Abstract
The marine cyanobacterium Trichodesmium is ubiquitous in tropical and subtropical seas and is an important contributor to global N and C cycling. We sought to characterize metabolic uptake patterns in individual Trichodesmium IMS-101 cells by quantitatively imaging 13C and 15N uptake with high-resolution secondary ion mass spectrometry (NanoSIMS). Trichodesmium fix both CO2 and N2 concurrently during the day and are, thus, faced with a balancing act: the O2 evolved during photosynthesis inhibits nitrogenase, the key enzyme in N2 fixation. After performing correlated transmission electron microscopy (TEM) and NanoSIMS analysis on trichome thin-sections, we observed transient inclusion of 15N and 13C into discrete subcellular bodies identified as cyanophycin granules. We speculate that Trichodesmium uses these dynamic storage bodies to uncouple CO2 and N2 fixation from overall growth dynamics. We also directly quantified both CO2 and N2 fixation at the single cell level using NanoSIMS imaging of whole cells in multiple trichomes. Our results indicate maximal CO2 fixation rates in the morning, compared with maximal N2 fixation rates in the afternoon, bolstering the argument that segregation of CO2 and N2 fixation in Trichodesmium is regulated in part by temporal factors. Spatial separation of N2 and CO2 fixation may also have a role in metabolic segregation in Trichodesmium. Our approach in combining stable isotope labeling with NanoSIMS and TEM imaging can be extended to other physiologically relevant elements and processes in other important microbial systems.
Keywords: NanoSIMS, stable isotope labeling, cyanophycin
The marine cyanobacterium Trichodesmium is ubiquitous in tropical and subtropical seas and is an important contributor to global N and C cycling (1). As a diazotrophic cyanobacterium, Trichodesmium is capable of both CO2 and N2 fixation. Studies have estimated that it may leak up to 30–50% of its newly fixed N (2), providing a valuable source of bioavailable N to other nondiazotrophic phytoplankton species cohabitating in the N-limited subtropical gyres. Capone et al. (3) estimate that it contributes ≈5.7 Tmol new N y−1 in the North Atlantic Ocean, which is comparable with the rate of NO3− that diffuses from depth into oligotrophic upper ocean ecosystems (3, 4). Although Trichodesmium is not the sole diazotrophic cyanobacteria in the open ocean (5–7), it is the most conspicuous and well-studied. Also, it has been in culture since 1992 (8), allowing numerous studies of its physiology and response to different limiting and controlling factors. Trichodesmium is often included in ecosystem models that attempt to describe CO2 and N2 fixation in open ocean systems (9). A better understanding of CO2 and N2 fixation in Trichodesmium is critical, because this species has a large role in global C and N cycling in the open ocean.
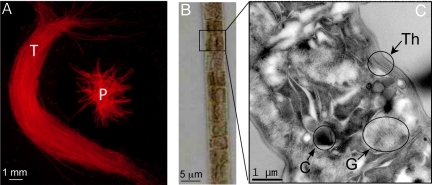
Commonly referred to as “saw dust” on the surface of the sea (10), Trichodesmium grows in filaments (referred to as trichomes) that can have 100–200 cells (Fig. 1B). In the field, Trichodesmium may be found in its colony form, as either puff-shaped or tuft-shaped colonies (Fig. 1A) (11). Colonies typically have 100–200 trichomes. In some systems, free trichomes may predominate (12). Trichodesmium are high-light adapted, and have gas vesicles that keep them near the surface of the ocean (Fig. 1C) (13). They often form thick blooms visible in satellite imagery (14). Trichodesmium grown in culture usually occurs as free trichomes of ≈80–100 cells per trichome during exponential phase, and aggregates during stationary phase.
Fig. 1.
Images of Trichodesmium filaments at 3 levels of magnification. (A) Image of Trichodesmium tuft (T) and puff (P) taken under green excitation (510–560 nm); (B) image of single Trichodesmium trichome by using light microscopy at 20× magnification; and (C) TEM image of individual Trichodesmium cell, demonstrating a cyanophycin granule (C), gas vesicles (G), and thylacoid membranes (Th).
Diazotrophy is a significant challenge for unicellular microorganisms, because the O2 produced from CO2 fixation is inhibitory to nitrogenase, the key enzyme in N2 fixation. Therefore, diazotrophs have developed different behavioral, biochemical, and physical strategies to protect nitrogenase from the O2 evolved during photosynthesis. Certain cyanobacteria, such as Gloeothece spp., temporally segregate the processes over a diel cycle by fixing CO2 during the day and fixing N2 at night (15). Others, such as Anabaena spp., have terminally differentiated cells, termed heterocysts, with thickened cell walls, and reduced PS II and rubisco activity (16). These cells serve to spatially segregate the 2 processes, with N2 fixation occurring in the heterocysts, whereas oxygenic photosynthesis and CO2 fixation occurs in vegetative cells. Trichodesmium is unique in that it is a nonheterocystous cyanobacteria that fixes both CO2 and N2 concurrently during the day (1). There is considerable current debate among researchers as to how these processes co-occur. Current theories, and sometimes conflicting data, suggest increased O2 consumption within cells protects nitrogenase [e.g., increased hydrogenase (17), Mehler (18, 19), and superoxide dismutase activity (20)], in addition to both spatial (21–23) and temporal segregation of CO2 and N2 fixation (19, 24, 25). Much of the current debate revolves around the metabolic potential of individual cells in a trichome, and how those capabilities may differ among cells in a trichome or within individual cells over time (26).
