Abstract
Despite substantial evidence that nitric oxide (NO) and/or endogenous S-nitrosothiols (SNOs) exert protective effects in a variety of cardiovascular diseases, the molecular details are largely unknown. Here we show that following left coronary artery ligation, mice with a targeted deletion of the S-nitrosoglutathione reductase gene (GSNOR−/−) have reduced myocardial infarct size, preserved ventricular systolic and diastolic function, and maintained tissue oxygenation. These profound physiological effects are associated with increases in myocardial capillary density and S-nitrosylation of the transcription factor hypoxia inducible factor-1α (HIF-1α) under normoxic conditions. We further show that S-nitrosylated HIF-1α binds to the vascular endothelial growth factor (VEGF) gene, thus identifying a role for GSNO in angiogenesis and myocardial protection. These results suggest innovative approaches to modulate angiogenesis and preserve cardiac function.
Keywords: angiogenesis, HIF-1α, myocardial infarction, nitric oxide, S-nitrosylation
Nitric oxide (NO) is a potent signaling molecule that has pleiotropic effects in the cardiovascular system. Recently, a role for NO in cellular signaling has been revealed whereby it covalently modifies cysteine residues found at active or allosteric sites of effector proteins (S-nitrosylation). A major subset of these S-nitrosylated proteins (SNO-proteins) are in equilibrium with S-nitrosogluathione (GSNO), a low molecular weight S-nitrosothiol (SNO), which acts as a second messenger to transduce NO bioactivity (1, 2). S-nitrosylation is regulated by the enzyme GSNO reductase (GSNOR), which degrades GSNO and lowers the levels of SNO-proteins (3, 4).
S-nitrosylation of proteins is increasingly implicated in critical aspects of cardiovascular physiology. In the heart, S-nitrosylation of essential regulators of β-adrenergic receptor signaling (e.g., G protein receptor kinase 2, β-arrestin 2) and calcium cycling (e.g., the L-type calcium channel and ryanodine receptor calcium release channel) help to maintain cardiac contractility (5–7). In the peripheral vasculature, S-nitrosylation of caspases (8), dynamin (9) and N-ethylmaleimide sensitive factor (10) mitigate inflammation and apoptosis, whereas S-nitrosylation of hemoglobin regulates blood flow and oxygen delivery (11, 12). SNO-proteins have also been implicated in neovascularization and transducing hypoxic signals (13), but their exact roles in these processes have not been elucidated.
Genetic elimination of GSNOR has enabled the elucidation of functions of SNOs in bacteria, plants, and mammals (14–16), the latter involving cardiovascular roles for SNO-Hb (3), GRK2 (4) and β arrestin 2 (7). To test whether SNO bioavailability, and in particular S-nitrosylation by GSNO, play an essential role in cardiac angiogenesis, we examined the functional consequences of left anterior descending (LAD) coronary artery ligation in mice deficient in the S-nitrosoglutathione reductase gene (GSNOR−/−) (3). We now report that HIF-1 is constitutively S-nitrosylated in normoxic GSNOR−/− mice, and that S-nitrosylated HIF-1 exhibits increased binding to the target gene, VEGF, a potent mediator of angiogenesis. Consistent with these observations, GSNOR−/− mice have increased myocardial capillary density, which preserves tissue oxygenation and cardiac function after an ischemic insult. Taken together, our studies suggest that S-nitrosylation of HIF-1 by endogenous GSNO mediates cardiac angiogenesis, and they suggest strategies to protect from myocardial injury.
Results
Myocardial Infarct Size Is Reduced in GSNOR−/− Mice Following Acute Coronary Ligation.
