Abstract
The immense range of human behaviours is rooted in the complex neural networks of the cerebrum. The creation of these networks depends on the precise integration of specific neuronal subtypes that are born in different regions of the telencephalon. Here, using the mouse as a model system, we review how these proliferative zones are established. Moreover, we discuss how these regions can be traced back in development to the function of a few key genes, including those that encode fibroblast growth factors (FGFs), sonic hedgehog (SHH), bone morphogenetic proteins (BMPs), forkhead box G1 (FoxG1), paired box 6 (PAX6) and LIM homeobox protein 2 (LHX2), that pattern the early telencephalon.
Despite the complexity of the adult cerebrum, the telencephalon starts off as a simple neuroepithelium at the anterior end of the neural plate. The elaborate process by which this primordial sheet of cells progresses into a mature brain can be divided into several discrete phases and has been best studied in the mouse. The initial phases involve assigning anterior–posterior identity in the neuraxis, on the basis of antagonistic signalling between the organizer and the anterior visceral endoderm1–3. Once the telencephalic anlagen is established, it is subdivided into different territories through the actions of morphogens that induce specific transcription factors. Following these early patterning events, the embryonic telencephalon gives rise to a dorsally positioned cortical ventricular zone and a series of ventral eminences that are positioned along the rostral–caudal axis. Each of these progenitor domains produces specific types of neurons that ultimately coalesce in the mature telencephalon and form connections that are then refined and modulated with experience. The neocortex is the major component of the dorsal telencephalon. It is largely comprised of excitatory glutamatergic principal cells (pyramidal neurons) and GABA (γ-aminobutyric acid)-ergic inhibitory interneurons. Pyramidal neurons are generated in the cortical ventricular proliferative zone. By contrast, interneurons originate in distinct ventral eminences and subsequently migrate dorsally into the developing cortex.
Here we focus exclusively on the steps in telencephalic development during which the telencephalic neuroepithelium is patterned into molecularly distinct proliferative zones that generate the neuronal diversity of the adult cerebrum. Specifically, we restrict our attention to the events that take place between embryonic day (E) 8.5 and E11 in the mouse, after the telencephalic primordium has been specified. We outline the events in this period that are critical for later telencephalic patterning and neurogenesis. Numerous reviews have focused on the roles of specific signalling pathways and associated transcription factors, such as sonic hedgehog (SHH), fibroblast growth factors (FGFs) and members of the SIX3 and NKX families, in neural development4–7. Moreover, anterior–posterior specification of the neuraxis, including the forebrain, has recently been considered3,8. However, to date there have been few attempts to provide a description of the critical genetic interactions that link the extrinsic and intrinsic events by which the cerebrum is patterned. Here we examine recent insights into the genetic processes by which this is achieved.
Although some understanding of these events has been obtained from the study of lower vertebrates or invertebrates, we focus on findings in the mouse because the events that are unique to higher mammals are likely to be the most relevant for human telencephalic development. We start by discussing how the orchestrated interplay between extrinsic and intrinsic signals, including interactions between SHH and GLI3, GLI3 and forkhead box G1 (FOXG1), FOXG1 and FGFs, and FGFs and SHH (even in biology, what goes around comes around), results in the emergence of defined dorsal and ventral telencephalic territories. We then go on to examine how the major divisions are further refined, with a focus on the pallial–subpallial boundary and the dorsal subdivisions. We suggest that taken together these observations provide the basis for a coherent model of telencephalic patterning. Our aim is to provide clarity in what is a complex field: readers should be aware that in many cases alternative interpretations certainly exist.
Early divisions in the telencephalon
Early in its development, the anterior neural plate gives rise to the prosencephalon, which is subsequently subdivided into the telencephalon and the diencephalon. The mechanisms that underlie these early processes have been reviewed elsewhere2,3,8, so in this review we confine ourselves to stages after the telencephalic primordium has been specified. This occurs at approximately E8.5 in mice, when expression of the forkhead transcription factor gene Foxg1 is initiated in this region9,10. At this stage the telencephalon is still a neuroepithelium of single-cell thickness. Immediately following Foxg1 expression the telencephalon becomes subdivided into several distinct territories, which are first indicated by the expression of specific molecular markers and which shortly afterwards can be distinguished on the basis of regional differences in their levels of proliferation.
Genetic studies using model organisms such as the mouse and the zebrafish have identified the key transcription factors that regulate embryonic telencephalic patterning. These transcription factors define four broad areas that generate different cell types and develop into functionally distinct adult structures. The embryonic dorsal telencephalon generates primarily glutamatergic neurons and can be divided into two main regions: an anterior and lateral region that gives rise to the neocortex, and a posterior and medial area that develops into the hippocampus, the cortical hem and the choroid plexus. The ventral telencephalon can also be divided into two main regions: a medial domain that is known as the medial ganglionic eminence (MGE), and two posterior and lateral regions that are designated the lateral ganglionic eminence (LGE) and the caudal ganglionic eminence (CGE). Each of these regions contributes neurons to discrete populations in the basal ganglia and to associated limbic structures, including the amygdala and the nucleus accumbens. In addition, the MGE produces somatostatin and parvalbumin subclasses of GABAergic interneurons (as well as a population of neuropeptide Y (NPY)-expressing interneurons) that ultimately reside in the basal ganglia and the cortex, whereas the CGE primarily produces calretinin- and/or vasoactive intestinal peptide (VIP)-expressing interneurons. By contrast, the LGE produces a substantial population of olfactory bulb interneurons, as well as inhibitory projection neurons that populate several ventral regions including the striatum and limbic areas11–13.
