Abstract
Localizing individual sound sources under reverberant environmental conditions can be a challenge when the original source and its acoustic reflections arrive at the ears simultaneously from different paths that convey ambiguous directional information. The acoustic parasitoid fly Ormia ochracea (Diptera: Tachinidae) relies on a pair of ears exquisitely sensitive to sound direction to localize the 5-kHz tone pulsatile calling song of their host crickets. In nature, flies are expected to encounter a complex sound field with multiple sources and their reflections from acoustic clutter potentially masking temporal information relevant to source recognition and localization. In field experiments, O. ochracea were lured onto a test arena and subjected to small random acoustic asymmetries between 2 simultaneous sources. Most flies successfully localize a single source but some localize a ‘phantom’ source that is a summed effect of both source locations. Such misdirected phonotaxis can be elicited reliably in laboratory experiments that present symmetric acoustic stimulation. By varying onset delay between 2 sources, we test whether hyperacute directional hearing in O. ochracea can function to exploit small time differences to determine source location. Selective localization depends on both the relative timing and location of competing sources. Flies preferred phonotaxis to a forward source. With small onset disparities within a 10-ms temporal window of attention, flies selectively localize the leading source while the lagging source has minimal influence on orientation. These results demonstrate the precedence effect as a mechanism to overcome phantom source illusions that arise from acoustic reflections or competing sources.
Keywords: cocktail party problem, sound localization, temporal window of attention
A fundamental task in hearing is to identify individual sources despite complex acoustic conditions that may mask relevant information. This is especially true in reverberant environmental conditions where sounds reflecting from acoustic clutter may produce multiple coherent copies that the auditory system must differentiate from the originating source for recognition and localization (1). For 2 spatially separated coherent signals of equal intensity that arrive at the ears simultaneously, vertebrate auditory systems experience a psychophysical phenomenon known as summing localization and perceive these inputs as a single “phantom” source located between the actual sources (1–7). Shifts in timing or loudness of sources result in systematic changes in the perceived phantom source location (2). With delays between 2 sources smaller than a minimum time interval a precedence effect is observed and only the leading source is perceived. Summing localization causes both signals to be impossible to localize individually (3, 4, 8) while the precedence effect allows for the leading source to be resolved (1, 2, 9, 10).
Acoustically orienting insects encounter an analogous situation in nature where the scattering of sound by vegetation may result in multiple acoustic reflections that degrade directional cues for sound localization (11). This is further complicated by species that communicate simultaneously and commonly in aggregations (12) rendering signals hard to identify and localize (11). We show that the acoustic parasitoid fly Ormia ochracea (Diptera: Tachinidae) may use the precedence effect to disambiguate source location under similar conditions. Ormia faces a complex auditory scene, where attractive calls of multiple host crickets may overlap in time and space. Nevertheless, Ormia must accomplish the task of finding a source with a much simpler auditory system compared to vertebrate acoustic specialists (13–15) and under the physical constraints on sound localization facing small animals (16). Hearing is critical to reproductive success for Ormia as their larvae must develop as internal parasites in nocturnally singing insects (crickets) that female flies locate by sound (17, 18). Ormia thus face 2 important auditory tasks: (1) correctly identifying the song of a suitable host and (2) successfully locating the potential host. Behavioral evidence indicates that flies rely on temporal parameters within songs to identify suitable host crickets (19–23). Recognition of host calling songs initiates phonotactic behavior in gravid female Ormia. They make their approach by flight and descend within the vicinity of the attractive sound source (18, 21, 24). Upon landing, females localize and approach their hosts to deposit larvae that remain to burrow and feed within host crickets (18).
Both localization and recognition of appropriate host species depend on the highly stereotyped characteristics of cricket songs (14, 15, 25). Under optimal conditions (single source), the auditory system of Ormia is well-suited to simultaneously processing time differences between consecutive sound pulses to detect species-specific pulse rates (recognition) and small time differences in the arrival of the song between both ears (localization). This is accomplished with specialized auditory receptors that fire precisely and only at pulse onsets, with consistent intensity-dependent firing latencies (14, 15). Acoustic interaural time differences (ITDs), the only directional cue available (26), vary according to sound position relative to the midline axis such that they decrease in magnitude with decreasing angular deviations from the midline. The jitter (variation in spike timing) of individual auditory receptors approximate to 70 μs but the ability of Ormia to localize a source to 2° azimuthal accuracy corresponds to a mean ITD of just 7 μs, a sensory threshold significantly lower than properties of individual auditory receptors (14). This directional hyperacuity is derived from the amplification of acoustic ITDs by direction-sensitive tympanal mechanics (27) and subsequently translated into increasingly larger interaural latency differences that encode sound direction (14). Previous studies have demonstrated the ability of Ormia to accurately localize single sources is based on a strategy of pooling responses of auditory receptors to derive a hyperacute measure of direction-dependent ITDs (14, 28). But under natural conditions this may be difficult as crickets commonly sing in aggregations and within vegetation (29, 30). Flies are thus expected to be within earshot of multiple overlapping sound sources and acoustic reflections with their auditory system required to segregate individual sound pulses that correspond to the same source and to measure the temporal disparity between both ears to determine sound direction. Overlapping sound sources and associated reverberations may severely mask both temporal parameters, leading to failed recognition and/or source localization.
It is unknown how Ormia respond to acoustic conditions that may mask acoustic cues for recognition and localization. To investigate the role of hyperacute directional hearing in resolving single-sound sources we examined walking phonotaxis in Ormia to multiple coherent sound sources that mimic a source and its reflections. We tested the hypothesis that exploitation of small time differences between competing sources allows for a precedence effect, resulting in localization dominance of the leading source to be resolved individually. We first describe the effects of temporal overlaps and whether or not they generate ambiguous directional cues as in summing localization. Secondly, we vary source separation and the relative position of simultaneous sources to examine if directional advantages arise to disambiguate locations of a source from its reflection. Collectively, these results suggest that Ormia experience both summing localization and localization dominance. Furthermore, our results demonstrate the precedence effect as a solution to resolving phantom sound-source illusions.
