Abstract
Summer conditions in the Mediterranean Sea are characterized by high temperatures and low food availability. This leads to “summer dormancy” in many benthic suspension feeders due to energetic constraints. Analysis of the most recent 33-year temperature time series demonstrated enhanced stratification due to global warming, which produced a ≈40% lengthening of summer conditions. Many biological processes are expected to be affected by this trend, culminating in such events as mass mortality of invertebrates. Climatic anomalies concomitant with the occurrence of these events represent prolonged exposure to warmer summer conditions coupled with reduced food resources. Simulation of the effects of these conditions on a model organism demonstrated a biomass loss of >35%. Losses of this magnitude result in mortality similar to that noted in field observations during mass mortality events. These results indicate that temperature anomalies are the underlying cause of the events, with energetic constraints serving as the main triggering mechanism.
Keywords: climatic anomalies, summer lengthening, energetic constraints, benthic suspension feeders, extreme events
In a rapidly warming world, marine and terrestrial biodiversity and ecosystems are responding through shifts in distribution and changes in abundance, phenology, structure, and functioning (1–3). Furthermore, global environmental change is expected to lead to a higher frequency and shorter return time of extreme events (4). An example of this is the occurrence of mass mortality events, which are affecting an increasing number of marine species and ecosystems worldwide (5, 6). These events have been well documented in coral reef ecosystems, where they have produced dramatic phase shifts in community structure (7–9). In recent years, the coralligenous community, one of the most diverse in the Mediterranean Sea (≈1,666 species; ref. 10), where suspension feeders are dominant, has been strongly affected by several mass mortality events [supporting information (SI) Table S1]. Engineer species, including gorgonians and sponges, have been the most affected taxa down to depths of 45 m (11–13). The impact of these events on populations is similar to that observed in the 1980s and 1990s in the Caribbean Sea (14, 15). But despite the wide extent of these effects on Caribbean populations, identification of the causes has not always been possible; however, some were associated with temperature increases (15), and others were associated with diseases (14). Understanding mass mortality events is becoming increasingly important, given the escalating presence of anthropogenic stressors that affect marine ecosystems (5, 6, 16).
The causes of mass mortality events in the Mediterranean remain unknown, and our ability to predict the effects of these events depends on characterizing them and elucidating trends exhibited by potentially causative factors. Current hypotheses about the causes of these events in the Mediterranean focus on their relationship with the occurrence of distinctive climatic anomalies in late summer and early fall (17). Climatic conditions coincident with the occurrence of the 2 largest mass mortality events (in 1999 and 2003) included temperatures ≈3–4 °C above average and prolonged water column stability in late summer (11, 17, 18). To determine whether these climatic anomalies could be considered the main cause of the mass mortality events, we need to determine the mechanisms that could possibly link this thermal phenomenon with the mass mortality of benthic suspension feeders. The occurrence of such climatic anomalies in late summer implies prolonged exposure of organisms dwelling above the thermocline to summer conditions (high temperatures and low food availability). In this framework, the main hypotheses regarding the causes of invertebrate mass mortality events are related to the exposure of benthic organisms to (i) temperatures above their level of thermal tolerance, (ii) temperatures below critical temperatures but producing physiological stress, and (iii) virulence of microorganisms (11, 12, 17, 19–22). But although lethal temperatures should not be disregarded, they did not characterize the environmental framework of the events (17), and microorganisms present on colonies during occurrence of the events were interpreted as opportunistic (11, 19; but see ref. 21). The fact that mass mortality events preferentially affect taxa that exhibit energy shortages, such as suspension-feeding gorgonians and sponges (12, 20), suggests that energetic constraints due to high metabolic activity and prolonged low levels of food should be explored as a potential determinant of mass mortality events.
In this study, we addressed the hypothesis of physiological stress due to energetic constraints. This hypothesis relies on the fact that seasonal dynamics of different suspension-feeding taxa show that summer is an energetically unfavorable period due to high temperature (and thus high respiratory demand) and low food availability, resulting from thermal stratification of the water column and leading to limited energy. Synergy of both factors in the summer produces an energetic constraint underlying a pattern of decreased activity that has been described as “summer dormancy” (20, 23). The hypothesis is that organisms can withstand the adverse conditions in summers of normal duration but cannot resist anomalous prolongation of summer conditions, particularly when combined with higher-than-normal temperatures. We used a 2-step approach to address the hypothesized relationships among climatic anomalies, energetic constraints, and mass mortality events. In the first step, we examined environmental conditions by analyzing a temperature time series of data recorded over the last 33 years over a 90-m depth range. In the second step, we conducted a controlled experiment in the laboratory to test the effects of normal and anomalous environmental conditions on benthic suspension feeders.
