Abstract
At −1.8 °C, the waters of Antarctica pose a formidable physiological barrier for most ectotherms. The few taxa that inhabit this zone have presumably made specific adjustments to their neuromuscular function and have enhanced their metabolic capacity. However, support for this assertion is equivocal and the details of specific compensations are largely unknown. This can generally be attributed to the fact that most Antarctic organisms are either too distantly related to their temperate relatives to permit direct comparisons (e.g., notothenioid fishes) or because they are not amenable to neuromuscular recording. Here, as a comparative model, we take advantage of 2 pelagic molluscs in the genus Clione to conduct a broadly integrative investigation on neuromuscular adaptation to the extreme cold. We find that for the Antarctic congener aerobic capacity is enhanced, but at a cost. To support a striking proliferation of mitochondria, the Antarctic species has shed a 2-gear swim system and the associated specialized neuromuscular components, resulting in greatly reduced scope for locomotor activity. These results suggest that polar animals have undergone substantial tissue-level reorganizations to accommodate their environment, which may reduce their capacity to acclimate to a changing climate.
Keywords: Antarctica, Clione antarctica, Clione limacina, temperature adaptation, mitochondria
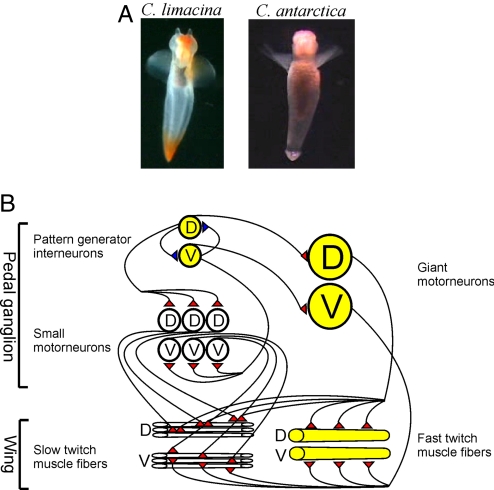
Clione antarctica, which passes its complete life cycle below 0 °C, and Clione limacina (Fig. 1A), normally found between ≈5 and 12° C in the north Atlantic and Pacific, occupy nearly identical ecological niches (1) despite their presumed separation by the circumpolar current >30 million years ago (2). They both feed exclusively on the thecosomatous pteropod Limacina helicina (3) and, although planktonic, rely on 2 wing-like parapodia for locomotion. The predatory arsenal of C. limacina, which has been studied in great detail, includes a multigeared neuromuscular swimming system that drives the parapodia to sweep through an arc of ≈180° (4, 5). During routine swimming, animals shift between slow (≈1–2 Hz) and fast (2–5 Hz) modes, based on their wing-beat frequency (5, 6). The present study was prompted by the simple observation that C. antarctica has never been seen swimming in the fast mode (4, 6) (Movies S1–S3).
Fig. 1.
The swim system of C. limacina. (A) Photos of C. limacina and C. antarctica. (Approximate magnification is 0.25× for C. limacina and 1× for C. antarctica.) (B) Cartoon circuit diagram of the swim system of C. limacina. D, elements involved in dorsal wing flexions; V, elements involved in ventral wing flexions. Excitatory synaptic connections are in red and inhibitory ones in blue. For simplicity, inhibitory connections between interneurons and motoneurons, and all connections to the contralateral pedal ganglion, have been left out. Elements that are reconfigured or recruited into activity during fast swimming are colored in yellow.
For C. limacina, the cellular underpinnings of the locomotory system, and its ability to shift speeds, have been uncovered by using a reduced preparation that contains only the parapodia, the wing nerves, and the 2 pedal ganglia (Fig. 1B), structures sufficient to generate the entire swim cycle (6, 7). Neural control of swimming is generated within the pedal ganglia. A pair of interneurons establish the basic wing-beat frequency (7, 8), one controlling dorsal phase flexions of the parapodia and the other ventral phase flexions. They inhibit each other by the process of postinhibitory rebound (9). Each interneuron is connected to multiple small motoneurons that drive small fields of slow-twitch, mitochondria-rich muscle fibers in the parapodia (10). During slow swimming, a long-duration action potential from one of the interneurons inhibits the small motoneurons from the opposite phase while exciting those of the same phase. Serotonin induces fast swimming by reducing the cycle period between action potentials in the interneurons and by recruiting 2 new neuromuscular elements into play. First, a pair of giant motoneurons is activated (6–8, 10–13). These cells innervate the entire assemblage of slow-twitch muscle fibers, and an equally abundant complement of fast-twitch, mitochondria-poor fibers that were previously quiescent (10). These 2 new elements effectively enhance contractility to create more powerful wingbeats.
