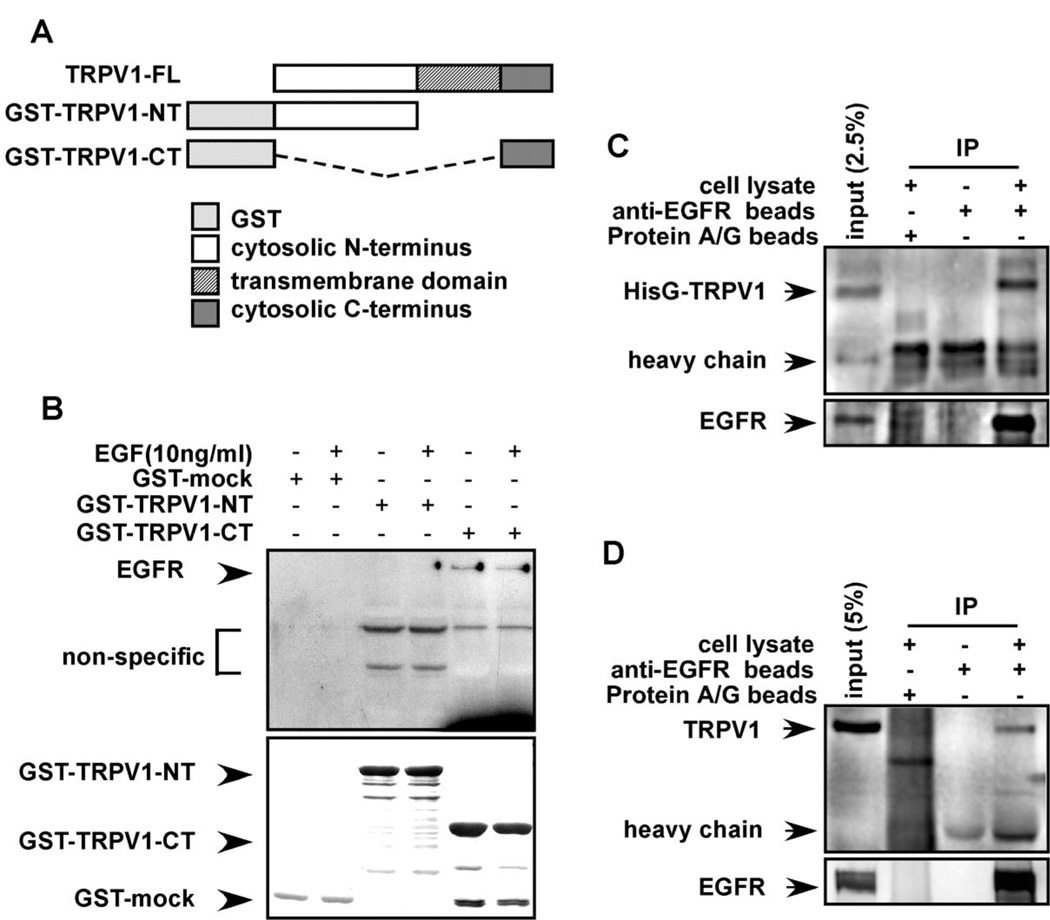
To examine this idea, we constructed GST-fusion proteins expressing full length (TRPV1-FL), the N-terminal (GST-TRPV1-NT) or C-terminal cytoplasmic domain (GST-TRPV1-CT) of TRPV1 (Fig. 2A). GST-fusion proteins were incubated with membrane fractions prepared from A431 human skin cells and binding with EGFR was assessed. Results confirmed that the C-terminal cytosolic domain, but not the N-terminal domain, of TRPV1 interacted with EGFR in vitro (Fig. 2B, upper panel, lanes 5, 6). Stimulation with EGF had no additional effect on the interaction between EGFR and TRPV1, suggesting that binding occurs without stimulation. Membrane fractions were then prepared from pcDNA4-HisG-TRPV1-transfected HEK293 cells (Fig. 2C) or HaCaT cells (Fig. 2D). Proteins were immunoprecipitated with beads pre-conjugated with anti-EGFR and results showed that the TRPV1 immunoprecipitated with EGFR ex vivo (Fig. 2C,D), indicating that TRPV1 and EGFR associate ex vivo and also confirming that TRPV1 is expressed in HaCaT cells.
Figure 2.
The TRPV1 and EGFR interact in vitro and ex vivo. (A) GST-fusion protein constructs were created as follows: TRPV1-FL (full length TRPV1); GST-TRPV1-NT (N-terminal cytoplasmic domain of TRPV1); and GST-TRPV1-CT (C-terminal cytoplasmic domain of TRPV1). (B) The EGFR binds to the C-terminal cytoplasmic domain of the TRPV1. The GST-mock, GST-TRPV1-NT, or GST-TRPV1-CT protein was mixed with membrane fractions from A431 human skin cells and incubated overnight. Anti-EGFR was used to detect binding of the EGFR to respective GST-TRPV1 proteins. The top panel illustrates the binding interaction and the bottom panel verifies input. (C) The membrane proteins from HEK293 cells transfected with pcDNA4-HisG-TRPV1 were immunoprecipitated with beads pre-conjugated with anti-EGFR and the co-immunoprecipitated TRPV1 was visualized by Western blotting with anti-HisG. (D) The membrane proteins from HaCaT cells were immunoprecipitated with beads pre-conjugated with anti-EGFR and the co-immunoprecipitated TRPV1 was visualized by Western blotting with anti-TRPV1. For C and D, immunoprecipitated EGFR is shown in the lower panel.