Summer conditions in the Mediterranean are characterized by high water column stability and high temperatures, resulting in a strong stratification of the water column. This stratification is responsible for the exhaustion of dissolved surface nutrients. Furthermore, particle sinking results in a severe depletion of suspended food resources (24, 25). Development of water column stratification due to solar radiation in the spring and summer results in greater differences in the annual temperature variation at the sea surface (≈12 °C) compared with that in deep layers (<4 °C at 80 m). Throughout the annual cycle, this produces a period of vertical homogeneity between November and April, with stratification between May and October. Wind episodes from late summer through fall contribute to the vertical mixing. The annual cycle of stratification is the main driver of the 2 distinct modes displayed by natural food conditions (24, 25). Previous studies allowed us to establish that the period of Secchi values >18 m after the spring bloom (days Secchi >18 m) correspond to the low food availability mode that characterizes the summer period (see SI Methods). Given that the reported warming trend of the northwestern Mediterranean during the 1974–2001 period was greater for surface waters (0.040 °C/year) than at 80 m (0.025 °C/year; ref. 26), we examined whether this warming trend affects stratification of the water column. We used temperature differences between the surface (0.5 m) and 80 m (T0–80) and between 20 and 80 m (T20–80) as proxies of stratification. We analyzed the T0–80 and T20–80 series and their anomalies (observed minus average, Ta0–80, Ta20–80) to detect deviations from the long-term average. The 20-m depth is above the thermocline in the summer, and we chose it to examine the effects of environmental conditions on the coralligenous community because mass mortality events have affected the community at least from this depth upward (11, 12, 13, 27).
Results and Discussion
Temperature Time Series Analysis.
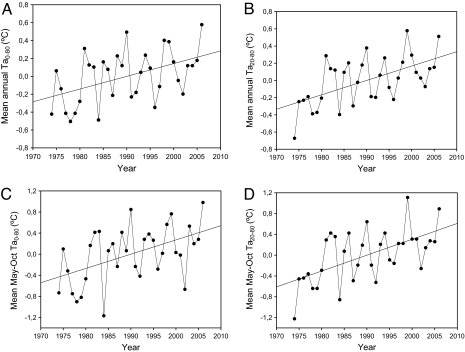
Between 1974 and 2006, the mean annual Ta0–80 exhibited a 3- to 6-year oscillatory pattern along with an increasing trend of 0.014 °C/year (Fig. 1A). The mean annual Ta20–80 displayed similar patterns of oscillation and increase (0.017 °C/year; Fig. 1B). However, in months when T0–80 and T20–80 were >1 °C (May–October; Fig. 2A), the increasing trend was twice that (0.027–0.030) of the mean annual value (Fig. 1 C and D; Table 1A). Furthermore, in contrast to the minimum mean monthly winter temperature, which did not increase over time at any depth, the maximum mean monthly temperature at the surface and at a depth of 20 m increased at a rate of 0.05 °C/year in the summer (Table 1A). This pattern correlated with the increases in the mean May–October Ta0–80may–oct and Ta20–80may–oct values (Table 1A). Thus, the increasing temperature anomaly, together with its oscillatory pattern and its positive correlation with maximum summer values, increase the probability of extreme temperature events. This finding is in accordance with the increased frequency of extreme temperature events observed at the study site over the past several decades (Table S2). But although descriptors of extreme events that include surface temperature, occurred with the same frequency during the last 2 time periods (1985–1995 and 1996–2006), descriptors of extreme events that include temperature at 20 m but temperature maxima, occurred only during the last time period (Table S2).
Fig. 1.
Trends exhibited by the 1974–2006 temperature time series. (A) Anomaly (observed minus average) of the mean annual temperature difference between 0.5 m and 80 m over time. (B) Anomaly of the mean annual temperature difference between 20 m and 80 m over time. (C) Anomaly of the mean temperature difference between 0.5 m and 80 m from May–October (the time period during which T0–80 was >1 °C) over time. (D) Anomaly of the mean temperature difference between 20 m and 80 m from May–October (the time period during which T20–80 was >1 °C) over time.
Fig. 2.
Main effects of temperature anomaly between depths of 20 m and 80 m on water column stratification. (A) Temperature differences between 20 m and 80 m over the annual cycle during the first 5 years of the series (1974–1978) versus those during the last 5 years (2002–2006), showing the increased intensity in stratification of the water column. (B) Rate of increase (days/year) in the number of days that the temperature difference between 20 m and 80 m was >0.5–8.5 °C. The shaded area indicates the range of temperature difference between 20 m 80 m in which the rate of increase was significant (see Table S3). (C) Number of days that temperature was >18 °C at 20 m in 1974–2006.
Table 1.
