Abstract
Phytochromes are a widespread family of photosensory proteins first discovered in plants, which measure the ratio of red to far-red light to control many aspects of growth and development. Phytochromes interconvert between red-absorbing Pr and far-red-absorbing Pfr states via photoisomerization of a covalently-bound linear tetrapyrrole (bilin) chromophore located in a conserved photosensory core. From recent crystal structures of this core region, it has been inferred that the chromophore structures of Pr and Pfr are conserved in most phytochromes. Using circular dichroism spectroscopy and ab initio calculations, we establish that the Pfr states of the biliverdin-containing bacteriophytochromes DrBphP and PaBphP are structurally dissimilar from those of the phytobilin-containing cyanobacterial phytochrome Cph1. This conclusion is further supported by chromophore substitution experiments using semisynthetic bilin monoamides, which indicate that the propionate side chains perform different functional roles in the 2 classes of phytochromes. We propose that different directions of bilin D-ring rotation account for these distinct classes of red/far-red photochemistry.
Keywords: bilin amides, biliprotein, circular dichroism, photoisomerization
Phytochromes comprise a class of red/far-red biliprotein photoreceptors that regulate photomorphogenesis, shade avoidance, and development in higher plants (1, 2). Also found in bacteria and fungi, phytochromes characteristically photoconvert between red-absorbing Pr and far-red-absorbing Pfr states (3, 4). Conversion between these 2 states involves Z/E photoisomerization of the 15/16 double bond of their linear tetrapyrrole (bilin) chromophores. Photosensory signaling by phytochromes relies on a conserved core comprising PAS (PER, ARNT, SIM), GAF (cGMP phosphodiesterase, adenylate cyclase, FhlA), and PHY (phytochrome-specific GAF-related) domains (5), with the bilin bound within a conserved pocket of the GAF domain (1, 6). The precise structure of the bilin chromophore, the nature of its covalent linkage to cysteine (Cys) side chains in the protein, and the location of those Cys residues vary among phytochromes (7), and 2 distinct subclasses can be distinguished on these grounds.
Phytobilin-containing phytochromes, which include the plant (Phys) and cyanobacterial (Cph1) phytochromes, are found exclusively in oxygenic photosynthetic organisms. The chromophore precursor for these phytochromes is either phytochromobilin or phycocyanobilin (PCB), both of which possess reduced ethylidene-containing A-rings. For these phytochromes, covalent linkage forms between a GAF-domain Cys and the α-carbon of the A-ring ethylidene (Fig. S1A). The much more widespread biliverdin-containing phytochromes (4), which include the bacteriophytochromes (BphPs) and fungal phytochromes, possess covalent linkages between a Cys residue upstream of the PAS domain and the β-carbon of the A-ring endo-vinyl group of biliverdin IXα (BV; Fig. S1B) (8). Pr is commonly the thermally stable dark state for both classes of phytochromes, with Pfr exhibiting slow thermal reversion to Pr (dark reversion). However, a small subset of biliverdin phytochromes, known as bathyphytochromes, have large amounts of Pfr at thermal equilibrium (9–11), and rare cases of atypical wavelength sensitivity have also been reported (12, 13). Such photosensory diversity suggests that rare divergent photochemical mechanisms have evolved, but most phytochromes retain spectrally characteristic Pr and Pfr states and hence are thought to share common Pr and Pfr structural features.
Microbial phytochromes have emerged as robust targets for biochemical, spectroscopic, and crystallographic analyses. These include the cyanobacterial phytochrome Cph1 from Synechocystis sp. PCC 6803, the BphPs DrBphP from Deinococcus radiodurans and RpBphP3 from Rhodopseudomonas palustris, and the bathyphytochrome PaBphP from Pseudomonas aeruginosa (11, 12, 14, 15). Structural information for photosensory cores of these phytochromes is now available (6, 8, 16–18). Cph1, RpBphP3, and DrBphP appear to share a common C5-Z,syn C10-Z,syn C15-Z,anti chromophore configuration for their Pr states (Fig. S1). The bilin chromophores of all 3 phytochromes also adopt an α-facial disposition for their D-rings (D-αf; Fig. S1C), which contrasts with the D-βf disposition observed for phycobiliproteins (19, 20). Only one Pfr structure has been reported to date, that of PaBphP crystallized as a thermally stable Pfr dark state (18). PaBphP's Pfr chromophore possesses a C5-Z,syn C10-Z,syn C15-E,anti configuration and adopts an α-facial disposition, suggesting that the facial disposition of BphP D-rings does not change upon Pr to Pfr interconversion.