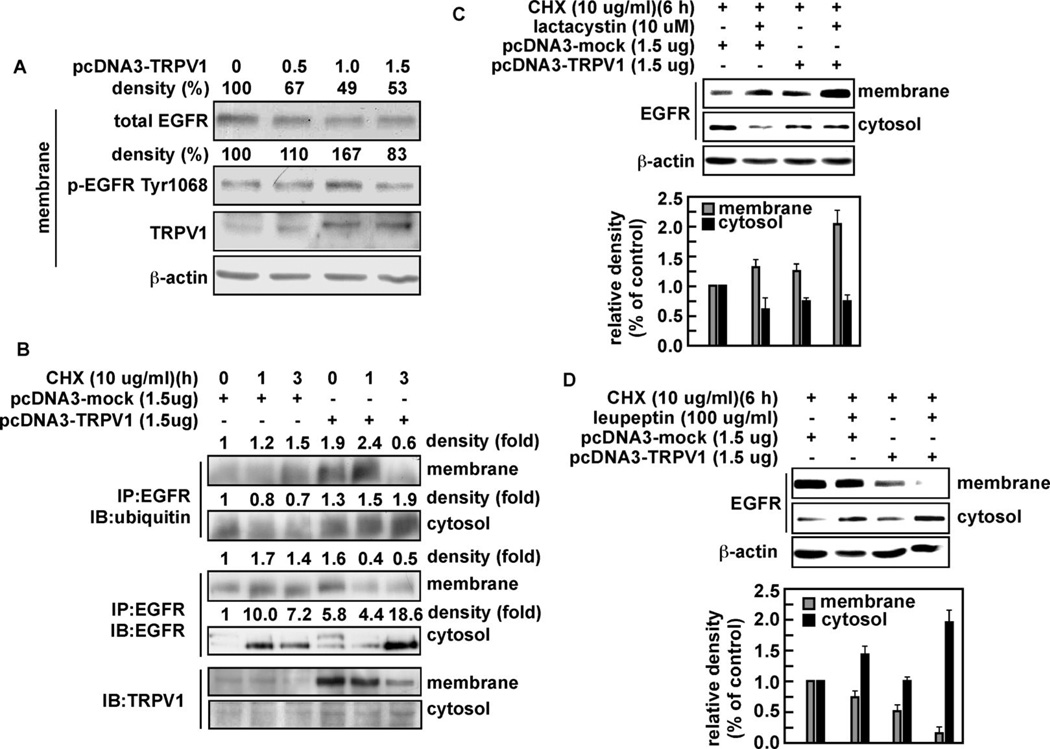
TRPV1 expression increases EGFR ubiquitylation and lysosomal degradation
A major deactivation pathway for EGFR involves ubiquitylation and ligand-induced internalization through endocytosis and degradation in the lysosomes (16). We hypothesized that the interaction of TRPV1 with EGFR might lead to ubiquitylation and degradation of the EGFR. To examine this, A431 human skin cells were transfected with increasing amounts of pcDNA3-TRPV1 and the effect on total and phosphorylated (Tyr1068) EGFR level was analyzed in membrane fractions. The findings indicated that total EGFR in membrane fractions was decreased dose-dependently in the presence of TRPV1 (Fig. 3A). The phosphorylation of EGFR (Tyr1068) is associated with the recruitment of Cbl, a protein that ubiquitylates the EGFR and targets it for degradation (17). Our results showed an increase in phosphorylation of Tyr1068 (Fig. 3A) in the presence of TRPV1. To examine a potential role for TRPV1 in the ubiquitylation of EGFR, we transfected pcDNA3-mock or pcDNA3-TRPV1 into A431 cells. Cells were cultured and treated with cycloheximide (CHX; 10 µg/ml) to prevent new protein synthesis, harvested at various times, and cytosolic and membrane fractions prepared. The respective fractions were used for immunoprecipitation with anti-EGFR and ubiquitylation of the EGFR was detected using anti-ubiquitin (Fig. 3B). The results indicated that ubiquitylation of EGFR was increased in membrane and cytosolic fractions isolated from cells transfected with TRPV1 compared with mock-transfected cells (Fig. 3B, upper 2 panels). Furthermore, total EGFR was decreased in membrane fractions but increased in cytosolic fractions of TRPV1-transfected cells compared with mock cells (Fig. 3B, middle 2 panels), suggesting that TRPV1 induces ubiquitylation of EGFR. TRPV1 levels also changed correspondingly (Fig. 3B, bottom 2 panels). These data support the idea that down-regulation of EGFR in the presence of TRPV1 is associated with an increase in ubiquitylated EGFR, corresponding to increased endocytosis and degradation of EGFR.
Figure 3.
The TRPV1 induces ubiquitylation and degradation of the EGFR protein through the lysosomal pathway. (A) A431 human skin cells were transfected with increasing amounts of TRPV1 and total and phosphorylated (Tyr1068) levels of EGFR and TRPV1 proteins were analyzed from membrane fractions by Western blotting using specific antibodies. β-Actin was used as a loading control to verify equal protein loading. (B) A431 human skin cells were transfected with pcDNA3-mock or pcDNA3-TRPV1 and then treated 48 h later with cycloheximide (10 µg/ml; CHX) to prevent additional protein synthesis. Cells were harvested and cytosolic and membrane fractions prepared. Ubiquitylation of the EGFR was detected in the membrane and cytosolic fractions (upper 2 panels). Total EGFR was immunoprecipitated and detected in the membrane and cytosolic fractions by Western blot analysis (middle 2 panels). The TRPV1 was detected in the membrane with a TRPV1 specific antibody by Western blotting (bottom 2 panels). A431 cells were transfected as described for (A) and treated for 6 h with CHX (10 µg/ml) and 10 µM lactacystin, a proteosomal pathway inhibitor (C), or with 100 µg/ml of leupeptin, a lysosomal pathway inhibitor (D). The EGFR protein level was detected in membrane and cytosolic fractions by Western blot (upper panels) and analyzed by densitometer (lower panels). The histograms illustrate the relative density of each band compared to a value of 1.0 for mock control; and β-actin was used to verify equal protein loading. IP: immunoprecipitation; IB: immunoblot.