Statistical support of the trends exhibited by the 1974–2006 temperature time series: Pearson product moment correlations (A) between temperature (°C) and time (years) and between the different descriptors of the temperature time series, and (B) between the number of days that temperature was >18°C and time and between the date that temperature reached 18°C in spring and dropped below 18°C in the fall
| Pearson product moment correlation | °C/year | R | P | n |
|---|---|---|---|---|
| (A) | ||||
| Mean annual Ta0–80 versus time | 0.014 | 0.475 | .048 | 33 |
| Mean annual Ta20–80 versus time | 0.017 | 0.578 | .002 | 33 |
| Mean Ta0.5–80 May–October versus time | 0.027 | 0.494 | .032 | 33 |
| Mean Ta20–80 May–October versus time | 0.030 | 0.587 | .002 | 33 |
| Maximum mean monthly 0.5-m summer temperature versus time | 0.052 | 0.498 | .029 | 33 |
| Maximum mean monthly 20-m summer temperature versus time | 0.052 | 0.547 | .006 | 33 |
| Maximum mean monthly 0.5-m summer temperature anomaly versus Ta0.5–80 May–October | 0.656 | <.001 | 33 | |
| Maximum mean monthly 20-m summer temperature anomaly versus Ta20–80 May–October | 0.661 | <.001 | 33 | |
| Maximum mean monthly 20-m summer temperature anomaly versus days at > 18°C at 20 m | 0.514 | .013 | 33 | |
| Minimum mean monthly 0.5-m winter temperature versus time | 0.230 | >.9 | 33 | |
| Minimum mean monthly 20-m winter temperature versus time | 0.295 | >.9 | 33 | |
| Minimum mean monthly 80-m winter temperature versus time | 0.282 | >.9 | 33 | |
| (B) | ||||
| Days that temperature was >18°C at 20 m versus time | 1.25 | 0.620 | <.001 | 33 |
| Day that temperature was >18°C at 20 m in spring versus time | −0.71 | 0.658 | <.001 | 33 |
| Day that temperature was <18°C at 20 m in fall versus time | 0.53 | 0.395 | .023 | 33 |
Surface (0.5 m)–80 m and 20–80 m refer to water depth in meters. Bonferroni correction has been applied to probability levels.
The only sampling point older than l'Estartit with a regular weekly sampling is at Villefranche-sur-mer (Ligurian Sea). This temperature time series started in 1957 and exhibited no significant trend between 1957 and 1978 (28). A posterior analysis of this series up to 1995 demonstrated a relative surface cooling from 1966 to 1974, followed by a rapid warming from 1975 onward (29). Such behavior would explain the absence of an overall trend up to 1978 found previously. However, because the reported standard deviations of temperature up to 1978 were 2 °C at surface but only 0.3 °C at 20 m (28), this cooling period might have been restricted to the surface layer. Moreover, despite the cooling period, the annual maxima of surface temperature from the Villefranche series up to 2005 demonstrated a significant warming trend (30). These results indicate that the cooling period observed during the 1960s and early 1970s in the English Channel (31) and in the northeastern Atlantic Ocean in general (4) also affected the surface layer in the western Mediterranean. Therefore, the trends detected in our series may be slightly overestimated, because the observations started at around the coldest years. But despite the cooling period, the final values for Villefranche-sur-mer are clearly higher than those in the late 1950s (29, 30), and thus the observed extreme events have no precedents in the last 50 years in the western Mediterranean.
Higher temperatures had 2 main effects on water column stratification. First, a strong stratification developed earlier, during the late spring to summer (achievement of 2.5–4.5 °C of T20–80, corresponding to an advancement of spring of 0.4–0.7 days/year; Fig. 2A; Table S3). Second, throughout the same period, T20–80 increased over time (Fig. 2 A and B; Table S3). Higher temperatures then led to stronger thermal stratification and increased stability of the water column. Within the affected range (2.5–4.5 °C), the rate of earlier development of strong stratification increased with increasing T20–80 (Fig. 2B; Table S3). The 4.7 °C value of T20–80 was used to examine the effect of higher temperature anomalies on the duration of stratification. This value approximately corresponded with a temperature of 18 °C at a depth of 20 m [T20–80: 4.67 ± 0.05 (mean ± SE)] that was reached on June 21 (± 2 days), which is near the summer solstice. The achievement of 18 °C at 20 m is a good indicator of summer conditions, because it represents a threshold above which stratification consolidates and becomes stable until the end of summer (Fig. 2B). The number of days that the temperature was >18 °C at 20 m (days T20 >18 °C) varied from 75 to 164 days over the study period, with a trend of an increase of 1.25 days/year (Fig. 2C), due mainly to the advancement of spring (Table 1B). The timing and duration of the descriptor of low food availability (days that Secchi value was >18 m: 113.46 ± 3.21 days, starting on June 18 ± 2 days) approximately corresponded with that of days T20 >18 °C (119.32 ± 3.39 days). Both of the main descriptors of summer conditions—periods of high temperature (days T20 >18 °C) and of low food availability (days Secchi >18 m)—were correlated over the study period (r = 0.3771; P <.0333; n = 33). We can conclude that on average, the observed warming trend between 1974 and 2006 has caused a 40% (i.e., 40 days) increase in the time during which the coralligenous community is exposed to summer conditions. No change in wind pattern affecting autumnal overturn has been observed. Lengthening of summer conditions correlated with an increase in maximum monthly summer temperature at 20 m (Table 1A). The observed lengthening of summer conditions, together with the oscillatory pattern of temperature anomalies, accentuates the probability of occurrence of extremely long summer periods.
