Abstract
VEGF-B, a homolog of VEGF discovered a long time ago, has not been considered an important target in antiangiogenic therapy. Instead, it has received little attention from the field. In this study, using different animal models and multiple types of vascular cells, we revealed that although VEGF-B is dispensable for blood vessel growth, it is critical for their survival. Importantly, the survival effect of VEGF-B is not only on vascular endothelial cells, but also on pericytes, smooth muscle cells, and vascular stem/progenitor cells. In vivo, VEGF-B targeting inhibited both choroidal and retinal neovascularization. Mechanistically, we found that the vascular survival effect of VEGF-B is achieved by regulating the expression of many vascular prosurvival genes via both NP-1 and VEGFR-1. Our work thus indicates that the function of VEGF-B in the vascular system is to act as a “survival,” rather than an “angiogenic” factor and that VEGF-B inhibition may offer new therapeutic opportunities to treat neovascular diseases.
Keywords: apoptosis, vascular survival, ocular neovascularization
Although current antiangiogenic reagents have shown clinical efficacy, immerging issues such as drug resistance, adverse side effects, selective usage/up-regulation of other angiogenic factors, etc., warrant the identification of other molecules important to antiangiogenic therapy. Further, it is known that blood vessels that have acquired pericyte (PC) or smooth muscle cell (SMC) coverage are more resistant to antiangiogenic therapy and are particularly difficult to prune (1–3). Antiangiogenic strategies targeting not only vascular endothelial cells (ECs), but also PCs and SMCs are therefore desired for greater antiangiogenic efficacy.
Among the 5 VEGF family members (VEGF-A, -B, -C, -D, and PlGF) VEGF-B is probably the least studied thus far. VEGF-B binds to VEGFR-1 and NP-1 (4, 5); Because of its high sequence homology and similar receptor binding patterns to VEGF-A (6), VEGF-B has naturally been considered as an angiogenic factor. However, studies along this line have only led to inconsistent results. VEGF-B was reported to be angiogenic in some studies (7–10) but not in others (11–15). VEGF-B was reported to play a role in pulmonary hypertension (16), but this was unconfirmed by other studies (17). One study reported abnormal coronary artery vasculature and smaller heart in VEGF-B deficient mice (18), while others failed to observe the same (11). Yet another puzzle in understanding the function of VEGF-B has been its' seeming lack of an obvious function. In contrast to VEGF-A or VEGF-C deficient mice (19–21), VEGF-B deficient mice appear largely healthy under normal conditions (11, 12, 17, 18). Unlike other VEGF family members, in most conditions VEGF-B does not affect angiogenesis and blood vessel permeability (9, 11–15, 17, 22–25). Moreover, the cellular and molecular targets of VEGF-B remain thus far unclear. Taken together, data derived from previous studies on VEGF-B have not positioned it as an important target in antiangiogenic therapy. Instead, VEGF-B has received little attention from the field thus far.
In this study, using different animal models and multiple types of cultured vascular cells, we found that even though VEGF-B has a negligible role in inducing blood vessel growth, it is critical for blood vessel survival under pathological conditions. Importantly, the survival effect of VEGF-B is not only on vascular EC but also on vascular SMCs and PCs. VEGF-B is therefore a pleiotropic vascular survival factor affecting all 3 types of vascular cells. In vivo, VEGF-B targeting inhibited both choroidal and retinal neovascularization, implicating VEGF-B as an important target in antiangiogenic therapy.
Results
Dispensable Role of VEGF-B in Blood Vessel Growth.
To test the role of VEGF-B in blood vessel growth, we first used the mouse cornea pocket assay. Exogenously supplied VEGF-A- and bFGF-induced blood vessel growth in VEGF-B deficient and wild-type mouse cornea to a similar extent (Fig. 1A, Upper, n = 8), indicating that in the presence of sufficient amount of other angiogenic factors, VEGF-B is dispensable for blood vessel growth. In the rabbit hind limb ischemia model, VEGF-B167 adenoviral gene delivery did not induce blood vessel growth (Fig. S1 A–D, n = 12, P > 0.05), while VFGF-A adenoviral gene delivery-induced robust angiogenesis in the ischemic hind limb muscles (Fig. S1 A–D, n = 12, P < 0.001). BrdU incorporation assay showed that VEGF-B167 adenoviral gene transfer did not affect cell proliferation (Fig. S1E, n = 12, P > 0.05), while VEGF-A adenoviral gene transfer increased the number of proliferating cells in ischemic muscles (Fig. S1E, n = 12, P < 0.05), further indicating a dispensable role of VEGF-B in inducing blood vessel growth. In cultured bovine aortic endothelial (BAEC) and human vascular SMCs (HVSMS), VEGF-B167 protein did not affect their migration at different concentrations tested (Fig. S1 F–I, n = 4, P > 0.05), while VEGF-A-induced BAEC migration (Fig. S1 F and G, n = 4, P < 0.05) and PDGF-B-induced HUSMC migration (Fig. S1 H and I, n = 4, P < 0.001). Thus, different model systems showed that VEGF-B has a dispensable role in inducing blood vessel growth.