A431 human skin cells transfected with pcDNA3-mock or pcDNA3-TRPV1 were cultured and treated with CHX (10 µg/ml) in combination with either lactacystin (10 µM, Fig. 3C), to inhibit proteosomal degradation, or with leupeptin (100 µg/ml, Fig. 3D), to inhibit degradation through the lysosomal pathway. Membrane and cytosolic fractions were prepared and used for detection of EGFR. As indicated earlier, cycling of EGFR involves ubiquitylation, endocytosis, and lysosomal degradation. Results indicated that when the proteosomal pathway was suppressed, the EGFR protein accumulated in the membrane in the presence of the TRPV1, but was relatively unchanged in the cytosolic fraction (Fig. 3C). This indicated that TRPV1-induced ubiquitylation and endocytosis of EGFR were suppressed, but also suggested that the proteosomal pathway was not involved. In contrast, suppression of the lysosomal pathway resulted in decreased EGFR in the membrane fraction indicating that TRPV1-induced ubiquitylation and endocytosis of EGFR proceeded as expected (Fig. 3D). However, the cytosolic EGFR protein levels increased, suggesting that the lysosomal pathway was the primary route for TRPV1-induced degradation of EGFR (Fig. 3D). These results revealed that TRPV1 induced EGFR ubiquitylation and degradation that was mediated through the lysosomal degradation pathway.
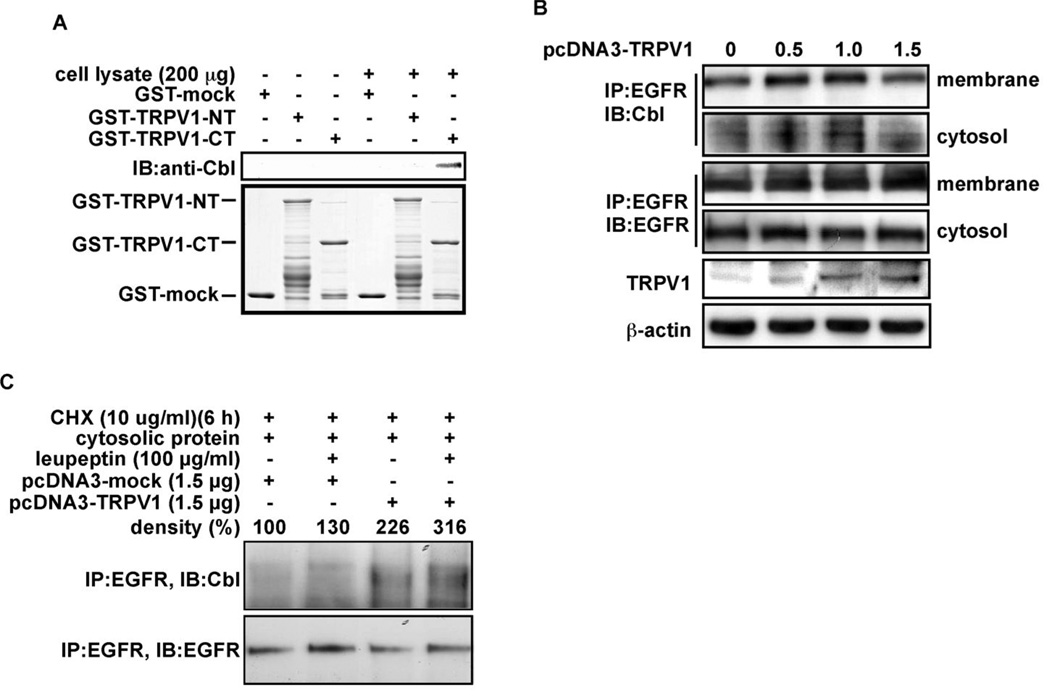
The C-terminal domain of the TRPV1 recruits Cbl
Results thus far showed that the down-regulation of EGFR in the presence of TRPV1 corresponds with increased ubiquitylated EGFR and endocytosis followed by lysosomal degradation of the EGFR. However, TRPV1 does not possess ubiquitin ligase activity and therefore must act through an alternative mechanism, perhaps by recruiting an ubiquitylation enzyme, such as Cbl, to EGFR. Cbl phosphorylation and activation results in ubiquitylation, endocytosis, and degradation of the EGFR (17). We therefore determined whether the TRPV1 interacts with Cbl. GST-mock, GST-TRPV1-CT, or GST-TRPV1-NT fusion proteins were incubated with A431 human skin cell lysates for GST pull-down and the Cbl protein level was detected by Western blot. Results indicated that the GST-TRPV1-CT fusion protein interacted with Cbl (Fig. 4A). We then hypothesized that if TRPV1 could recruit the Cbl protein to EGFR, then Cbl binding with EGFR should be enhanced with increasing amounts of TRPV1. This was confirmed by immunoprecipitation of EGFR and detection of Cbl in A341 cells transfected with increasing amounts of TRPV1 (Fig. 4B). In addition, blocking the lysosomal pathway resulted in an increased association of EGFR and Cbl in the cytosol of cells overexpressing TRPV1 compared with mock-transfected or cells not treated with the lysosomal inhibitor (Fig. 4C). Overall these results suggested that TRPV1 plays an important role in the down-regulation of EGFR by recruiting the Cbl ubiquitin ligase to EGFR to target the EGFR for degradation.
Figure 4.
TRPV1 interacts with the Cbl protein for the down-regulation of the EGFR. (A) A431 human skin cells were combined with GST-mock, GST-TRPV1-NT, or GST-TRPV1-CT. A GST pull down assay was performed and Cbl was detected by Western blot analysis. (B) A431 human skin cells were transfected with increasing amounts of pcDNA3-TRPV1, and the EGFR was immunoprecipitated from membrane or cytosolic fractions. Cbl was detected by Western blot analysis (top 2 panels). Total EGFR was immunoprecipitated and detected in the membrane and cytosolic fractions by Western blot analysis (middle 2 panels). Expression of TRPV1 was detected in the membrane with a TRPV1 specific antibody by Western blotting (2nd panel from bottom). (C) A431 human skin cells were transfected with pcDNA3-mock or pcDNA3-TRPV1 and cultured for 48 h. Cells were then treated with CHX and leupeptin as for Fig. 3D. The EGFR was immunoprecipitated from cytosolic fractions and Cbl (top panel) or EGFR (lower panel) was detected by Western blotting. IP: immunoprecipitation; IB: immunoblot.