Stratification of the water column is a key control of marine productivity (32) that affects the flux of carbon and temperature of the coupled ocean–atmosphere system (33, 34), a main determinant of global climate (35). The Mediterranean Sea is been affected by warming (26) similarly to other areas (36). Although under this scenario, the upper sea should become more stratified, the outcome of warming on stratification is uncertain, because of the trade-off between events producing water column stabilization and destabilization events (4). Furthermore, this outcome may vary regionally, and long-term time series of direct observations are lacking (37). Our results provide evidence that enhanced stratification of coastal waters due to global warming is occurring in the northwestern Mediterranean.
Many biological processes are expected to be affected by increased stratification intensity, which produces longer and warmer summers (Table S4), similar to recent evidence of the early onset of spring events (38) and the prolonged growing season in terrestrial ecosystems (39) and alterations of marine pelagic phenology (40), which also have been attributed to climate warming. Associating changes in biological processes, such as mass mortality events, with their responsible environmental causes is one of the most challenging aspects of understanding biological variability. The frequency of mass mortality events has increased over the last several decades in the northwestern Mediterranean from the occurrence of local-scale events (several tens of meters or kilometers) to regional-scale events (several hundreds of kilometers) in 1999 and 2003 (Table S1). These regional-scale events coincided with the years displaying extreme events in terms of the highest number of days that T20–80 >6.5 °C (the greatest T20–80 exhibiting a significant pattern of increase over time; Table S3) over the period 1974–2006 (Table S2). Extreme events in terms of the highest mean May–October Secchi disk measurements occurred in 2003, 1997, and 1999. Then the number of days that T20–80 >6.5 °C is the descriptor of extreme events that better accounts for the main factors of the energetic constraint hypothesis, because it integrates high temperature (high respiratory demand), high stratification (low food availability), and time of exposure to both factors. We examined the potential consequences of the trend of warming and lengthening of summer conditions on the Mediterranean coralligenous community by examining the effects on a well-studied model organism, the gorgonian Paramuricea clavata, a large contributor to the community structure and biomass that has been strongly affected by the events (11–13, 27).
Experimental Work on a Model Organism.
We collected apical branches of P. clavata from different colonies and kept them in flow-through seawater tanks. After acclimation, the branches were exposed to 4 different combinations of temperature (ambient and high) and food concentration (ambient and high), to examine the performance of the organisms under the observed climatic anomalies (high-temperature–ambient-food treatment; see ref. 16) using normal summer natural conditions as a reference (ambient-temperature–ambient-food treatment; see Methods). This allowed us to explore whether energetic constraints could be the mechanism through which anomalous climatic conditions induce mass mortality of benthic suspension feeders. It is important to note that the simulated high temperature (23 °C) is within the temperature range that the organisms experience under natural conditions (26). Partial mortality, surface area, and biomass of the branches were monitored periodically. Furthermore, field P. clavata samples were collected monthly over an annual cycle and from affected and unaffected colonies during a mass mortality event.
Appearance of Partial Mortality.
None of the colonies in either ambient-temperature treatment displayed partial mortality over the duration of the experiment. In contrast, colonies in both high-temperature treatments displayed partial mortality. The development of damaged tissue was the first sign of partial mortality. Damaged tissue exhibited a color change from normal red or yellow to grayish and black. This color change, which was due to tissue cell death, was conducive to sclerite exposure and subsequently led to axis denudation. This process was similar to that documented in situ (12). The first signs of partial mortality appeared in the high-temperature–ambient-food treatment, 49 days after the start of the experiment. From there on, the proportion of colonies with partial mortality increased up to 83%. In contrast, in the high-temperature–high-food treatment, only 55% of the colonies showed partial mortality at day 130, and no colony was affected until day 84 (Fig. 3A). Thus, supplemental food resulted in a ≈45% delay in the appearance of partial mortality.