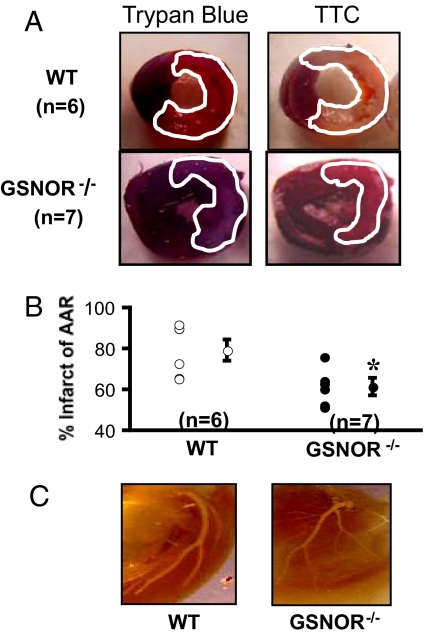
To determine the effect of increased SNO bioavailability on myocardial responses to ischemia, we ligated the LAD coronary artery of wild type (WT) and GSNOR−/− mice. Forty-eight hours following ligation, hearts were explanted and infused with trypan blue to demarcate the ischemic area susceptible to infarction, defined as the area at risk (AAR), and counterstained with triphenyltetrazolium chloride (TTC) to identify infarcted regions within the AAR (Fig. 1A). Despite similar AARs between the 2 groups, GSNOR−/− hearts demonstrated a significantly smaller proportion of infarcted myocardium compared to WT mice (60 ± 5% vs. 80 ± 10%, respectively; *, P = 0.02; Fig. 1B). To rule out aberrant left coronary anatomy as the etiology of reduced infarct size in the GSNOR−/− mice, silicone casts were made of the mice hearts that revealed similar coronary artery anatomies (Fig. 1C).
Fig. 1.
GSNOR−/− mice have smaller infarctions following coronary ligation. (A) Representative cross sections of wild type and GSNOR−/− hearts 48 h following LAD ligation. Before harvesting the heart from the euthanized mouse, the ascending aorta was cannulated to infuse trypan blue for demarcation of the area at risk (AAR, outlined in white). Following removal of the heart and sectioning, each myocardial section was photographed and then incubated in triphenyltetrazolium chloride (TTC) for demarcation of viable versus infarcted myocardium (outlined in white). (B) For each trypan blue myocardial segment, the corresponding TTC stained segment was photographed and used to calculate infarcted percentage of the AAR. The GSNOR−/− mice experienced an approximate 20% reduction in the infracted percentage of the AAR in comparison with the WT mice (*, P = 0.02, GSNOR−/− vs. WT). (C) Structural determination of coronary artery anatomy was performed using silicone casts, digestion of the myocardium, and digital photography. WT and GSNOR−/− mice exhibited similar coronary anatomy with a left main coronary artery that gives off a circumflex branch proximally and terminates in a bifurcation near the LV apex.
GSNOR−/− Mice Show Smaller Infarcts and Preserved Left Ventricular Function 12 Weeks After Myocardial Infarction.
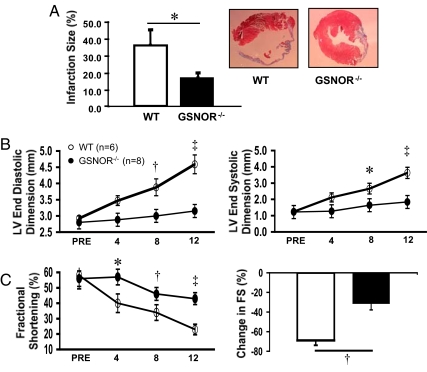
To determine whether the decrease in infarct size at 48 h in the GSNOR−/− mice would result in long-term benefits, we measured the amount of scar tissue by Masson trichrome staining of myocardial sections 12 weeks following infarction. Consistent with the results of acute myocardial ischemia, late infarct size was also significantly smaller in GSNOR−/− mice compared to WT mice (17 ± 3% vs. 35 ± 10% respectively; *, P = 0.03; Fig. 2A). We next determined whether the smaller infract size in the GSNOR−/− mice translated into improved left ventricular function. Following MI, GSNOR−/− mice showed reduced deterioration in LV end-diastolic (EDD) and end-systolic dimensions (ESD), and improved fractional shortening (FS) over the course of 12 weeks (*, P ≤ 0.03; †, P < 0.01; ‡, P < 0.001, respectively; Figs. 2 B and C). These data indicate preservation of cardiac function over time in GSNOR−/− mice compared to WT mice.