The nascent anterior neural plate expresses several transcription factor genes in a region that includes the cells that are destined to form the telencephalon14. Among these genes are Gli3, Foxg1 and paired box 6 (Pax6). As discussed below, each of these genes has a role in dividing the telencephalon into its dorsal and ventral sections.
SHH and GLI3 specify dorsal and ventral domains
The initial subdivision that defines what will later become the dorsal and the ventral telencephalon is regulated at least in part by the dorsalizing effects of Gli3 expression and the ventralizing influence of Shh expression. Gli3 encodes a zinc-finger transcription factor that was first identified as the gene that is disrupted in the classical mouse mutation ‘extra toes’ (Xt)15. Gli3 is initially expressed broadly throughout the telencephalic anlagen and then is progressively downregulated in the ventral portion16,17 (FIG. 1). In the absence of Gli3, the development of the dorsal telencephalon is disrupted: the choroid plexus, the cortical hem and the hippocampus fail to form and the development of the neocortex is progressively compromised18–21.
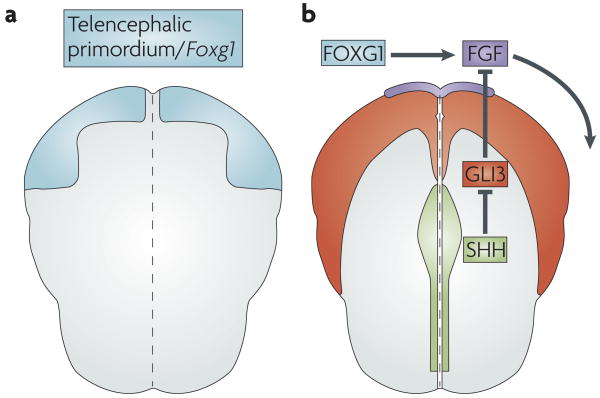
Figure 1. Key extrinsic and intrinsic cellular factors establish the dorsal and ventral subdivisions of the telencephalon.
Schematics of the anterior neural plate in a mouse embryo (dorsal view, five somites stage, anterior is up). a | The region that will become the telencephalon is defined by expression of forkhead box G1 (Foxg1) (shown in blue). b | FoxG1 and sonic hedgehog (SHH; green) promote fibroblast growth factor (FGF; purple) expression in the anterior neural ridge. This patterns the nascent telencephalon (indicated by the curved arrow). SHH promotes FGF expression indirectly by inhibiting the repressor activity of GLI3 (expression of GL13 is shown in red). Consequently, SHH promotes the formation of a ventral telencephalic subdivision by inhibiting the dorsalizing effects of GLI3.
The Shh gene encodes a secreted signalling protein that is related to the Drosophila protein hedgehog. Shh is expressed in the midline of the nascent neural plate and continues to be expressed along the ventral midline of the CNS throughout development22 (FIG. 1). In Shh−/− mouse embryos, the telencephalon is reduced in size and ventral cell types are lost17,23–25 (BOX 1). In double-mutant mice that lack both Shh and Gli3, however, ventral patterning is largely rescued16,26,27. Therefore, SHH restricts the dorsalizing function of GLI3 and controls the positioning of the dorsoventral boundary. Hence, SHH promotes ventral identity by preventing dorsalization of the telencephalon, rather than by directly promoting ventral cell character. The rescue of ventral development in the Shh mutant through compound removal of Gli3 indicates that other genes, acting independently or downstream of Shh, function positively to generate ventral cell types26.
Box 1 | Sonic hedgehog’s role as a morphogen
Morphogens are molecules that bestow cell identity in a concentration-dependent manner81. As such, they are known to be essential for CNS patterning. Of the extrinsic signals that have been implicated as morphogens, the regulation of the activity of sonic hedgehog (Shh) is the best understood. Shh signalling is modulated by numerous factors, including GLI effectors, which have been shown to be involved in establishing ventral identity throughout the developing CNS16,26,82. GLI effectors come in two forms: constitutively cleaved forms that act as transcriptional repressors in the absence of Shh signalling, and uncleaved forms that function as transcriptional activators in the presence of Shh ligands. however, the precise utilization of different components of the Shh signalling pathway is level-specific. For instance, whereas GLI activators are critical to ventral patterning in the spinal cord, GLI repressors are primarily required in the telencephalon26,82. In the forebrain, Shh signalling also requires other factors for ventral development, such as smoothened (SMO; the obligate downstream effector of Shh), low-density lipoprotein receptor-related protein 2 (LRP2, also known as megalin) and the multifunctional transmembrane protein CDO83–86.
Gli3 and Foxg1 maintain the telencephalon
FOXG1, through its ability to induce expression of the extracellular signal FGF8 (see below), is probably the main source of positive regulation of ventral telencephalic identity. Foxg1 is expressed in the early anterior plate cells that are destined to form the telencephalon10,28,29 (FIG. 1). Its expression is independent of that of Shh: although Foxg1 expression is impaired in Shh−/− mice, experiments using Shh−/−;Gli3−/− double-mutant mice revealed that this impairment occurs as a result of increased GLI3 repressor function27. Disruption of Foxg1 expression results in a loss of ventral cell types30–32. However, unlike in the Shh−/− phenotype, ventral cells are not rescued in the Foxg1−/− mutants by removal of Gli3 (REF. 33). In fact, the telencephalon is completely lost in the Foxg1−/−;Gli3−/− double mutant, indicating that Gli3 and Foxg1 are essential for generating and maintaining the dorsal and ventral subdivisions of the telencephalon, respectively (FIG. 2).
