Abstract
Improved survival is likely linked to the ability to generate stable memories of significant experiences. Considerable evidence in humans and mammalian model animals shows that steroid hormones, which are released in response to emotionally arousing experiences, have an important role in the consolidation of memories of such events. In insects, ecdysone is the major steroid hormone, and it is well characterized with respect to its essential role in coordinating developmental transitions such as larval molting and metamorphosis. However, the functions of ecdysone in adult physiology remain largely elusive. Here, we show that 20-hydroxyecdysone (20E), the active metabolite of ecdysone that is induced by environmental stimuli in adult Drosophila, has an important role in the formation of long-term memory (LTM). In male flies, the levels of 20E were found to be significantly increased after courtship conditioning, and exogenous administration of 20E either enhanced or suppressed courtship LTM, depending on the timing of its administration. We also found that mutants in which ecdysone signaling is reduced were defective in LTM, and that an elevation of 20E levels was associated with activation of the cAMP response element binding protein (CREB), an essential regulator of LTM formation. Our results demonstrate that the molting steroid hormone ecdysone in adult Drosophila is critical to the evolutionarily conserved strategy that is used for the formation of stable memories. We propose that ecdysone is able to consolidate memories possibly by recapturing molecular and cellular processes that are used for normal neural development.
Keywords: courtship conditioning, CREB, ecdysone receptor, steroid hormone, 20-hydroxyecdysone
Memories are not created equal; some are long-lasting, whereas others are short-lived. Various clinical and experimental analyses in humans and mammalian model animals have provided convincing evidence that steroid hormones that are released in response to a stressful experience (e.g., cortisol) have an important role in the consolidation of memory (1). Gonadal steroid hormones are also known to influence memory function in vertebrates (2, 3). Although previous studies have revealed the involvement of certain neural circuits and neurotransmitter systems in steroid-mediated regulation of memory in vertebrates (4–6), the underlying molecular mechanisms are still poorly understood. Also, it remains unclear how prevalent a memory consolidation process involving steroids is among evolutionarily divergent animal species.
As one of the most versatile model organisms for behavioral genetics, the fruit fly Drosophila melanogaster has been extensively used to identify genes and signaling cascades that are critical for the processes of learning and memory (7). It has become evident from these studies that the fundamental molecular and cellular mechanisms that underlie learning and memory are astonishingly well conserved between the fruit fly and higher vertebrates. Noticeably, however, little is known whether steroid hormone signaling is involved in the process of memory consolidation in this species, as in the case of vertebrate animals. The aim of the present study was to address this issue by investigating whether the steroid hormones that are induced by environmental stimuli have a role in regulating memory in adult Drosophila.
The major steroid hormone in insects is ecdysone, and its functions during development have been extensively studied in Drosophila, as well as in other insects (8). Ecdysone has essential roles in coordinating major developmental transitions, such as larval molting and metamorphosis, through its active metabolite 20-hydroxyecdysone (20E) (9); 20E signaling also regulates the rate of tissue growth during development by impeding insulin signaling (10). Although ecdysone is known to be present throughout life in both male and female Drosophila (11), its functions in adult physiology are largely unknown. In fully mature adult females, ecdysone signaling controls oogenesis (12, 13). Interestingly, 20E levels are significantly affected by environmental conditions, and changes in 20E levels influence the regulation of oogenesis. For example, high temperature and nutritional shortage result in an increase in the 20E titer (14, 15), and induce apoptosis in oocytes, preventing oogenesis under these unfavorable conditions (15). It has also been reported that mutations that reduce the strength of ecdysone signaling in adults extend life span of Drosophila males and females by 40 to 50% (16), indicating the importance of the ecdysone hormonal pathway for adult physiology.