All previous studies of the C. limacina swim system were conducted at room temperature. By controlling the experimental temperature, we could expand on these results and ask 3 basic questions related to adaptation of the Clione swim system to the extreme cold. First, how does the swim system of C. limacina operate across its native temperature range? Second, how would it operate at the low temperatures experienced by C. antarctica? Third, even if C. antarctica is never observed swimming fast, can its nervous system generate the signals for fast swimming and if so, why doesn't it swim fast? To answer these questions, we recorded the output from swim motoneurons in reduced preparations from each species between −1.8 °C and 20 °C. These recordings allowed us to monitor the temperature dependence of the swim cycle frequency (both fast and slow) in both species without damaging the interneurons that control it. The electrophysiological results were surprising and led us to examine the specific neuromuscular elements that are required for fast swimming in C. limacina. These data suggest that there is a tradeoff between the increased aerobic capacity required for routine locomotion in the cold and the ability to generate fast swimming.
Results
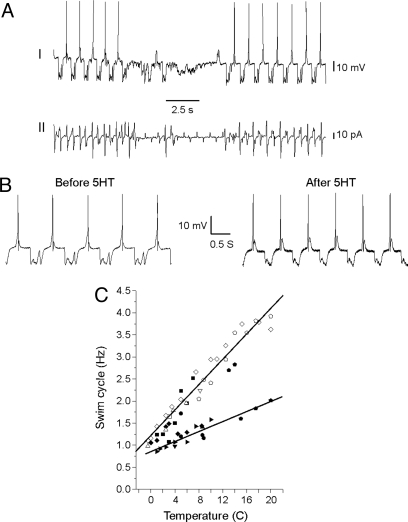
As a first step to examining adaptation in the Clione nervous system, we measured the neural output that drives swimming in C. limacina over a broad range of temperatures. Animals for these studies were collected in Logy Bay, Newfoundland, Canada, where the water temperature was ≈7 °C. Others have shown that the swim system spontaneously shifts between slow and fast modes, presumably due to the intermittent release of endogenous serotonin. The application of exogenous serotonin forces the preparation exclusively into the fast mode. Fig. 2A shows an intracellular recording (I) from a swim motoneuron from C. limacina measured at 4 °C and a simultaneous recording from a motion detector electrode (II). Clearly, the neuron's firing pattern and cyclical resting potential matches wing movements, making it easy to identify as a swim motoneuron. Fig. 2B shows recordings from the same cell at an expanded time scale, immediately before and after the application of serotonin. The resting potential approximates a sine wave with a single action potential fired at its apex. In this case, serotonin increases the swim cycle frequency from ≈1 Hz to 1.3 Hz. The increase was greater at higher temperatures, in agreement with previous studies (6). Fig. 2C shows swim cycle vs. temperature data for many cells, with and without serotonin. Two swim cycle speeds are evident, particularly at higher temperatures. C. limacina live at ≈7–10 °C, thus their swim speed would shift between ≈1.25 Hz and 2.5 Hz. An interesting feature of the slow and fast swim cycles is that they have different temperature dependencies (Q10slow = 1.6, Q10fast = 2.2). This difference has an important consequence: below ≈2 °C fast and slow swim cycles are basically the same. Presumably, the separation of the Antarctic continent and the development of the circumpolar current ≈30 million years ago, C. antarctica have lived at a temperature that would prohibit fast swimming for C. limacina. To be able to change gears and swim fast, have they adapted?
Fig. 2.
Microelectrode recordings from swim motoneurons of C. limacina. Specimens were collected from Logy Bay, Newfoundland. (A) An example of a simultaneous recording from a swim motoneuron (I) and a motion sensor electrode placed against the wing (II) at 4 °C. (B) The same cell immediately before and after the bath application of 50 μM serotonin (5-hydroxytryptamine, 5HT). (C) Cumulative temperature vs. swim cycle data. The swim cycle was measured between the peaks of successive action potentials. Each shape represents a different swim motoneuron. Filled symbols were measured in filtered seawater, open symbols were measured in filtered seawater with 50 μM serotonin. Lines are linear fits to the slow or fast data. See Methods for explanation on how slow and fast swimming were separated. The bursting properties of the swim motoneurons also varied with temperature. At temperatures above ≈12 °C, the cells tended to fire bursts of 4–7 action potentials. At temperatures below ≈7 °C, they generally fired only 1–2.