To investigate the dynamics of C and N metabolism at this level, we combined tracer-level additions of inorganic 13C and 15N with high-resolution secondary ion mass spectrometry (NanoSIMS; Cameca). NanoSIMS imaging allowed us to track C and N stable isotope incorporation rates and subsequent cellular fates by mapping distributions of isotopes (27) in multiple trichomes. The results of our NanoSIMS investigations are compared with bulk isotopic analyses and related to existing models of Trichodesmium metabolism.
Results and Discussion
NanoSIMS imaging was performed on Trichodesmium trichomes spiked with NaH13CO3 and 15N2, and harvested sequentially during 24 h (see Materials and Methods). Both whole- and thin-sectioned samples were imaged to quantify the distribution of newly fixed 13C and 15N within trichomes. The distribution of newly fixed 13C and 15N was determined by simultaneously imaging C and N isotopes and calculating quantitative 13C/12C and 15N/14N ratio images. Each 10-μm2 image includes 2 to 3 cells, and was scanned with 150-nm resolution to capture intercellular variability. Multiple adjacent cells along multiple trichomes were analyzed to quantify cell–cell variability. For thin-sectioned trichomes, we used transmission electron microscopy (TEM) imaging to morphologically map samples before NanoSIMS analysis.
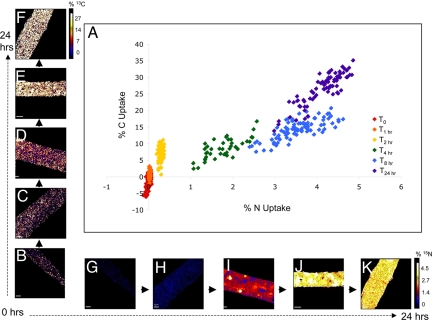
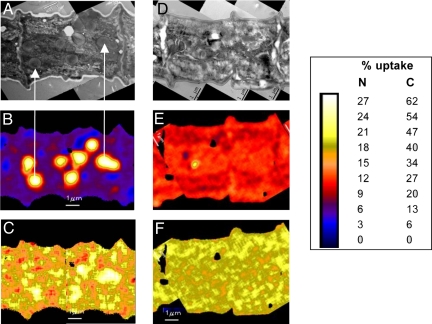
NanoSIMS analysis showed substantial subcellular spatial variability in 15N and 13C enrichment along Trichodesmium trichomes and with depth through individual cells (Figs. 2, 3, and 4); 13C/12C and 15N/14N ratio images generated from sectioned trichomes, along with correlated TEM maps, provide direct evidence of subcellular uptake localization within cells ≈8 h, and the redistribution of that enrichment after 24-h incubation (Fig. 3). In both sectioned and whole cells, we observed discrete hotspots enriched in 15N and 13C at 4 h, with increased density at 8 h (Figs. 2 I and J, and 3 B and C). These features are likely cyanophycin granules, a type of N-rich storage vacuole comprised of an asparagine and aspartate polymer found in many cyanobacteria (28–30), including Trichodesmium (21, 31). NanoSIMS analyses of Trichodesmium sections from 8 h indicate that the 13C:15N ratio of the cyanophycin granules is consistent with a mixture of asparagine (2:1) and aspartate (4:1), providing chemical evidence to identify these granules as cyanophycin. Also, these 15N hotspots correspond directly to vacuole-like structures identified as cyanophycin granules in corresponding TEM images (Fig. 3 A and B). The hotspots were no longer evident after 24-h incubation, at which point cells were uniformly enriched with newly fixed 15N (Figs. 2K and 3E). These results demonstrate the dynamic nature of these subcellular features, where it appears that newly fixed 15N is stored during the daytime (when N2 fixation is active), and is subsequently used at night for cellular biosynthesis (when N2 fixation has ceased). As might be expected, elevated levels of 13C are also associated with these cyanophycin granules (Fig. 3C).
Fig. 2.