Bilin chromophores are strongly active in circular dichroism (CD) spectroscopy; hence, CD has long been exploited to assess the structure and environment of linear tetrapyrroles in biliproteins (21). For PCB, we have shown that the CD sign of the red transition arises from the facial disposition of the D-ring (22). CD spectra of phytobilin- and BV-containing phytochromes in their Pr states share strong negative rotation for the red absorbance band (22–25). This band exhibits weakly negative rotation in the Pfr state of the BphP Agp1 assembled with a synthetic chromophore (25), consistent with structural results suggesting that the D-ring remains α-facial during Pr/Pfr interconversion of biliverdin phytochromes (8, 16, 18). By contrast, CD spectra of plant and cyanobacterial (Cph1) phytochromes show strong inversion of the red band upon Pr to Pfr photoconversion (23, 24), which raises the possibility that the Pfr states of BV-containing phytochromes differ from those of phytobilin-containing phytochromes.
Additional support for structurally-distinct subclasses of Pfr comes from mutagenesis of a conserved Tyr residue proximal to the chromophore D-ring in the Pr state (Tyr-176 in Cph1 and DrBphP). Substitution of His for this Tyr residue in both Cph1 and plant phytochromes yields intensely red-fluorescent holoproteins that are nearly photoinactive (26–28). The equivalent substitution in DrBphP and PaBphP does not block Pfr formation, despite the observation (Fig. S1) that the Tyr side chain directly contacts the BV C-ring 12-propionate side chain in the crystal structure of the PaBphP Pfr state (18, 27, 29).
The present work was undertaken to test the hypothesis that BV- and phytobilin-containing phytochromes possess distinct photochemical reaction mechanisms. Using CD spectroscopy, we establish that the Pfr state of Cph1 differs from those of the BphPs DrBphP and PaBphP. We further show that the structural requirements for Pr/Pfr interconversion in Cph1 are distinct from those of DrBphP by reconstitution experiments with synthetic chromophore 12-monoamides. Our studies provide compelling support for the conclusion that BV- and phytobilin-containing phytochromes exhibit different photochemical reaction trajectories. A mechanistic formulation of the 2 distinct red/far-red photochemical pathways is proposed.
Results
Cph1 and DrBphP Adopt Different D-Ring Facial Dispositions in the Pfr State.
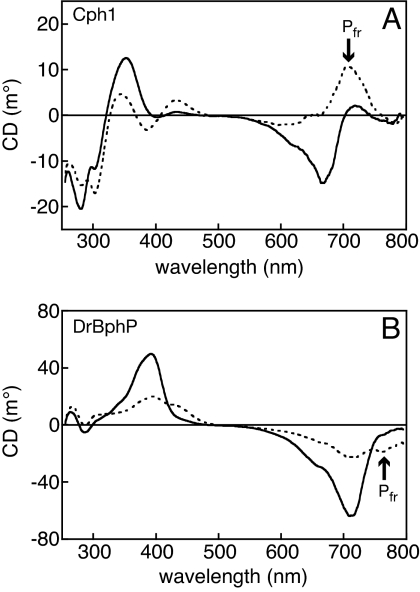
Previous results have raised the possibility that phytobilin and biliverdin phytochromes have distinct Pfr states. To test this hypothesis, we examined Cph1, DrBphP, and PaBphP, for which crystal structures have been reported. Both Cph1 and DrBphP exhibit negative CD for the red absorbance band in the Pr state (Fig. 1) and positive CD for the blue absorbance band, consistent with the D-αf disposition (22). The CD spectrum for the red-light-generated Pr/Pfr photoequilibrium of Cph1 displayed positive CD at wavelengths corresponding to the red Pfr absorbance band and more complicated behavior in the blue absorbance band (Fig. 1A). These results are in good agreement with those previously obtained for Cph1 (24) and confirm that Cph1 photochemistry proceeds with inversion of CD.
Fig. 1.
The Pfr states of Cph1 and DrBphP are distinct. (A) CD spectra of 21 μM Cph1 in the Pr state (solid line) and at Pr/Pfr photoequilibrium (dashed line). (B) CD spectra of 17 μM DrBphP in the Pr state (solid line) and at Pr/Pfr photoequilibrium (dashed line). The far-red absorbance of Pfr is indicated.
In contrast with Cph1, the CD of the Pfr red absorbance band of DrBphP remained negative (Fig. 1B). The signal strength of the Pfr state of DrBphP was notably weaker than that of Pr, indicating that the DrBphP Pfr chromophore is more planar than Pr. This observation is consistent with the weak negative CD reported for a synthetic BV chromophore bound to the BphP Agp1 in the Pfr state (25). We also observed negative CD for the thermal Pr/Pfr equilibrium of the bathyphytochrome PaBphP across the entire red absorbance band (Fig. S2). Both BphP Pfr spectra exhibited negative far-red CD.
The different signs of rotation for the Pfr states of Cph1 and BphPs demonstrate that the Pfr chromophore configurations of phytobilin and biliverdin phytochromes are distinct. We reported that red band CD of PCB chromophores reports the facial disposition of the bilin D-ring relative to the B- and C-rings (22). We extended these calculations to models for BV chromophores and to 15-E,anti configurations appropriate for Pfr (Table 1). Although the results preclude unambiguous assignment of the red band to a single calculated transition, the qualitative trend established previously is reproduced: every D-αf configuration has negative red CD, and every D-βf configuration has positive red CD. This finding is consistent with the D-αf disposition observed for PaBphP (18). We thus assign Pfr in Cph1 as D-βf; the similar inversion of CD reported for oat phytochrome (23, 24) would imply a D-βf Pfr for plant phytochrome as well.