Fig. 3.
Experimental work on the model organism P. clavata (Cnidaria: Octocorallia). (A) Variation over time in the proportion of colonies with partial mortality under different treatments and replicates. Arrows depict appearance of the first signs of partial mortality 49 days and 84 days after the beginning of the experiment in the high-temperature–ambient-food treatment and in the high-temperature–high-food treatment, respectively. None of the colonies under either ambient temperature treatment exhibited partial mortality. (B) Percentage of biomass loss at the first appearance of partial mortality of the colonies undergoing both high-temperature treatments. Because none of the colonies in either ambient-temperature treatment displayed partial mortality, the biomass loss in these treatments was estimated as the difference in biomass between the first day and the last day of the experiment. Colonies in the ambient-temperature–high-food treatment did not display biomass loss. Solid squares, ambient-temperature–high-food; Open squares, ambient-temperature–ambient-food;. Solid circles, high-temperature–high-food;Open triangles circles, high-temperature–ambient-food. Vertical bars denote SE.
Biomass.
In those treatments for which the branches exhibited partial mortality (i.e., both high-temperature treatments), the variation in biomass was calculated as the difference in biomass between the beginning of the experiment and the day of the first appearance of partial mortality. This allowed us to determine that reaching a biomass loss threshold of ≈35.6% ± 1.3% (Fig. 3B) was the main determinant of the appearance of partial mortality in the colonies. This threshold also can be described in terms of reaching a biomass of ≈0.026 ± 0.001 gDW/cm of branch length.
In those treatments for which the branches did not exhibit partial mortality (i.e., both ambient-temperature treatments), the variation in biomass was calculated as the difference in biomass between the first day and the last day of the experiment. Biomass did not change in the colonies subjected to the ambient-temperature–high-food treatment (Fig. 3B). At ambient-food conditions, the rate of biomass loss was ≈2.9 times greater in the branches exposed to high temperatures than those exposed to ambient temperatures (0.14% ± 0.01%/day vs. 0.40% ± 0.01%/day), explaining the early appearance of partial mortality. Supplemental food reduced the rate of biomass loss at ambient temperature but not at high temperature (0.32% ± 0.01%/day; Table S5). Thus, variation in the timing of occurrence of partial mortality in the different treatments was due to the differences in the rate of biomass loss. In terms of dry weight, branches in the ambient-temperature–ambient-food treatment exhibited a 14% decrease in dry weight (0.030 vs. 0.035 gDW/cm initial biomass; Fig. 3B). Branches in the high-temperature–high-food treatment lost about 31% of their biomass (0.024 gDW/cm), and those in the high-temperature–ambient-food treatment lost about 49% of their biomass (0.018 gDW/cm).
The 2 main effects observed in the experiment were a 14% decrease in biomass (0.030 gDW/cm) in the treatment simulating normal summer conditions and that a biomass loss of ≈35.6% determined the appearance of partial mortality (0.026 gDW/cm). These experimental results were similar to field observations, where (i) under normal summer conditions, the biomass of gorgonian branches varied over the annual cycle, higher in the spring (0.042 ± 0.001 gDW/cm) and minimal at the end of summer (0.031 gDW/cm; 1-way ANOVA; F3,313 = 18.974; P <.00001; Scheffé′s contrast test), and (ii) the biomass of gorgonian branches affected by a mass mortality event (0.022 ± 0.001 gDW/cm) differed from that of unaffected colonies (1-way ANOVA; F2,58 = 22.956; P <.000001; Scheffé′s contrast test) and was below the threshold value determined above.
The fact that the ambient-temperature–ambient-food treatment produced a biomass loss similar to that observed in gorgonians in the field during normal summer conditions indicates that lengthening of summer conditions would lower the colonies' capability to withstand anomalous conditions. According to the energetic constraints hypothesis, the loss in biomass would be the end result of a metabolic imbalance between the high energy expenditure due to respiration at high summer temperatures combined with low energy income due to low food availability in the summer. The experimental results conform to the field observations, and both the experimental and field results support the energetic constraints hypothesis, because (i) the biomass of colonies affected by a mortality event was below the threshold experimentally observed to determine the appearance of partial mortality, (ii) the variation in the timing of occurrence of partial mortality in the treatments was due to differences in the rate of biomass loss, (iii) the loss of biomass in the ambient-temperature–ambient-food treatment was similar to that observed in situ in late summer, (iv) the loss of biomass did not occur when supplemental food was provided at ambient temperatures, and (v) the alternative hypothesis related to the exposure to lethal temperature and/or to pathogenic microorganisms causes tissue cell death without biomass loss (21, 22).