Fig. 2.
GSNOR−/− mice have less pathological remodeling and preserved left ventricular function 12 weeks after myocardial infarction. (A) At 12 wks following LAD ligation, mice were euthanized and hearts were harvested for infarction size determination. The GSNOR−/− mice displayed a markedly reduced infarction size compared to WT mice (17 ± 3% vs. 35 ± 10%, respectively; *, P = 0.03). Representative histological sections of infarcted LV myocardium in WT and GSNOR−/− mice demonstrate the decreased infarction size in a knockout heart. (B) WT and GSNOR−/− mice were evaluated with serial echocardiography immediately before, and for 12 wks following LAD ligation. Relative to the WT mice, which exhibited pathologic left ventricular remodeling with steady increase in LV end diastolic and end systolic dimensions (LV EDD, LV ESD), the GSNOR−/− mice experienced little increase in chamber dimensions after MI. (C) LV function, as determined by the percentage of fractional shortening and percent change in fractional shortening, declined to a significantly greater degree in the WT mice during the 12 wks following LAD ligation (*, P ≤ 0.03; †, P < 0.01; ‡, P < 0.001).
Twelve weeks after myocardial infarction (MI), WT and GSNOR−/− mice also underwent hemodynamic assessment to measure intrinsic cardiac contractility using a conductance catheter to obtain pressure-volume loops. These results were consistent with the echocardiographic measures, as GSNOR−/− mice had smaller end-systolic and end-diastolic volumes (ESV and EDV) and significantly increased indices of contractility and diastolic function compared to WT mice (Table 1). Together, these results indicate that increased SNO bioavailability results in reduced susceptibility to myocardial injury.
Table 1.
Hemodynamic assessment 12 weeks following LAD ligation
| Parameter | WT (n = 6) | GSNOR−/− (n = 6) |
|---|---|---|
| HR, bpm | 343 ± 28 | 355 ± 18 |
| ESV, μL | 46 ± 4 | 26 ± 4* |
| EDV, μL | 57 ± 4 | 41 ± 4* |
| ESP, mm Hg | 85 ± 3 | 103 ± 5* |
| EDP, mm Hg | 6 ± 1 | 4 ± 2 |
| Stroke volume, μL | 15 ± 2 | 17 ± 2 |
| EF, % | 26 ± 4 | 42 ± 6* |
| Cadiac Output, μL/min | 5,074 ± 905 | 6,038 ± 678 |
| Stroke Work, mmHg*μl | 838 ± 183 | 1,358 ± 180 |
| dP/dt max, mmHg/s | 5,961 ± 613 | 7,433 ± 786 |
| dP/dt min, mmHg/s | −5,001 ± 462 | −5,403 ± 294 |
| Tau Weiss, ms | 11 ± 1 | 9 ± 1* |
| Maximal power, mWatts | 4 ± 1 | 6 ± 1* |
| Ees | 5 ± 1 | 8 ± 1* |
| Slope-EDPVR | 1 ± 0.2 | 0.4 ± 0.1* |
HR, heart rate; ESV, end-systolic volume; EDV, end-diastolic volume; ESP, end-systolic pressure; EDP, end-diastolic pressure; EF, ejection fraction; Ees, end-systolic elastance; EDPVR, end-diastolic pressure-volume relationship.
*, P < 0.05 versus WT.
GSNOR−/− Mice Have Increased Baseline Capillary Density and Preserved Myocardial Tissue Oxygenation After Ligation of the LAD Artery.