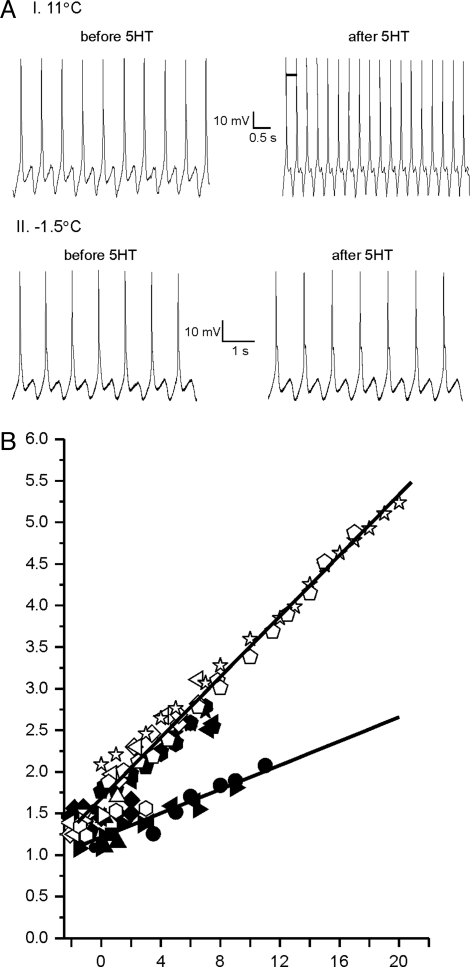
To address this question we performed identical experiments on C. antarctica collected from the partially frozen waters surrounding McMurdo Station, Antarctica. Fig. 3AI shows records from a swim motoneuron at 11 °C, before and after bath application of serotonin. As with C. limacina, the swim cycle accelerates, in this case between 1.7 Hz and 3.25 Hz. Also like C. limacina, at −1.5 °C serotonin has no visible effect: the swim cycle remains at ≈1.1 Hz after application (Fig. 3AII). In Fig. 3B, the cumulative results for many experiments are plotted. Qualitatively, the pattern resembles that of C. limacina. Slow and fast swim cycles are apparent only at higher temperatures. Again, because the swim cycles have different temperature dependencies, below ≈0 °C there is essentially no difference between the two. Thus, for C. antarctica at their native temperature, a shift between fast and slow modes would not accelerate swimming. The absolute rates of the fast and slow swim cycles show a modest up-regulation in C. antarctica, in partial compensation for the temperature difference between habitats of the congeners (see Fig. S1).
Fig. 3.
Microelectrode recordings from swim motoneurons of C. antarctica. (A) An example of a recording from a swim motoneuron before and after the application of serotonin at 11 °C (I.) and at −1.5 °C (II.). (B) Cumulative temperature vs. swim cycle data. Each shape represents a different swim motoneuron. Filled symbols were measured in filtered seawater, open symbols were measured in filtered seawater with 50 μM serotonin. Lines are linear fits to the slow or fast data.
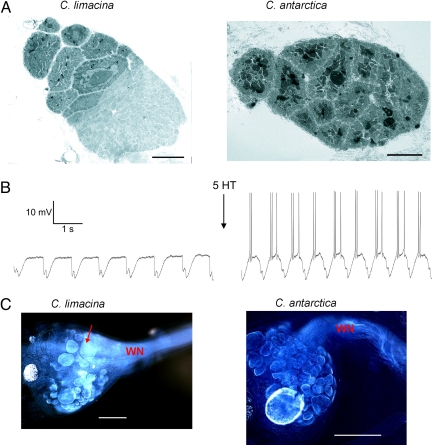
If C. antarctica are unable to generate fast swimming in their native environment, what has become of the specialized neuromuscular elements that drive this process in C. limacina (Fig. 1B)? In C. limacina wing musculature is composed of 4 layers of muscle bundles (2 dorsal and 2 ventral), each consisting of approximately equal proportions of oxidative, slow-twitch (mitochondria-rich) fibers and anaerobically-poised, fast-twitch (mitochondria-poor) fibers (10). Our electron micrographs on wing cross-sections confirm this pattern (Fig. 4A). Oxidative fibers contain a core of mitochondria that is darkly stained and clearly visible, whereas fast-twitch fibers are lighter. In C. limacina the 2 muscle types occupy distinct regions of the muscle bundle such that the oxidative fibers (upper left) and anaerobic fibers (lower right) are readily discernible by the eye. Anaerobic fibers occupy 55.3 ± 3.4% of the muscle bundle in C. limacina. In similar micrographs on C. antarctica, however, the pattern is very different. Each muscle bundle is made up entirely of oxidative fibers with distinct mitochondrial cores, the fast-twitch fibers being conspicuously absent. In C. antarctica there is also a 2-fold increase in mitochondrial abundance.