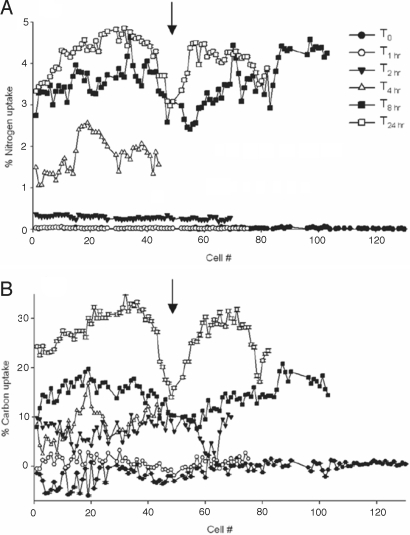
Percentage 13C and 15N uptake by Trichodesmium trichomes at 6 time points and corresponding NanoSIMS images. (A) Percentage 13C and 15N uptake at 5 time points in Trichodesmium cells analyzed by NanoSIMS. Along the x axis are representative NanoSIMS images of 15N enrichment in ≈2.5 cells at (B) 0 h, (C) 2 h, (D) 4 h, (E) 8 h, and (F) 24 h. Along the y axis are corresponding images of 13C enrichment at (G) 0 h, (H) 2 h, (I) 4 h, (J) 8 h, and (K) 24 h. Data points represent average values for individual cells measured along a single trichome by NanoSIMS. [Scale bar, 1 μm; image enrichment scale ranges from 0 to 5% uptake (N), and 0 to 30% uptake (C), moving from black-blue-red-yellow-white.]
Fig. 3.
TEM images of ≈2 cells from a Trichodesmium filament after (A) 8-h and (D) 24-h incubation with H13CO3 and 15N2. Correlated NanoSIMS images demonstrate percentage fixed 15N after (B) 8 h and (E) 24 h, percentage fixed 13C after (C) 8 h and (F) 24 h. Arrows indicate correlation between cyanophycin granules identified by TEM and 15N enriched hotspots evident in NanoSIMS image. (Scale bar, 1 μm.) Because NanoSIMS analysis is a destructive process, distinct cells were imaged for the 2 different time points.
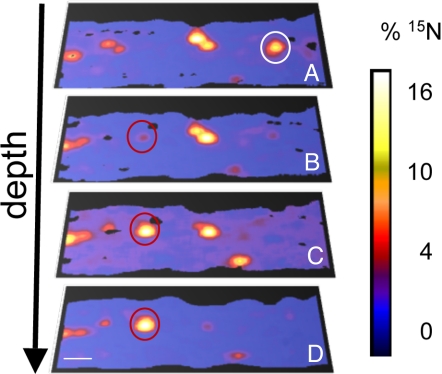
Fig. 4.
Images of cyanophycin granules, as evidenced by hotspots of 15N/14N accumulation (percentage 15N uptake), with depth through ≈2 individual cells. The white circle in A represents a cyanophycin granule that does not appear in the subsequent images. The red circles in B–D indicate a cyanophycin granule that becomes more prominent with depth through the cell. (Scale bar, 1 μm.)
NanoSIMS analysis also allowed us to examine subcellular isotope enrichment in the vertical dimension of cells. Based on analyses of multiple cells examined from multiple trichomes and time points, we determined that cyanophycin granules can comprise up to 6.4% (± 0.7%) of cell area/volume, and are evenly distributed along trichomes. In depth profile analyses of whole cells, as the NanoSIMS analyzed deeper and deeper layers, individual cyanophycin granules became apparent, and then disappeared as the ion beam sputtered through them (Fig. 4), suggesting that the granules are randomly distributed throughout the cell volume. It has been hypothesized that certain nondifferentiated cells, called “diazocytes”, along a trichome contain nitrogenase and are the sole sites of N2 fixation (21, 22, 32). Fredriksson and Bergman (21) found that only 24% of these diazocyte cells contained cyanophycin granules, compared with 61% of non-N2-fixing cells. We did not find evidence for this theory in our samples. When cells were examined in TEM thin sections (0-, 8-, and 24-h incubations), and with NanoSIMS depth profiling (4- and 8-h incubations), cyanophycin granules were observed at similar densities in all cells.
Previous studies have examined the transient nature of cyanophycin granules in single-celled and heterocystous diazotrophs. Sherman et al. (30) used immunocytochemical analysis to demonstrate the localization of cyanophycin at the polar plugs of mature heterocysts in Anabaena sp. PCC7120. Mackerras et al. (33) used cultures of Anabaena cylindrica (a heterocystous cyanobacteria) and Synechocystis 6308 (a unicellular cyanobacteria that temporally segregates CO2 and N2 fixation) grown in media with limited ammonium concentration ([NH4+]). They demonstrate that synthesis and subsequent degradation of cyanophycin granules in these 2 organisms as [NH4+] in the media is drawn down, indicating that the 2 cyanobacteria produce cyanophycin granules in N-replete conditions and use the stores during N-deplete conditions. Our study demonstrates the dynamic nature of the cyanophycin pool in Trichodesmium grown under diazotrophic conditions. Transient accumulation of cyanophycin indicates an uncoupling of CO2 and N2 fixation with cell growth. Interestingly, recent modeling of Trichodesmium metabolism (34) suggested that storage pools of C and N are dynamic over the diel cycle, accumulating during the day and consumed during the dark period. Our results provide direct evidence for this prediction.