TRPV1 negatively regulates tumor promoter-induced cell transformation
We hypothesized that the ability of TRPV1 to down-regulate EGFR might have a role in malignant cell transformation and skin carcinogenesis. To examine this hypothesis, we established JB6 Cl41 cells that stably overexpress TRPV1 (JB6 TRPV1-OE). These cells were compared to wildtype JB6 Cl41 (JB6 WT) cells, which express low levels of TRPV1, in an anchorage-independent cell transformation assay. In this assay, cells were cultured and stimulated with EGF (Supp. Fig. 1A, left) or 12-O-tetradecanoylphorbol-13-acetate (TPA; Supp. Fig. 1A, right) to induce colony formation. Results indicated that overexpression of TRPV1 significantly inhibited malignant cell transformation either with or without a tumor promoter. Moreover, treatment of cells with AG1478 (5 µM), an EGFR inhibitor, totally blocked EGF-induced colony formation in soft agar in both mock and TRPV1 stably transfected JB6 Cl41 cells (Supp. Fig. 1B), confirming that colony formation in soft agar by either cell line is dependent on signaling through the EGFR.
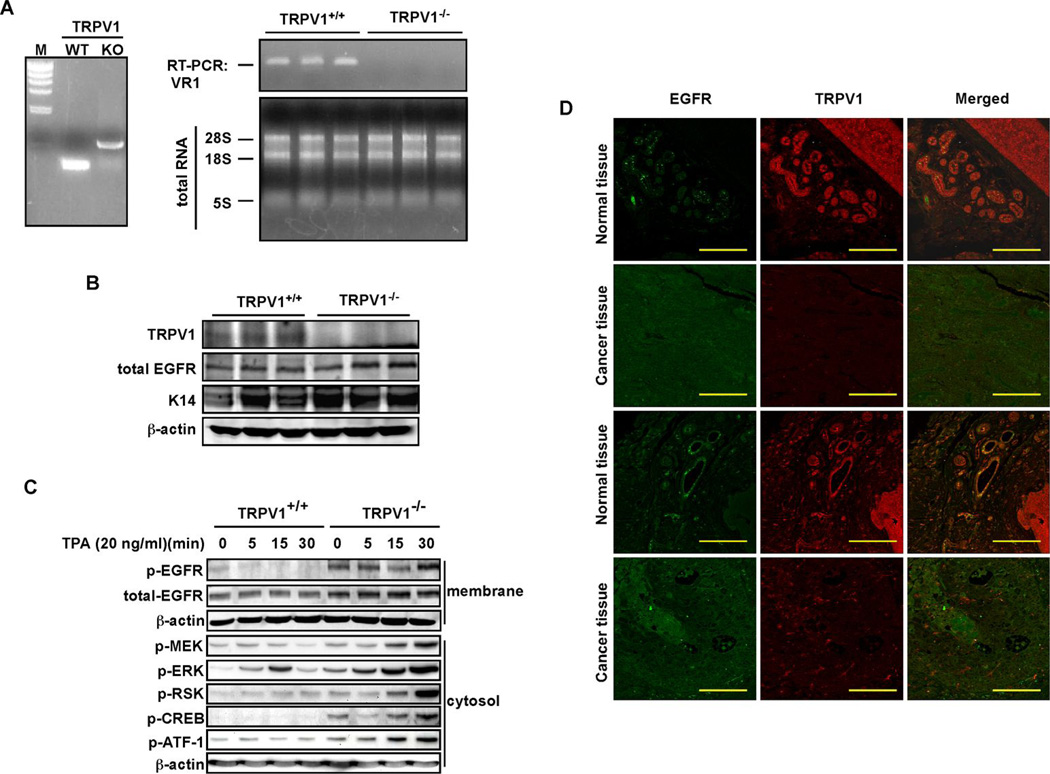
The next step was to confirm the presence of TRPV1 in mouse skin epidermis. The genotype of TRPV1 wildtype (TRPV1+/+) and knockout (TRPV1−/−) mice was first confirmed by PCR in skin (Fig. 5A, left) and RT-PCR was used to confirm the absence of TRPV1 gene expression in TRPV1−/− skin compared to TRPV1+/+ skin (Fig. 5A, right). Dorsal skin was isolated from these mice and examined for the relationship between TRPV1 and EGFR. Results confirmed that TRPV1 was expressed in wildtype skin but not in TRPV1−/− skin (Fig. 5B). Furthermore, in the absence of TRPV1, total EGFR protein was increased (Fig. 5B).
Figure 5.
TRPV1 suppresses EGFR signaling and cell transformation. (A, left) PCR detection of genomic TRPV1 in TRPV1 wildtype (WT) and knockout (KO) mouse skin. (A, right) RT-PCR detection of TRPV1 in wildtype (TRPV1+/+) and KO (TRPV1−/−) mouse skin. Mouse skin (0.1 g) was homogenized and total RNA extracted. The RT was conducted with random hexa-primers, and TRPV1 was amplified by PCR and visualized by ethidium bromide staining (upper panel). Total RNA (2 µg) was resolved by electrophoresis and visualized as an internal control. (B) The TRPV1 protein is expressed in TRPV1+/+ mouse skin, but not in TRPV1−/− skin. Dorsal skin from TRPV1+/+ and TRPV1−/− mice was homogenized and membrane proteins were extracted by ultracentrifugation. Membrane proteins (40 µg) were used for Western blotting with specific antibodies as indicated. The K14 protein was used as a skin sample marker and β-actin was used to verify equal protein loading. (C) TPA markedly induces EGFR signaling in TRPV1−/− MEFs compared to TRPV1+/+ MEFs. The signaling cascades known to be associated with TPA-induced cell transformation were examined in TRPV1+/+ and TRPV1−/− MEFs. The proteins were visualized by Western blotting using specific antibodies and β-actin was used to verify equal protein loading. (D) TRPV1 downregulation and EGFR upregulation is observed in human skin cancer tissues. A human skin tissue array, including matched normal skin and cancer tissues, was purchased, deparaffinazed and stained with anti-EGFR or anti-TRPV1 as primary antibodies and secondary antibodies conjugated with Alexa Fluor 488 or 647, respectively. The tissue array slide was observed by laser scanning confocal microscopy (NIKON C1si Confocal Spectral Imaging System) using a CFI Plan Fluor 20x objective. Scale bar = 100 microns.