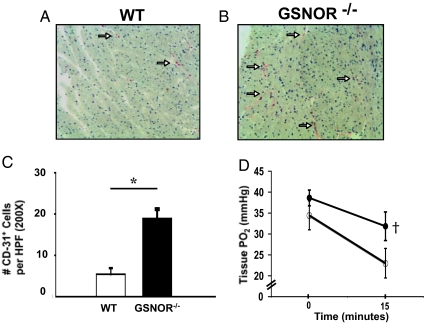
To investigate the mechanisms by which maintained NO bioavailability might reduce myocardial injury, we quantified myocardial capillary density in GSNOR−/− and WT mice. In the basal state, CD31 immunohistochemical staining was significantly greater in GSNOR−/− mice compared to WT mice (*, P = 0.002; Fig. 3A-C,). This finding suggests that chronic elevation in GSNO increases basal myocardial vascularity, thus rendering GSNOR−/− mice less susceptible to ischemic injury.
Fig. 3.
GSNOR−/− mice have increased baseline capillary density and preserved myocardial tissue oxygenation after ligation of the LAD artery. Myocardial immunohistochemical staining for capillary density using CD-31 antibody was performed in (A) WT and (B) GSNOR−/− mice. These representative sections show a higher level of positive CD-31 staining (white arrows) in the GSNOR−/− mouse heart, indicating increased capillary density. (C) Immunohistochemical analysis revealed a significantly greater number of CD-31 positive cells in the myocardium of GSNOR−/− mice (*, P = 0.002). (D) Myocardial partial pressure oxygen (pO2, mm Hg) measurements were recorded in WT and GSNOR−/− mice before and 15 min after ligation of the LAD artery (WT mice n = 10; GSNOR−/− mice, n = 8). Time-points represent averages of continuous readings taken over 10 seconds at each time interval. After 15 min of myocardial ischemia/infarct, WT mice experienced a significant decrease in myocardial pO2 compared to GSNOR−/− mice (†, P = 0.045).
To verify that increased vascularity of GSNOR−/− mice resulted in preserved tissue oxygenation, we measured myocardial oxygen tension before and after ligation of the LAD artery (Fig. 3D). Although pO2 at basal conditions was not significantly different between mice, after 15 min of LAD occlusion, tissue oxygenation was relatively preserved in GSNOR−/− mice compared to WT (22 ± 10 mm Hg vs. 32 ± 8 mm Hg, respectively; P = 0.045). Thus, the increased vascularity in the myocardium of GSNOR−/− mice mitigated tissue hypoxia and preserved ventricular function after myocardial infarction.
GSNOR−/− Mice Have Enhanced Myocardial HIF-1 Transcriptional Activity Under Normoxia.
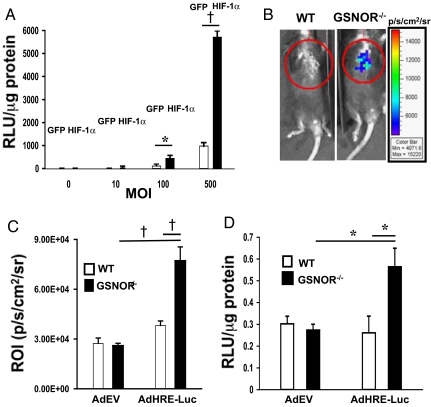
We next evaluated potential mechanisms of increased basal vascularity in GSNOR−/− mice. HIF-1α is a master transcriptional regulator of the cellular response to hypoxia and induces genes such as VEGF that regulate angiogenesis. Although HIF-1α is degraded and undetectable in normoxic tissue, recent in vitro reports indicate that NO and GSNO can stabilize HIF-1α in normoxic conditions (17–19). We therefore hypothesized that deletion of GSNOR might lead to accumulation of HIF-1α in vivo under basal conditions. To test this hypothesis a reporter assay was used. HeLa cells infected with an adenovirus encoding luciferase under the control of a hypoxia response element (HRE) promoter (AdHRE-Luc) were transfected with a plasmid encoding a stabilized HIF-1α mutant or GFP control. Increasing titers of AdHRE-Luc resulted in incremental luciferase activity, whereas transfection with GFP alone had only a minimal effect on luciferase activity (*, P ≤ 0.008; †, P < 0.001, respectively; Fig. 4A). These studies therefore demonstrate a reliable tool to detect HIF-1α transcriptional activity.