Table 1.
Calculated spectral parameters
| Bilin | C15 configuration | D-ring facial disposition | λvert | fL | Rv |
|---|---|---|---|---|---|
| PCB | Z, anti | D-αf* | 631 | 0.69 | −332 |
| PCB | Z, anti | D-βf* | 628 | 0.62 | +274 |
| PCB | E, anti | D-αf | 656, 642 | 0.14, 0.49 | −112, −263 |
| PCB | E, anti | D-βf | 640 | 0.45 | +141 |
| BV | Z, anti | D-αf | 705 | 0.84 | −315 |
| BV | Z, anti | D-βf | 752, 643 | 0.24, 0.59 | +85, +129 |
| BV | E, anti | D-αf | 750, 665 | 0.31, 0.41 | −187, −200 |
| BV | E, anti | D-βf | 721 | 0.33 | +288 |
*Values from ref. 22.
Apophytochromes Assemble with Chromophore Monoamides.
In the PaBphP crystal structure, Tyr-163 (Tyr-176 in DrBphP and Cph1) adopts a side-chain rotamer not observed in Pr crystal structures (Fig. S1) (6, 8, 16–18). This places the Tyr-163 side chain under the α-facial D-ring, permitting an interaction between the Tyr-163 hydroxyl and the 12-propionate side chain of the BV chromophore. Such an interaction is not plausible for Tyr-176 in the Cph1 Pfr state, because a D-βf Pfr chromophore would sterically disfavor this Tyr rotamer. We therefore expect that the roles of the 12-propionate and this Tyr residue in Pr/Pfr interconversion will differ in these 2 classes of phytochromes. Previous studies have shown that Tyr-176 indeed performs distinct roles in the photochemistry of phytobilin and biliverdin phytochromes (8, 26–28). We tested the roles of the 12-propionate in phytochrome photochemistry by preparing 12-monoamide derivatives of BV and PCB (BV12MA and PCB12MA).
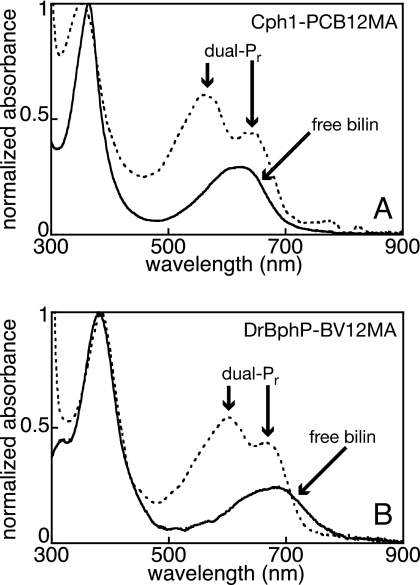
12-Monoamide chromophores were assembled with purified Cph1 or DrBphP apoprotein, dialyzed to remove free chromophore, and analyzed by spectroscopy. Both apoproteins formed covalent adducts with appropriate monoamide chromophore analogs (Fig. S3) and exhibited enhanced red absorbance relative to the free chromophores (Fig. 2). Unlike adducts with the free acids, both holoprotein spectra exhibited a pair of absorption maxima between 500 and 750 nm (Fig. 2). Both absorption bands were blue-shifted relative to the Pr peak observed with diacid chromophores (Table S1). These results indicate that bilin 12-monoamides exhibit at least 2 spectrally-distinct, thermally-stable populations on this time scale (≈0.2–0.5 s). The common “dual-Pr” behavior of Cph1 and DrBphP suggests that the 12-propionate plays similar roles in bilin binding and Pr formation in both classes of phytochromes. The reason for the presence of these 2 Pr bands is not clear, although it is possible that they represent protonated and deprotonated populations.
Fig. 2.
Apophytochromes incorporate chromophore 12-monoamides. (A) PCB12MA (solid line) was assembled with apoCph1 to give the PCB12MA adduct Cph1-PCB12MA, which has a dual-Pr spectrum (dashed line; peaks indicated). (B) BV12MA (solid line) was assembled with apoDrBphP to give the BV12MA adduct DrBphP-BV12MA, which has a similar dual-Pr spectrum (dashed line; peaks indicated). Spectra are normalized to the blue/UV (Soret) band.
Cph1 Requires the 12-Propionate for Pfr Formation, Whereas DrBphP Does Not.