Fig. 4.
C. antarctica lack giant swim motoneurons and fast-twitch muscle fibers. (A) Representative transmission electron micrographs (magnification 1,500×) of an individual muscle bundle from C. limacina and C. antarctica. (Scale bar, 10 μm.) (B) Intracellular recordings from the same General Excitor motorneuron of C. limacina, before and after bath application of 50 uM serotonin. (C) Examples of cobalt backfills of the wing nerve from each species. WN, the junction of the pedal ganglion and the wing nerve. The dorsal surface of each pedal ganglion is shown. The arrow points to a giant swim neuron in C. limacina. Image colors have been inverted for clarity. As is common with this technique, not all of the swim motoneurons (small and large) were labeled in each preparation. For C. limacina, in some cases only 1 giant was visible, and in a few cases none were visible. However, more frequently both were apparent. For C. antarctica, the giant cells were never observed. (Scale bar, 100 μm.)
Interestingly, neural elements have also been lost in C. antarctica. In C. limacina, the 2 giant swim motoneurons used for fast swimming are the most readily identifiable cells in the pedal ganglion. They are found on the dorsal surface, one near the emergence of the wing nerve and the other a little farther back (6, 10). Because they are so clearly visible under a dissecting microscope, they make ready targets for microelectrode penetration and can be easily identified by their electrophysiological output (10) (Fig. 4B). During slow swimming their resting potential cycles with the swim cycle, but they rarely fire action potentials. During fast swimming (induced by serotonin), they fire action potentials during each cycle. In C. antarctica these cells were not evident under the dissecting microscope. As an illustration, Fig. 4C shows an example of a cobalt backfill of the wing nerve from each species. A giant motoneuron for C. limacina is marked with an arrow. In a total of 10 preparations from C. antarctica, the giants were never observed. In 7 of 8 C. limacina preparations, one or both were seen. Furthermore, extensive microelectrode penetrations throughout the pedal ganglia of C. antarctica never revealed the characteristic electrophysiological properties of the giant motoneurons. The small swim motoneurons, however, were frequently encountered. In C. limacina, the vast majority of microelectrode penetrations were in the giants. The data suggest that in C. antarctica, the giant swim motoneurons either are not present or at least have become sufficiently small that they are not readily distinguishable from other swim motoneurons.
Discussion
Two key elements for fast swimming, the fast-twitch muscle fibers and giant swim motoneurons, are missing in C. antarctica. Why have they been lost? There are at least 2 reasonable explanations. First, because the fast swim cycle has a steeper temperature dependence than that for slow swimming, there is no difference between at −1.8 °C, the temperature where C. antarctica lives. Without the ability to generate the fast swim cycle, these 2 specialized anatomical elements serve no purpose and have not been maintained by evolution. If this argument is valid then, for an unknown reason, the neural output of the central pattern generator necessary to generate fast swimming at −1.8 °C has not evolved. The swim cycle is set by a single broad action potential followed by postinhibitory rebound in the central pattern generator interneurons (14, 15). Possibly, there is a temperature-dependent limitation to the gating of one of the underlying channels or there simply has not been sufficient selective pressure for the system to adjust. An alternative explanation for the lost neuromuscular features is based on the assumption that, to compensate for depressed ATP synthesis, C. antarctica has had to enhance production capacity by increasing the number of mitochondria. The temperature sensitivity (Q10) of ATP production, as indicated by whole-animal oxygen consumption rates, is 3.60 and 4.26 for C. limacina and C. antarctica, respectively (16). In C. antarctica wing muscle there is a 2-fold increase in mitochondrial abundance (Fig. 4A) that will at least partially compensate for the ≈10 °C difference in habitat temperature of these 2 congeners. This was accomplished by completely displacing fast-twitch fibers with slow-twitch fibers, making giant motoneurons unnecessary. Past work has shown that mitochondrial proliferation is a common feature of cold adaptation (17), in some cases reaching levels theoretically high enough to displace contractile machinery (18, 19). Here we find direct evidence for this hypothesized tradeoff. In addition, others have hypothesized that giant neurons in molluscs have arisen as an adaptive response to large synaptic fields (20, 21). These cells are usually polyploid and therefore have the production capacity to supply a large number of synapses. Without the fast-twitch fibers, these cells no longer need to be giant, if they exist at all.