Fig. 4.
GSNOR−/− mice exhibit enhanced HIF-1 transcriptional activity in normoxia. HeLa cells were transfected with either GFP or HIF-1α, 24 h before infection with Ad-HRE-luciferase at multiplicities of infection (MOIs) of 0, 10, 100, and 500. (A) The following day, the reporter assay was performed for luciferase activity, which demonstrated greatly increased luciferase activity with increased MOI of Ad-HRE-luciferase. Conversely, transfection with GFP did not induce an appreciable change in luciferase activity relative to HIF-1α (*, P ≤ 0.008; †, P < 0.001, HIF-1α vs. GFP). (B) On day 0, mice underwent single LV injection of empty adenovirus or Ad-HRE-luciferase, and, on day 3, they underwent in vivo bioluminescence evaluation. These representative images depict the significantly increased bioluminescence observed in the GSNOR−/− mice injected with Ad-HRE luciferase compared to WT mice (n = 3 per group). (C) In vivo quantification of emitted photons from these mice demonstrated a marked augmentation of luciferase activity in the GSNOR−/− mice injected with Ad-HRE-luciferase (†, P < 0.001, Ad-HRE-luciferase GSNOR−/− vs. WT; and GSNOR−/− Ad-HRE-luciferase vs. AdEV). (D) Ex vivo quantification of luciferase activity on myocardial tissue harvested on day 6 following viral injection revealed substantially increased relative light units (normalized for protein concentration) in the GSNOR−/− mice injected with Ad-HRE luciferase (*, P ≤ 0.008, Ad-HRE-luciferase GSNOR−/− vs. WT; and GSNOR−/− Ad-HRE-luciferase vs. AdEV).
Using this assay we examined HIF-1-mediated transcriptional activity in vivo following direct intramyocardial injection of AdHRE-luc or a control empty virus in WT and GSNOR−/− mice. Three days after myocardial injection, bioluminescence (in vivo luciferase activity) was measured in a photon detection chamber. GSNOR−/− mice exhibited a significant increase in in vivo bioluminescence (P < 0.001; Fig. 4 B and C) and ex vivo luciferase activity (*, P ≤ 0.008; Fig. 4D) compared to WT mice. HIF-1 transcriptional activity is thus increased in GSNOR−/− mice.
GSNO Stabilizes HIF-1α Expression Under Normoxia.
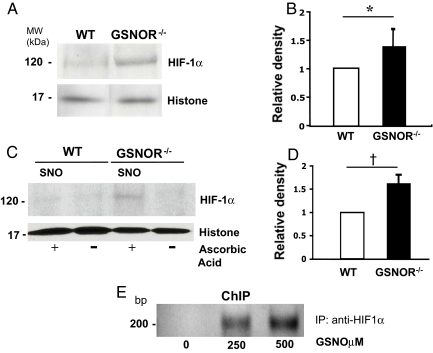
To verify that the enhanced transcriptional activity of HIF-1 under basal conditions corresponded to increased HIF-1α stabilization, we analyzed the expression of HIF-1α in nuclear extracts from the myocardium of WT and GSNOR−/− mice. We discovered an increase in HIF-1α:histone signal in GSNOR−/− mice compared to WT (Fig. 5 A and B; *, P = 0.03; the relative density in GSNOR−/− mice was 1.41 ± 0.28; the ratio of HIF-1α:histone in WT mice was arbitrarily defined as 1). It is important to note that expression of NOS isoforms and downstream effectors of HIF-1α were not changed in cardiac tissues of the WT and GSNOR−/− mice (Fig. S1). Taken together, these data support the notion that increased expression of HIF-1α under normoxic conditions is uniquely regulated by GSNO.
Fig. 5.