To closely study the signaling pathway associated with the inhibitory effect of the TRPV1 on cell transformation, we used TRPV1+/+ and TRPV1−/− MEFs and examined EGFR downstream signaling molecules. Results showed a marked difference in phosphorylation of various kinases and transcription factors involved in EGFR signaling in TRPV1+/+ MEFs compared to TRPV1−/− MEFs (Fig. 5C). In particular, phosphorylation of MEK, ERKs, RSK, CREB, and ATF-1 was not only increased by TPA stimulation, but basal levels were also higher in TRPV1−/− MEFs compared with TRPV1+/+ MEFs (Fig. 5C). The constitutive activation of these downstream EGFR signaling proteins agrees with the observed enhanced level of total and phosphorylated EGFR in the absence of the TRPV1.
CREB–dependent transactivation is dependent on EGFR signalling and plays an important role in skin cancer development. Therefore, we examined the effect of TRPV1 expression on the transactivation activity of CREB 2 or 3 using a 5xGal4-luciferase reporter plasmid (Supp. Fig. 2A, upper panel) and increasing amounts of TRPV1. Results indicated that TRPV1 suppressed CREB2 or CREB3 transactivation (Supp. Fig. 2A, lower panel). To examine the effect of TRPV1 on CREB2- or CREB3-induced cell transformation, we introduced CREB2 or CREB3 with constitutively-active Ras (RasG12V) into NIH3T3 cells and conducted a focus-forming assay (Supp. Fig. 2B). Results indicated that CREB2 or CREB3 enhanced foci formation mediated by Ras and that TRPV1 inhibited CREB-induced foci formation (Supp. Fig. 2B). Taken together, these results clearly demonstrated that TRPV1 is a negative regulator for EGFR-mediated cell transformation in vitro. Furthermore, results from a human skin tissue array (U.S. Biomax, Inc.), including cancer tissues and matched normal tissue, revealed that TRPV1 was down-regulated and EGFR was up-regulated in skin cancer compared to matched normal tissues (Fig. 5D), suggesting that TRPV1 is deregulated in skin cancer when EGFR is highly expressed.
TRPV1 knockout mice are highly susceptible to TPA-induced skin carcinogenesis
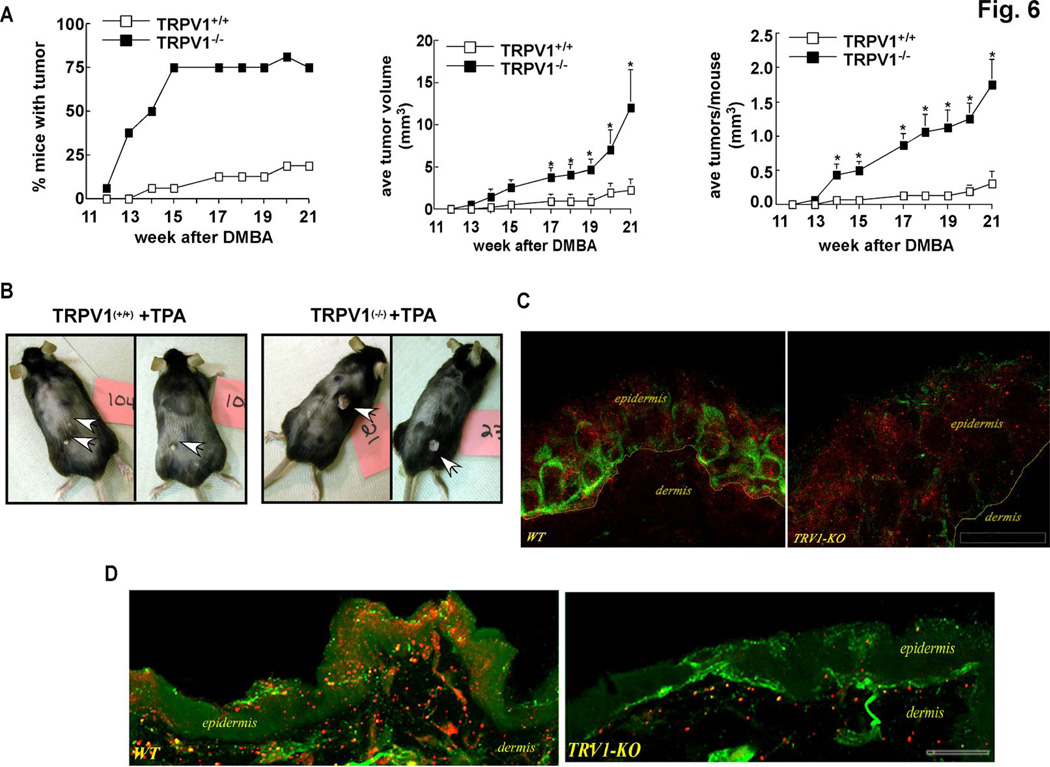
Based on transformation assay results and the finding that TRPV1 expression is associated with decreased EGFR expression, we hypothesized that the TRPV1 might have a suppressive effect on TPA-induced skin cancer formation in mice. To test this idea, TRPV1−/− and age- and gender-matched TRPV1+/+ mice were subjected to a two-stage skin carcinogenesis experiment. TRPV1+/+ and TRPV1−/− mice were initiated with 7,12-dimethyl benz(a)anthracene (DMBA, 200 nmol) and promoted with TPA (17 nmol) over a period of 21 weeks. Results indicated substantial tumor development that was significantly enhanced in mice lacking the TRPV1 (Fig. 6A). Compared to TRPV1+/+ mice, almost all TRPV1−/− mice developed papillomas (Fig. 6A, left) and the tumors were larger (Fig. 6A, middle) and more numerous (Fig. 6A, right). DMBA alone had no effect on tumor development in either TRPV1+/+ or TRPV1−/− mice (data not shown). These results suggested that the TRPV1 might have a protective effect in preventing DMBA/TPA-induced mouse skin carcinogenesis (Fig. 6B). Because EGFR seemed to play a more prevalent role in skin carcinogenesis in the absence of TRPV1, we determined whether inhibition of EGFR would have a differential effect on skin carcinogenesis in TRPV1+/+ and TRPV1−/− mice. These mice were treated as before with DMBA and TPA except that some groups were treated with the EGFR inhibitor, AG1478, 1 h before application of TPA. Results indicated that TRPV1−/− mice again showed increased susceptibility to TPA-induced tumorigenesis compared with TRPV1+/+ mice (data not shown). However, treatment with the EGFR inhibitor before TPA treatment significantly suppressed tumorigenesis in both TRPV1+/+ and TRPV1−/− mice, but the inhibitory effect was substantially greater in TRPV1−/− mice compared to TRPV1+/+ mice (Supp. Fig. 3). Notably, compared to mice treated with only TPA, the average number of TPA-induced papillomas in TRPV1−/− mice pretreated with the EGFR inhibitor was decreased by 50% compared to a 22% reduction in similarly treated TRPV1+/+ mice. Further, the average volume of papillomas was decreased by about 75% in TRPV1−/− mice pretreated with AG1478 compared to an approximate 50% reduction in TRPV1+/+ mice similarly treated (Supp. Fig. 3), supporting the idea that the EGFR might play a more prevalent role in carcinogenesis in the absence of TRPV1. Mice treated with only AG1478 in the absence of TPA did not develop tumors (data not shown).