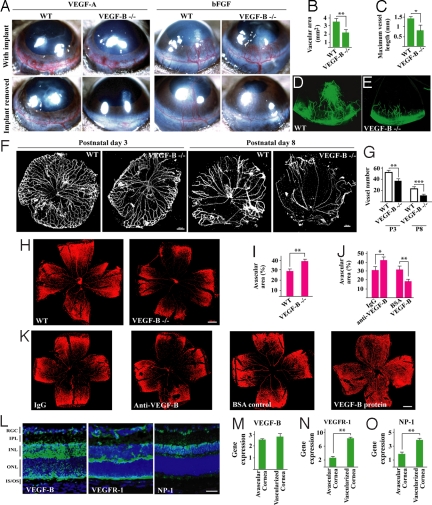
Fig. 1.
VEGF-B is required for blood vessel survival in pathological conditions. (A) VEGF-A- and bFGF-induced blood vessel growth in wild-type (WT) and VEGF-B deficient (VEGF-B −/−) mouse cornea to a similar extent (Upper). After removing the growth factors, blood vessels degenerated faster in the VEGF-B −/− cornea (Lower). (B–E) Three weeks after removing the bFGF implants, fewer vessels were left in VEGF-B deficient cornea. (F and G) VEGF-B −/− hyaloid blood vessels (HBVs) displayed poorer survival at postnatal day 3 and 8 (P3, P8). (Scale bar: 200 μm.) (H and I) VEGF-B deficiency led to more blood vessel regression (bigger avascular areas) in the hyperoxia-induced retinal blood vessel degeneration model measured by Alexa568-isolectin GS-IB4 (IB4) staining. (Scale bar: 300 μm.) (J and K) VEGF-B neutralizing antibody intravitreal injection exacerbated blood vessel regression; VEGF-B167 protein intravitreal treatment inhibited blood vessel regression measured by IB4 staining. (Scale bar: 300 μm.) (L) VEGF-B, VEGFR-1, and NP-1 (green color) were expressed in P8 retina. RGC: retinal ganglion cell layer; IPL: inner plexiform layer; INL/ONL: inner/outer nuclear layer; IS/OS: inner/outer segment. (Scale bar: 50 μm.) (M–O) VEGF-B, VEGFR-1, and NP-1 were expressed in avascular and vascularized cornea measured by real-time PCR (arbitrary unit normalized against beta-actin), with the later 2 up-regulated in vascularized cornea. *, P < 0.05, **, P < 0.01, ***, P < 0.001.
VEGF-B Is Critical for Blood Vessel Survival Under Pathological Conditions.
We next used the mouse cornea pocket assay to test the potential role of VEGF-B in blood vessel survival. When VEGF-A or bFGF implanted in the cornea was removed, the VEGF-A- or bFGF-induced blood vessels in the cornea degenerated faster in VEGF-B deficient cornea compared with those in wild-type cornea (Fig. 1A, lower panel, n = 8). Three weeks after removal of bFGF there were many fewer vessels left in the VEGF-B deficient cornea (Fig. 1 B–E, n = 8, P < 0.01 or 0.05), indicating that although VEGF-B is dispensable for blood vessel growth, it is critical for blood vessel survival. Indeed, VEGF-B deficient hyaloid blood vessels (HBVs) displayed poorer survival at different time points (Fig. 1 F and G, n = 12–18, P < 0.01 or 0.001). Moreover, in the oxygen-induced retinal blood vessel regression model, VEGF-B deficiency led to a greater degree of blood vessel regression compared with wild-type mice (Fig. 1 H and I, n = 10, P < 0.01). Further, intravitreal injection of VEGF-B neutralizing antibody exacerbated blood vessel regression (Fig. 1 J and K, n = 8, P < 0.05; similar to VEGF-B shRNA treatment in Fig. S2), while intravitreal administration of VEGF-B167 protein inhibited blood vessel regression (Fig. 1 J and K, n = 8, P < 0.01; also in Fig. S3 and Fig. S4). VEGF-B, VEGFR-1, and NP-1 were highly expressed in the developing retina (P8) measured by immunohistochemical staining (Fig. 1L, green fluorescence). Real-time PCR showed that VEGF-B, VEGFR-1, and NP-1 were expressed in both normal avascular cornea and vascularized cornea (Fig. 1 M–O), with the later 2 up-regulated in vascularized cornea (Fig. 1 N and O, n = 6, P < 0.01). Thus, different model systems demonstrated that VEGF-B is critically required for blood vessel survival in pathological conditions.