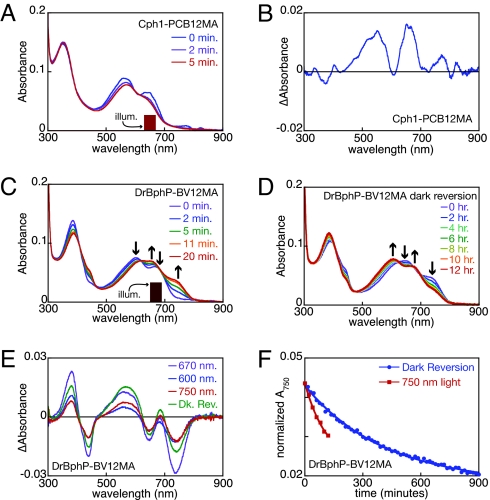
To examine the role of the 12-propionate in Cph1 photoconversion, we illuminated the PCB12MA adduct with red light matching the longer dual-Pr peak and collected spectra as a function of illumination time (Fig. 3A). We observed slow loss of red absorbance without appearance of obvious photoproducts. Loss of both dual-Pr peaks upon irradiation could arise from an equilibrium between the 2 Pr populations or spectral overlap of the shorter red absorbance peak with the illuminating light. Control samples assembled with authentic PCB exhibited characteristic peak/trough pairs in the blue and red/far-red regions of the difference spectra (Fig. S3C), but samples assembled with PCB12MA displayed only very small positive peaks in the difference spectra (Fig. 3B). We cannot rule out formation of a photoproduct with a peak wavelength of ≈600 nm, but such a product would not correspond to Pfr (Table S1). We therefore conclude that the 12-propionate is essential for formation of stable Pfr by Cph1.
Fig. 3.
Distinct roles for the 12-propionate in Cph1 and DrBphP photochemistry. (A) Cph1-PCB12MA was illuminated with 650 ± 20 nm light (indicated), and spectra were taken as a function of time. (B) The photochemical difference spectrum is plotted for the data in A. (C) DrBphP-BV12MA was illuminated with 670 ± 20 nm light (indicated) as in A. Arrows indicate peak/trough regions in the difference spectrum. (D) Dark reversion of the dual-Pfr photoequilibrium was examined. Arrows are as in C. (E) Difference spectra are shown for conversion of DrBphP-BV12MA from Pr to Pfr with 670 ± 20 nm light (blue), for subsequent conversion of the same sample with 600 ± 5 nm light (green), for conversion of the 670-nm photoequilibrium mixture with 750 ± 20 nm (red), and for dark reversion of the 600-nm photoequilibrium mixture to the Pfr state (purple). (F) Normalized absorbance at 750 nm is shown for conversion of the dual-Pr/dual-Pfr photoequilibrium mixture of DrBphP-BV12MA to dual-Pr either via dark reversion (blue) or in the presence of 750 ± 20 nm light (red). Illum, illumination.
Pfr formation was readily detectable upon illumination of the BV12MA adduct of DrBphP (Fig. 3). The resulting Pfr exhibited a slight blue-shift from 750 to 738 nm by comparison with wild-type DrBphP. An additional photoproduct with a peak wavelength falling in between the 2 Pr peaks was also formed for the BV12MA adduct of DrBphP (Table S1). Dual-Pr thus gives rise to dual-Pfr. Moreover, irradiation of either Pr peak depleted both Pr peaks to yield both Pfr peaks (Fig. 3C and Fig. S3E), indicating that the dual-Pr states are in equilibrium. These results demonstrate that the 12-propionate is not necessary for Pfr formation by DrBphP.
Spontaneous thermal reversion of these dual-Pfr photoproducts to dual-Pr could be observed over a time scale of several hours (Fig. 3D), which indicates that dark reversion, 1 of 2 routes for conversion of Pfr to Pr in DrBphP, can still occur with the BV12MA adduct. Illumination of the dual-Pfr photoequilibrium mixture with 750-nm light (Fig. S3F) resulted in formation of both dual-Pr species, suggesting that the dual-Pfr states are also in equilibrium. The difference spectra for dual-Pfr formation and dual-Pr formation show similar features (Fig. 3E): a Pr/Pfr doublet in the blue and a pair of Pr/Pfr doublets in the red/far-red, with very similar peak wavelengths (Table S1). Dual-Pr formation by 750-nm illumination was faster than dark reversion (Fig. 3F), indicating that the BV12MA adduct is competent for photochemical conversion of Pfr to Pr. DrBphP therefore does not require the 12-propionate side chain of BV for interconversion of Pr and Pfr states, whereas Cph1 does.
Discussion
We present evidence that the red/far-red photocycles of the phytochrome superfamily can be divided into at least 2 classes that correlate to the BV- and phytobilin-containing phytochrome subfamilies. Both classes exhibit red/far-red photoconversion, and their Pr states are structurally similar as assayed by both CD spectroscopy and crystallography (8, 17). The roles of the bilin 12-propionate side chain during assembly of the Pr state seem similar in these 2 classes as well (Fig. 2). However, Pfr formation in phytobilin phytochromes proceeds with inversion of CD and requires the free 12-propionate side chain, whereas Pfr formation by biliverdin phytochromes does not lead to CD sign inversion and does not require the 12-propionate. These 2 classes also possess distinct requirements for a conserved Tyr residue in stable Pfr formation (27, 29). We therefore conclude there are structurally-distinct variants of red/far-red photochemistry within the phytochrome superfamily.