Figure 6.
TPA-induced skin carcinogenesis is markedly enhanced in TRPV1−/− mice compared to TRPV1−/− mice. (A, left) Percentage of TRPV1+/+ and TRPV1−/− mice developing papillomas over a 21-week period. (A, middle) Average papilloma volume per mouse measured by caliper and calculated as [(short diameter2/long diameter)/2]. (A, right) Average number of papillomas per mouse. Data are represented as the respective means +/− S.E. and the asterisk (*) indicates a significantly (*, p < 0.05) greater average papilloma volume (middle) or average number of papillomas per mouse (right) in TRPV1−/− mice compared to TRPV1+/+. Representative photographs of TPA-treated TRPV1+/+ (B, left) or TPA-treated TRPV1−/− (B, right) mice. (C) TRPV1 (green) immunoreactivity is abundant in epidermal keratinocytes from TRPV1+/+ mouse skin (left) compared to TRPV1−/− (right); whereas EGFR (red) immunoreactivity is more abundant in TRPV1−/− compared to TRPV1+/+. Scale bar = 10 microns. (D) TRPV1 (red) immunoreactivity is abundant in epidermal keratinocytes from TRPV1+/+ mouse skin (left) compared with TRPV1−/− skin (right). Collagen IV (green color) is used as a marker to differentiate between dermis and epidermis.
In addition, immunohistofluorescence analysis of normal skin isolated from TRPV1+/+ or TRPV1−/− mice verified that the TRPV1 protein was highly expressed in skin from TRPV1+/+ mice (Fig. 6C, green, left panel), but not in TRPV1−/− mouse skin (Fig. 6C, green, right panel). Notably, the EGFR protein level in TRPV1−/− mouse skin (Fig. 6C, red, right panel) was markedly increased compared with the amount of EGFR expressed in normal skin from TRPV1+/+ mice (Fig. 6C, red, left panel). Furthermore, TRPV1 immunoreactivity was abundant in epidermal keratinocytes from TRPV1+/+ (Fig. 6D, red, left panel) compared to TRPV1−/− epidermis (Fig. 6D, red, right panel). Collagen (Fig. 6D, green color) was used as a marker to differentiate between dermis and epidermis.
DISCUSSION
The capsaicin-sensitive TRPV1 has been purported to be exclusively expressed on polymodal primary sensory neurons (18), and the effects of capsaicin on non-neuronal cells were generally considered to be nonspecific (18). However, TRPV1 expression has since been detected in keratinocytes (3, 4, 7, 19), vascular endothelial cells (20), hepatocytes (21), T cells (22), and mast cells (23). Even though ion channels, such as TRPV1, were viewed to function as passive responders to extracellular or intracellular biochemical events, evidence suggests that these channels directly influence biochemical events in a manner that is independent of their function as ion channels (24). For example, TRPV6, which is found in pancreas, rat small intestine and colon, and placenta, mediates cell proliferation (25) and heat-shock-induced matrix metalloproteinase-1 (MMP-1) expression is mediated by activation of TRPV1 in human keratinocytes (19). Little doubt remains that TRPV1 is expressed in epidermal skin cells but its function is still elusive. In the present work, we found that TRPV1 is expressed in mouse epidermis and keratinocytes in opposition to the EGFR. We showed that the C-terminal portion of the TRPV1 directly interacted with the EGFR and increasing expression of the TRPV1 was associated with a substantial down-regulation of EGFR. The down-regulation was mediated by the interaction of TRPV1 and the Cbl ubiquitin ligase, which corresponded with increased ubiquitylation and lysosomal degradation of EGFR. Furthermore, we found that the absence of TRPV1 corresponded not only with increased total EGFR protein, but also to an enhanced activation of downstream signaling components of the EGFR pathway, including MEK1/2, ERKs, RSK, and CREB. Importantly, we found that skin papilloma incidence and multiplicity was markedly increased in TRPV1−/− mice compared to TRPV1+/+ mice.