In more than half of the trichomes analyzed from the 8- and 24-h incubations, we observed a marked reduction in 13C and 15N enrichment in cells located near the middle of trichomes (Fig. 5); this pattern may be related to programmed cell death (PCD) and/or trichome splitting. In Trichodesmium, as cells divide and the trichome increases in length, it is hypothesized that cells at the center of the trichome begin to have decreased metabolic activity and CO2 and N2 fixation. Eventually, the trichome splits at this point, yielding 2 shorter trichomes. Although PCD has been described for Trichodesmium (24, 35), we cannot confirm whether zones of reduced 13C and 15N uptake we observed are undergoing PCD, or whether the reduction in activity is due to other factors. However, secondary electron images we obtained before SIMS analysis provide visual evidence of thinner cells at these points (Fig. S1), suggesting that the trichome is stressed and about to break.
Fig. 5.
Percentage isotope uptake in individual cells along the full length of Trichodesmium trichomes (filaments) at increasing time intervals for 15N (A) and 13C (B). Each data point represents average enrichment (measured by NanoSIMS) in a single cell, and data are plotted in the order found in the analyzed trichome. For example, the midpoint of the T24 trichome is found at approximately cell 50. Different symbols indicate trichomes from successive harvest points after addition of stable isotope labels. An arrow marks the point of proposed trichome division. Error bars represent 2 sigma error, but are small enough as to be obscured by the data symbols.
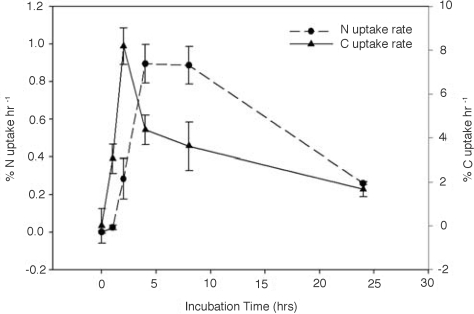
We used NanoSIMS analysis of whole cells to calculate uptake rates of CO2 and N2 fixation at the single cell level. These data were averaged for 40–120 contiguous cells located in multiple trichomes from each time point (Fig. 6), and were comparable to rates calculated from samples harvested in parallel, and bulk-analyzed by isotope ratio mass spectrometry (Fig. S2). Results from both approaches indicate that CO2 fixation rates were highest in the morning (≈10 AM), whereas N2 fixation rates were highest in the afternoon (≈12 PM; Fig. 6 and Fig. S2). These results provide evidence for a temporal decoupling of CO2 and N2 fixation in Trichodesmium, and corroborate earlier findings where Berman-Frank et al. (24) used fast repetition rate fluorescence (FRRF), O2 production, 14CO2 uptake, and acetylene reduction, to show maximal CO2 fixation rates in the morning (≈11 AM) and afternoon (≈3 PM). Maximal N2 fixation rates were measured at midday (≈12 PM), when CO2 fixation was down-regulated. Küpper et al. (25), using fluorescence kinetic microscopy, provided further evidence for temporal segregation within individual cells, and suggest that there is a finely regulated shift between photosynthetic activity states throughout the course of the day that contributes to the protection of nitrogenase from O2 evolved during photosynthesis. Together, our findings and these aforementioned studies build on the theory that increased O2-consumptive activities within individual cells (17–20) enable them to temporally segregate the incompatible processes of CO2 and N2 fixation.
Fig. 6.
15N and 13C fixation rates (percentage uptake hour−1) in Trichodesmium cells during a 24-h isotope labeling experiment. Data are generated from single cell NanoSIMS analysis of multiple filaments, and are averaged by time point (average n = 73 cells per time point). Error bars represent 2 sigma error.
A controversial and evolving area of debate in the field of Trichodesmium physiology has been the suggestion that CO2 and N2 fixation within Trichodesmium are spatially segregated between different cells. The earliest microautoradiography studies suggested that spatial segregation happens at the level of the colony, with anoxic microzones occurring near the center of aggregates, where N2 fixation was localized and photosynthesis down-regulated (36, 37). This theory was later questioned, when it was demonstrated that individual trichomes from disrupted colonies still fix both N2 and CO2 (38). More recently, conflicting immunochemical studies have examined the distribution of nitrogenase proteins along trichome cells. Some studies (23, 39) indicate that nitrogenase is dispersed throughout all cells in colonies, whereas other research suggests that only ≈15% of cells along a trichome contain nitrogenase (21, 22), and that these cells occur in clusters along the trichome (21, 32). These clusters, referred to as diazocytes, suggest a spatial segregation of N2 fixation and CO2 fixation. It should be noted that cells that contain nitrogenase are not necessarily photosynthetically inactive (15).