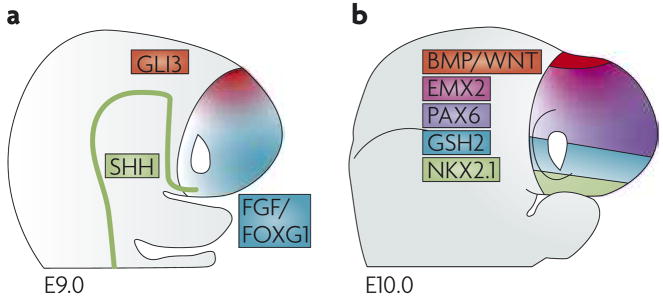
Figure 2. The dorsal and ventral telencephalic regions are subdivided into four major domains.
The dorsal and ventral subdivisions of the embryonic mouse telencephalon at embryonic day (E) 9.0 (a), and the four broad subdivisions at E10.0 (b). In both schematics, dorsal is up, ventral is down. The Gli3-expressing dorsal region at E9.0 is split, by E10.0, into a bone morphogenetic protein (BMP)- and Wingless/Int protein (WNT)-expressing medial region and a more lateral cortical region that expresses countergradients of empty spiracles homeobox 2 (Emx2) and paired box 6 (Pax6). The ventral region at E9.0 is split, by E10.0, into medial Nkx2.1-expressing domains and lateral Gsh2-expressing domains. At E10.0 the expression domain of Gsh2 overlaps with that of Nkx2.1; this is not represented for the sake of illustrative simplicity. Similarly, sonic hedgehog (Shh), fibroblast growth factor (FGF) and forkhead box G1 (Foxg1) expression at E10.0 is omitted.
FOXG1 and FGFs act cooperatively
In generating the ventral telencephalon, FOXG1 acts in concert with FGF signalling. FOXG1 is required for Fgf8 expression32 and, conversely, Foxg1 expression might itself be regulated by FGF signalling. FGF8-soaked beads can ectopically induce expression of Foxg1 (REF. 10), and Foxg1 expression is reduced in Fgf8−/− mutants34, leading to the hypothesis that FGFs and FOXG1 form a positive-feedback loop. Whether FGF signalling is essential for Foxg1 expression remains unclear owing to potential functional compensation by other members of the FGF family in the FGF signalling mutants that have been examined to date.
FGF signalling, like SHH signalling, is essential for the generation of ventral cell types in the telencephalon34–38. Moreover, there are data that indicate that FGFs act in a dose-dependent manner to pattern the ventral telencephalon. Disruption of the gene that encodes FGF receptor 1 (Fgfr1) leads to a loss of expression of LIM homeobox protein 6 (LHX6) and LHX7, two LIM-domain transcription factors that are expressed in the MGE and are necessary for the differentiation of MGE-derived interneurons38–40. Disruption of both Fgfr1 and Fgfr2 results in an even more severe loss of ventral precursor cells38. In these mutants, both the homeobox gene Nkx2.1, which is normally expressed in the MGE and is necessary for its development41, and Gsh2, which is necessary for LGE development42,43, are no longer expressed. Similarly, the phenotypes of severely hypomorphic and null alleles of Fgf8 reveal a progressive loss of ventral markers (including Nkx2.1 expression) and associated structures with diminishing levels of Fgf8 expression34.
FGFs downstream of Shh specify ventral identity
Several lines of evidence indicate that FGFs operate downstream of Shh to generate ventral cell types. SHH maintains the expression of Fgf3, Fgf8, Fgf15, Fgf17 and Fgf18 in the anterior medial telencephalon, and FGF receptors are required for the ventralizing effect of SHH16,25,27,38. Moreover, FGF8-soaked beads can induce ventral gene expression in cultured dorsal telencephalic explants when SHH signalling is inhibited21.
Why are ventral cells still generated in the Shh−/−;Gli3−/− double mutant if SHH is required to maintain the expression of FGF genes? The answer again is that expression of these FGF genes is only indirectly promoted by SHH, through its ability to negatively regulate GLI3’s repressor function (FIG. 1). In the Shh−/− mutant there is no FGF expression, and ventral cell types are lost because of the unchecked repressive action of GLI3. In the Shh−/−;Gli3−/− double mutant, FGF expression is no longer attenuated by GLI3, and so ventral development is restored. FGF expression is similarly expanded in both the Gli3−/− and the Shh−/−;Gli3−/− mutants16,19,21,27, confirming that SHH promotes FGF expression indirectly by attenuating GLI3’s repressor function. loss of Gli3 does not rescue the dorsalized phenotype that results from a loss of FGF receptors, consistent with the notion that GLI3 acts upstream of FGF signalling38 (FIG. 1).
Because SHH specifies fates in the ventral CNS in a dose-dependent manner, it meets the strict definition of a morphogen (BOX 1). However, in the telencephalon at least, it does so indirectly through its regulation of FGFs. Similarly, it seems that much of SHH’s mitogenic effects in the telencephalon are also FGF-mediated. This indicates that FGFs are the obligate effectors of SHH signalling and, by implication, that FGFs are both mitogens and morphogens in their own right.