HIF-1α protein stabilization and S-nitrosylation is increased in GSNOR−/− mice in normoxia. (A) Nuclear extracts from GSNOR−/− and WT myocardial tissue were analyzed for basal HIF-1α expression (n = 8) by western blot analysis. Normoxic HIF-1α expression was consistently higher in the GSNOR−/− mice compared to WT. (B) Densitometric analysis demonstrates a significant increase in the relative ratio of HIF-1α to histone in the GSNOR−/− mice compared to WT. The relative ratio of HIF-1α to histone in WT mice is arbitrarily presented as 1 (*, P = 0.03, relative density in GSNOR−/− mice 1.41 ± 0.28). (C) S-Nitrosylation of HIF-1α was determined in GSNOR−/− vs. WT mice by the biotin switch technique. S-nitrosylated HIF-1α (+ ascorbic acid, which enables labeling of SNO-protein with biotin) was consistently higher in the nuclear extracts of GSNOR−/− mice compared to WT in normoxic environments. (D) Densitometric analysis demonstrates a significant increase in the relative ratio of S-nitrosylated-HIF-1α to histone in the GSNOR−/− mice compared to WT. The relative ratio of S-nitrosylated HIF-1α to histone in WT mice is arbitrarily defined as 1 (†, P = 0.007, relative density in GSNOR−/− mice 1.62 ± 0.19). (E) Chromatin immunoprecipitation (ChIP) analysis of HIF-1α binding to the promoter region of the VEGF gene in W293 cells treated with increasing concentrations of GSNO. Immunoprecipitation of the HIF-1α-DNA complex revealed increases after treatment with GSNO.
HIF-1α Is S-nitrosylated in Normoxic Myocardium of GSNOR−/− Mice.
To elucidate the mechanism of enhanced HIF-1α stability in GSNOR−/− mice, we performed biotin switch assays on myocardial samples from GSNOR−/− and WT mice as previously described (20). Because of the relatively low concentrations of HIF-1α protein under normoxic conditions, nuclear extracts obtained from the myocardium of 5 mice were combined for each analysis (n = 5 per group, performed in triplicate). Under basal conditions, SNO proteins purified from the myocardium of GSNOR−/− mice and immunoblotted for HIF-1α consistently demonstrated increased S-nitrosylation compared to WT mice (Fig. 5 C and D; †, P = 0.007, the relative density in GSNOR−/− mice was 1.62 ± 0.19; the relative ratio of S-nitrosylated-HIF-1α:histone in WT mice was arbitrarily defined as 1). These data provide a potential mechanism for the increased vascularity found in GSNOR−/− mice under basal conditions.
S-nitrosylated HIF-1α Binds to the Promoter of the VEGF Gene.
To assess whether S-nitrosylation of HIF-1 induces transcription of target genes responsible for angiogenesis, we performed a chromatin immunoprecipitation (ChIP) assay for binding of HIF-1α to the VEGF promoter. W293 cells were treated with increasing concentrations of GSNO for 5 h and then harvested as previously described (21). PCR amplification of the VEGF promoter region occupied by HIF-1α revealed increased expression of the PCR product after treatment with GSNO (Fig. 5E). This data suggests that in response to GSNO, S-nitrosylated HIF-1α binds to the promoter of VEGF, a prototypical angiogenic gene that may account for the increased vascularity observed in GSNOR−/− mice at basal conditions.
Discussion
The results of the present study demonstrate that endogenous S-nitrosothiols confer dramatic protection against myocardial ischemic injury. This effect may result from S-nitrosylation of the transcription factor HIF-1α, which is stabilized in hearts of GSNOR−/− mice, under normoxic conditions. S-nitrosylated HIF-1α, in turn, increases neovascularization to create an oxygen reserve that protects the myocaridum from ischemic injury. These results suggest that endogenous SNOs regulate cardiac HIF independently of O2 tension, and thus expand a paradigm in which SNOs are hypoxia-mimetic (13).