In our study, after 2 h of exposure to labeled isotopes, newly fixed 13C and 15N was apparent and relatively evenly distributed in all cells along trichomes (Fig. 2). Although depleted regions did develop by 8 and 24 h, uptake of both C and N was simultaneously lower, and these regions corresponded to morphologically anomalous “thinning” spots thought to be prebreakage points (see discussion above). However, in our experiment, the lack of clear spatial differences in uptake patterns could be attributed to rapid redistribution of recently fixed C and N. Wolk et al. (40), using autoradiography with 13N-N2 gas, demonstrated in the cyanobacteria Anabaena variabilis that N2 fixing cells are able to fix and redistribute newly fixed N to neighboring cells in <1.5 min. More recently, in experiments with the heterocystous cyanobacteria Anabaena oscillariodes, extant heterocysts were depleted in 15N relative to vegetative cells (41), despite the fact that the heterocysts are the indisputable sites of N2 fixation. This phenomenon may be due to the loss of diffusible ions and small molecules in the cytosol that are not fixed in place by cell fixatives used before NanoSIMS analysis (e.g., gluteraldehyde) (42). If a similar mechanism occurred in Trichodesmium cells, it could account for soluble metabolites formed in the early time points being undetectable. Although our data seems to suggest otherwise, it is possible that CO2 and N2 fixation did not occur concurrently within all cells.
Nonetheless, the accumulation of recently fixed 13C and 15N in individual cells appears uniform, and is significantly correlated with an average r2 = 0.4 (Fig. 2), suggesting that these Trichodesmium trichomes do not contain diazocyte-like regions. We come to this conclusion by comparing our results with NanoSIMS analyses of Anabaena that contain specialized N2-fixing heterocyst cells where the fate of recently fixed 13C and 15N is directly linked to cell physiology and development (41). In Anabaena, heterocysts fall outside the pattern of 13C and 15N correlation seen among vegetative cells (Fig. S3). If diazocyte cells of Trichodesmium behave similarly to heterocysts of Anabaena, and specialize in N2 fixation at the expense of CO2 fixation, we would expect to see a similar 13C and 15N anticorrelation in adjacent groups of Trichodesmium cells. In contrast, the relationship between 13C and 15N enrichment in Trichodesmium was consistent in all cells along a trichome at all time points. Because there are no groups of spatially adjacent cells that diverge from the mean in our Trichodesmium dataset, we take this result as evidence against specialized N2 fixation regions (diazocytes), and conclude that temporal segregation may have a more dominant role in segregating CO2 and N2 fixation. However, the lack of observed enrichment at early (< 2 h) time points, and the possibility that rapid redistribution of enriched metabolites occurred between cells, precludes us from a firm conclusion on this important point. Studies using higher levels of isotope enrichment, in parallel with immunofluoretic methods, will hopefully provide a resolution to this current dilemma.
Conclusion
In summary, the NanoSIMS approach has allowed us to examine in detail the distribution and uptake ratio of CO2 and N2 in individual cells of a Trichodesmium trichome, and to compare patterns of distribution and uptake among cells in the trichome and over the diel cycle. We observed the uptake of newly fixed CO2 and N2 within all cells along a Trichodesmium trichome, as well as the dynamic nature of cyanophycin granules, which accumulate in cells during the light period and are metabolized and assimilated into cellular biomass during the dark period. These patterns suggest a temporal uncoupling of metabolic processes operating over the diel period in Trichodesmium. We have also provided direct evidence at a cellular level for a temporal decoupling of CO2 and N2 fixation in the trichome, with maximal CO2 fixation occurring early in the day and maximal N2 fixation occurring later in the day. We did not find evidence for specialized diazocyte cells; and if there is a “division of labor” in Trichodesmium trichomes, it is very transient, or occurs by some other mechanism. Unraveling the ecological and physiological complexities of this globally important organism remains an ongoing challenge. As present investigations reveal, a combined analysis approach, incorporating stable isotope labeling, modern imaging technologies (NanoSIMS, TEM, and SEM), and in situ molecular identification, may be critical to advanced studies of microbial metabolism.
Materials and Methods
Trichodesmium IMS-101 cultures were grown at 27 °C in 2-L polycarbonate culture bottles with YBCII liquid media (43) at an irradiance of 80 μE m−2 s−1 on a 12-h light/12-h dark cycle beginning at 8 AM. Subsamples of 165 mL were incubated in sealed 165 mL of serum vials. To each vial, we injected 0.07 mL NaH13CO3 (≈99 atom percentage 13C, 0.04 M, final 13C enrichment 1.9 atom percentage DIC; Cambridge Isotope Laboratories) and 0.3 mL 99 atom percentage 15N2 gas (Isotech Associates) (final N2 enrichment 16.2 atom percentage). There was no headspace in the bottles to ensure no dilution of the 15N2 added with atmospheric 15N2. Subsamples were returned to the incubator, and kept in the conditions described above until their predetermined harvest time. At 8 time points (0 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 24 h), 1 mL of sample was harvested and preserved in 2% glutaraldehyde for SIMS analysis. For the first time point (0 min), isotopes were injected, and then the sample was immediately harvested (i.e., within <30 s of injection). Experiments were initiated at 10 AM.