Results
Field Trials.
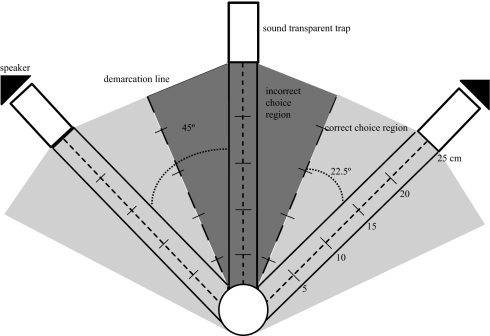
We attracted gravid female Ormia to our experimental arena by broadcasting continuous Gryllus rubens trills from a lure speaker located beneath an 8-cm diameter start circle (Fig. 1). Three funnel traps were positioned in front of the arena at 0° and ± 45° azimuth (forward, right and left positions). Test speakers behind the left and right traps (test speakers separated by 2 m) broadcast sound in the direction of the start zone. The arena was divided into 3 regions, each with lanes and demarcation lines marked with distance measurements in 5-cm increments that extended from the margin of the start circle to the trap. This allowed us to record distance traveled in each direction, distance at which files crossed demarcation lines (i.e., crossed from 1 choice lane to another), and final choice region. We tested a total of 41 flies. Flies either landed directly within the starting circle, or elsewhere on the arena and subsequently walked into the starting region. No attempt was made to control the precise position or orientation of flies within the start circle so that precise phase relationships between test speakers varied randomly among flies tested. Once a fly was within the start circle, the lure stimulus was discontinued, at which point flies became stationary, and a single test speaker (single source condition) or both left and right test speakers (simultaneous condition) were turned on to broadcast identical continuous trills. Phonotactic responses were scored as correct if flies reached sound traps in front of an active speaker while those that walked to the centre (silent) trap were scored as misdirected phonotaxis.
Fig. 1.
Experimental arena for field phonotaxis trials. A lure speaker orientated upwards was positioned at the 8-cm circular start region underneath the arena. Two test speakers (black triangles) orientated horizontally were separated by 90° and positioned behind sound traps (rectangles). A third (silent) trap was placed between the 2 speakers (0° from the start region). Demarcation lines divided the test arena into 3 choice regions: 2 correct choice regions (light gray) and an incorrect choice region (dark gray).
Single-source Trials.
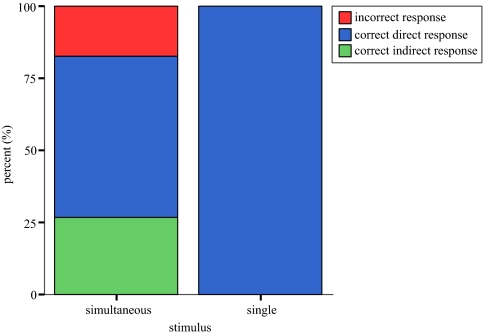
All flies that were presented with a single source turned and walked directly to the active speaker (100%) (Fig. 2). In no case were flies unable to resolve source location (n = 23 flies).
Fig. 2.
Phonotaxis to simultaneous and single sources under field conditions. Direct responses. Percentage of correct responses with direct approach to source one location (blue). Indirect responses. Percentage of correct responses with initial misdirected walking trajectories to a phantom source (0° heading) (green). Incorrect responses. Percentage of unsuccessful phonotaxis (red) with walking trajectories misdirected toward a phantom source location (0° heading). Most flies were able to resolve an individual source while a smaller proportion of flies walked toward a phantom source location.
Simultaneous-source Trials.
Flies presented with 2 simultaneous sources showed 3 response patterns. 1) Direct responses. A majority of flies (23/41, 56.1%) walked directly to 1 of the speakers similarly to single-source trials (Fig. 2). Flies oriented to both speakers with equal frequency [χ2 (1) = 0.043, P = 0.835]. 2) Indirect responses. For some flies (11/41, 26.83%), initial walking trajectories were toward the centre (silent) trap, but flies then turned toward 1 of the active sound sources. These flies walked an average of 12.30 ±2.91 cm toward the centre trap before deviating to 1 side and crossed the demarcation line at 17.75 ± 4.32 cm (mean ± SD). Flies chose either source with equal frequency [χ2 (1) = 2.273, P = 0.132] but flies always continued in the direction of their initial exit from the centre lane. In these indirect phonotactic responses, flies traveled a significantly greater total distance (indirect response Mdn = 30.9 cm, direct response Mdn = 25 cm, U = 192.00, P = 0.01, r = −0.96) to the source and their walking trajectories were composed of a significantly greater forward-directed component (indirect response Mdn = 12.5 cm, direct response Mdn = 0 cm, U = 0.00, P < 0.001, r = −0.96) compared to direct phonotaxis. 3) Incorrect responses. A smaller proportion of flies (7/41, 17.07%) oriented to the centre “silent” trap and walked to the perimeter of the arena without deviating from the centre lane (Fig. 2).
Laboratory Trials.
We examined the flies' directional responses to competing sources in more detail in the laboratory. We recorded phonotactic responses of freely walking flies to a single presentation of 2 competing stimuli under free-field acoustic conditions. In these trials, flies were placed on top of a piece of clear acetate whereby moving the acetate allowed for precise positioning of flies on a calibrated starting point equidistant (40 cm) from 3 surrounding speakers. This allowed for control of the phase and timing relationships of competing stimuli. Responses to such combined stimuli were tested under 2 speaker arrangements: 1) both speakers located either symmetrically on either side of the fly (180° angular separation), or 2) 1 speaker forward (on the midline axis) and the other lateral (90° angular separation).