VEGF-B Promotes Survival of Not Only Vascular EC, but also PCs, SMC, and Their Stem/Progenitor Cells.
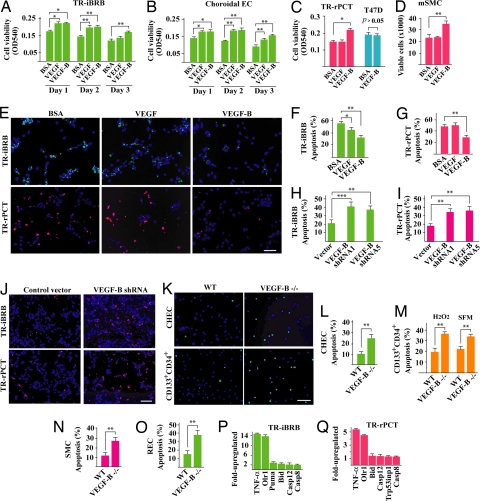
To pinpoint the cellular target of VEGF-B, we starved different types of vascular cells in serum-free medium and investigated their survival status. VEGF-B167 protein treatment (100 ng/ml) increased survival of not only vascular ECs [Fig. 2 A and B, immortalized rat retinal EC (TR-iBRB), choroidal ECs, n = 6, P < 0.01 or 0.05], but also that of PCs [Fig. 2C, immortalized rat retinal EC (TR-rPCT), n = 6, P < 0.05] and SMCs [Fig. 2D, primary mouse aortic SMC (mSMC), n = 6, P < 0.01]. Real-time PCR showed that the TR-iBRB, choroidal EC, TR-rPCT, and mSMC cells expressed both VEGFR-1 and NP-1 (data not shown). VEGF-B167 protein treatment did not increase survival of T47D human ductal breast carcinoma epithelial tumor cells (Fig. 2C, n = 6, P > 0.05), which expressed minimum level of VEGFR-1 and NP-1 measured by real-time PCR (data not shown), indicating that the survival effect of VEGF-B was specific. In ECs, the survival effect of VEGF-B167 was as potent as that of VEGF (Fig. 2 A and B). In PCs and mSMCs, VEGF-B167, but not VEGF, increased cell survival (Fig. 2 C and D). VEGF-B167 treatment inhibited serum deprivation-induced apoptosis in both ECs and PCs, while VEGF inhibited apoptosis only in ECs measured by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (Fig. 2 E–G, n = 8, P < 0.05 or 0.01). The other VEGF-B isoform, VEGF-B186, displayed a similar survival effect as VEGF-B167 on vascular ECs, PCs, and SMCs using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (Fig. S5, n = 6, P < 0.05). Two different VEGF-B shRNA treatment, which decreased VEGF-B expression to about 50% of normal level (Fig. S2C, n = 3, P < 0.01), increased apoptosis in both ECs and PCs (Fig. 2 H–J n = 8, P < 0.001 or 0.01). VEGF-B deficient primary choroidal ECs (CHEC), retinal ECs (REC), primary adult bone marrow-derived CD133+CD34+ vascular progenitor/stem cells, and primary aortic SMCs isolated from VEGF-B deficient mice displayed an increased apoptosis, when cultured in serum-free medium or under H2O2-inducedoxidative stress (Fig. 2 K–O, n = 8, P < 0.01). VEGF-B167 protein treatment (100 ng/ml) reduced apoptosis in different types of VEGF-B deficient cells (Fig. S6, n = 8, P < 0.001, or 0.01, or 0.05), even though the rescue effect did not reach wild-type level, which might be explained by a suboptimal concentration of VEGF-B167 protein, a requirement of both VEGF-B167 and VEGF-B186 protein, or the altered genetic composition of the VEGF-B deficient cells. VEGF-B shRNA knock-down increased expression level of TNF-α and Olr1, 2 potent cell death/apoptosis inducers in vascular ECs and SMCs, as well as other proapoptotic genes (Fig. 2 P and Q). VEGF-B thus promoted survival of multiple types of vascular cells.
Fig. 2.