The TRP family of proteins exhibits differential expression in cancer tissues. Rather than mutations, changes in expression of TRP proteins appear to be related to alterations in wildtype protein level, which might be associated with specific stages of cancer. However, whether alterations in TRP protein expression are causative or secondary to other changes is not known. The TRPV6 and TRPM8 are overexpressed in advanced prostate cancer (26, 27), whereas TRPM1 expression was decreased in tumorigenic melanocytes (28). TRPV1 expression was altered in prostate, colon, and pancreatic cancers (29–31). Interestingly, the TRPV1 was shown to exhibit a gradual loss of expression in the urothelium as transitional cell carcinoma stage increased and cell differentiation decreased (30). Specifically, low-grade papillary carcinoma showed strong expression of TRPV1, whereas TRPV1 expression was absent in invasive carcinoma (30). In our study, we found that TRPV1 was substantially depleted in skin cancer tissues compared to normal matched skin tissues and, importantly, loss of TRPV1 corresponded with an increase in mouse skin papilloma formation in vivo.
Constitutive activation of EGFR in cancer occurs through overexpression or mutational activation and its anomalous activation was linked to increased proliferation, angiogenesis, and metastasis of cancer cells (32–35). The regulation of EGFR is very well understood, and in general, ligand binding (e.g., EGF) to EGFR leads to dimerization and autophosphorylation of the EGFR. The ubiquitin ligase, Cbl, binds to the activated EGFR and promotes monoubiquitylation of the activated EGFR at the plasma membrane. Monoubiquitylation of EGFR at multiple sites results in endocytosis and degradation of the receptor (36). We found that the C-terminal of TRPV1 directly interacted with EGFR and Cbl, which was associated with increased ubiquitylation and degradation of the EGFR through the lysosomal pathway.
The EGFR is highly expressed in the skin and is strongly amplified and overexpressed in many human tumors of epithelial origin (37). We found that when EGFR is expressed at high levels, TRPV1 is substantially decreased in epidermal skin cells. Dysregulation of the EGFR is associated with increased neoplastic growth (38) and evidence suggests that decreased expression or knockout of the EGFR causes reduced skin cancer development (39). For example, transgenic mice expressing EGFR and a constitutively active form of the Ras activator, Son of Sevenless (SOS), in the basal cells of the epidermis easily develop skin papillomas. However, if these mice were bred into an EGFR-deficient background, tumor formation was substantially retarded (40). In addition, EGFR-deficient cells were resistant to transformation by SOS-F or RasV12 (40). When injected into immunocompromised mice, EGFR-deficient keratinocytes that express v-rasHa result in smaller papillomas (39). Furthermore, Ha-Ras transgenic animals that express a dominant-negative EGFR in basal keratinocytes developed smaller tumors compared to controls (41). These studies all suggest that EGFR is important in skin cancer development.
At least one other protein, mitogen-inducible gene 6 (Mig6 or RALT), was reported as a specific negative regulator of EGFR signaling in skin development (42, 43). Mig6-deficient mice exhibited hyperactivation of the EGFR that caused increased proliferation and impaired differentiation of epidermal keratinocytes. Notably, these mice spontaneously developed tumors and were highly susceptible to chemically induced skin cancer (42, 43). This suggested that Mig6 acts as a tumor suppressor in opposition to EGFR and its expression is down-regulated in various human cancers (42, 43). These findings strongly support the hypothesis that TRPV1 could interact with EGFR to suppress skin carcinogenesis.
The DMBA/TPA two-stage carcinogenesis model is the most classical and well-accepted model for studying skin carcinogenesis. Topical application of tumor promoters such as TPA in the two-stage skin carcinogenesis model leads to elevated levels of EGFR (44) and TPA was clearly shown to exert its major effects in the skin in an organ specific manner, regardless of administration route (45). Even though mice lacking the TRPV1 seem to have a benign phenotype, evidence showed that mice with homogenous deletion of the TRPV1 exhibit some abnormalities (46–48). Our results using the DMBA/TPA mouse model indicated that TRPV1−/− mice showed an increased susceptibility to TPA-induced skin carcinogenesis compared to TRPV1+/+ mice, and high expression of the TRPV1 suppressed tumor promoter-induced cell transformation. Compared to TRPV+/+ mice, tumor promotion in the TRPV1−/− mice was also more effectively suppressed in the presence of an EGFR inhibitor, which suggests a greater role for the EGFR in skin carcinogenesis in the absence of the TRPV1.
Compounds, like capsaicin, that interact with the TRPV1 eventually result in the desensitization of this channel receptor and the mechanism appears to involve a down-regulation of the TRPV1 in response to prolonged treatment (15). Chronic blockade of the TRPV1 has been suggested to be an effective therapeutic approach to pain relief and a great deal of effort has been expended by pharmaceutical companies in the attempt to develop new, high affinity, orally active TRPV1 antagonists that might be useful for treating chronic pain. The TRPV1 is more ubiquitously expressed than was originally believed and our understanding of the normal physiological or biological functions of TRPV1 and other TRP proteins in non-neuronal cells is incomplete. Thus the effect of chronic blockade on the non-neuronal functions of TRPV1 is very unclear. Our results suggest a protective role for the TRPV1 against some diseases, including cancer, and might indicate that TRPV1 antagonists could cause more diverse and severe side effects than initially believed.
ACKNOWLEDGEMENTS
The authors have no commercial affiliations or conflicts of interest to disclose. The authors thank Dr. David Julius (USCSB, Santa Barbara, CA) for the kind gifts of the B6, 129S4-Trpv1 (TRPV1 KO) mice and cDNA plasmids. These studies were supported by the Hormel Foundation and the National Institutes of Health grants CA120388, CA111536, CA088961, CA081064, CA027502, and CA007646.