CD spectroscopy of photoactive biliproteins is most easily interpreted in terms of the facial disposition of the D-ring (22): negative CD for the long-wavelength transition is associated with the D-αf disposition, whereas positive CD corresponds to D-βf. This interpretation is consistent not only with all available phytochrome crystal structures (6, 8, 16–18), but also with structural data for α-phycoerythrocyanin (α-PEC), a phycobiliprotein that exhibits inversion of CD upon Z/E photoisomerization (20, 30, 31). Although confirmation will require crystallographic information on the Cph1 Pfr state, we assign the Pfr states of phytobilin phytochromes as D-βf, distinct from the D-αf Pfr predicted for DrBphP and observed for PaBphP.
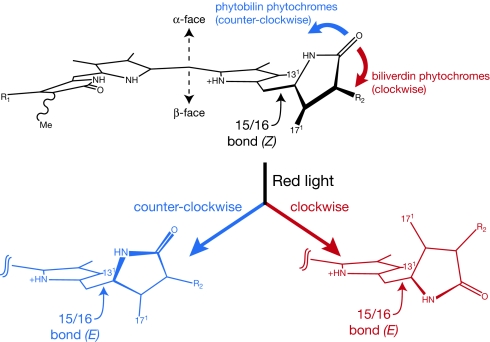
We propose that different directions of rotation in BphPs and Cph1 account for the formation of their distinct Pfr states. As an initial test of this hypothesis, we modeled the motions involved in such rotations by manually displacing a model compound for the C15-Z,anti chromophore of the DrBphP Pr state in either direction, followed by semiempirical geometry optimization (Movies S1 and S2). If D-ring rotation were counterclockwise about the 15/16 bond (as viewed from the C16 side: Fig. 4), the resulting C15-E,anti geometry would be quite twisted. A significant steric clash would arise between the bilin methyl groups at C131 and C171, repelling the D-ring down to the β-face of the bilin ring system and resulting in a D-βf Pfr state (Movie S1). By contrast, an initial clockwise rotation would move the D-ring nitrogen further toward the B/C plane (Fig. 4). This would move the D-ring NH proton into a steric clash with the C131 methyl group. Energy to overcome this barrier could be gained by relaxing the strained C15/C16 dihedral angle observed in BphP Pr structures but not in Cph1 (6, 8, 16, 17). Once this barrier is overcome, the D-ring is free to continue rotating and does not encounter a similar steric clash between methyl groups (Movie S2). The resulting geometry retains a D-αf disposition that closely resembles the observed D-αf Pfr of PaBphP. Although these simulations should not be considered accurate representations of the reactions occurring in phytochrome, they illustrate that different directions of rotation can account for the distinct Pfr states of Cph1 and DrBphP.
Fig. 4.
Different directions of D-ring rotation in phytochrome photochemistry. (Upper) The ring skeleton for the Pr chromophore is shown. Rotation of the D-ring to a formally E,anti configuration will lead to a strained 15/16 bond. (Lower Left) Counterclockwise rotation of the α-facial D-ring about the 15/16 bond (blue) will result in a steric clash between the indicated methyl groups at C131 and C171 as the 15/16 dihedral relaxes. This steric repulsion causes the D-ring to slump to the bilin β-face and results in a D-βf C15-E,anti configuration (Movie S1). (Lower Right) Clockwise rotation of the D-ring (red) does not produce this clash upon 15/16 dihedral relaxation and permits further rotation to a D-αf C15-E,anti configuration (Movie S2).
The structural differences between these Pfr states are also consistent with the distinct phenotypes of GAF-domain tyrosine mutants in the 2 classes of phytochromes. The interaction between GAF-domain Tyr-163 and the C12-propionate in the PaBphP Pfr state (18) would be sterically disfavored in the D-βf Pfr state of Cph1. The role of Tyr-176 in Cph1 would thus have to be different from that found in BphPs, and indeed Y176H and equivalent mutations have profoundly different effects in the 2 classes of phytochromes (26–29). In Cph1 (and plant phytochromes), the Y176H mutation results in loss of photochemistry and supports intense red fluorescence (26, 27). This phenotype could be explained were the mutant His residue to adopt the side-chain rotamer observed in PaBphP's Pfr dark state, disfavoring movements required for formation of the normal D-βf Pfr state of Cph1. Residual Pfr formation observed for Y176H Cph1 (26, 27) could arise either from formation of aberrant D-αf Pfr or from a population of holoprotein with His-176 in the rotamer observed in wild-type Cph1. In biliverdin-containing phytochromes, equivalent mutations neither ablate Pr/Pfr interconversion nor enhance fluorescence (27, 29). Assembly of DrBphP with BV12MA also does not ablate photochemistry or dark reversion. Perhaps neither substitution of this Tyr with His nor introduction of the amide moiety on the C-ring ablates the interaction between Tyr-163 and the C12-propionate seen in the PaBphP structure.