VEGF-B is a survival factor for multiple types of vascular cells and their progenitors. (A–D) VEGF-B167 protein treatment increased survival of rat retina-derived ECs (A) TR-iBRB, mouse choroidal ECs (B) choroidal EC, rat retina-derived vascular pericytes (C) TR-rPCT, and mouse aortic SMCs (D) mSMC cultured in serum-free medium. In TR-iBRB and choroidal ECs, the survival effect of VEGF-B167 was as potent as VEGF. In TR-rPCT pericytes and mSMC cells, VEGF-B167, but not VEGF, promoted their survival (C and D). VEGF-B167 did not increase survival of T47D human tumor cells (C), which expressed minimum level of VEGFR-1 and NP-1 (data not shown). (E–G) VEGF-B167 treatment inhibited serum starvation-induced apoptosis in TR-iBRB and TR-rPCT cells [TUNEL staining at day 6 after serum starvation, (E Upper and Lower) green and red fluorescein-labeled dUTP incorporations, respectively]. VEGF had an effect only on the TR-iBRB cells. (Scale bar: 20 μm.) (H–J) Two different VEGF-B shRNA treatment increased apoptosis in both TR-iBRB and TR-rPCT cells cultured in serum free medium (day 6 after serum starvation). (Scale bar: 20 μm.) (K–M) VEGF-B deficient CHEC and adult bone marrow derived CD133+CD34+ vascular progenitor/stem cells displayed an increased apoptosis when cultured in serum-free medium (day 6 after serum starvation) or under H2O2-inducedoxidative challenge. (Scale bar: 20 μm.) (N and O) VEGF-B deficient aortic SMCs and retinal ECREC displayed an increased apoptosis when cultured in serum-free medium. (P and Q) VEGF-B shRNA treatment up-regulated the expression of many apoptotic/cell death-related genes in TR-iBRB and TR-rPCT cells measured by real-time PCR. *, P < 0.05, **, P < 0.01, ***, P < 0.001.
Genome-Wide Gene Profiling Reveals That VEGF-B Up-Regulates the Expression of Numerous Vascular Prosurvival Genes.
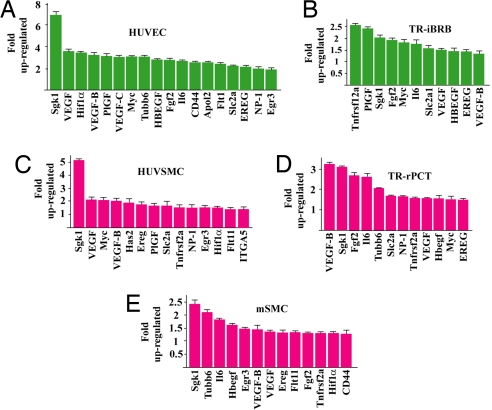
To understand the molecular mechanism underlying the vascular survival effect of VEGF-B, we performed genome-wide gene expression profiling. VEGF-B167 protein treatment of the mouse primary aortic SMCs under a hypoxic condition (1% oxygen) up-regulated the expression of numerous prosurvival genes, such as Sgk1, by more than 2-fold (Table S1). We validated the microarray data using 5 different types of vascular cells, which expressed NP-1 and VEGFR-1 measured by real-time PCR (data not shown). VEGF-B treatment up-regulated the expression of many prosurvival genes in the cells tested (Fig. 3 A–E). Thus, the vascular survival effect of VEGF-B is achieved, at least partially, by up-regulating the expression of numerous prosurvival genes.
Fig. 3.
VEGF-B up-regulates the expression of many prosurvival genes in vascular cells. VEGF-B167 protein treatment up-regulated the expression of many prosurvival genes in vascular ECs, (A) human umbilical vein EC (HUVEC) and (B) TR-iBRB; vascular pericytes, (D) TR-rPCT; and SMCs, (C) human umbilical vein SMC (HUVSMC) and (E) (mSMC) in hypoxia (1% oxygen) measured by real-time PCR.
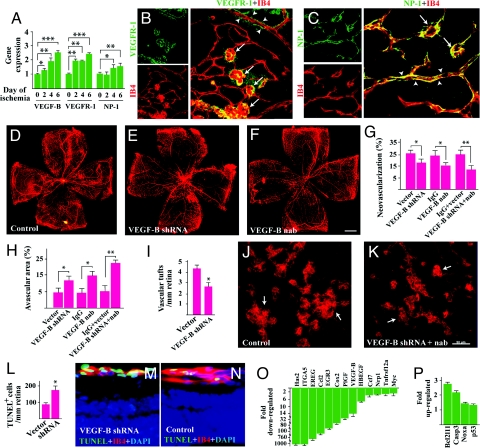
VEGF-B Targeting Inhibits Choroidal Neovascularization (CNV).