Based on these observations, we hypothesized that the steroid molting hormone 20E, which is induced by environmental stimuli in adult flies, has an important role in consolidating memories into a stable, long-lasting form. To investigate this hypothesis, we have used an ethologically relevant, associative learning paradigm in Drosophila known as courtship conditioning. In this paradigm, male flies that have courted unreceptive, nonvirgin females subsequently suppress their courtship behavior, even toward receptive virgin females (17, 18). The period of courtship memory retention (as evidenced by a reduction in courtship activities in males that have been aversively “trained” with nonvirgin females) varies in length depending on the training conditions (17, 19, 20). One-hour training creates memories that are detectable for a few hours (17), whereas 7-h training results in long-term memory (LTM), lasting at least 5 days (20). Here, we show that ecdysone is induced in male flies after an experience with unreceptive, nonvirgin females, and that ecdysone signaling has a critical role in the formation of LTM. Our findings demonstrate that the steroid molting hormones in insects are vital to the evolutionarily conserved strategy for the formation of stable memories.
Results
Extended Training for Courtship LTM Leads to an Increase in 20E Levels.
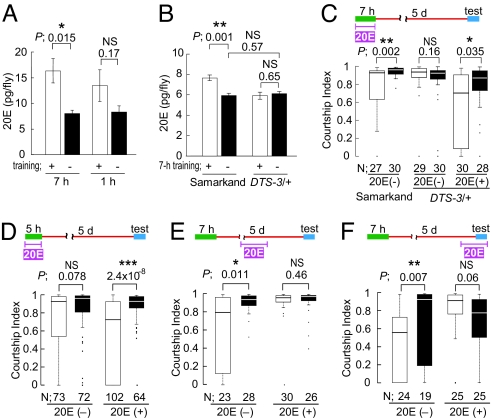
We first investigated whether the levels of the steroid hormone 20E are increased after male flies are trained with nonvirgin females; 20E levels were compared immediately after training between naive wild-type male flies and males that had been paired with a nonreceptive, mated female under the conditions used for training in courtship conditioning assays. As shown in Fig. 1A, a 7-h training bout led to a considerable elevation of 20E levels (P = 0.015). This increase in 20E levels is similar to those previously reported for flies subjected to thermal stress (38 °C) (21). Although 1 h of training tended to lead to an increase in 20E levels as well, the effect did not reach statistical significance (P = 0.17) (Fig. 1A). To investigate the functional consequences of the observed increase in 20E levels with respect to memory formation, we used Dominant temperature-sensitive 3 (DTS-3), a mutation that specifically affects the ecdysone synthetic pathway (22, 23). Heterozygosity for DTS-3 (DTS-3/+) results in developmental lethality at the restrictive temperature (29 °C) due to the low 20E titer (for more detailed explanation of DTS-3, see SI Materials and Methods) (24). At 25 °C, DTS-3/+ males are viable, and the 20E levels in these adults were comparable with those in the parental wild-type strain Samarkand, as reported previously (Fig. 1B, P = 0.57) (24). However, at 25 °C, DTS-3/+ males showed a clear mutant phenotype; unlike the relevant wild-type control Samarkand flies, DTS-3/+ males failed to exhibit an increase in 20E levels after 7-h of training (Fig. 1B, P = 0.65). Therefore, 25 °C is a nonpermissive temperature for DTS-3/+ males with respect to up-regulation of 20E after 7-h of training. In parallel with the failure in 20E induction after 7-h of training, DTS-3/+ males failed to show courtship LTM (Fig. 1C Center, P = 0.16). In contrast, Samarkand males subjected to the same conditions exhibited LTM (Fig. 1C Left). The impaired LTM in DTS-3/+ males is likely due to an insufficient increase in 20E levels during training, because the defect was rescued by feeding DTS-3/+ males 20E during the training period (Fig. 1C Right).
Fig. 1.