Simultaneous Stimuli.
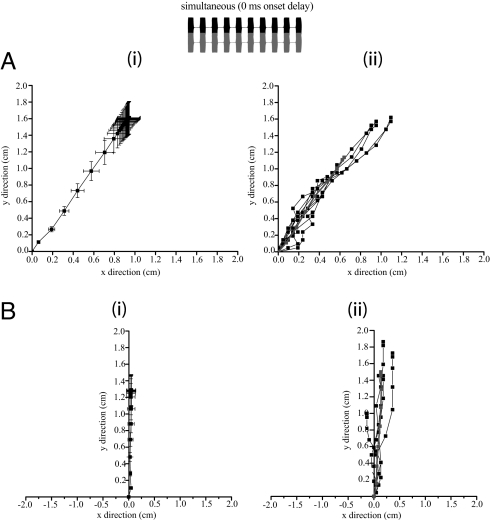
When flies were presented with synchronous sources (directly overlapping in-phase chirps, Fig. 3Inset) separated by 90°, they did not orient to an individual source location. Walking responses were closely timed with stimulus duration. Following stimulus onset, flies accelerated to a maximum velocity of 0.31 ± 0.02 cm/s (n = 10 flies), covered a total distance of 1.77 ± 0.19 cm, and came to a full stop at stimulus offset. Phonotactic responses were directed to a location between both speakers (“phantom source”: angular heading: 31.32 ± 2.40°; n = 10 flies, 10 run/fly; Fig. 3A).
Fig. 3.
Phonotaxis to simultaneous sources under laboratory conditions. Two synthetic sound sources broadcast synchronously (0-ms onset delay) to produce overlapping chirps (Inset). (A) Average walking trajectory of free-walking flies to sources separated by 90°. (i) Average response (n = 10 flies). (ii) Individual responses of a single representative fly. (B) Average walking response of flies to sources separated by 180°. (i) Average response (n = 9 flies). (ii) Individual responses of a single representative fly. Gray line within individual response plots (ii) indicate average responses. Square symbols (i) and horizontal/vertical projections from symbols indicate the mean and confidence intervals of the location of flies at 0.03-s intervals. Flies responded to simultaneous sources by directing phonotaxis to a phantom source location between broadcasting speakers (≈45° heading in 90° condition, ≈0° heading in 180° condition).
With a 180° speaker separation, walking responses were similar to sources that were separated by 90° in that responses were closely timed with stimulus onset and offset, flies walked a similar total distance [1.428 ± 0.18 cm, t (9) = 1.113, P = 0.295], and flies failed to resolve a single source location. Responses were consistently directed between both speakers with an angular heading of 3.34 ± 2.83° (n = 9 flies, 10 run/fly; Fig. 3B).
Asynchronous Stimuli.
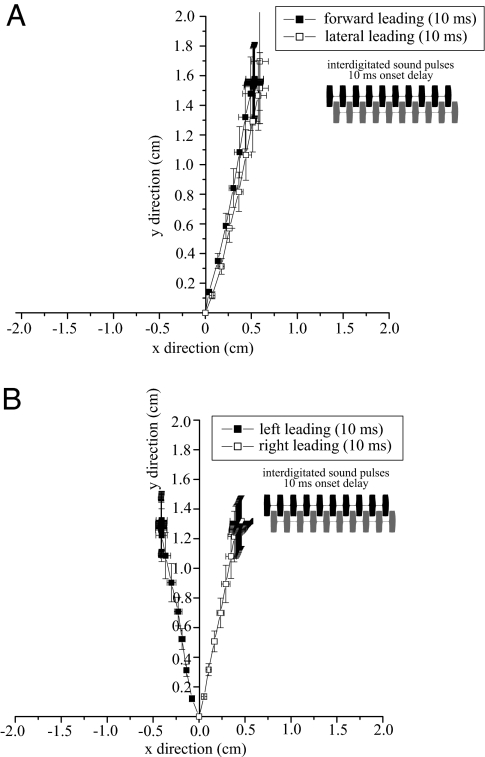
We varied the temporal overlap in the combined stimuli. A delay of 10 ms (Fig. 4Inset) between competing sound sources generated an interdigitated temporal pattern where 1 source was leading by a single pulse and subsequent pulses occurred between pulses from the second source and the combined stimulus had a doubled pulse rate with alternating pulses from separate speakers. When sources were separated by 90° (1 speaker in the forward direction), flies were still unable to localize a single source and walked in an intermediate direction, but showed an overall bias toward the forward direction regardless of which speaker delivered the leading pulse (initial pulse from forward source: 23.31 ± 2.17°, initial pulse from lateral (right) source: 29.06 ± 3.06°, n = 10 flies, 5 run/fly, Watson's U = 0.055, P > 0.10, Fig. 4A). When interdigitated sources were separated by 180°, flies again directed their walking response toward an intermediate location but with a bias toward the source that delivered the leading pulse (angular headings: 180° speaker separation—initial pulse from left source, −30.69 ± 5.16°, initial pulse from right source, 20.54 ± 4.81°, n = 10 flies, 5 run/fly, Watson's U = 0.425, P < 0.001, Fig. 4B).
Fig. 4.