By contrast with BphPs, Cph1 requires both Tyr-176 and the 12-propionate for efficient photoconversion of Pr to Pfr. Indeed, no other residue can compensate for Tyr at this position in Cph1 (27), and PCB12MA does not support Pfr formation (Fig. 3). That said, the 12-propionate side chain appears to regulate formation of the protonated, extended C15-Z,anti configuration in similar ways in both classes, as revealed by the dual-Pr spectra of both 12MA adducts. The native propionate side chain may thus modulate the pKa of the bilin ring system.
Structurally-distinct red/far-red photocycles within the phytochrome superfamily have implications for engineering of plant phytochromes for agricultural purposes. In particular, the chromophore–protein interactions of plant phytochrome Pfr are likely to be quite different from those of PaBphP. However, it is conceivable that such differences could be offset by changes in the structure of the protein, such that the conformational changes in the protein that lead to changes in the signaling state are still conserved. Such offsetting changes may well occur in the “tongue” linking the PHY domain to the chromophore (17, 18), permitting distinct Pfr states to proceed through a single, conserved signal transmission pathway to the various phytochrome output domains.
Materials and Methods
Bilin Pigments.
PCB and BV were prepared for in vitro assembly reactions as described (32–34). BV12MA was prepared by acid scrambling of appropriate precursors and was used to prepare PCB12MA via enzymatic conversion.
Holophytochrome Expression and Purification.
CD experiments used full-length phytochromes including histidine kinase domains. Strep-tagged PaBphP was purified as described (27). Full-length Cph1 was coexpressed with PCB biosynthetic machinery in Escherichia coli (35) from plasmid pBAD-Cph1FL-CBD, which contains the pBAD promoter and Cph1 reading frame described (14, 27, 35) fused to the intein/chitin-binding-domain region of pTYB2 (NEB). Cph1 holoprotein was purified as a chitin-binding fusion in accordance with the manufacturer's directions, followed by overnight dialysis into TKKG buffer [25 mM TES·KOH (pH 7.5), 25 mM KCl, 10% glycerol]. A full-length DrBphP construct (gift of Richard Vierstra, University of Wisconsin, Madison) was subcloned into pTYB2 (NEB), and the resulting DrBphPFL-CBD fusion was subcloned into plasmid pBAD-Cph1FL-CBD to yield pBAD-DrBphPFL-CBD. Expression and purification of DrBphP proceeded as for Cph1 using the ho1-expressing plasmid pAT-BV, derived by excising PcyA from pAT103 (36), to drive BV production.
Spectroscopy and Photoconversion.
Absorption spectra were recorded at 480 nm/min at 25 °C in a Cary 50 UV/Vis spectrophotometer equipped with a temperature-controlled cuvette holder. A 75-W Xe source (PTI) passed through a water filter and an appropriate bandpass interference filter was used to irradiate sample solutions in the Cary 50 via a liquid light guide. Bandpass filters used for triggering photochemistry were 650-nm center/40-nm width, 700 nm/40 nm, 750 nm/40 nm, 670 nm/40 nm, and 600 nm/10 nm. This apparatus permitted efficient and saturating photoconversion (Fig. S2). CD spectra were acquired on a Jasco J-720 at room temperature and are presented as the smoothed average of 2–3 scans with buffer subtraction.
In Vitro Assembly Reactions.
Truncated Cph1Δ (amino acids 1–514) and full-length DrBphP were expressed and purified as apoproteins by omitting plasmids encoding chromophore biosynthetic machinery. Apoprotein concentrations were estimated by using a modified Bradford assay (Bio-Rad). Chromophore (4–5 nmol, in DMSO) and apoprotein (16–20 nmol) were mixed in 1 mL of TKKG buffer with 1 mM Tris(chloroethyl)phosphine. Final DMSO levels were <2% vol/vol. After 2 h, reactions were dialyzed overnight in TKKG buffer to remove free chromophore. Presence of covalently attached chromophore after dialysis and competence of apoproteins for forming active holophytochrome were confirmed in control experiments (Fig. S3).
Ab Initio Calculations.
Model compounds lacked propionate side chains and thioethers as described (22). Gas-phase B3LYP/6–31+G* geometries were used for excited-state BLYP/6–311+G* calculations (22). BV in the C15-Z,anti D-αf configuration was adjusted manually to match the DrBphP crystal structure. Oscillator strength fL is reported in the length representation; rotational strength Rv is reported in the velocity representation as (esu2 cm2) × 1040. Movement of the model compound to different C15-E,anti geometries was examined by manually rotating the 15/16 dihedral angle of the BV Pr geometry from +35.8° to −100° or +103° (formally E). The resulting structures were then optimized at the AM1 level in GAMESS (37). Movies were made in MacMolPlt (38).
Supplementary Material
Acknowledgments.