Training for courtship memory leads to an increase in the level of endogenous 20E, and the application of exogenous 20E promotes LTM. (A) The 20E levels in wild-type flies with or without 1- or 7-h training; n = 8. (B) The 20E levels in Samarkand and DTS-3/+ flies with or without 7-h training; n = 5. (A and B) Each bar represents mean ± SEM. Student's t test was applied. (C) Courtship indices 5 days after 7-h training in Samarkand and DTS-3/+ flies, represented by box plot. (D–F) Courtship indices 5 days after 5- or 7-h training with or without 20E treatment; 5-h training generates LTM with 20E treatment during the training period (D). LTM formed after 7-h training is disrupted by 1-day treatment of 20E between training and test (E), or immediately before the memory test (F). Schematic representation (above each graph), statistical significance (P), sample number (N), and fly genotypes are shown. □, trained; ■, naive. Mann–Whitney U test was applied. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
Exogenous Administration of 20E Has Context-Dependent Effects on LTM.
It is known that in mammals, new memory traces can be reinforced by the administration of appropriate steroid hormones immediately after training (1, 25). We tested whether 20E treatment had a similar memory-enhancing effect in Drosophila. To detect possible memory enhancement in courtship conditioning, we used a 5-h training protocol. As we previously reported (20), training for 5 h using our experimental protocol is not sufficient for male flies to develop robust courtship LTM (P = 0.078, Fig. 1D Left). However, when males were fed 20E during the 5-h training period, LTM was detected on day 5 (P = 2.4 × 10−8, Fig. 1D Right), suggesting that 20E reinforces the memories that are formed during the “insufficient” 5-h training period. However, as has also been observed in the case of mammalian steroid hormones (26), 20E does not always have a positive effect on LTM. We found that memories induced by 7-h training were disturbed or erased when 20E feeding was not associated with the training period (Fig. 1 E and F). These results indicate that steroid hormone signaling in Drosophila indeed affects LTM, and that it does so in a context-dependent manner.
Heterozygous Mutants for the Ecdysone Receptor (EcR) Gene Are Defective in Courtship LTM.
Ecdysone signaling is primarily mediated by EcRs, which are ligand-activated transcription factors and members of an evolutionarily conserved nuclear hormone receptor family (27, 28). Flies homozygous for the loss-of-function EcR mutations do not survive development. Thus, we tested heterozygotes (EcR/+) to determine whether a 50% reduction in EcR expression affects the regulation of courtship memory. The EcR mutant alleles used in this study are the following: EcRF288Y and EcRC300Y, each of which has a point mutation in the DNA binding domain; EcRA483T and EcRV559fs, which carry a point mutation and a small deletion in the ligand binding domain, respectively (Fig. S1A) (29); and EcRNP2237 and EcRNP5219, each of which has a P-element insertion in an EcR gene intron and exhibits reduced EcR protein levels in the adult heads (Fig. S1 B and C).
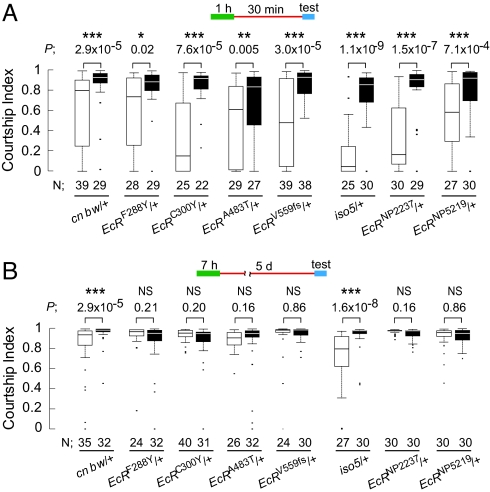
When EcR/+ males were trained for 1 h with a mated female and then tested for 30-min courtship memory, all trained EcR heterozygous mutants, as well as appropriate control flies, displayed significantly reduced levels of courtship activity toward a virgin test female, compared with the naive flies (P < 0.05, Fig. 2A). This result demonstrated that EcR/+ males can detect the sensory stimuli that are important for the acquisition of courtship memory, store the memory for at least 30 min, and retrieve it when exposed to a test female target. However, when EcR/+ males were tested for 5-day LTM after 7-h training, both trained and naive males displayed equivalent levels of courtship activity toward a test female (P > 0.05, Fig. 2B). This result indicated that the EcR/+ males, in which the EcR expression level is reduced to ≈50% of that in control flies, are defective in LTM. Under the same conditions, trained control flies (cn bw/+ or iso5/+) exhibited LTM; the courtship activity of these males was significantly lower than that of naive males (P = 2.9 × 10−5 for cn bw/+ and P = 1.6 × 10−8 for iso5/+, Fig. 2B).