Phonotaxis to interdigitated (10 ms onset delay) sources. Two synthetic sound sources broadcast with a 10-ms onset delay to produce interdigitated chirps (Inset). (A) Average walking response of flies (n = 10 flies) to sources separated by 90°. (B) Average walking response of flies to sources separated by 180° (n = 10 flies). Square symbols and horizontal/vertical projections from symbols indicate the mean and confidence intervals of the location of flies at 0.03-s intervals. Filled symbols indicate walking responses to forward (A) or left (B) leading sound sources while open symbols indicate walking response to lateral or right leading sound sources. Flies responded to interdigitated sources by directing phonotaxis to a phantom source location between the broadcasting speakers (45° heading in 90° condition, 0° heading in 180° condition) but with a slight bias toward leading sources.
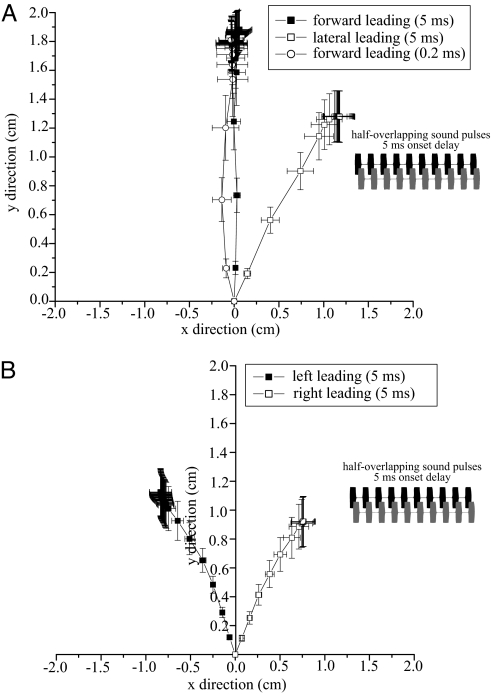
Shorter time differences, however, allowed flies to resolve source location ambiguity when the leading source was in the forward direction. A 5-ms onset delay (Fig. 5Inset), created half-overlapping sound pulses within chirps. When sources were separated by 90° (one speaker in the forward direction), flies correctly oriented to the forward speaker when it was the leading source, but oriented to an intermediate location when the lateral speaker was leading (angular headings: 90° speaker separation - forward source leading: 12.76 ± 1.59°, lateral source leading: 46.72 ± 3.05°, n = 10 flies, 5 run/fly, Watson's U = 0.425, P < 0.001, Fig. 5A, square symbols). When both speakers were lateral (180° speaker separation), flies showed a stronger bias toward the leading speaker (compared with interdigitated stimuli), but still oriented to intermediate directions (180° speaker separation angular headings - left source leading, −29.00 ± 4.76°, right source leading, 39.59 ± 3.03°, n = 10 flies, 5 run/fly, Watson's U = 0.4035, P < 0.001, Fig. 5B).
Fig. 5.
Phonotaxis to overlapping sources with small onset time differences (5- and 0.2-ms onset delay). Two synthetic sound sources broadcast with a 5-ms onset delay to produce half-overlapping sound pulses (Inset, A and B), or a 0.2-ms onset delay to produce overlapping stimuli out of phase by 1 cycle of the 5-kHz carrier frequency. (A) Average walking responses (square symbols, 5-ms onset delay; circular symbols, 0.2-ms onset delay) of flies to sources spatially separated by 90° (n = 10 flies). (B) Average walking responses of flies to sources spatially separated by 180° (n = 10 flies). Symbols and horizontal/vertical projections from symbols indicate the mean and confidence intervals of the location of flies at 0.07- (A) and 0.03-s (B) intervals. Filled symbols indicate walking responses to forward (A) or left (B) leading sources while open symbols indicate walking response to lateral (A) or right (B) leading sources. Small onset time differences between sources (0.2 ms) allowed flies to resolve source location ambiguity to the leading source.
With the 90° speaker separation, we also tested flies with a 0.2-ms onset delay (i.e., forward speaker leading, stimuli out of phase by 1 cycle of the 5 kHz carrier frequency). Angular headings were correctly oriented to the leading (forward) speaker and were significantly different from the incorrectly directed responses to simultaneous sources (0.2 delay angular heading: 11.37 ± 4.89°, mean ± SE, n = 10 flies, 10 run/fly, Watson's U = 0.2725, P < 0.01, Fig. 5A, circular symbols).
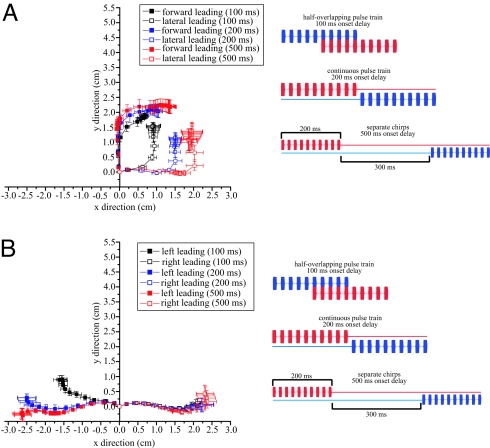
For larger interstimulus delays, flies were presented with a pair of pulse trains, with the first pulse train leading the second pulse train by 100, 200, or 500 ms. When competing sources were delayed by 100 ms (Fig. 6Inset), the latter half of the leading train of pulses overlapped (simultaneous with) the first half of the lagging train. An onset delay of 200 ms (Fig. 6 Inset) resulted in an overall stimulus with a continuous train of pulses 400 ms in duration that switched speakers halfway through stimulus presentation. An onset delay of 500 ms (Fig. 6 Inset) created 2 distinct chirps separated by 300 ms of silence. For these larger inter-stimulus delays flies walked initially toward the leading source and they maintained this directional heading until after the onset of the second source. Following the onset of the second source, flies changed their directional heading to track the second source with a latency corresponding to the inter-stimulus delay (Fig. 6A). For 100- and 200-ms inter-stimulus delays, flies tracked the 2 sound sources in a single phonotactic response. Responses to a 500-ms inter-stimulus delay (2 independent sources from different locations separated by 300 ms of silence) further demonstrate a prominent effect of a forward source. Flies responded to this situation with 2 separate walking responses where they initially tracked the leading source (90° speaker separation—front source leading: −13.08 ± 1.85°, right source leading: 88.36 ± 5.93°, Fig. 6A; 180° speaker separation—left source leading: −83.55 ± 2.66°, right source leading: 84.09 ± 5.13°, n = 7 flies, 5 run/fly, Fig. 6B), paused during the silent interval (290.61 ± 6.11 ms, n = 7 flies) and subsequently engaged in a second walking response to the lagging source. However, responses were strikingly weaker with smaller turn angles toward the lagging source in the 180° speaker configuration than compared to 90° speaker separation (90° speaker separation—front source leading: 56.88 ± 4.11°, right source leading: −49.48 ± 9.33°, Fig. 6A; 180° speaker separation—left source leading: 11.90 ± 4.38°, right source leading: 18.50 ± 7.84°. Fig. 6B).