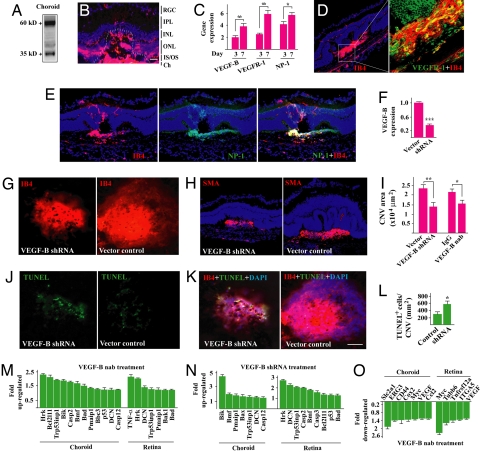
It remains thus far unknown whether VEGF-B targeting could inhibit CNV. VEGF-B was highly expressed in the choroid measured by Western blot (Fig. 4A). Immunohistochemical staining showed that 7 days after laser-induced CNV, VEGF-B was highly expressed in the CNV area (Fig. 4B, lined). The expression level of VEGF-B, VEGFR-1, and NP-1 was increased during CNV development measured by real-time PCR (Fig. 4C, n = 8, P < 0.01 or 0.05). Immunofluorescent staining confirmed the abundant expression of VEGFR-1 and NP-1 in the CNV area and their colocalization with IB4+ staining (EC marker) (Fig. 4 D and E). Intravitreal injection of VEGF-B shRNA, which reduced VEGF-B expression in the retina to about 37% of normal level (Fig. 4F, n = 8, P < 0.001), decreased CNV area significantly (Fig. 4 G and I, n = 7, 8, P < 0.001) and reduced smooth muscle α-actin (SMA)+ (SMC marker) area (Fig. 4H). VEGF-B neutralizing antibody (nab) intravitreal injection also reduced CNV area (Fig. 4I, n = 7, P < 0.05). TUNEL staining showed that VEGF-B shRNA treatment led to more TUNEL+ cells within the CNV area (Fig. 4 J–L, n = 7, 8, P < 0.05). Further, VEGF-B inhibition by shRNA and neutralizing antibody increased the expression of numerous proapoptotic genes in both choroids and retinae with CNV (Fig. 4 M and N) and decreased the expression of many prosurvival genes measured by real-time PCR (Fig. 4O). Thus, VEGF-B targeting inhibited choroidal neovascularization.
Fig. 4.
VEGF-B targeting inhibits CNV. (A) Western blot showed that VEGF-B was highly expressed in adult mouse choroid. (B) Immunofluorescent staining showed that VEGF-B was highly expressed in the choroidal neovascularization area (lined). RGC: retinal ganglion cell layer, IPL: inner plexiform layer, INL/ONL: inner/outer nuclear layer, IS/OS: inner/outer segment; Ch: choroid. (Scale bar: 20 μm.) (C) Real-time PCR (arbitrary unit normalized against beta-actin) showed that VEGF-B, VEGFR-1, and NP-1 expression increased at day 7 after laser treatment in choroid. (D and E) Immunofluorescent staining showed that VEGFR-1 (green, D) and NP-1 (green, E) were expressed in the CNV area and colocalized with IB4 staining (red). (F) VEGF-B shRNA intravitreal treatment decreased VEGF-B transcript level in adult retina measured by the real-time PCR. (G and I) VEGF-B shRNA and neutralizing antibody (nab) intravitreal treatment reduced CNV area. (H) VEGF-B shRNA treated CNV displayed less SMA staining. (J and L) VEGF-B shRNA treatment led to more apoptotic cells (TUNEL staining) within the CNV area. (K) The TUNEL positive cells within the CNV area were colocalized with IB4 staining. (Scale bar: 50 μm.) (M–O) VEGF-B inhibition by neutralizing antibody (nab) or shRNA up- and down-regulated the expression of many apoptotic/cell death-related (M and N) and prosurvival (O) genes respectively in choroids and retinae measured by real-time PCR. *, P < 0.05, **, P < 0.01, ***, P < 0.001.
VEGF-B Targeting Inhibits Ischemia-Induced Retinal Neovascularization.