We thank Prof. Richard Vierstra for the original DrBphP construct, Prof. Stephanie Dungan (University of California, Davis) for use of the Jasco CD spectrometer, and Abigail Jang, Rima Woods, and Keenan Taylor for technical assistance. This work was supported by National Institutes of Health Grant GM068552 (to J.C.L.) and National Science Foundation Center for Biophotonics Science and Technology Subcontract PHY-0120999 (to J.C.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902370106/DCSupplemental.
References
- 1.Rockwell NC, Su YS, Lagarias JC. Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol. 2006;57:837–858. doi: 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharrock RA. The phytochrome red/far-red photoreceptor superfamily. Genome Biol. 2008;9:230. doi: 10.1186/gb-2008-9-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gärtner W, Braslavsky SE. The phytochromes: Spectroscopy and function. In: Batschauer A, editor. Photoreceptors and Light Signaling, Comprehensive Series in Photochemical and Photobiological Sciences. Vol 3. Cambridge, UK: Royal Society of Chemistry; 2003. pp. 136–180. [Google Scholar]
- 4.Karniol B, Vierstra RD. Structure, function, and evolution of microbial phytochromes. In: Schäfer E, Nagy F, editors. Photomorphogenesis in Plants and Bacteria: Function and Signal Transduction Mechanisms. 3rd Ed. Dordrecht, The Netherlands: Springer; 2005. pp. 65–98. [Google Scholar]
- 5.Montgomery BL, Lagarias JC. Phytochrome ancestry: Sensors of bilins and light. Trends Plant Sci. 2002;7:357–366. doi: 10.1016/s1360-1385(02)02304-x. [DOI] [PubMed] [Google Scholar]
- 6.Wagner JR, Brunzelle JS, Forest KT, Vierstra RD. A light-sensing knot revealed by the structure of the chromophore binding domain of phytochrome. Nature. 2005;438:325–331. doi: 10.1038/nature04118. [DOI] [PubMed] [Google Scholar]
- 7.Lamparter T. Evolution of cyanobacterial and plant phytochromes. FEBS Lett. 2004;573:1–5. doi: 10.1016/j.febslet.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JR, Zhang J, Brunzelle JS, Vierstra RD, Forest KT. High-resolution structure of Deinococcus bacteriophytochrome yields new insights into phytochrome architecture and evolution. J Biol Chem. 2007;282:12298–12309. doi: 10.1074/jbc.M611824200. [DOI] [PubMed] [Google Scholar]
- 9.Giraud E, et al. Bacteriophytochrome controls photosystem synthesis in anoxygenic bacteria. Nature. 2002;417:202–205. doi: 10.1038/417202a. [DOI] [PubMed] [Google Scholar]
- 10.Karniol B, Vierstra RD. The pair of bacteriophytochromes from Agrobacterium tumefaciens are histidine kinases with opposing photobiological properties. Proc Natl Acad Sci USA. 2003;100:2807–2812. doi: 10.1073/pnas.0437914100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasler R, Moises T, Frankenberg-Dinkel N. Biochemical and spectroscopic characterization of the bacterial phytochrome of Pseudomonas aeruginosa. FEBS J. 2005;272:1927–1936. doi: 10.1111/j.1742-4658.2005.04623.x. [DOI] [PubMed] [Google Scholar]
- 12.Giraud E, et al. A new type of bacteriophytochrome acts in tandem with a classical bacteriophytochrome to control the antennae synthesis in Rhodopseudomonas palustris. J Biol Chem. 2005;280:32389–32397. doi: 10.1074/jbc.M506890200. [DOI] [PubMed] [Google Scholar]
- 13.Jaubert M, et al. A singular bacteriophytochrome acquired by lateral gene transfer. J Biol Chem. 2007;282:7320–7328. doi: 10.1074/jbc.M611173200. [DOI] [PubMed] [Google Scholar]
- 14.Yeh K-C, Wu S-H, Murphy JT, Lagarias JC. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]
- 15.Bhoo SH, Davis SJ, Walker J, Karniol B, Vierstra RD. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature. 2001;414:776–779. doi: 10.1038/414776a. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Stojkovic EA, Kuk J, Moffat K. Crystal structure of the chromophore binding domain of an unusual bacteriophytochrome, RpBphP3, reveals residues that modulate photoconversion. Proc Natl Acad Sci USA. 2007;104:12571–12576. doi: 10.1073/pnas.0701737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Essen LO, Mailliet J, Hughes J. The structure of a complete phytochrome sensory module in the Pr ground state. Proc Natl Acad Sci USA. 2008;105:14709–14714. doi: 10.1073/pnas.0806477105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Kuk J, Moffat K. Crystal structure of Pseudomonas aeruginosa bacteriophytochrome: Photoconversion and signal transduction. Proc Natl Acad Sci USA. 2008;105:14715–14720. doi: 10.1073/pnas.0806718105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerring M, Huber R, Bode W, Ruembeli R, Zuber H. Refined 3-dimensional structure of phycoerythrocyanin from the cyanobacterium Mastigocladus laminosus at 2.7 Å. J Mol Biol. 1990;211:633–644. doi: 10.1016/0022-2836(90)90270-v. [DOI] [PubMed] [Google Scholar]