The increased vascularity characteristic of GSNOR−/− mice suggests that angiogenic regulators may be subject to S-nitrosylation, and multiple lines of evidence point to HIF as a possible candidate. In the classic context, hypoxia induces HIF-1α stabilization and translocation to the nucleus where, together with HIF-1β, it binds to hypoxia-response elements in the promoter regions of numerous genes that regulate angiogenesis, such as VEGF. Under normoxic conditions HIF-1α is hydroxylated by oxygen-dependent prolylhydroxylases, ubiquitinated by the E3 ubiquitin ligase pVHL, and then rapidly degraded in the proteasome. However, the notion that HIF-1 is only active in hypoxia has been brought into question by in vitro studies showing that S-nitrosylation stabilizes the protein in normoxia (19, 22). Notably these studies were performed in various tumor cell lines or HIF-1-overexpressing cells in vitro, hence the physiological significance of these findings is not clear. Our findings provide an in situ demonstration of HIF-1α S-nitrosylation and, importantly, link this process to myocardial angiogenesis in normoxia.
In addition to its effects on HIF-1α, increased SNO bioavailability may enact neovasculogenesis through other mechanisms. For instance, it is known that vascular progenitor cells are mobilized from the bone marrow and home to areas of ischemia (23), whereas eNOS-deficient mice demonstrate decreased progenitor cell mobilization and impaired neovascularization after either myelosuppression or coronary artery ligation (24–26). Furthermore, ex vivo treatment of impaired BM-derived mononuclear cells with an eNOS transcriptional enhancer resulted in improved neovascularization in a murine model of hind limb ischemia (27). Consistent with these reports, we found that GSNOR−/− mice exhibit an increased number of bone marrow-derived hematopoietic stem cells (Fig. S2). Although our studies did not pursue this line of investigation, the implications of this observation are far-reaching as they not only support the concept that SNOs subserve vasculogenesis, but more generally indicate a role for S-nitrosylation-based pathways in stem cell biology.
It is important to appreciate that the increase in capillary density likely represents developmental responses to increased SNO bioavailability. Short-term administration of NO donors would not be anticipated to recapitulate these effects (Fig. S3). Thus, our findings support the potential of therapeutically targeting the GSNOR pathway in myocardial ischemia for periods adequate to promote revascularization, and they suggest that unexpected benefits may be derived from chronic NO-based therapies. Additional consequences of GSNOR inhibition include an improvement in β-adrenergic contractility that results from S-nitrosylation of G protein-coupled receptor kinase 2 and β-arrestin 2 (4). Furthermore, S-nitrosothiols within red blood cells—which increase in GSNOR null mice (3)—have been shown to vasodilate the pulmonary, coronary, and systemic vasculature, thereby reducing both right and left ventricular afterload as well as enhancing myocardial oxygen delivery (28–30).
In summary, we demonstrate that GSNOR−/− mice exhibit a unique cardioprotective characteristic in which S-nitrosylation and stabilization of HIF-1α is associated with an increase in myocardial capillary density under basal conditions. This feature maintains tissue oxygen concentrations after coronary artery ligation, which likely accounts in main part for the reduction in infarct size and improvement in myocardial function. Agents that increase tissue SNOs may provide a therapeutic strategy for the management of acute myocardial infarction, coronary artery disease, and congestive heart failure.
Methods
Animals.
Generation and characterization of GSNOR−/− mice have been described previously (3). WT C57BL/6 mice were obtained from Jackson Laboratories and were age-matched to GSNOR−/− mice in each experiment. Animals were handled according to animal welfare regulations of the Duke University Institutional Animal Care and Use Committee.
Experimental Model of Myocardial Infarction.
Adult male mice weighing 23—30 g were anesthetized and intubated as previously described (31). Myocardial infarction was produced by ligating the proximal LAD coronary artery with an 8-O proline suture as previously described (31).
Transthoracic Echocardiography.
Transthoracic echocardiography was performed in conscious mice and myocardial measurements were obtained as described previously (32), using a 30 MHz transducer (Vevo, VisualSonics).