VEGF-B is abundantly expressed in the retina (Fig. 1L). However, it remained unknown whether VEGF-B targeting could inhibit retinal neovascularization. VEGF-B, VEGFR-1, and NP-1 expression was up-regulated in ischemic retinae measured by real-time PCR (Fig. 5A, n = 6, P < 0.05, or 0.01, or 0.001). Immunofluorescent staining showed that both VEGFR-1 and NP-1 were expressed by retinal blood vessels (Fig. 5 B and C, arrowheads) with a much higher expression level by the newly formed neovascular tufts (Fig. 5 B and C, arrows). VEGF-B shRNA and neutralizing antibody intravitreal injection at P12 reduced retinal neovascularization at P17 (Fig. 5 D–G, n = 8, P < 0.05 or 0.01) and led to fewer neovascular tufts (Fig. 5 I–K, n = 8, P < 0.05). VEGF-B shRNA treatment decreased VEGF-B expression level to about 40% of normal level in the retina (Fig. S2 A and B, n = 6, P < 0.01). The VEGF-B shRNA and neutralizing antibody treated retinae displayed greater avascular areas, at least partially, due to less neovascular formation (Fig. 5 D–F, H, n = 8, P < 0.01). VEGF-B inhibition led to more TUNEL+ cells colocalized with IB4+ staining (vascular EC marker, Fig. 5 L–N, n = 8, P < 0.05). Further, VEGF-B inhibition suppressed considerably the expression of numerous prosurvival genes (Fig. 5O), and increased the expression of several proapoptotic genes in the neovascular retina (Fig. 5P). Thus, VEGF-B targeting inhibited retinal neovascularization.
Fig. 5.
VEGF-B targeting inhibits retinal neovascularization. (A) VEGF-B, VEGFR-1, and NP-1 expression was up-regulated in retinal ischemia measured by real-time PCR (arbitrary unit normalized against beta-actin). (B and C) VEGFR-1 and NP-1 were expressed (immunofluorescent staining) in both normal retinal vasculature (arrowheads) and in retinal neovascular tufts (arrows) with a higher expression level in the later (arrows). (Scale bar: 50 μm.) (D–H) VEGF-B shRNA and neutralizing antibody (nab) intravitreal injection reduced retinal neovascularization and led to greater avascular areas in the retinae. VEGF-B nab + shRNA treatment inhibited retinal neovascularization to a greater extent. (Scale bar: 300 μm.) (I–K) VEGF-B shRNA and nab treatment reduced neovascular tuft numbers in the retina. (Scale bar: 50 μm.) (L–N) VEGF-B shRNA treatment led to more TUNEL+ cells colocalized with IB4 staining in the tuft area. (O and P) Real-time PCR showed that VEGF-B inhibition (nab + shRNA) suppressed and increased the expression of many prosurvival (O) and cell death-related/apoptotic (P) genes respectively in the ischemic retinae. *, P < 0.05, **, P < 0.01, ***, P < 0.001.
Both NP-1 and VEGFR-1 Mediate the Vascular Survival Effect of VEGF-B.
VEGF-B binds to NP-1 (5). To verify whether NP-1 mediates the vascular survival effect of VEGF-B, we screened for an NP-1 neutralizing antibody that can block VEGF-B binding to NP-1 using a surface plasmon resonance assay. We found that the antiNrP-1B neutralizing antibody to a certain extent blocked VEGF-B167 binding to NP-1 (Fig. S3 A and B). In cultured TR-rPCT cells, antiNrP-1B treatment largely abolished the regulatory effect of VEGF-B167 on the expression of many prosurvival genes (Fig. S3 C, n = 3, P < 0.001 or 0.01). In the oxygen-induced retinal blood vessel regression model, antiNrP-1B intravitreal injection abolished, to a certain extent, the survival effect of VEGF-B167 on retinal vasculature (Fig. S3 D–G, n = 8, P < 0.01 or 0.05). VEGF-B also binds to VEGFR-1 (4). VEGFR-1 was detected in TR-iBRB cells using Western blot assay (Fig. S4A). VEGF-B167 stimulation (100 ng/ml) resulted in VEGFR-1 activation in these cells in hypoxia (1% oxygen) (Fig. S4A). VEGFR-1 neutralizing antibody treatment largely abolished the regulatory effect of VEGF-B167 on the expression of many prosurvival genes in TR-rPCT cells (Fig. S4B, n = 6, P < 0.001, or 0.01, or 0.05). In vivo, VEGFR-1 neutralizing antibody intravitreal injection to a large extent abolished the survival effect of VEGF-B167 on retinal vasculature in the oxygen-induced blood vessel regression model (Fig. S4 C–F, n = 7, P < 0.01). Thus, both NP-1 and VEGFR-1 play a role in mediating the vascular survival effect of VEGF-B.