- 20.Duerring M, Schmidt GB, Huber R. Isolation, crystallization, crystal structure analysis, and refinement of constitutive C-phycocyanin from the chromatically adapting cyanobacterium Fremyella diplosiphon at 1.66-Å resolution. J Mol Biol. 1991;217:577–592. doi: 10.1016/0022-2836(91)90759-y. [DOI] [PubMed] [Google Scholar]
- 21.Falk H. The Chemistry of Linear Oligopyrroles and Bile Pigments. Vienna: Springer; 1989. [Google Scholar]
- 22.Rockwell NC, et al. A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus. Biochemistry. 2008;47:7304–7316. doi: 10.1021/bi800088t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litts JC, Kelly JM, Lagarias JC. Structure–function studies on phytochrome: Preliminary characterization of highly purified phytochrome from Avena sativa enriched in the 124-kDa species. J Biol Chem. 1983;258:11025–11031. [PubMed] [Google Scholar]
- 24.Borucki B, et al. Mechanism of Cph1 phytochrome assembly from stopped-flow kinetics and circular dichroism. Biochemistry. 2003;42:13684–13697. doi: 10.1021/bi035511n. [DOI] [PubMed] [Google Scholar]
- 25.Seibeck S, et al. Locked 5Zs-biliverdin blocks the Meta-Ra to Meta-Rc transition in the functional cycle of bacteriophytochrome Agp1. FEBS Lett. 2007;581:5425–5429. doi: 10.1016/j.febslet.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 26.Fischer AJ, Lagarias JC. Harnessing phytochrome's glowing potential. Proc Natl Acad Sci USA. 2004;101:17334–17339. doi: 10.1073/pnas.0407645101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer AJ, et al. Multiple roles of a conserved GAF domain tyrosine residue in cyanobacterial and plant phytochromes. Biochemistry. 2005;44:15203–15215. doi: 10.1021/bi051633z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su YS, Lagarias JC. Light-independent phytochrome signaling mediated by dominant GAF domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants. Plant Cell. 2007;19:2124–2139. doi: 10.1105/tpc.107.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner JR, et al. Mutational analysis of Deinococcus radiodurans bacteriophytochrome reveals key amino acids necessary for the photochromicity and proton exchange cycle of phytochromes. J Biol Chem. 2008;283:12212–12226. doi: 10.1074/jbc.M709355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao KH, Scheer H. Type I and type II reversible photochemistry of phycoerythrocyanin α-subunit from Mastigocladus laminosus both involve Z, E isomerization of phycoviolobilin chromophore and are controlled by sulfhydryls in apoprotein. Biochim Biophys Acta Bioenerg. 1995;1228:244–253. [Google Scholar]
- 31.Schmidt M, Patel A, Zhao Y, Reuter W. Structural basis for the photochemistry of α-phycoerythrocyanin. Biochemistry. 2007;46:416–423. doi: 10.1021/bi061844j. [DOI] [PubMed] [Google Scholar]
- 32.McDonagh AF. Bile pigments: Bilatrienes and 5,15-biladienes. In: Dolphin D, editor. The Porphyrins. Vol VI. New York: Academic; 1979. pp. 293–491. [Google Scholar]
- 33.Arciero DM, Bryant DA, Glazer AN. In vitro attachment of bilins to apophycocyanin. I. Specific covalent adduct formation at cysteinyl residues involved in phycocyanobilin binding in C-phycocyanin. J Biol Chem. 1988;263:18343–18349. [PubMed] [Google Scholar]
- 34.Elich TD, McDonagh AF, Palma LA, Lagarias JC. Phytochrome chromophore biosynthesis. Treatment of tetrapyrrole-deficient Avena explants with natural and non-natural bilatrienes leads to formation of spectrally active holoproteins. J Biol Chem. 1989;264:183–189. [PubMed] [Google Scholar]
- 35.Gambetta GA, Lagarias JC. Genetic engineering of phytochrome biosynthesis in bacteria. Proc Natl Acad Sci USA. 2001;98:10566–10571. doi: 10.1073/pnas.191375198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tooley AJ, Cai YA, Glazer AN. Biosynthesis of a fluorescent cyanobacterial C-phycocyanin holo-α subunit in a heterologous host. Proc Natl Acad Sci USA. 2001;98:10560–10565. doi: 10.1073/pnas.181340998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt MW, et al. General atomic and molecular electronic structure system. J Comput Chem. 1993;14:1347–1363. [Google Scholar]
- 38.Bode BM, Gordon MS. MacMolPlt: A graphical user interface for GAMESS. J Mol Graphics Model. 1998;16:133–138. doi: 10.1016/s1093-3263(99)00002-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.