FGF signalling acts as a telencephalic organizer
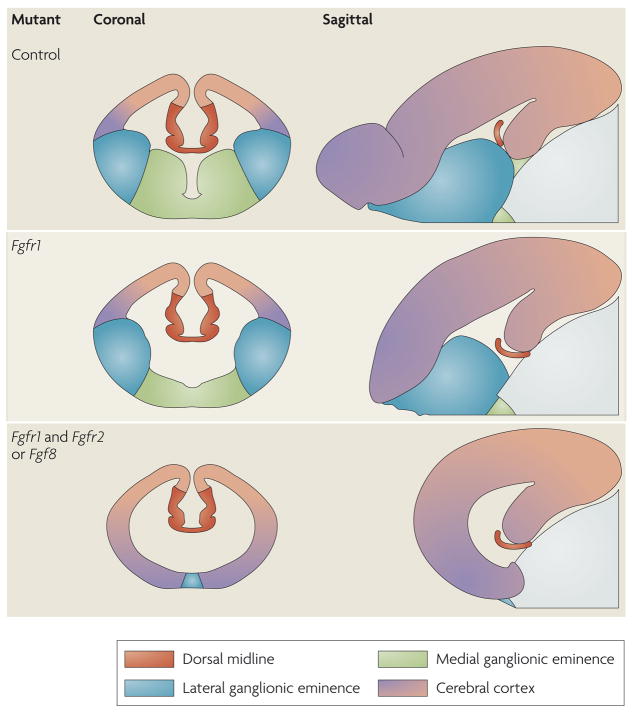
The dose-dependent patterning of the telencephalon by FGF signalling extends beyond the ventral regions into the dorsal regions. In embryos in which FGF signalling is only partially reduced by ectopic expression of a dominant-negative receptor, or in moderately hypomorphic Fgf8 mutants, the transcription-factor gradients in the cortical neuroepithelium shift anteriorly44,45. In these mutants, the areal territories that are normally positioned in the more posterior regions of the cortex, such as the visual and sensory areas, are expanded at the expense of anterior areal regions, such as the prelimbic cortex. Moreover, as the levels of FGF signalling are progressively reduced, loss of anterior cortical regions is accompanied by a diminution of the ventral telencephalon (FIG. 3). Although only the ventromedial cell types (those that arise from the MGE) are lost in Fgfr1−/− mutants, in Fgfr1−/−;Fgfr2−/− double mutants all ventral cells are lost38,46. Similarly, in severely hypomorphic and null Fgf8 mutants, not only are ventral precursors lost but the neocortex is also progressively diminished in size, with a preferential loss of anterior–lateral markers34. Moreover, preliminary results indicate that when the expression of all three of the FGF receptor genes that are normally expressed in telencephalic precursors (Fgfr1, Fgfr2 and Fgfr3) is lost, both the ventral telencephalon and the cerebral cortex are lost (J.H., unpublished observations). Taken together, these findings suggest that, as in other tissues (BOX 2), FGF signalling acts as an organizer for the telencephalon.
Figure 3. FGF signalling as an organizer for the telencephalon.
Schematics of coronal and sagittal sections in the telencephalon between embryonic day (E) 12.5 and E15.5 in various mutant mice. The colours represent areas that contain cells with different telencephalic identities. Progressively deleting more fibroblast growth factor receptor (FGFR) genes specifically in the anterior neural plate leads to diminished FGF signalling and gradually more severe truncations of telencephalic regions. Anterior ventral regions are lost first, followed by posterior dorsal regions34,38,46.
Box 2 | FGFs mediate organizer activity in multiple developmental contexts
The experiments conducted by Spemann and Mangold in 1924 defined an organizer as a group of cells that can induce neighbouring cells to change their fate and reorganize into a new, normally patterned tissue87. Fibroblast growth factors (FGFs), and in particular FGF8, are expressed in several organizers in the developing embryo, including the apical ectodermal ridge (at the tips of the limb buds) and the isthmus at the midbrain–hindbrain boundary88. In these locations FGF8 itself is likely to mediate organizer activity, as it is both necessary and sufficient (when it is ectopically expressed) to induce the formation of a normally structured limb and midbrain89–95. FGFs are also expressed in the anterior neural ridge (FIG. 1), a group of cells that forms the border between the most anterior part of the ectoderm and the neuroectoderm and that patterns the telencephalon10,88,96. The loss of parts of the telencephalon that is observed in mutants that lack combinations of the three FGF receptors in the anterior neural plate suggests that FGFs that emanate from the anterior neural ridge might mediate organizer activity for the telencephalon38. These results, together with some preliminary findings in mutants lacking Fgfr1, Fgfr2 and Fgfr3 (J.h., unpublished observations), indicate that, unlike in the midbrain–hindbrain boundary and the limb, loss of FGF8 can be compensated for by other FGF ligands in organizing the telencephalon34,38. FGFs are also likely to be sufficient to induce the telencephalon in the context of the early anterior neural plate, as FGF8 can induce forkhead box G1 (Foxg1) expression in the early anterior neural plate10 and can cause mirror-image duplications (a hallmark of organizer activity) of somatosensory whisker barrels later in the development of the dorsal telencephalon44. hence, the combined effect of several FGFs that emanate from the anterior ridge seems to mediate organizer activity to generate and pattern all but the posterior medial telencephalon (FIG. 3).
Pax6 establishes cortical regions
Although FOXG1 is absolutely required for the establishment of ventral identity in the telencephalon, it might also collaborate with FGFs in partitioning the dorsal telencephalon into the anterior–lateral neocortex and the posterior–medial territories. In Foxg1−/− embryos, although the ventral telencephalon is most severely affected, the cortex is also reduced in size30. This is due at least in part to the premature differentiation of precursor cells into layer-1 Cajal–retzius neurons47. However, it is also partially due to the loss of anterior lateral precursor cells, as the remaining cortical field seems to be caudalized in Foxg1−/− mutants33,48. Hence, both FOXG1 and FGFs are required for normal cortical development, despite their ventralizing functions.