Fig. 2.
Heterozygous EcR mutants are defective in 5-day memory, but not in 30-min memory. Courtship indices in EcR mutant and control flies (A) 30 min after 1-h training, or (B) 5 days after 7-h training, represented by box plot. Schematic representation (above each graph), P, N, and fly genotypes are shown. □, trained; ■, naive. Mann–Whitney U test was applied. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
Courtship LTM Requires Functional EcRs in Adult Neurons.
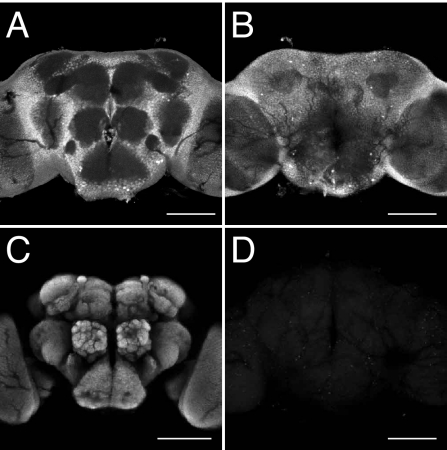
Impaired LTM in EcR/+ males could potentially be due to a decrease in the strength of the ecdysone signal during development, and a consequent malfunction of the adult nervous system. Alternatively, the defect in LTM could be caused by inefficient ecdysone signaling in the physiological process that is involved in the memory formation, storage, or retrieval of courtship memory. As shown in Fig. 1 D–F, 20E administration to adult males had a significant influence on courtship LTM. Also, the LTM defects in EcR/+ males (see Fig. 4A), as well as those in DTS-3/+ males (Fig. 1C), were rescued by feeding the animals 20E during the training period. These observations suggested that the defective LTM in EcR mutants is not a result of anomalous development of the nervous system, but of insufficient EcR-mediated signaling during the physiological process that is assessed by courtship conditioning assays. Consistent with a possible function for EcRs in the adult nervous system, EcRs were found to be widely expressed in the adult Drosophila brain (Fig. 3 A and B). The synaptic neuropil regions marked with monoclonal antibody nc82 (Fig. 3C) (30) were devoid of EcR proteins, in agreement with the expected function of EcRs as nuclear transcription factors (see also Fig. S2).
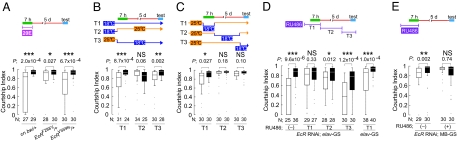
Fig. 4.
Courtship LTM is affected by manipulation of ecdysone signaling in adults. Courtship indices in flies with altered EcR activity were measured 5 days after training. (A) LTM was observed in EcRF288Y/+ (DNA-binding domain mutation) and EcRV559fs/+ (ligand-binding domain mutation) flies that were treated with 20E during the training period. (B and C) Temperature-sensitive EcR mutant (EcRA483T/+) shows a temperature- and treatment timing-dependent defect in LTM. (D and E) LTM is impaired by conditional expression of EcR RNAi using RU486-dependent Gal4 drivers that are specific to neurons (elav-GS-Gal4, D) or the MBs (MB-GS-Gal4, E). Schematic representation (above each graph), P, N, and fly genotypes are shown. □, trained; ■, naive. Mann–Whitney U test was applied. **, P < 0.01; ***, P < 0.001; NS, not significant.