For NanoSIMS microanalysis, multiple whole Trichodesmium trichomes were filtered, washed with Milli-Q H2O, and dried onto a silica chip. SIMS was performed at Lawrence Livermore National Laboratory by using a Cameca NanoSIMS 50 instrument, according to previously described methods (41). Briefly, a ≈2pA Cs+ primary beam focused to ≈150 nm was scanned across a 256 × 256 pixel 10–15 μm2 raster to generate secondary ions in ≈20 serial quantitative secondary ion images. Electron multiplier detectors collected 12C−, 13C−, 12C14N−, 12C15N−, and 31P− ions, and secondary electrons were simultaneously imaged. The NanoSIMS was tuned for ≈7,000 mass resolving power to resolve isobaric interferences. Samples were presputtered to at least 100 nm to achieve sputtering equilibrium; depth of measurement analysis ranged from 200 to 350 nm. For each time point, 4–5 trichomes were analyzed. We include data in Figs. 2 and 5 where we were able to analyze at least 40–120 contiguous cells. Data were processed as previously described (41) by using custom software. Briefly, each cell was defined as a region of interest using a 30% 12C threshold; individual cells are easily identified in NanoSIMS secondary electron, 12C and 14N images. The isotopic composition for each ROI was calculated, corrected with reference standards, and converted to percentage uptake (Figs. 2 and 5) (41). Reference standards used included finely ground bovine liver (NIST SRM 1577b) (41) and a Bacillus subtilis spore preparation (44). Measurement precision, σ(internal), was 0.4–1.4% (2σ) for individual 13C/12C and 15N/15N measurements, and replicate analyses of the standard yielded an analytical precision, s(std), of 2.1% (2σ) for an individual measurement. Repeated measurements with depth on selected cells and on multiple filaments within a time point were used to ensure measurement accuracy. Rates of N and C fixation (Fig. 6) are the change C or N percentage uptake hour−1 relative to the initial measurement. TEM analysis (Tecnai G2) was conducted on Trichodesmium filaments from the 0-, 8-, and 24-h time points, dehydrated in a series of ethanol washes, embedded in LR White epoxy, and microtomed to 150 nm. Correlated NanoSIMS analysis was then performed on the filaments imaged by TEM. Percentage area of cyanophycin granules were measured relative to whole-cell area in 65 cells from 6 distinct trichome thin-sections taken from the 0-, 8-, and 24-h time points. NanoSIMS analyses of Anabaena filaments (Fig. S3) are described in Popa et al. (41).
For each time point, a sample (50–75 mL) of bulk cell biomass was filtered onto precombusted GF/F filters and dried. The isotope ratio of these samples was analyzed at the University of Southern California Stable Isotope Facility on a VG IsoPrime interfaced to an elemental analyzer run in continuous flow mode.
Supplementary Material
Acknowledgments.
We thank John Waterbury (Wood Hole Oceanographic Institution, Woods Hole, MA) for providing us his Trichodesmium culture, IMS-101; Rachel Foster (University of California Santa Cruz) and Ed Carpenter (San Francisco State University) for their aid in identifying cyanophycin in the TEM images; Larry Nittler (Carnegie Institute of Washington) for software development; Christina Ramon [Lawrence Livermore National Laboratory (LLNL)] for assistance with sample preparation for NanoSIMS and TEM analysis; and 3 anonymous reviewers, who provided valuable comments that helped improve the manuscript. This work was supported in part by the U.S. Department of Education Office of Biological and Environmental Research Genomics Genomes to Life Research Program (J.P.-R. and P.K.W.), and the National Science Foundation Ocean Science Program Grants OCE 0452765 and OCE 0753218. LLNL was supported by U.S. Department of Energy Contract DE-AC52-07NA27344.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810547106/DCSupplemental.