Pressure-Volume Loop Analyses.
Cardiac catheterizations of anesthetized and intubated mice were performed as previously described (32). Steady-state pressure and volume measurements were recorded at baseline and during an increase in afterload that was generated by gently pulling on a suture to transiently constrict the aorta. Data were recorded digitally at 1,000 Hz and analyzed with PVAN analysis software (Millar Instruments).
Assessment of LV Area at Risk, Histological Evaluation of Infarct Size, Miniosmotic Pump Implantation, Myocardial Oxygenation Measurements, Progenitor Cell Cytometry Analysis, Coronary Anatomy Silicone Casts, and Other Biochemical, Immunohistochemical, and Molecular Assays.
The protocols for the above procedures including the biotin switch and ChIP assays are described in the SI Materials and Methods.
Statistical Analysis.
Data are expressed as the mean ± SEM. Multigroup comparisons were performed using 1-way ANOVA with Bonferroni correction. Paired comparisons for were performed using paired 2-tailed Student's t test. For all analyses, P ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgments.
This work was supported by the National Institutes of Health grant P01-HL075443 to Howard Rockman and Jonathan Stamler. We thank Dr. Lan Mao for her assistance in the microsurgical procedures.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901043106/DCSupplemental.
References
- 1.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 2.Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv. 2003;3:253–263. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 4.Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 5.Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol. 2003;35:719–729. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, et al. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa K, et al. S-nitrosylation of b-arrestin regulates b-adrenergic receptor traficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannick JB, et al. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 9.Kang-Decker N, et al. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- 10.Calvert JW, et al. Inhibition of N-ethylmaleimide sensitive factor protects against myocardial ischemia/reperfusion injury. Circ Res. 2007;101:1247–1254. doi: 10.1161/CIRCRESAHA.107.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frehm EJ, Bonaventura J, Gow AJ. S-nitrosohemoglobin: An allosteric mediator of NO group function in mammalian vasculature. Free Radic Biol Med. 2004;37:442–453. doi: 10.1016/j.freeradbiomed.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Singel DJ, Stamler JS. Blood traffic control. Nature. 2004;430:297. doi: 10.1038/430297a. [DOI] [PubMed] [Google Scholar]
- 13.Palmer LA, et al. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 15.Feechan A, et al. A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci USA. 2005;102:8054–8059. doi: 10.1073/pnas.0501456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tada Y, et al. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carver DJ, Gaston B, Deronde K, Palmer LA. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am J Respir Cell Mol Biol. 2007;37:255–263. doi: 10.1165/rcmb.2006-0289SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 21.Yu P, Kodadek T. Dynamics of the hypoxia-inducible factor-1-vascular endothelial growth factor promoter complex. J Biol Chem. 2007;282:35035–35045. doi: 10.1074/jbc.M707557200. [DOI] [PubMed] [Google Scholar]
- 22.Sumbayev VV, Budde A, Zhou J, Brune B. HIF-1 alpha protein as a target for S-nitrosation. FEBS Lett. 2003;535:106–112. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 23.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 24.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 25.Landmesser U, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 26.Jones SP, et al. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci USA. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki K, et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103:14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds JD, et al. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 30.Bin JP, et al. Effects of nitroglycerin on erythrocyte rheology and oxygen unloading: Novel role of S-nitrosohemoglobin in relieving myocardial ischemia. Circulation. 2006;113:2502–2508. doi: 10.1161/CIRCULATIONAHA.106.627091. [DOI] [PubMed] [Google Scholar]
- 31.Curcio A, et al. Competitive displacement of phosphoinositide 3-kinase from beta-adrenergic receptor kinase-1 improves postinfarction adverse myocardial remodeling. Am J Physiol Heart Circ Physiol. 2006;291:H1754–H1760. doi: 10.1152/ajpheart.01199.2005. [DOI] [PubMed] [Google Scholar]
- 32.Perrino C, et al. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.