Regardless of the ultimate cause, C. antarctica lacks the neuromuscular capacity necessary to generate fast swimming. This regression results in a reduced scope for activity that is apparent at the behavioral level. When disturbed, C. antarctica displays a whole-body withdrawal reflex, whereas C. limacina displays an escape response that involves fast swimming. Only in C. limacina does prey capture rely on fast swimming (1, 4).
The selective advantage of mitochondrial proliferation is clear: greater production capacity compensates for the low-temperature depression of the ATP synthesis rate (22). However, the associated cost, reduced scope for activity, was unexpected. Low temperature could diminish the ability of C. antarctica to capture prey, a situation that presumably has far greater fitness consequences for a highly specialized predator such as Clione than it would for a generalist (16, 23). Therefore we expected strong selection for the maintenance of locomotory capacity in C. antarctica at polar temperatures. The apparent absence of such selective pressure for fast swimming is surprising, as is the ability of C. antarctica to capture prey and avoid predators by using a regressed locomotory system (24). The ability of C. antarctica to feed by using a regressed locomotory system highlights what may be a coevolved response of its prey to a similar selective regime, and investigation into the neuromuscular system of Limacina helicina is warranted. As stated aptly by Montgomery and Clements (25), “the best evidence of loss is the subsequent adaptive recovery.” Predator avoidance in C. antarctica is achieved through a unique “antifeedant” compound, pteroenone (26), which compensates effectively for the loss of burst swimming. In fact, Antarctic pteropods are sometimes “abducted” by amphipods—themselves seeking to avoid predation by carrying a noxious cargo (27). For C. limacina, neither amphipod abductions nor noxious compounds have been reported, although their presence cannot be ruled out. In any case, these data show that to colonize the Antarctic, Clione had to undergo large-scale rearrangements of neuromuscular elements. This is surprising because the temperature difference between the seas at Newfoundland and Antarctica is modest. Accordingly, we predict that a small increase in temperature, such as that predicted from anthropogenic forcing of climate by the Intergovernmental Panel on Climate Change, could have a disproportionate effect on the fitness of organisms adapted to the extreme cold.
Methods
Specimen Collection.
C. antarctica were collected from the waters surrounding McMurdo Station, Antarctica (Cape Bird, Cape Evans, or Cape Royds) and held for 1–3 days in aquaria at −1 °C. C. limacina were collected from Logy Bay and held for 1–4 days in aquaria at 7 °C. In some cases, animals were shipped overnight from Newfoundland to Los Angeles, where they were then held at 7 °C.
Electrophysiological Measurements.
All recordings from C. antarctica were performed on site at McMurdo Station. Recordings for C. limacina were performed either in Logy Bay or at the University of California, Los Angeles. Reduced preparations were made by dissecting specimens of each species in filtered seawater so that only the wings, wing nerves, and the central ring ganglia remained. These structures were pinned out in a recording chamber coated with Sylgard by using cactus spines. The chamber temperature was controlled by using Peltier devices. All experiments were performed in filtered seawater with or without 50 μM serotonin. Individual neurons in the intact pedal ganglion were impaled with high resistance (≈5 MΩ) glass electrodes filled with 3 M KCl. Analog signals were filtered at 20 kHz before digitizing at 100 kHz. Data were analyzed by using software developed in house. All swim cycle data collected in the presence of serotonin were considered “fast.” In the absence of serotonin, data that were within 2 standard deviations of the serotonin data were considered “fast” and the rest was considered “slow.”
Histology.