Our experimental results together with the field observations help clarify the mechanisms that trigger mass mortality events by establishing a cause-and-effect relationship between the environmental factors that characterize summer conditions and biomass loss and the occurrence of partial mortality in the laboratory and in situ. The results indicate that positive temperature anomalies are the underlying cause of mass mortality, with energetic constraints being the main triggering mechanism. Under the current warming trend, the relationship between maximum monthly summer temperatures and the lengthening of the summer period due to enhanced stratification increases the probability of longer and warmer summers (extreme events; Table S2) and thus of the occurrence of mass mortality events similar to what has been observed in coral reef ecosystems (41, 42). Our findings are consistent with the occurrence of the regional-scale episodes of mass mortality affecting populations of benthic invertebrates in the northwestern Mediterranean over the last decade (Table S1). Such mortality events were not reported previously. Our results also are in accordance with the findings of studies on microorganisms as a factor related to these events, because biomass loss is a sign of physiological stress that makes colonies more susceptible to opportunistic, residential, and/or pathogenic microorganisms (11, 19, 21), which has been observed in corals (43). But under the observed trend of increasing extreme temperature events, increased contributions of lethal temperature and increased virulence of microorganisms to the events should be expected in the near future. The link between global warming and mass mortality supports the current trend of increasing frequency of mass mortality events, which would induce profound changes in the present benthic community composition in littoral areas and may lead to the demise of populations of the coralligenous community situated above the summer level of the thermocline. Thus, further work relating changing physical oceanographic conditions to mass mortality events is crucial to determine the causes and mechanisms of future events that are likely to occur.
The examination of temporal trends in environmental conditions, coupled with the experimental establishment of cause-and-effect relationships, is crucial to understanding the consequences of global warming on marine ecosystems. Our study provides evidence of enhanced stratification of the water column due to global warming and may contribute to an understanding of the extent to which the Mediterranean coralligenous community may or may not be able to acclimate to the gradual pattern of change in environmental conditions and to the consequences of increases in the frequency and magnitude of extreme events that global warming implies.
Methods
Temperature Time Series.
Temperature was measured 1.7 km offshore of the Medes Islands (northwestern Mediterranean, 42°3′N, 3°13′15′E) over a 33- year period (1974–2006) in a 90-m depth range. The seawater temperature was measured with calibrated Richter and Weise reversing thermometers at the surface (0.5) and at depths of 20, 50, and 80 m approximately weekly (50–60 times per year). The data were averaged to obtain monthly estimates of temperature. Temperature differences between the surface and 80 m (T0–80) and between 20 and 80 m (T20–80) were used as proxies of stratification. The depth of 20 m was used to examine the effects of environmental conditions on the coralligenous community because all mass mortality events have affected the community at least from this depth upward (11–13). We characterized the long-term series in the interannual variation of T0–80 and T20–80 and their anomalies (observed minus average temperature) to examine whether a trend exists and if so, whether it affects the stratification of the water column. By interpolating the data set, we determined the date in the spring that T20–80 reached 0.5 °C and the date in fall that it dropped below 0.5 °C. Next, we estimated the duration of stratification by calculating the time period during which T20–80 was >0.5 °C every year and determining whether this period varied over time. We performed the same estimate for 2 °C intervals of T20–80 between 0.5 and 8.5 °C. We performed Secchi disk measurements simultaneously with the temperature measurements. Salinity was not systematically recorded at the location before 1991. The mean salinity over the annual cycle was 37.71% ± 0.06%, indicating that salinity plays only a minimal role in the stability of the water column. Typically, Mediterranean tides are very low. Tides were measured at the study site since 1990, demonstrating a mean amplitude of 18.3 ± 0.1 cm, with some occasional storm surges that reach higher values but never above 70 cm. This indicates that tides exert little effect to counter solar radiation as the main forcing mechanism of water column stratification.
Sample Collection and Experimental Setup.