Fig. 6.
Phonotaxis to overlapping sources with large onset delays (100 ms, 200 ms, and 500 ms). Two synthetic sound sources broadcast with 100 ms onset delay (half-overlapping pulse train), 200 ms onset delay (continuous pulse train), and 500 ms onset delay (2 distinct chirps separated by 300 ms of silence) (Insets, A and B). (A) Average walking responses of flies (n = 7 flies) to sources spatially separated by 90° with onset delays of 100 ms (black), 200 ms (blue), and 500 ms (red). (B) Average walking responses (n = 7 flies) of flies to sources spatially separated by 180° with onset delays of 100 ms (black), 200 ms (blue), and 500 ms (red). Square symbols and horizontal/vertical projections from symbols indicate the mean and confidence intervals of the location of flies at 0.07-ms (A) and 0.03-s (B) intervals. Filled symbols indicate walking responses to forward (A) or left (B) leading sources while open symbols indicate walking response to lateral (A) or right (B) leading sources. Under all temporal overlaps tested, flies initially turned and walked toward the leading source followed by a secondary turning response to the lagging source. Secondary turning responses were weaker for sources separated by 180°.
Discussion
We examined the ability of Ormia to localize a single source under conditions that mimic reverberant environments with multiple sources. Temporal overlap of competing signals greatly affected overall walking orientation. Complete overlap (synchrony) of attractive stimuli led to misdirected phonotaxis to a “phantom” source. We observed similar behavior for stimuli that were simultaneous but with pulses out of phase (interdigitated). More importantly, our study demonstrates the precedence effect as a solution for overcoming conditions (echoes or competing sources) that generate ambiguous directional information. Small positional and temporal asymmetries were sufficient for correct phonotactic responses and are thus important for source localization.
Solving the “Phantom Source” Problem.
Sensory illusions are well-known in studies of human perception and psychophysics (2, 31, 32). Illusions are more difficult to demonstrate in non-human animals, but have also been used to study sensory mechanisms in a variety of taxa that include monkeys (33), birds (34, 35), and honey bees (36). In addition, a well-known sensory illusion is the phenomenon of summing localization, where 2 spatially separated sources broadcasting identical stimuli elicit the perception of a fused single source located midway between 2 speakers (2), a problem that may be due to constraints in neural function for processing simultaneous signals. This phenomenon has also been demonstrated in some non-human animals. For example, barn owls orient to a central location in response to identical lateral sources presented simultaneously (3, 37). This behavioral response has been associated with space-specific auditory neurons that signal the location of a single phantom-source between 2 actual source locations (4). A variety of species including owls (37), rats (38), cats (5, 39), crickets (10), and katydids (40–42) seem to solve this problem using a common strategy; exploiting small time differences between competing sources such that perception of directional information is predominantly conveyed by the leading source while directional information from lagging sources is suppressed (precedence effect). Several electrophysiology studies suggest that this occurs as neural responses to lagging sources are suppressed by responses to the leading source (1, 5, 6, 10, 43).
Our results show similar phenomena in Ormia hearing, although with some differences that may be relevant to the question of how these computations are carried out by simple auditory systems. With synchronous stimuli, flies respond to a phantom source. With a small arrival-time difference between 2 stimuli, flies selectively localize the leading source (localization dominance). In Ormia, however, the precedence effect appears to operate only within a small time frame when temporal disparities fall within the duration of a sound pulse. Flies orient with a bias to the leading source when pulses are half-overlapping (5-ms onset delay), but track the leading source only weakly when disparities are beyond the duration of a pulse (i.e., inter-digitated condition). Localization accuracy may be expected to improve with greater temporal separation, but responses to inter-digitated stimuli are actually worse than responses to half-overlapping pulses. In addition, selective localization only occurs when the leading source is closer to the midline than the lagging source. When the 2 sources are symmetrically located relative to the midline axis, the leading source is more attractive but responses to the lagging source are not fully suppressed. Under single-source conditions, Ormia show more vigorous phonotaxis (high walking speed, longer distance walked) for a forward source than for an equivalent stimulus from a lateral source (28). Thus source localization in Ormia depends on both the relative timing and location of competing sources.
A breakdown (release from suppression) of the precedence effect may occur due to changes in the lagging stimulus (1). Preference to a leading source can be compensated and even reversed with sufficient increases in sound intensity of the lagging source, as reported in katydids and frogs (42, 44, 45). Sound intensity between competing sources was balanced at the start position of flies tested in our laboratory experiments, but perceived relative intensities are expected to change as flies make their approach to a source. Therefore, observed responses reflect initial differences of lead-lag relationships between sources and reinforcing changes in perceived relative sound intensities.