What enables FOXG1 and FGFs to specify ventral telencephalon in one context and anterior–lateral cortex in another? The answer, at least in part, involves PAX6, a paired-box transcription factor that is essential for setting up the sharp border that distinguishes the ventral telencephalon from the dorsal telencephalon. The embryonic patterning role of PAX6 was initially identified following genetic mapping of the classical ‘small eye’ (sey) mouse mutant49. At the neural plate stage Pax6 is expressed throughout the telencephalic anlagen14. At the neural tube stage its expression is downregulated in the region that will become the ventral telencephalon, concomitant with the upregulation of Nkx2.1 in this region17. As a result, the boundary between Pax6 and Nkx2.1 expression initially demarcates the dorsoventral border. Slightly later in development (at E9.5 in mice), the Pax6- and Nkx2.1-expressing regions become separated by a domain of Gsh2 expression17 (FIG. 2). As a result, the pallial–subpallial boundary becomes defined by the intersection of Pax6 and Gsh2 expression (the expression domains overlap slightly).
Structurally, the pallial–subpallial boundary occurs at the interface between the ventral cortical primordium and the most dorsal portion of the subpallium, designated the dorsal LGE. This is a remarkable boundary: at this border, the ordered laminar arrangement of the neurons in the cortex shifts to the apparently chaotic nuclear structure of the subpallium. Pax6 and Gsh2 help to establish this boundary and show a mirrored pattern of expression, with Pax6 being expressed in a dorsal (low) to ventral (high) gradient and Gsh2 being expressed in a ventral (low) to dorsal (high) gradient. loss-of-function analysis of these two genes demonstrates that their mutual antagonism is required for the positioning of the boundary. In Pax6−/− embryos the most ventral area of the cortical primordium becomes the dorsal LGE, whereas in Gsh2−/− embryos the reverse occurs and the dorsal LGE is transformed into the ventral cortex50–53.
In a phenotype that is reminiscent of the broader rescue of dorsoventral patterning that is seen in Gli3−/−;Shh−/− double mutants, patterning of the pallial–subpallial boundary is partially restored in Pax6−/−;Gsh2−/− double mutant embryos. This suggests that these genes form only part of a broader genetic network that maintains the positioning of this boundary52. Consistent with this notion, loss of Pax6 function, like loss of Gli3, partially compensates for the loss of Shh, suggesting that GLI3 and PAX6 interact to promote dorsal fates54. In fact, although GLI3 is not necessary for initial Pax6 expression, it is partially required to maintain Pax6 expression16,19,21. Determining whether loss of Pax6 can suppress the dorsalization of the telencephalon that is observed in FGF-gene mutants and Foxg1 mutants should help us to elucidate Pax6’s position in the dorsal–ventral regulatory cascade. Moreover, even though SHH and FGFs seem to be the key ventralizing signals, it is evident that other factors, such as TLX and retinoic acid, probably participate in setting up the boundary between the cortex and the ventral telencephalon55,56.
Some of the cooperative dorsalizing actions of PAX6 and GLI3 seem to be mediated through empty spiracles homeobox 2 (EMX2), a transcription factor that is related to empty spiracles protein. With regards to cortical patterning, EMX2 seems to lie genetically downstream of GLI3, as indicated by the loss of Emx2 expression in Gli3 mutants57. Furthermore, Pax6−/−; Gli3−/− double mutants resemble Pax6−/−; Emx2−/− double mutants, in that both compound mutants exhibit an expansion of the cortical hem and the subpallium at the expense of the isocortex54,58. One copy of any of these genes in either compound mutant is sufficient to maintain cortical identity.
In addition to acting cooperatively to specify telencephalic precursor cells as cortical, EMX2 and PAX6 act individually and antagonistically to further pattern the cortical neuroepithelium59–63. These proteins, among numerous other factors including the nuclear receptors coup-TF1 and TLX, the zinc-finger transcription factor SP8 and the basic helix–loop–helix proteins neurogenin 1 and 2, are also likely to be important in patterning cortical precursors35,64–70. The fact that certain factors are required for both cortical specification and progenitor proliferation underscores the need for developmental coordination of areal and laminar patterning in the assembly of cortical architecture.
Partitioning the dorsal telencephalon
The dorsal telencephalon is split into two broad domains: the cerebral cortex, which gives rise mainly to the neocortex and the hippocampus; and the dorsal midline, which gives rise to the choroid plexus and the cortical hem. LHX2, a LIM homeodomain transcription factor that is expressed in the cortical primordium, is crucial in specifying cells as cortical rather than dorsal midline. In Lhx2−/− embryos, the entire area that normally becomes the cortex is lost at the expense of an expanded choroid plexus and cortical hem71. In fact, experiments that used chimaeras and mosaics demonstrated that Lhx2 acts as a classic selector gene to specify a cortical fate and inhibit a hem or anti-hem fate72 (BOX 3). FOXG1 also has a role in confining the development of the dorsal midline, as in Foxg1−/− mutants dorso-medial markers (such as bone morphogenetic protein 4 (Bmp4) expression) are expanded well beyond the length of their normal domain31,32.