In late June, an apical branch was collected by scuba divers from each of 240 healthy P. clavata gorgonian colonies from a depth of 15–20 m at the Medes Islands. The gorgonians were transported in large seawater containers to the Experimental Aquarium Zone (ZAE) of the Institute of Marine Sciences in Barcelona within 1.5 h of collection. Branches were placed on artificial substrata using an inert mastic compound and distributed randomly among 8 large tanks (150 cm long × 75 cm wide × 25 cm deep). After the acclimation period, the colonies were gradually subjected to 4 different treatments combining temperature (ambient and high) and food concentration (ambient and high), with 2 replicate tanks used for each treatment. Ambient temperature followed the normal summer cycle of temperature at a depth of 20 m, which did not differ from that of the mean summer temperature. (The mean value over the course of the experiment, 18.9 ± 0.1 °C, was similar to the mean July–October temperature at 20 m at the sampled site, 19.6 °C; see ref. 26.) The high temperature was set at 3–4 °C above the ambient temperature; the mean value over the course of the experiment was 23.2 ± 0.1 °C. This high temperature was selected to represent the temperatures occurring during mass mortality events (17). Temperature was continuously monitored with Hobo pendant sensors (UA-002–64; Onset Computer Corp). Food concentration was estimated as total particulate organic carbon and zooplankton density. Ambient food concentration was 321 ± 49 mg C/L and 0.3 zooplankters/L, and high food concentration was 452 ± 72 mg C/L and 3.2 zooplankters/L. These values mimicked natural food conditions that exhibit 2 modes through the year at the study area (25). The treatments were maintained for 130 days to mimic the average number of days that the temperature was >18 °C over the last 15 years (128 ± 5 days, 1992–2006). Surface area and partial mortality were monitored periodically by visual and photographic inspection. In the figures, error bars represent SE. Details regarding tank maintenance, acclimation, experimental conditions, monitored parameters, field colony weight, and data analysis are provided in SI Methods.
Supplementary Material
Acknowledgments.
Support for this work was provided by FPI fellowships from the Ministerio de Ciencia e Innovación (MCI) of Spain (to E.S. and E.J.) and by MCI Projects CTM 2006–01463 and CGL2007–66757-C02–01/BOS. We thank R. A. Kinzie, E. Calvo, C. Pelejero, and E. Ballesteros for their valuable comments on different versions of the manuscript; the ZAE staff at the Institute of Marine Science for technical assistance; and C. Linares and A. Gori for assistance with field colony weight estimates. The authors are part of the Marine Biogeochemistry and Global Change research group from the Generalitat de Catalunya.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805801106/DCSupplemental.
References
- 1.Stenseth NH, et al. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 2.Helmuth B, Mieszkowska N, Moore P, Hawkins SJ. Living on the edge of two changing worlds: Forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst. 2006;37:373–404. [Google Scholar]
- 3.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 4.Intergovernmental Panel on Climate Change. Climate Change 2007: The Physical Sciences Basis. Cambridge, UK: Cambridge Univ Press; 2007. Contribution of Working Group I to the Fourth Assessment Report to the Intergovernmental Panel on Climate Change. [Google Scholar]
- 5.Harvell CD, et al. Emerging marine diseases: Climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 6.Harvell CD, et al. The rising tide of ocean diseases: Unresolved problems and research priorities. Front Ecol Environ. 2004;2:375–382. [Google Scholar]
- 7.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 8.Gardner TA, Côte IM, Gill JA, Gran A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 9.Knowlton N. Multiple “stable” states and the conservation of marine ecosystems. Prog Oceanogr. 2004;60:387–396. [Google Scholar]
- 10.Ballesteros E. Mediterranean coralligenous assemblages: A synthesis of the present knowledge. Oceanogr Mar Biol Annu Rev. 2006;44:123–195. [Google Scholar]
- 11.Cerrano C, et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (NW Mediterranean), summer 1999. Ecol LeTT. 2000;3:284–293. [Google Scholar]
- 12.Perez T, et al. Mortalité massive d'invertébrés marins: Un événement sans précédent en Méditerranée nord-occidentale. CR Acad Sci Paris III. 2000;323:853–865. doi: 10.1016/s0764-4469(00)01237-3. [DOI] [PubMed] [Google Scholar]
- 13.Garrabou J, et al. Mass mortality in the NW Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Global Change Biol. 2009 in press. [Google Scholar]
- 14.Kim K, Harvell CD. The rise and fall of a 6-year coral-fungal epizootic. Am Nat. 2004;164:S52–S63. doi: 10.1086/424609. [DOI] [PubMed] [Google Scholar]
- 15.Lasker HR. Gorgonian mortality during a thermal event in the Bahamas. Bull Mar Sci. 2005;76:155–160. [Google Scholar]