References
- 1.Capone DG, Zehr J, Paerl H, Bergman B, Carpenter EJ. Trichodesmium: A globally significant marine cyanobacterium. Science. 1997;276:1221–1229. [Google Scholar]
- 2.Glibert PM, Bronk DA. Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp. Appl Environ Microbiol. 1994;60:3996–4000. doi: 10.1128/aem.60.11.3996-4000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capone DG, et al. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and sub-tropical North Atlantic Ocean. Global Biogeochem Cycles. 2005;19 GB2024. [Google Scholar]
- 4.Karl D, et al. The role of nitrogen fixation in biogeochemical cycling in the subtropical north Pacific ocean. Nature. 1997;388:533–538. [Google Scholar]
- 5.Zehr J, et al. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature. 2001;412:635–638. doi: 10.1038/35088063. [DOI] [PubMed] [Google Scholar]
- 6.Falcón LI, Carpenter EJ, Cipriano F, Bergman B, Capone DG. N2 fixation by unicellular bacterioplankton from the Atlantic and Pacific Oceans: Phylogeny and in situ rates. Appl Environ Microbiol. 2004;70:765–770. doi: 10.1128/AEM.70.2.765-770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montoya JP, et al. High rates of N2-fixation by unicellular diazotrophs in the oligotrophic Pacific. Nature. 2004;430:1027–1031. doi: 10.1038/nature02824. [DOI] [PubMed] [Google Scholar]
- 8.Prufert-Bebout L, Paerl HW, Lassen C. Growth, nitrogen fixation and spectral attenuation in cultivated Trichodesmium species. Appl Environ Microbiol. 1993;59:1367–1375. doi: 10.1128/aem.59.5.1367-1375.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood RR, Christian JR. In: Nitrogen in the Marine Environment. Capone DG, Bronk D, Mulholland M, Carpenter EJ, editors. New York: Elsevier; 2008. pp. 1445–1495. [Google Scholar]
- 10.Furnas M. In: Nitrogen in the Marine Environment. Carpenter EJ, Capone DG, editors. New York: Academic; 1992. pp. 265–272. [Google Scholar]
- 11.Carpenter EJ, Subramaniam A, Capone DG. Biomass and primary productivity of the cyanobacterium, Trichodesmium spp, in the southwestern tropical N Atlantic Ocean. Deep-Sea Res PT I. 2004;51:173–203. [Google Scholar]
- 12.Letelier RM, Karl DM. Role of Trichodesmium spp. in the productivity of the subtropical north Pacific ocean. Mar Ecol-Prog Ser. 1996;133:263–273. [Google Scholar]
- 13.Carpenter EJ, Scranton MI, Novelli PC, Michaels A. Validity of N2 fixation rate measurements in marine Oscillatoria (Trichodesmium) J Plankton Res. 1987;9:1047–1056. [Google Scholar]
- 14.Carpenter EJ, Harvey HR, Fry B, Capone DG. Biogeochemical tracers of the marine cyanobacterium Trichodesmium. Deep-Sea Res. 1997;44:27–38. [Google Scholar]
- 15.Gallon JR. Reconciling the incompatible: N2 fixation and O2. Tansley Review No. 44. New Phytol. 1992;122:571–609. [Google Scholar]
- 16.Stewart WDP. Nitrogen fixation by photosynthetic microorganisms. Annu Rev Microbiol. 1973;27:283–316. doi: 10.1146/annurev.mi.27.100173.001435. [DOI] [PubMed] [Google Scholar]
- 17.Saino T, Hattori A. Aerobic nitrogen fixation by the marine non-heterocystous cyanobacterium Trichodesmium (Oscillatoria) spp.: Its protective mechanism against oxygen. Mar Biol. 1982;70:251–254. [Google Scholar]
- 18.Kana TM. Rapid oxygen cycling in Trichodesmium thiebautii. Limnol Oceanogr. 1993;38:18–24. [Google Scholar]
- 19.Milligan AJ, Berman-Frank I, Gerchman Y, Dismukes GC, Falkowski PG. Light-dependent oxygen consumption in nitrogen-fixing Cyanobacteria plays a key role in nitrogenase protection 1. J Phycol. 2007;43:845–852. [Google Scholar]
- 20.Cunningham KA, Capone DG. In: Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs. Carpenter EJ, editor. Netherlands: Kluwer Academic; 1992. pp. 331–341. [Google Scholar]
- 21.Fredriksson C, Bergman B. Ultrastructural characterisation of cells specialised for nitrogen fixation in a non-heterocystous cyanobacterium, Trichodesmium spp. Protoplasma. 1997;197:76–85. [Google Scholar]
- 22.Lin S, Henze S, Lundgren P, Bergman B, Carpenter E. Whole-cell immunolocalization of nitrogenase in marine diazotrophic cyanobacteria, Trichodesmium spp. Appl Environ Microbiol. 1998;64:3052–3058. doi: 10.1128/aem.64.8.3052-3058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohki K. Intercellular localization of nitrogenase in a non-heterocystous cyanobacterium (cyanophyte) Trichodesmium sp. NIBB1067. J Oceanogr. 2008;64:211–216. [Google Scholar]
- 24.Berman-Frank I, et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science. 2001;294:1534–1537. doi: 10.1126/science.1064082. [DOI] [PubMed] [Google Scholar]