Discussion
In this study, using different animal models and multiple cultured vascular cells, we found that VEGF-B is a vascular survival factor rather than an angiogenic factor. Further, the vascular survival effect of VEGF-B is not only on vascular ECs, but also on vascular SMCs and PCs. VEGF-B targeting inhibited both choroidal and retinal neovascularization in vivo, implicating VEGF-B as an important target in antiangiogenic therapy.
We showed in this study that VEGF-B deficiency or protein/gene delivery did not affect blood vessel growth. Instead, VEGF-B deficiency/inhibition led to poorer blood vessel survival in the cornea pocket assay after growth factor withdrawal, fewer surviving hyaloid vessels in postnatal mouse eyes, and greater oxygen-induced retinal vascular degeneration in neonatal mice. In vitro, VEGF-B deficient vascular cells displayed greater apoptosis when challenged by serum deprivation or oxidative stress. Conceivably, VEGF-B treatment rescued blood vessel survival both in vitro and in vivo under stressed conditions. Consistent with these findings, genome-wide gene profiling revealed that VEGF-B up-regulated the expression of numerous prosurvival genes in different types of vascular cells. Thus, although VEGF-B is dispensable for blood vessel growth, it is critical for blood vessel survival in pathological conditions. Our data therefore advocates a conceptual change of the function of VEGF-B in the vascular system as a “survival” rather than an “angiogenic” factor. This explains the controversies on the “angiogenic” nature of VEGF-B in previous studies. That is, as a survival factor, VEGF-B has a negligible role in inducing blood vessel growth, explaining why in many conditions VEGF-B protein treatment or gene delivery did not induce blood vessels growth (11–14, 26). On the other hand, under certain pathological conditions when blood vessels are stressed, VEGF-B is then critically required and VEGF-B treatment can indeed lead to more (survived) blood vessels (7, 8, 26, 27). Under such conditions, VEGF-B may appear to be “angiogenic” (7, 8, 26, 27). However, this “angiogenic” effect is likely due to its vascular survival activity.
Another important finding in this study is that the survival effect of VEGF-B is on multiple types of vascular cells, including vascular ECs, PCs, and SMCs. This was observed in both isolated primary vascular cells and established vascular cell lines, using both loss- and gain-of-function assays. It is known that once blood vessels acquire PC or SMC coverage and become more mature, they are more resistant to antiangiogenic reagents and difficult to prune (1, 3). Antiangiogenic strategies targeting not only vascular ECs, but also PCs and SMCs simultaneously are much desired. Our data showed that VEGF-B may be one such pleiotropic molecule to be inhibited. Indeed, VEGF-B targeting by genetic deletion, neutralizing antibody or shRNA inhibited both choroidal and retinal neovascularization. VEGF-B therefore may be an important target in antiangiogenic therapy and VEGF-B blockade may have clinical implications in treating different types of vascular malformations, such as hemangioma (28, 29).
In summary, we showed in this study that although VEGF-B is dispensable for blood vessel growth, it is critical for their survival. Our work advocates a conceptual change of the function of VEGF-B in the vascular system as a “survival,” rather than an “angiogenic” factor. Importantly, the vascular survival effect of VEGF-B is pleiotropic and on multiple types of vascular cells. VEGF-B inhibition may therefore offer new therapeutic opportunities to treat different types of neovascular diseases.
Materials and Methods
Blood Vessel Survival, Retinal Neovascularization, and Rabbit Hind Limb Ischemia Models.
All animal experiments were approved by the Animal Care and Use Committee at the National Eye Institute/National Institutes of Health (animal study protocol #06–553) and were performed according to the NIH guidelines. VEGF-B deficient mice were described previously and were bred onto C57Bl6 strain for more than 6 generations (25). Blood vessel survival, retinal neovascularization, and rabbit hind limb ischemia models are described in SI Materials and Methods.
Primary Cell Isolation, Culture, and Survival/Migration/Real-Time PCR Assays.
Primary mouse retinal/choroidal ECs, aortic artery SMCs, and bone marrow CD133+CD34+ cells were isolated and cultured as described in SI Materials and Methods. All cell culture experiments were performed in triplicates and repeated at least twice. Primers used for real-time PCR are listed in Table S2.
Laser-Induced CNV Model and Surface Plasmon Resonance (SPR) Assay.
The laser-induced CNV model was described previously (25) and in SI Materials and Methods. The SPR assay used to screen for an NP-1 neutralizing antibody to block VEGF-B binding to NP-1 was described previously (30) and in SI Materials and Methods.