- 16.Ward JR, Lafferty KD. The elusive baseline of marine disease: Are diseases in ocean ecosystems increasing? PLoS Biol. 2004;2:542–547. doi: 10.1371/journal.pbio.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano JC, Bensoussan N, Younes WAN, Arlhac D. Anomalies thermiques dans les eaux du golfe de Marseille durant l'été 1999: Une explication partielle de la mortalité d'invertébrés fixés. CR Acad Sci Paris III. 2000;323:415–427. doi: 10.1016/s0764-4469(00)00141-4. [DOI] [PubMed] [Google Scholar]
- 18.Sparnocchia S, Schiano ME, Picco P, Bozzano R, Cappelletti A. The anomalous warming of summer in the surface layer of the central Ligurian Sea (western Mediterranean) Ann Geophys. 2006;24:443–452. [Google Scholar]
- 19.Martin Y, Bonnefont JL, Chancerelle L. Gorgonians mass mortality during the 1999 late summer in French Mediterranean coastal waters: The bacterial hypothesis. Water Res. 2002;36:779–782. doi: 10.1016/s0043-1354(01)00251-2. [DOI] [PubMed] [Google Scholar]
- 20.Coma R, Ribes M. Seasonal energetic constraints in Mediterranean benthic suspension feeders: Effects at different levels of ecological organization. Oikos. 2003;101:205–215. [Google Scholar]
- 21.Bally M, Garrabou J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: A new case of emerging disease linked to climate change. Global Change Biol. 2007;13:2078–2088. [Google Scholar]
- 22.Torrents O, Tambutté E, Caminiti N, Garrabou J. Upper thermal thresholds of shallow vs. deep populations of the precious Mediterranean red coral Corallium rubrum (L.): Assessing the potential effects of warming in the NW Mediterranean. J Exp Mar Biol Ecol. 2008;357:7–19. [Google Scholar]
- 23.Coma R, Ribes M, Gili JM, Zabala M. Seasonality in coastal benthic ecosystems. Trends Ecol Evol. 2000;15:448–453. doi: 10.1016/s0169-5347(00)01970-4. [DOI] [PubMed] [Google Scholar]
- 24.Estrada M. Primary production in the northwestern Mediterranean. Sci Mar. 1996;60:55–64. [Google Scholar]
- 25.Ribes M, Coma R, Gili JM. Seasonal variations of POC, DOC and the contribution of microbial communities to the live POC in a shallow near-bottom ecosystems at the northwestern Mediterranean Sea. J Plankton Res. 1999;21:1077–1100. [Google Scholar]
- 26.Salat J, Pascual J. The oceanographic and meteorological station at L'Estartit (NW Mediterranean) In: Briand F, editor. Tracking Long-Term Hydrological Change in the Mediterranean Sea. Monaco: CIESM Workshop; 2002. pp. 29–32. [Google Scholar]
- 27.Linares C, et al. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Mar Ecol Prog Ser. 2005;305:127–137. [Google Scholar]
- 28.Béthoux N, Etienne M, Ibanez F, Rapaire JL. Spécificités hydrologiques des zones littorales: Analyse chronologique par la méthode du Census II appliquée à la baie de Villefranche-sur-mer. Ann Ins Océanogr. 1980;56:81–95. [Google Scholar]
- 29.Buecher E. Distribution and abundance of Pleurobrachia rhodopis (Cydippid ctenophore) in the Bay of Villefranche-sur-Mer (northwestern Mediterranean) studied using three different planktonic time series. Ann Inst Oceanogr. 1997;72:173–184. [Google Scholar]
- 30.Diaz-Almeda E, Marbà N, Duarte CM. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Global Change Biol. 2007;13:224–235. [Google Scholar]
- 31.Hawkins SJ, Southward AJ, Genner MJ. Detection of environmental change in a marine ecosystem: Evidence from the western English Channel. Sci Total Environ. 2003;310:235–256. doi: 10.1016/S0048-9697(02)00645-9. [DOI] [PubMed] [Google Scholar]
- 32.Behrenfeld MJ, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444:752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 33.Sarmiento JL, Hughes TMC, Stouffer RJ, Manabe S. Simulated response of the ocean carbon cycle to anthropogenic climate warming. Nature. 1998;393:245–249. [Google Scholar]
- 34.Le Quéré C, Aumont O, Monfray P, Orr J. Propagation of climatic events on ocean stratification, marine biology, and CO2: Case studies over the 1979–1999 period. J Geophys Res. 2003;108:3375–3389. [Google Scholar]
- 35.Rahmstorf S, Ganopolski A. Long-term global warming scenarios computed with an efficient coupled climate model. Clim Change. 1999;43:353–367. [Google Scholar]
- 36.Levitus S, Antonov J, Boyer T. Warming of the world ocean, 1955–2003. Geophys Res Lett. 2005;32:L02604. [Google Scholar]
- 37.Sharples J, Ross ON, Scott BE, Greenstreet SPR, Fraser H. Inter-annual variability in the timing of stratification and the spring bloom in the northwestern North Sea. Continent Shelf Res. 2006;26:733–751. [Google Scholar]
- 38.Whalter GR, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 39.Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- 40.Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson C, et al. Ecological and socioeconomic impacts of 1998 coral mortality in the Indian Ocean: An ENSO impact and a warning of future changes? AMBIO. 1999;28:188–196. [Google Scholar]
- 42.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshw Res. 1999;50:839–866. [Google Scholar]
- 43.Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Gulberg O. Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol. 2007;346:36–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.