REFERENCES
- 1.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 2.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 3.Bodo E, Biro T, Telek A, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodo E, Kovacs I, Telek A, et al. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol. 2004;123:410–413. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Koizumi S, Fuziwara S, Denda S, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002;291:124–129. doi: 10.1006/bbrc.2002.6393. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Lee SA, Yun SJ, et al. Expression of vanilloid receptor 1 in cultured fibroblast. Exp Dermatol. 2006;15:362–367. doi: 10.1111/j.0906-6705.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 7.Stander S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 9.Park KK, Surh YJ. Effects of capsaicin on chemically-induced two-stage mouse skin carcinogenesis. Cancer Lett. 1997;114:183–184. doi: 10.1016/s0304-3835(97)04657-0. [DOI] [PubMed] [Google Scholar]
- 10.Park KK, Chun KS, Yook JI, Surh YJ. Lack of tumor promoting activity of capsaicin, a principal pungent ingredient of red pepper, in mouse skin carcinogenesis. Anticancer Res. 1998;18:4201–4205. [PubMed] [Google Scholar]
- 11.Toth B, Gannett P. Carcinogenicity of lifelong administration of capsaicin of hot pepper in mice. In Vivo. 1992;6:59–63. [PubMed] [Google Scholar]
- 12.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science (New York, NY. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 13.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 14.Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39:1348–1354. doi: 10.1016/s0959-8049(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 15.Eglen RM, Hunter JC, Dray A. Ions in the fire: recent ion-channel research and approaches to pain therapy. Trends Pharmacol Sci. 1999;20:337–342. doi: 10.1016/s0165-6147(99)01372-3. [DOI] [PubMed] [Google Scholar]
- 16.Peschard P, Park M. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell. 2003;3:519–523. doi: 10.1016/s1535-6108(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 17.Levkowitz G, Waterman H, Ettenberg SA, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 18.Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. General pharmacology. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- 19.Li WH, Lee YM, Kim JY, et al. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J Invest Dermatol. 2007;127:2328–2335. doi: 10.1038/sj.jid.5700880. [DOI] [PubMed] [Google Scholar]
- 20.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 21.Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell calcium. 2004;36:19–28. doi: 10.1016/j.ceca.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz EC, Wolfs MJ, Tonner S, et al. TRP channels in lymphocytes. Handb Exp Pharmacol. 2007:445–456. doi: 10.1007/978-3-540-34891-7_26. [DOI] [PubMed] [Google Scholar]
- 23.Biro T, Maurer M, Modarres S, et al. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–1340. [PubMed] [Google Scholar]
- 24.Kaczmarek LK. Non-conducting functions of voltage-gated ion channels. Nature reviews. 2006;7:761–771. doi: 10.1038/nrn1988. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz EC, Wissenbach U, Niemeyer BA, et al. TRPV6 potentiates calcium-dependent cell proliferation. Cell calcium. 2006;39:163–173. doi: 10.1016/j.ceca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Wissenbach U, Niemeyer BA, Fixemer T, et al. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- 27.Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 28.Duncan LM, Deeds J, Cronin FE, et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol. 2001;19:568–576. doi: 10.1200/JCO.2001.19.2.568. [DOI] [PubMed] [Google Scholar]
- 29.Hartel M, di Mola FF, Selvaggi F, et al. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55:519–528. doi: 10.1136/gut.2005.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazzeri M, Vannucchi MG, Spinelli M, et al. Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. Eur Urol. 2005;48:691–698. doi: 10.1016/j.eururo.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez MG, Sanchez AM, Collado B, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 32.D'Agnano I, D'Angelo C, Savarese A, et al. DNA ploidy, proliferative index, and epidermal growth factor receptor: expression and prognosis in patients with gastric cancers. Lab Invest. 1995;72:432–438. [PubMed] [Google Scholar]
- 33.Harper ME, Goddard L, Glynne-Jones E, et al. Multiple responses to EGF receptor activation and their abrogation by a specific EGF receptor tyrosine kinase inhibitor. Prostate. 2002;52:59–68. doi: 10.1002/pros.10069. [DOI] [PubMed] [Google Scholar]
- 34.Jonjic N, Kovac K, Krasevic M, et al. Epidermal growth factor-receptor expression correlates with tumor cell proliferation and prognosis in gastric cancer. Anticancer Res. 1997;17:3883–3888. [PubMed] [Google Scholar]
- 35.Kedar D, Baker CH, Killion JJ, Dinney CP, Fidler IJ. Blockade of the epidermal growth factor receptor signaling inhibits angiogenesis leading to regression of human renal cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 2002;8:3592–3600. [PubMed] [Google Scholar]
- 36.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 37.Derynck R. The physiology of transforming growth factor-alpha. Advances in cancer research. 1992;58:27–52. doi: 10.1016/s0065-230x(08)60289-4. [DOI] [PubMed] [Google Scholar]
- 38.Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 39.Dlugosz AA, Hansen L, Cheng C, et al. Targeted disruption of the epidermal growth factor receptor impairs growth of squamous papillomas expressing the v-ras(Ha) oncogene but does not block in vitro keratinocyte responses to oncogenic ras. Cancer Res. 1997;57:3180–3188. [PubMed] [Google Scholar]
- 40.Sibilia M, Fleischmann A, Behrens A, et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 41.Casanova ML, Larcher F, Casanova B, et al. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 2002;62:3402–3407. [PubMed] [Google Scholar]
- 42.Ballaro C, Ceccarelli S, Tiveron C, et al. Targeted expression of RALT in mouse skin inhibits epidermal growth factor receptor signalling and generates a Waved-like phenotype. EMBO reports. 2005;6:755–761. doi: 10.1038/sj.embor.7400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferby I, Reschke M, Kudlacek O, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006;12:568–573. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 44.Kiguchi K, Beltran L, Rupp T, DiGiovanni J. Altered expression of epidermal growth factor receptor ligands in tumor promoter-treated mouse epidermis and in primary mouse skin tumors induced by an initiation-promotion protocol. Molecular carcinogenesis. 1998;22:73–83. [PubMed] [Google Scholar]
- 45.Zhong SP, Ma WY, Quealy JA, Zhang Y, Dong Z. Organ-specific distribution of AP-1 in AP-1 luciferase transgenic mice during the maturation process. Am J Physiol Regul Integr Comp Physiol. 2001;280:R376–R381. doi: 10.1152/ajpregu.2001.280.2.R376. [DOI] [PubMed] [Google Scholar]
- 46.Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 47.Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]