For cobalt backfills, the main portion of the wing nerve was dissected away from the wing musculature but was left connected to the pedal ganglion. The cut end of the nerves, which was separated from the rest of the preparation by Vaseline, was immersed in 0.5 M CoCl2. The rest of the preparation was immersed in filtered seawater. Cobalt was allowed to backfill for 12–15 hr at 4 °C. The preparation was then incubated in 2% ammonium sulfide in sea water for 10 minutes followed by fixation in Carnoy's fixative (70% ethanol, 20% chloroform, 10% acetic acid) and then dehydration in an ethanol series. Tissue was then cleared by using oil of wintergreen and mounted with Permount.
Parapodial (wing) muscles of C. limacina and C. antarctica were preserved in 2% glutaraldehyde in 0.1 M cacodylic acid for electron microscopy. Fixed wings were processed for electron microscopy at JFE Enterprises. Cross-sections were sampled from the center of each individual wing, embedded in Spurr's resin, stained with uranyl acetate, poststained with lead citrate, and sectioned with an ultramicrotome. Thin sections (60–90 nm) were placed on grids and viewed at 80 kV with a JEOL 1200EX transmission electron microscope equipped with Tietz Video and an Image Processing Systems (TVIPS) high-resolution digital camera. Images were captured and analyzed according to standard guidelines of stereology (28). Individual bundles of fibers were viewed at a magnification of 1,200–1,500×.
Supplementary Material
Acknowledgments.
We thank the staff at McMurdo Station, Antarctica, and the Marine Institute of Memorial University, Newfoundland, for excellent support. This work was supported by NSF OPP Awards 9980360 and 0538479, NSF IBN Award 0344070, NIH GM Award 30376, and the University of Rhode Island Council for Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901321106/DCSupplemental.
References
- 1.Gilmer R, Lalli C. Bipolar variation in Clione, a gymnosomatous pteropod. Am Malacol Bull. 1990;8:67–75. [Google Scholar]
- 2.Aronson RB, et al. Climate change and the invisibility of the Antarctic benthos. Annu Rev Ecol Syst. 2007;38:129–154. [Google Scholar]
- 3.Lalli C, Gilmer R. Pelagic Snails: The Biology of Holoplanktonic Gastropod Molluscs. Stanford, CA: Stanford Univ Press; 1989. p. 259. [Google Scholar]
- 4.Borrell BJ, Goldbogen JA, Dudley R. Aquatic wing flapping at low Reynolds numbers: Swimming kinematics of the Antarctic pteropod, Clione antarctica. J Exp Biol. 2005;208:2939–2949. doi: 10.1242/jeb.01733. [DOI] [PubMed] [Google Scholar]
- 5.Satterlie RA, LaBarbera M, Spencer AN. Swimming in the pteropod mollusc, Clione limacina. I. Behaviour and morphology. J Exp Biol. 1985;116:189–204. [Google Scholar]
- 6.Arshavsky Yu I, Beloozerova IN, Orlovsky GN, Panchin Yu V, Pavlova GA. Control of locomotion in marine mollusc Clione limacina. I. Efferent activity during actual and fictitious swimming. Exp Brain Res. 1985;58:255–262. doi: 10.1007/BF00235307. [DOI] [PubMed] [Google Scholar]
- 7.Satterlie RL, Spencer AN. Swimming in the pteropod mollusc, Clione limacina. II. Physiology. J Exp Biol. 1985;116:205–222. [Google Scholar]
- 8.Arshavsky Yu I, Beloozerova IN, Orlovsky GN, Panchin Yu V, Pavlova GA. Control of locomotion in marine mollusc Clione limacina. II. Rhythmic neurons of pedal ganglia. Exp Brain Res. 1985;58:263–272. doi: 10.1007/BF00235308. [DOI] [PubMed] [Google Scholar]
- 9.Satterlie RA. Reciprocal inhibition and postinhibitory rebound produce reverberation in a locomotor pattern generator. Science. 1985;229:402–404. doi: 10.1126/science.229.4711.402. [DOI] [PubMed] [Google Scholar]
- 10.Satterlie RA. Neuromuscular organization in the swimming system of the pteropod mollusc Clione limacina. J Exp Biol. 1993;181:119–140. doi: 10.1242/jeb.181.1.119. [DOI] [PubMed] [Google Scholar]