Microarray Analysis, VEGFR-1 Expression/Activation Assay, and Statistics.
Microarray analysis using primary mouse aortic artery SMCs and VEGFR-1 expression/activation assay were described previously (25). Two tailed Student's t test was used for statistical analysis. Difference was considered statistically significant when P < 0.05. Data are presented as mean ± SEM of the number of determinations.
Supplementary Material
Acknowledgments.
We thank Dr. Ken-ichi Hosoya at the Faculty of Pharmaceutical Sciences, University of Toyama, Japan, for kindly providing the rat retinal cell lines. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging and the National Eye Institute.
Footnotes
Conflict of interest statement: A.N. and P.S. are employees of CSL Limited and A.N. holds stock options. R.J.W. and A.W.K. are employees of Genentech, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813061106/DCSupplemental.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development (Cambridge, UK)t. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 4.Olofsson B, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makinen T, et al. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol Chem. 1999;274:21217–21222. doi: 10.1074/jbc.274.30.21217. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. 2001;33:421–426. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 7.Silvestre JS, et al. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res. 2003;93:114–123. doi: 10.1161/01.RES.0000081594.21764.44. [DOI] [PubMed] [Google Scholar]
- 8.Wright CE. Effects of vascular endothelial growth factor (VEGF)A and VEGFB gene transfer on vascular reserve in a conscious rabbit hindlimb ischaemia model. Clin Exp Pharmacol Physiol. 2002;29:1035–1039. doi: 10.1046/j.1440-1681.2002.03773.x. [DOI] [PubMed] [Google Scholar]
- 9.Mould AW, et al. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res. 2005;97:e60–70. doi: 10.1161/01.RES.0000182631.33638.77. [DOI] [PubMed] [Google Scholar]
- 10.Yoon YS, Losordo DW. All in the family: VEGF-B joins the ranks of proangiogenic cytokines. Circ Res. 2003;93:87–90. doi: 10.1161/01.RES.0000084992.10766.36. [DOI] [PubMed] [Google Scholar]
- 11.Aase K, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Reichelt M, et al. Vascular endothelial growth factor-B and retinal vascular development in the mouse. Clin Experiment Ophthalmol. 2003;31:61–65. doi: 10.1046/j.1442-9071.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 13.Rissanen TT, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaj S, et al. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther. 2003;14:1451–1462. doi: 10.1089/104303403769211664. [DOI] [PubMed] [Google Scholar]
- 15.Malik AK, et al. Redundant roles of VEGF-B and PlGF during selective VEGF-A blockade in mice. Blood. 2006;107:550–557. doi: 10.1182/blood-2005-05-2047. [DOI] [PubMed] [Google Scholar]
- 16.Wanstall JC, et al. Vascular endothelial growth factor-B-deficient mice show impaired development of hypoxic pulmonary hypertension. Cardiovasc Res. 2002;55:361–368. doi: 10.1016/s0008-6363(02)00440-6. [DOI] [PubMed] [Google Scholar]
- 17.Louzier V, et al. Role of VEGF-B in the lung during development of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003;284:L926–L937. doi: 10.1152/ajplung.00247.2002. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo D, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 21.Karkkainen MJ, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 22.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: Role of endogenous PAF and NO synthesis. J Cell Biochem. 2007;100:727–737. doi: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 23.Abraham D, et al. VEGF-A and -C but not -B mediate increased vascular permeability in preserved lung grafts. Transplantation. 2002;73:1703–1706. doi: 10.1097/00007890-200206150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, et al. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol. 2008;28:1614–1620. doi: 10.1161/ATVBAHA.107.158725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wafai R, Tudor EM, Angus JA, Wright CE. Vascular Effects of FGF-2 and VEGF-B in Rabbits with Bilateral Hind Limb Ischemia. J Vasc Res. 2008;46:45–54. doi: 10.1159/000139132. [DOI] [PubMed] [Google Scholar]
- 28.Lapidoth M, Ben-Amitai D, Bhandarkar S, Fried L, Arbiser JL. Efficacy of topical application of eosin for ulcerated hemangiomas. J Am Acad Dermatol. 2009;60:350–351. doi: 10.1016/j.jaad.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Shirazi F, Cohen C, Fried L, Arbiser JL. Mammalian target of rapamycin (mTOR) is activated in cutaneous vascular malformations in vivo. Lymphat Res Biol. 2007;5:233–236. doi: 10.1089/lrb.2007.1012. [DOI] [PubMed] [Google Scholar]
- 30.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.