Box 3 | Signals from the midline subdivide the dorsal telencephalon
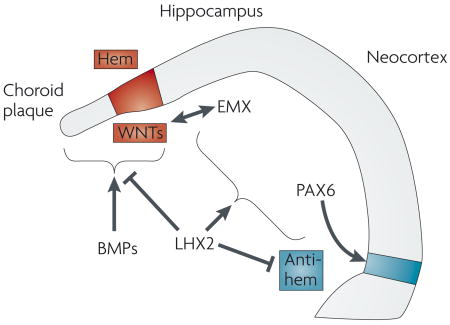
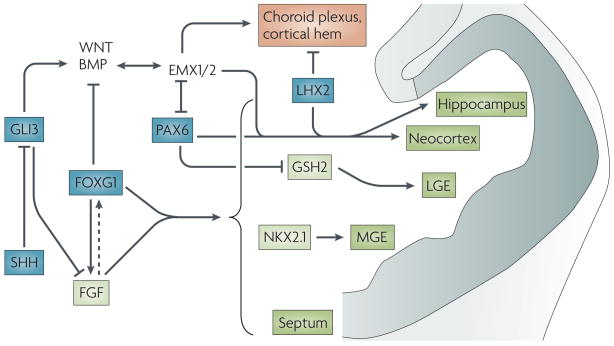
Bone morphogenetic proteins (BMPs), which are expressed in midline cells, are required for the formation of the choroid plaque (the precursor to the choroid plexus) and the cortical hem74,75. In turn, the cortical hem acts as an organizer for the hippocampus72. Wingless/INT proteins (WNTs) are likely to be important components of this organizer activity, as without secretion of WNT3a from the hem the hippocampus fails to develop77. Intracellular effectors of BMP and WNT signalling can bind to enhancer elements found upstream of empty spiracles homologue 2 (Emx2) and regulate its expression. WNT3a might therefore act together with BMPs to promote hippocampal formation by directly regulating the expression of EMX genes57,76. The neocortex, however, requires both EMX-gene expression and Pax6 expression to form58. In addition, LIM homeobox protein 2 (Lhx2) acts as a selector gene that cell-autonomously specifies cortical fates, both hippocampal and neocortical72. LHX2 also suppresses the infringement of flanking cell fates, by repressing expansion of the cortical hem and antihem, a band of cells at the lateral extreme of the pallium97. Note that Pax6, which is only partially regulated by LHX2, is required for formation of the antihem. At present the signals that regulate the expression of Lhx2 and Pax6 remain unknown. One candidate for a source of such signals is the antihem, which might be involved in patterning the dorsal telencephalon. These signalling pathways are illustrated in the figure.
What promotes the development of the dorsal midline itself? The dorsal midline is marked by the expression of two families of secreted signalling molecules: BMPs and Wingless/Int proteins (WNTs). BMP signalling can induce dorsal midline features both in vitro and in vivo. BMP-soaked beads placed on explants of cortical neuroepithelium induce midline features, such as apoptosis and Msx1 expression, and repress lateral features, such as proliferation and Foxg1 expression73. In addition, transgenic expression of an activated BMP receptor in the cortical neuroepithelium leads to an expansion of mid-line cell types at the expense of cortical ones74. Moreover, BMP signalling is required for the formation of the dorsal midline, as in mice that lack both BMP receptor 1a and BMP receptor 1b (Bmpr1a−/−;Bmpr1b−/− double mutants), the choroid plexus and cortical hem fail to form75.
In Gli3−/− embryos the dorsal midline also fails to form, presumably because the expression of BMP genes is lost18–21. Immediately adjacent to the dorsal midline is the hippocampal primordium, the development of which requires EMX1 and EMX2 (REF. 76). Emx1 and Emx2 expression throughout the cortical primordium depends on GLI319,20. This is likely to be an indirect dependency that requires BMPs and WNTs that emanate from the cortical hem, which acts as a hippocampal organizer57,72,77,78. Conversely, EMX2 might feedback and promote WNT-gene expression79,80. Hence, as with the other broad domains of the developing cerebral hemispheres, key players have been identified that regulate the development of dorso-medial structures.
Conclusion
Much progress has been made in the past decade in understanding the genetic pathways that are involved in patterning the early telencephalon. A small number of intrinsic and extrinsic cellular factors have been identified that interact to set up discrete telencephalic domains (FIG. 4). To fully understand the mechanisms behind telencephalic development, it will be necessary to further elucidate how the known patterning factors regulate each other’s function, what additional factors are involved, how multiple signals are integrated over time in precursor cells, and how this allows them to progressively restrict their identity. It is likely that additional roles for factors such as WNTs and BMPs will be revealed. Collectively, these developments will lead to an understanding of how the various factors impart individual neurons with appropriate regional and cell-type identities in closely adjacent telencephalic territories. Appreciation of the mechanisms that underlie the assembly of the telencephalon will also allow us to gain further insights into the molecular underpinnings of developmental brain disorders. A deeper understanding of how the fate of neural precursor cells is regulated in vivo will undoubtedly provide new diagnostic and prognostic tools and therapeutic targets for treating these conditions.
Figure 4. Key genetic pathways that interact to form and pattern the early telencephalon.
Factors that act early to establish broad telencephalic regions are shown in blue. Sonic hedgehog (SHH) ventralizes the telencephalon by antagonizing the dorsalizing effect of GLI3. By repressing Gli3, SHH, together with forkhead box G1 (FoxG1), activates fibroblast growth factor (FGF) expression. FGF might feedback and promote Foxg1 expression (dotted arrow). FoxG1 and FGF signalling are necessary for forming all regions of the telencephalon (shown in green), except for the dorsomedial region (shown in orange). Downstream transcription factors, such as GSH2 and NKX2.1, then form specific subdivisions. In the dorsal telencephalon, GLI3’s promotion of the expression of bone morphogenetic proteins (BMPs) and Wingless/Int proteins (WNTs) is required for EMX-gene expression. The products of the EMx genes, along with PAX6 and LHX2, further subdivide the dorsal telencephalon. LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence.