- 25.Küpper H, Ferimazova N, Setlik I, Berman-Frank I. Traffic lights in Trichodesmium. Regulation of photosynthesis for nitrogen fixation studied by chlorophyll fluorescence kinetic microscopy. Plant Physiol. 2004;135:2120–2133. doi: 10.1104/pp.104.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergman B. In: Marine Cyanobacteria. Charpy L, Larkum T, editors. Vol 19. Monaco: Institute Oceanographic; 1999. pp. 158–163. [Google Scholar]
- 27.Lechene C, et al. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J Biol. 2006;5:20. doi: 10.1186/jbiol42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon RD. The cyanobacteria. Amsterdam: Elsevier; 1987. pp. 199–225. [Google Scholar]
- 29.Simon RD. Cyanophycin granules from the blue-green alga Anabaena cylindrica: A reserve material consisting of copolymers of aspartic acid and arginine. Proc Natl Acad Sci USA. 1971;68:265–267. doi: 10.1073/pnas.68.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman DM, Tucker D, Sherman LA. Heterocyst development and localization of cyanophycin in N2-fixing cultures of Anabaena sp. PCC7120 (Cyanobacteria) J Phycol. 2000;36:932–941. [Google Scholar]
- 31.Romans KM, Carpenter EJ, Bergman B. Buoyancy regulation in the colonial diazotrophic cyanobacterium Trichodesmium tenue: Ultrastructure and storage of carbohydrate, polyphosphate and nitrogen. J Phycol. 1994;30:935–942. [Google Scholar]
- 32.El-Shehawy R, Lugomela C, Ernst A, Bergman B. Diurnal expression of hetR and diazocyte development in the filamentous non-heterocystous cyanobacterium Trichodesmium erythraeum. Microbiology. 2003;149:1139–1146. doi: 10.1099/mic.0.26170-0. [DOI] [PubMed] [Google Scholar]
- 33.Mackerras AH, Youens BN, Weir RC, Smith GD. Is cyanophycin involved in the integration of nitrogen and carbon metabolism in the cyanobacteria Anabaena cylindrica and Gloeothece grown on light/dark cycles? Gen Microbiol. 1990;136:2049–2056. [Google Scholar]
- 34.Rabouille S, Staal M, Stal L, Soetaert K. Modeling the dynamic regulation of nitrogen fixation in the cyanobacterium Trichodesmium sp. Appl Environ Microbiol. 2006;72:3217–3227. doi: 10.1128/AEM.72.5.3217-3227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG. The demise of the marine cyanobacterium, Trichodesmium spp, via an autocatalyzed cell death pathway. Limnol Oceanogr. 2004;49:997–1005. [Google Scholar]
- 36.Carpenter EJ, Price CC. Marine Oscillatoria (Trichodesmium): Explanation for aerobic nitrogen fixation without heterocysts. Science. 1976;191:1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- 37.Paerl HW. Spatial segregation of CO2 fixation in Trichodesmium spp.: Linkage to N2 fixation potential. J Phycol. 1994;30:790–799. [Google Scholar]
- 38.Carpenter EJ, et al. Re-evaluation of nitrogenase oxygen-protective mechanisms in the planktonic marine cyanobacterium Trichodesmium. Mar Ecol-Prog Ser. 1990;65:151–158. [Google Scholar]
- 39.Paerl HW, Priscu JC, Brawner DL. Immunochemical localization of nitrogenase in marine Trichodesmium aggregates: Relationship to N2 fixation potential. Appl Environ Microbiol. 1989;55:2965–2975. doi: 10.1128/aem.55.11.2965-2975.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolk CP, Austin SM, Bortins J, Galonsky A. Autoradiographic localization of 13N after fixation of 13N-labeled nitrogen gas by a heterocyst-forming blue-green alga. J Cell Biol. 1974;61:440–453. doi: 10.1083/jcb.61.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popa R, et al. Carbon and nitrogen fixation and metabolite exchange in and between individual cells of Anabaena oscillarioides. ISME J. 2007;1:354–360. doi: 10.1038/ismej.2007.44. [DOI] [PubMed] [Google Scholar]
- 42.Guerquin-Kern JL, Wu TD, Quintana C, Croisy A. Progress in analytical imaging of the cell by dynamic secondary ion mass spectrometry (SIMS microscopy) BBA-Gen Subjects. 2005;1724:228–238. doi: 10.1016/j.bbagen.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Chen YB, Zehr JP, Mellon M. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp IMS 101. J Phycol. 1996;32:916–923. [Google Scholar]
- 44.Behrens S, et al. Linking microbial phylogeny to metabolic activity at the single-cell level by using enhanced element labeling-catalyzed reporter deposition fluorescence in situ hybridization (EL-FISH) and NanoSIMS. Appl Environ Microbiol. 2008;74:3143–3150. doi: 10.1128/AEM.00191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.