In field experiments the flies' initial position was allowed to vary between the 2 sources potentially introducing small intensity and timing disparities. Most flies showed a direct response to a single source, suggesting that small binaural disparities may have permitted dominant representation of a single source. But these disparities were small enough that on average flies were significantly diverted from direct phonotaxis. In our field trials, fly starting positions varied within an 8-cm diameter circle around the centre point, so maximum arrival time differences due to flies being closer to 1 speaker were on the order of 100 μs. In laboratory trials, flies reliably oriented to a forward source when the only cue was a 200-μs time difference.
These results suggest that, in addition to sound localization per se, hyperacute auditory directionality in Ormia (14) may be an important part of the mechanism for selective responses to competing sources. Rapid, accurate orientation to a sound source places that source on the fly's midline axis, allowing it to dominate sources at other locations, whereas orientation with larger error angles [as in crickets (46)] would not. Within a small temporal window of attention, flies orient reliably to a leading source while a lagging source has minimal influence on orientation. For very short delays between competing sources, the bias in responses toward the leading source will lead to a more gradual movement of the leading source to the midline position. But the mechanism will be more effective for delays longer than the latency of phonotactic responses. Beyond the temporal window of attention there is an increasing attractive effect of the lagging source, especially if it is more forwardly located. This is demonstrated by our results for longer interstimulus delays (100–500 ms, Fig. 6). When both sources were lateral (180° separation), flies initiated phonotaxis to the leading source and showed little reorientation to the lagging stimulus. At the onset of phonotaxis flies orient to face the sound source (28), thus placing the leading source in the forward position and the lagging source rearward, resulting in disproportionate attraction to the leading source. Symmetrical stimulation of both ears from a rearward position also give rise to the same directional cues that correspond to a forward location (26, 27, 47). Consequently, flies sometimes responded to the rearward source by actually walking in the preferred forward direction and away from the lagging source. However, for sources separated by 90°(one forward and the other lateral), orientation to the leading source leaves the lagging source in a position that is still more attractive than a rearward source and consequently flies orient to the lagging source with comparatively stronger walking responses.
Our results suggest both the precedence effect and a forward preference as an effective strategy for isolating sources separated by small time-differences occurring within the duration of a pulse, but flies seem to use the strategy ‘forward is best’ when the precedence effect is weak (greater temporal separation). We have demonstrated this at the behavioral level and suggest hyperacute directional hearing has an important role in selective phonotaxis. Although the range of temporal overlaps between simultaneous sources in this study may not entirely reflect conditions that Ormia encounter in nature (12), our results serve to highlight their behavioral capacity and limitations in isolating individual sources. Further studies on the likelihood of encountering similar temporal overlaps as the combined effect of acoustic reflections and acoustic interactions among singing hosts are required to interpret the ecological relevance of our findings. Fine-grain measurements of auditory directionality to competing sources varying in spatial separation is needed to directly test for the role of temporal disparity and sound direction in source segregation. Furthermore, physiological measurements are needed to test for the sensory basis of these effects.
Materials and Methods
Animals.
Field experiments were carried out between 1,830 to 2,100 h in mid-October 2007 in an open pasture in Gainesville, Florida (USA). Laboratory experiments were conducted using gravid female O. ochracea derived from laboratory-reared populations that were originally collected at the same location. Laboratory-reared animals were maintained on a 12-h:12-h light:dark regime and fed nectar solution ad libitum.
Field study: Acoustic Stimuli.
Acoustic stimuli were simulated G. rubens calls (local host cricket for Ormia) consisting of a continuous trill of 5 kHz, 10-ms tone pulses, presented at 50 pulses/s for 20 min. Acoustic stimuli were synthesized in Matlab (Release 2007b), edited in Sony Soundforge (Version 8.0) and recorded onto an audio CD that was played with a portable CD player (Durabrand CD-566). Two test speakers were connected to the left and right channel of the CD player for simultaneous playback from both speakers. Stimuli were amplified with stereo circuit boards removed from powered computer speakers and broadcast from Radio Shack piezoelectric horn tweeters. Sound level of the lure speaker was calibrated (B&K Type 2231 Sound Level Meter, Type 4139 ¼“ microphone) to 104 dB SPL at the centre of the 8 cm circular start region while the 2 test speakers were calibrated to 94 dB SPL.
Laboratory Study.
Experiments were conducted under free-field conditions with the test arena surrounded by sound attenuating foam.
Acoustic Stimuli.
Acoustic stimuli were synthesized using Tucker-Davis Technologies (TDT) hardware (System 3) and custom scripts written in Matlab. The synthetic stimuli consist of a train of 5 kHz, 10-ms tone pulses, presented at 50 pulses/s, with 10 pulses/train giving an overall chirp duration of 200 ms. The stimuli were amplified (NAD S300), passed through a programmable attenuator (TDT model PA5) and broadcast from Radio Shack piezoelectric horn tweeters. Stimulus amplitude and timing were controlled by computer. Stimulus levels and timing were calibrated with a probe microphone (B&K Type 4182).
Data Analysis.
Phonotactic responses were recorded with a standard video camera (Panasonic WV-GP460; Matsushita Electric Industrial Co. or Sony DCR-HC65) mounted above the arena and VCR (Hitachi DA4). Analog video clips were captured and digitized at 15 or 30 frames/s using Adobe Premiere 6.0 and Cinepak compression codec (Radius). Digitized video clips were imported into motion analysis software (Midas 2.0, Xcitex) to extract distance, velocity and direction of movement.