Acknowledgments
We would like to apologize to the authors of the many studies that have not been mentioned owing to lack of space. We would like to thank the many members of the Hébert and Fishell laboratories who read and offered suggestions on this Review, especially Drs Rossignol, Machold, Sousa, Demarco and Karayannis. In addition we would like to thank J. Dasen and B. Rudy for their comments on earlier versions of this manuscript. This research was supported by US National Institutes of Health grants to J.H. and G.F., as well as by funding from the Simons Foundation to G.F. and from the McDonnell and Sinsheimer Foundations to J.H.
- Neuroepithelium
The embryonic ectoderm that develops into the nervous system
- Neural plate
The neural epithelial cells that form in the early embryo after neuronal induction and give rise to the nervous system
- Anlagen
An embryonic precursor of a more mature tissue
- Morphogen
A secreted factor that can induce two or more different cell fates in a concentration-dependent manner by forming a gradient
- Pallium
The roof of the telencephalon (but not synonymous with the cortex). It contains both cortical structures (for example, the hippocampus and the neocortex) and deep-lying nuclear structures (for example, the claustrum and parts of the amygdala)
- Cortical hem
A transient structure located in the dorso-medial area of the embryonic telencephalon, between the hippocampal anlagen and the choroid plexus. The cortical hem acts as a hippocampal organizer
- Mitogen
An agent that induces mitosis, usually resulting in cell division
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
calretinin | EMX2 | FGF8 | Fgfr1 | FoxG1 | GLI3 | Gsh2 | LHX6 | LHX7 | Nkx2.1 | NPY | parvalbumin | Pax6 | SHH | somatostatin | VIP
FURTHER INFORMATION
Gord Fishell’s homepage: http://saturn.med.nyu.edu/research/dg/fishelllab/
Jean Hébert’s homepage: http://neuroscience.aecom.yu.edu/faculty/primary_faculty_pages/hebert.html
All LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 3.Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nature Rev Neurosci. 2002;3:5031–5040. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- 4.Ingham P, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi D, et al. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- 6.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nature Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 7.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nature Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. This paper provided the first description of Foxg1−/− mice. It supplied strong evidence that Foxg1 is centrally involved in the specification of the telencephalon. [DOI] [PubMed] [Google Scholar]
- 10.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. This beautiful paper provided the first evidence that FGF8 can induce FOXG1 signalling and hence the specification of the telencephalon. [DOI] [PubMed] [Google Scholar]
- 11.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 12.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nature Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 13.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nature Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 14.Inoue T, Nakamura S, Osumi N. Fate mapping of the mouse prosencephalic neural plate. Dev Biol. 2000;219:373–383. doi: 10.1006/dbio.2000.9616. [DOI] [PubMed] [Google Scholar]
- 15.Hui CC, Joyner AL. A mouse model of Greig cephalo–polysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nature Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- 16.Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. The paper, which was published concurrently with reference 26, demonstrated that SHH signalling in the telencephalon represses the expansion of dorsal signals rather than directly specifies ventral identity (a role that seems to be primarily subserved by FGF signals). [DOI] [PubMed] [Google Scholar]
- 17.Corbin JG, Rutlin M, Gaiano N, Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development. 2003;130:4895–4906. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- 18.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. This was the first of a series of papers by this group which were seminal in elucidating the patterning of the cortical hem. Indeed, this paper was the first to introduce the concept of the cortical hem as a hippocampal organizing centre. [DOI] [PubMed] [Google Scholar]
- 19.Theil T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- 20.Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes. Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- 21.Kuschel S, Ruther U, Theil T. A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol. 2003;260:484–495. doi: 10.1016/s0012-1606(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 22.Echelard Y, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 23.Ericson J, et al. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. This paper provided the first indication that SHH signalling is directly required in the patterning of the ventral forebrain, in a manner that is analogous to its action in the spinal cord. [DOI] [PubMed] [Google Scholar]
- 24.Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. This paper provided the first description of the Shh−/− mutant and demonstrated that SHH is required for the establishment of ventral patterning throughout the nervous system, as previously predicted by the McMahon–Tabin and Jessell groups. [DOI] [PubMed] [Google Scholar]
- 25.Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 26.Rallu M, et al. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. This paper showed that ventral patterning in the telencephalon can be established independently of SHH signalling. It demonstrated that the dorsalization that is seen in Shh mutants is largely a result of increased GLI3 activator function. See also reference 16. [DOI] [PubMed] [Google Scholar]
- 27.Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for Sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 29.Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 30.Xuan S, et al. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 31.Dou CL, Li S, Lai E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb Cortex. 1999;9:543–550. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- 32.Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Hanashima C, Fernandes M, Hébert JM, Fishell G. The role of Foxg1 and dorsal midline signaling in the generation of Cajal-Retzius subtypes. J Neurosci. 2007;27:11103–11111. doi: 10.1523/JNEUROSCI.1066-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storm E, et al. Dosage dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 35.Shanmugalingam S, et al. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development. 2000;127:2549–2561. doi: 10.1242/dev.127.12.2549. [DOI] [PubMed] [Google Scholar]
- 36.Shinya M, Koshida S, Sawada A, Kuroiwa A, Takeda H. Fgf signalling through MAPK cascade is required for development of the subpallial telencephalon in zebrafish embryos. Development. 2001;128:4153–4164. doi: 10.1242/dev.128.21.4153. [DOI] [PubMed] [Google Scholar]
- 37.Walshe J, Mason I. Unique and combinatorial functions of Fgf3 and Fgf8 during zebrafish forebrain development. Development. 2003;130:4337–4349. doi: 10.1242/dev.00660. [DOI] [PubMed] [Google Scholar]
- 38.Gutin G, et al. FGF acts independently of SHH to generate ventral telencephalic cells. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. This paper provided the first definitive evidence that FGF signalling is the primary positive effector that establishes ventral identity in the telencephalon. The findings in this paper were complemented by those in reference 34, which demonstrated that FGF8 is central to this process. [DOI] [PubMed] [Google Scholar]