- 11.Arshavsky Yu I, Beloozerova IN, Orlovsky GN, Panchin Yu V, Pavlova GA. Control of locomotion in marine mollusc Clione limacina. IV. Role of type 12 interneurons. Exp Brain Res. 1985;58:285–293. doi: 10.1007/BF00235310. [DOI] [PubMed] [Google Scholar]
- 12.Arshavsky Yu I, Beloozerova IN, Orlovsky GN, Panchin Yu V, Pavlova GA. Control of locomotion in marine mollusc Clione limacina. III. On the origin of locomotory rhythm. Exp Brain Res. 1985;58:273–284. doi: 10.1007/BF00235309. [DOI] [PubMed] [Google Scholar]
- 13.Satterlie RA, Norekian TP. Serotonergic modulation of swimming speed in the pteropod mollusc Clione limacina. III. Cerebral neurons. J Exp Biol. 1995;198:917–930. doi: 10.1242/jeb.198.4.917. [DOI] [PubMed] [Google Scholar]
- 14.Satterlie RA. Reciprocal inhibition and postinhibitory rebound produce reverberation in a locomotor pattern generator. Science. 1985;229:402–404. doi: 10.1126/science.229.4711.402. [DOI] [PubMed] [Google Scholar]
- 15.Satterlie RA, Norekian TP, Pirtle TJ. Serotonin-induced spike narrowing in a locomotor pattern generator permits increases in cycle frequency during accelerations. J Neurophysiol. 2000;83:2163–2170. doi: 10.1152/jn.2000.83.4.2163. [DOI] [PubMed] [Google Scholar]
- 16.Seibel B, Dymowska A, Rosenthal J. Metabolic temperature compensation and coevolution of locomotory performance in pteropod molluscs. J Integ Comp Biol. 2007;47:880–891. doi: 10.1093/icb/icm089. [DOI] [PubMed] [Google Scholar]
- 17.Johnston I, Calvo J, Guderley H, Fernandez D, Palmer L. Latitudinal variation in the abundance and oxidative capacities of mitochondria in perciform fishes. J Exp Biol. 1998;201:1–12. doi: 10.1242/jeb.201.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Pörtner H. Physiological basis of temperature-dependent biogeography: Trade-offs in muscle design and performance in polar ectotherms. J Exp Biol. 2002;205:2217–2230. doi: 10.1242/jeb.205.15.2217. [DOI] [PubMed] [Google Scholar]
- 19.Weibel E. Design and performance of muscular systems: An overview. J Exp Biol. 1985;115:405–412. doi: 10.1242/jeb.115.1.405. [DOI] [PubMed] [Google Scholar]
- 20.Baltzley MJ, Lohmann KJ. Comparative study of TPep-like immunoreactive neurons in the central nervous system of nudibranch molluscs. Brain Behav Evol. 2008;72:192–206. doi: 10.1159/000157356. [DOI] [PubMed] [Google Scholar]
- 21.Gillette R. On the significance of neuron giantism in gastropods. Biol Bull. 1991;180:234–240. doi: 10.2307/1542393. [DOI] [PubMed] [Google Scholar]
- 22.Clarke A. Temperature and energetics: An introduction to cold ocean physiology. In: Portner H, Playle R, editors. Cold Ocean Physiology. Cambridge, UK: Cambridge University Press; 1998. pp. 3–29. Society for Experimental Biology Seminar Series 66. [Google Scholar]
- 23.Brodie EDI, Brodie EJ. Predator-prey arms races. Bioscience. 1999;49:557–568. [Google Scholar]
- 24.Fong D, Kane T, Culver D. Vestigialization and loss of nonfunctional characters. Annu Rev Ecol Syst. 1995;26:249–268. [Google Scholar]
- 25.Montgomery J, Clements K. Disaptation and recovery in the evolution of Antarctic fishes. Trends Ecol Evol. 2000;15:267–271. doi: 10.1016/s0169-5347(00)01896-6. [DOI] [PubMed] [Google Scholar]
- 26.Bryan PJ, Yoshida WY, McClintock JB, Baker BJ. Ecological role for pteroenone, a novel antifeedant from the conspicuous antarctic pteropod Clione antarctica (Gymnosomata: Gastropoda) Mar Biol. 1995;122:271–277. [Google Scholar]
- 27.McClintock JB, Janssen J. Pteropod abduction as a chemical defense in a pelagic amphipod. Nature. 1990;346:462–464. [Google Scholar]
- 28.Weibel ER. Stereological Methods. 2 Vol. London: Academic; 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.