At least 5 phonotactic responses per fly were recorded for all conditions tested. Data from repeated responses for individuals were averaged and individual averages were pooled across flies. Data are given as means ± SE unless otherwise stated. For comparison of responses under different stimulus conditions, overall response angles were calculated as in (28). Statistical analyses were carried out using Matlab (version R2007b) and R (version 2.6.1) software.
Acknowledgments.
We are in debt to TJ Walker for his generous help in providing assistance and support for our field collecting trip. We thank M. C. B. Andrade, M. M. Kasumovic, J. A. Stoltz, M. J. Kim Lee, and 2 anonymous reviewers for comments that greatly improved our manuscript. We thank M. Chu, P. Martis, A. Feng, M. Leung, and the Mason Lab Group for assistance in animal care. Funding was provided by grants from the NSERC Canada (238882 241419) to A.C.M., National Institutes of Health NRSA (1F32GM076091–01A1) to the Department of Energy, and Animal Behavior Society Student Research Award and NSERC PGS D3 to N.L.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Litovsky RY, Colburn HS, Yost WA, Guzman SJ. The precedence effect. J Acoustic Soc Am. 1999;106:1633–1654. doi: 10.1121/1.427914. [DOI] [PubMed] [Google Scholar]
- 2.Blauert J. Spatial Hearing: The Psychophysics of Human Sound Localization. Cambridge, MA: The MIT Press; 1997. [Google Scholar]
- 3.Keller CH, Takahashi TT. Binaural cross-correlation predicts the responses of neurons in the owl's auditory space map under conditions simulating summing localization. J Neurosci. 1996;16:4300–4309. doi: 10.1523/JNEUROSCI.16-13-04300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi TT, Keller CH. Representation of multiple sound sources in the owls auditory space map. J Neurosci. 1994;14:4780–4793. doi: 10.1523/JNEUROSCI.14-08-04780.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin TCT. Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat. J Neurosci. 1994;14:5170–5186. doi: 10.1523/JNEUROSCI.14-09-05170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tollin DJ, Populin LC, Yin TCT. Neural correlates of the precedence effect in the inferior colliculus of behaving cats. J Neurophysiol. 2004;92:3286–3297. doi: 10.1152/jn.00606.2004. [DOI] [PubMed] [Google Scholar]
- 7.Bee MA, Riemersma KK. Does common spatial origin promote the auditory grouping of temporally separated signal elements in grey treefrogs? Anim Behav. 2008;76:831–843. doi: 10.1016/j.anbehav.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best V, van Schaik A, Carlile S. Separation of concurrent broadband sound sources by human listeners. J Acoust Soc Am. 2004;115:324–336. doi: 10.1121/1.1632484. [DOI] [PubMed] [Google Scholar]
- 9.Tollin DJ, Yin TCT. Psychophysical investigation of an auditory spatial illusion in cats: The precedence effect. J Neurophysiol. 2003;90:2149–2162. doi: 10.1152/jn.00381.2003. [DOI] [PubMed] [Google Scholar]
- 10.Wyttenbach RA, Hoy RR. Demonstration of the precedence effect in an insect. J Acoust Soc Am. 1993;94:777–784. doi: 10.1121/1.408207. [DOI] [PubMed] [Google Scholar]
- 11.Romer H, Bailey WJ. Insect hearing in the field. Comp Biochem Physiol. 1990;97:443–447. [Google Scholar]
- 12.Cade WH. Field Cricket Spacing, and the Phonotaxis of Crickets and Parasitoid Flies to Clumped and Isolated Cricket Songs. J Comp Ethol. 1981;55:365–375. [Google Scholar]
- 13.Robert D, Gopfert MC. Novel schemes for hearing and orientation in insects. Curr Opin Neurobiol. 2002;12:715–720. doi: 10.1016/s0959-4388(02)00378-1. [DOI] [PubMed] [Google Scholar]
- 14.Mason AC, Oshinsky ML, Hoy RR. Hyperacute directional hearing in a microscale auditory system. Nature. 2001;410:686–690. doi: 10.1038/35070564. [DOI] [PubMed] [Google Scholar]
- 15.Oshinsky ML, Hoy RR. Physiology of the auditory afferents in an acoustic parasitoid Fly. J Neurosci. 2002;22:7254–7263. doi: 10.1523/JNEUROSCI.22-16-07254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michelsen A. In: Compartive Hearing: Insects. Hoy RR, Popper AN, Fay RR, editors. New York: Springer; 1998. pp. 18–62. [Google Scholar]
- 17.Adamo SA, Robert D, Perez J, Hoy RR. The response of an insect parasitoid, Ormia-Ochracea (Tachinidae), to the uncertainty of larval success during infestation. Behav Ecol Sociobiol. 1995;36:111–118. [Google Scholar]
- 18.Cade W. Acoustically orienting parasitoids - fly phonotaxis to cricket songs. Science. 1975;190:1312–1313. [Google Scholar]
- 19.Zuk M, Rotenberry JT, Simmons LW. Calling songs of field crickets (Teleogryllus oceanicus) with and without phonotactic parasitoid infection. Evolution. 1998;52:166–171. doi: 10.1111/j.1558-5646.1998.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 20.Walker TJ. Phonotaxis in female Ormia ochracea (Diptera, Tachinidae), a parasitoid of field crickets. J Insect Behav. 1993;6:389–410. [Google Scholar]
- 21.Muller P, Robert D. Death comes suddenly to the unprepared: Singing crickets, call fragmentation, and parasitoid flies. Behav Ecol. 2002;13:598–606. [Google Scholar]