- 39.Fragkouli A, et al. Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur J Neurosci. 2005;21:2923–2938. doi: 10.1111/j.1460-9568.2005.04141.x. [DOI] [PubMed] [Google Scholar]
- 40.Liodis P, et al. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 42.Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128:4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- 43.Yun K, Garel S, Fischman S, Rubenstein JLR. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes is required for histogenesis of the striatum and olfactory bulb and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461:151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- 44.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- 45.Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 46.Hébert JM, Lin M, Partanen J, Rossant J, McConnell SK. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development. 2003;130:1101–1111. doi: 10.1242/dev.00334. [DOI] [PubMed] [Google Scholar]
- 47.Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 48.Muzio L, Mallamaci AJ. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill RE, et al. Mouse Small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 50.Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- 51.Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toresson H, Potter S, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 53.Yun K, Potter S, Rubenstein JLR. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 54.Fuccillo M, Rutlin M, Fishell G. Removal of Pax6 partially rescues the loss of ventral structures in Shh null mice. Cereb Cortex. 2006;16(Suppl 1):i96–i102. doi: 10.1093/cercor/bhk023. [DOI] [PubMed] [Google Scholar]
- 55.Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 23:10568–1057. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marklund M, et al. Retinoic acid signalling specifies intermediate character in the developing telencephalon. Development. 2004;131:4323–4332. doi: 10.1242/dev.01308. [DOI] [PubMed] [Google Scholar]
- 57.Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. This is a rare example of a study that examined a direct link between extracellular signals and the regulation of key transcription factors; the authors found that effectors of BMP and WNT signalling directly bind upstream cis elements at the Emx2 locus to regulate its expression. [DOI] [PubMed] [Google Scholar]
- 58.Muzio L, et al. Conversion of cerebral cortex into basal ganglia in Emx2−/−Pax6Sey/Sey double-mutant mice. Nature Neurosci. 2002;5:737–745. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- 59.Bishop KM, Goudreau G, O’Leary DDM. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. This paper provided the first attempt to explain how expression gradients of two transcription factors, EMX2 and PAX6, establish area identity (that is, functional territories) in the telencephalon. Coup-TF1 has also now been shown to contribute to this process (see reference 69). Recently this model has been codified into the ‘cooperative concentration’ model (see reference 63). [DOI] [PubMed] [Google Scholar]
- 60.Mallamaci A, Muzio L, Chan C-H, Parnavelas J, Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2−/− mutant mice. Nature Neurosci. 2000;3:679–686. doi: 10.1038/76630. This was the first paper to show that the loss of a specific transcription factor results in a shift in areal identity in the neocortex. [DOI] [PubMed] [Google Scholar]
- 61.Bishop KM, Rubenstein JL, O’Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muzio L, et al. Emx2 and Pax6 control regionalization of the pre-neuronogenic cortical primordium. Cereb Cortex. 2002;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- 63.Hamasaki T, Leingartner A, Ringstedt T, O’Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Zhou C, Tsai SY, Tsai MJ. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 66.Stenman J, Yu RT, Evans RM, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130:1113–1122. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- 67.Roy K, et al. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuurmans C, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nature Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 70.Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Develop. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 72.Mangale VS, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. Although previous work by this group and others had demonstrated a requirement for LHX2 in the development of the neocortex, this paper definitively demonstrated a complete failure in neocortical specification in Lhx2−/− mice. The finding suggests that LHX2 acts high in the hierarchy of neocortical induction, and provides an entry point for understanding neocortical specification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furuta Y, Piston DW, Hogan BLM. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 74.Panchision DM, et al. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–2110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandes M, Gutin G, Alcorn H, McConnell SK, Hébert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- 76.Shinozaki K, Yoshida M, Nakamura M, Aizawa S, Suda Y. Emx1 and Emx2 cooperate in initial phase of archipallium development. Mech Dev. 2004;121:475–489. doi: 10.1016/j.mod.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 77.Lee SMK, Tole S, Grove E, McMahon AP. A local Wnt3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 78.Backman M, et al. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- 80.Muzio L, Soria JM, Pannese M, Piccolo S, Mallamaci A. A mutually stimulating loop involving Emx2 and canonical Wnt signalling specifically promotes expansion of occipital cortex and hippocampus. Cereb Cortex. 2005;15:2021–2028. doi: 10.1093/cercor/bhi077. [DOI] [PubMed] [Google Scholar]
- 81.Turing AM. The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 83.Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- 84.Spoelgen R, et al. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–414. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the Sonic hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Fernandes M, Hébert JM. The ups and downs of holoprosencephaly, dorsal versus ventral patterning forces. Clin Genet. 2008;73:413–423. doi: 10.1111/j.1399-0004.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 87.Spemann H, Mangold H. Über induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Wilhelm Roux Arch Entwicklungsmech Organ. 1924;100:599–638. [Google Scholar]
- 88.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 89.Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- 90.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nature Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 91.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 92.Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130:2633–2644. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- 93.Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 94.Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 95.Sato T, Joyner AL, Nakamura H. How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development? Dev Growth Differ. 2004;46:487–494. doi: 10.1111/j.1440-169x.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 96.Houart C, Westerfield M, Wilson SW. A small population of anterior cells patterns the forebrain during zebrafish gastrulation. Nature. 1998;391:788–792. doi: 10.1038/35853. [DOI] [PubMed] [Google Scholar]
- 97.Assimacopoulos S, Grove EA, Ragsdale CW. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]