- 22.Gray DA, Banuelos C, Walker SE, Cade WH, Zuk M. Behavioural specialization among populations of the acoustically orienting parasitoid fly Ormia ochracea utilizing different cricket species as hosts. Anim Behav. 2007;73:99–104. [Google Scholar]
- 23.Wagner WE. Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav Ecol. 1996;7:279–285. [Google Scholar]
- 24.Mueller P, Robert D. A shot in the dark: The silent quest of a free-flying phonotactic fly. J Exp Biol. 2001;204:1039–1052. doi: 10.1242/jeb.204.6.1039. [DOI] [PubMed] [Google Scholar]
- 25.Oshinsky M. L. Department of Neurobiology and Behavior. Ithaca: Cornell University; 1998. [Google Scholar]
- 26.Robert D, Miles RN, Hoy RR. Directional hearing by mechanical coupling in the parasitoid fly Ormia ochracea. J Comp Physiol. 1996;179:29–44. doi: 10.1007/BF00193432. [DOI] [PubMed] [Google Scholar]
- 27.Miles RN, Robert D, Hoy RR. Mechanically coupled ears for directional hearing in the parasitoid Ormia ochracea. J Acoust Soc Am. 1995;98:3059–3070. doi: 10.1121/1.413830. [DOI] [PubMed] [Google Scholar]
- 28.Mason AC, Lee N, Oshinsky ML. The start of phonotactic walking in the fly Ormia ochracea: A kinematic study. J Exp Biol. 2005;208:4699–4708. doi: 10.1242/jeb.01926. [DOI] [PubMed] [Google Scholar]
- 29.Greenfield M, Shaw K. In: Orthopteran mating systems. Sexual competition in a diverse group of insects. Gwynne D, Morris G, editors. Boulder, Colorado: Westview Press; 1983. pp. 1–27. [Google Scholar]
- 30.Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans: Common problems and diverse solutions. Chicago: The University of Chicago Press; 2002. [Google Scholar]
- 31.Bregman AS. Auditory Scene Analysis: The Perceptual Organization of Sound. Cambridge, Mass: Bradford Books, MIT Press; 1990. [Google Scholar]
- 32.Seghier ML, Vuilleumier P. Functional neuroimaging findings on the human perception of illusory contours. Neurosci Biobehav Rev. 2006;30:595–612. doi: 10.1016/j.neubiorev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Von Der Heydt R, Peterhans E, Baumgartner G. Illusory contours and cortical neuron responses. Science. 1984;224:1260–1262. doi: 10.1126/science.6539501. [DOI] [PubMed] [Google Scholar]
- 34.Nieder A, Wagner H. Perception and neuronal coding of subjective contours in the owl. Nat Neurosci. 1999;2:660–663. doi: 10.1038/10217. [DOI] [PubMed] [Google Scholar]
- 35.Niu YQ, Xiao Q, Liu RF, Wu LQ, Wang SR. Response characteristics of the pigeon's pretectal neurons to illusory contours and motion. J Physiol. 2006;577:805–813. doi: 10.1113/jphysiol.2006.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horridge GA, Zhang SW, Ocarroll D. Insect perception of illusory contours. Philos Trans R Soc Lond B Biol Sci. 1992;337:59–64. [Google Scholar]
- 37.Keller CH, Takahashi TT. Responses to simulated echoes by neurons in the barn owl's auditory space map. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1996;178:499–512. doi: 10.1007/BF00190180. [DOI] [PubMed] [Google Scholar]
- 38.Kelly JB. Localization of paired sound sources in rat - small time differences. J Acoust Soc Am. 1974;55:1277–1284. doi: 10.1121/1.1914697. [DOI] [PubMed] [Google Scholar]
- 39.Cranford JL. Localization of paired sound sources in cats - effects of variable arrival times. J Acoust Soc Am. 1982;72:1309–1311. doi: 10.1121/1.388409. [DOI] [PubMed] [Google Scholar]
- 40.Greenfield MD, Roizen I. Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature. 1993;364:618–620. [Google Scholar]
- 41.Greenfield MD, Siegfreid E, Snedden WA. Variation and repeatability of female choice in a chorusing katydid, Ephippiger ephippiger: An experimental exploration of the precedence effect. Ethology. 2004;110:287–299. [Google Scholar]
- 42.Snedden WA, Greenfield MD. Females prefer leading males: Relative call timing and sexual selection in katydid choruses. Anim Behav. 1998;56:1091–1098. doi: 10.1006/anbe.1998.0871. [DOI] [PubMed] [Google Scholar]
- 43.Spitzer MW, Takahashi TT. Sound localization by barn owls in a simulated echoic environment. J Neurophysiol. 2006;95:3571–3584. doi: 10.1152/jn.00982.2005. [DOI] [PubMed] [Google Scholar]
- 44.Fertschai I, Stradner J, Romer H. Neuroethology of female preference in the synchronously singing bushcricket Mecopoda elongata (Tettigoniidae; Orthoptera): Why do followers call at all? J Exp Biol. 2007;210:465–476. doi: 10.1242/jeb.02655. [DOI] [PubMed] [Google Scholar]
- 45.Dyson ML, Passmore NI. The combined effect of intensity and the temporal relationship of stimuli on phonotaxis in female painted reed frogs Hyperolius-marmoratus. Anim Behav. 1988;36:1555–1556. [Google Scholar]
- 46.Wyttenbach RA, Hoy RR. Spatial acuity of ultrasound hearing in flying crickets. J Exp Biol. 1997;200:1999–2006. doi: 10.1242/jeb.200.14.1999. [DOI] [PubMed] [Google Scholar]
- 47.Robert D, Miles RN, Hoy RR. Tympanal mechanics in the parasitoid fly Ormia ochracea: Coupling during mechanical vibration. J Comp Physiol. 1998;183:443–452. [Google Scholar]