Fig. 3.
Immunohistochemical detection of EcR in the adult brain. EcR localization in the adult brain was detected using DDA2.7 anti-EcR antibody. Anterior (A) and posterior (B) views of whole adult brain are shown. (C) Visualization of the synaptic neuropil regions with monoclonal antibody nc82. (D) The brain stained without primary antibody as a negative control. (Scale bar, 100 μm.)
To directly demonstrate the functional significance of EcRs in the physiological process required for LTM in adults, we used a temperature-sensitive EcR allele, EcRA483T, for which 18 and 25 °C are permissive and restrictive temperatures, respectively (13, 29). As shown in Fig. 2B, EcRA483T/+ males were defective in courtship LTM when they were raised and tested for courtship memory at 25 °C. As expected for a temperature-sensitive allele, EcRA483T/+ males reared and tested at 18 °C displayed LTM (Fig. 4B Left). To investigate the temporal requirements of functional EcRs for courtship LTM, we performed a set of temperature-shift experiments. LTM was impaired when EcRA483T/+ males were kept at 18 °C until eclosion and then shifted to 25 °C (Fig. 4B Center), whereas LTM was normal when males were kept at 25 °C until eclosion and then shifted to 18 °C (Fig. 4B Right). These results showed that the ability of male flies to form courtship LTM is not affected by a 50% reduction in EcR expression during development, but that this ability does require intact EcR activity in the adult. To further define the temporal requirements for EcRs in the establishment of LTM, we transferred EcRA483T/+ males kept at 25 to 18 °C at different time points, and tested courtship LTM. As shown in Fig. 4C, LTM was detected when EcRA483T/+ males were shifted to 18 °C during the training period, whereas a temperature shift to 18 °C after training did not improve the mutant LTM phenotype. These results suggest that EcR-mediated signaling is critical for LTM during the training period, which is when courtship LTM is likely formed.
We also manipulated EcR levels in adults by generating flies that carry both UAS-EcR RNAi and the RU486-inducible neuronal driver, elav-GS-Gal4 (GeneSwitch) (31, 32). RU486 treatment (500 μM, 3 days) of adult flies reduced EcR expression in the fly heads by ≈50% (Fig. S3). We found that male flies were defective in courtship LTM when the expression of the EcR-RNAi was induced by RU486 before and during the training period (Fig. 4D). This LTM defect was the result of RU486-induced EcR-RNAi expression, because flies carrying only elav-GS-Gal4 did not show a LTM defect, even when RU486 was applied (Fig. 4D Right). However, RU486-induced down-regulation of EcR after the training period did not have a severe effect on either courtship or LTM (Fig. 4D). The results with the EcR RNAi are consistent with those obtained using the temperature-sensitive EcR allele (Fig. 4 B and C). A similar LTM defect was observed when EcR RNAi was preferentially expressed in the adult mushroom bodies (MBs) using an MB-GS-Gal4 (Fig. 4E) (33). Together, these experiments demonstrate that EcR-mediated signaling is critical to the physiological process that generates courtship LTM. They also suggest that the courtship LTM requires normal levels of ecdysone signaling in adult neurons (particularly in the neurons within the MBs) during the training period.
The Elevation of 20E Reinforces CREB Activity.
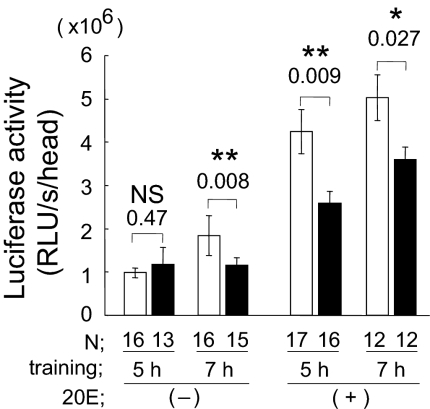
Studies in both vertebrates and invertebrates have revealed an essential role for the transcriptional regulator CREB in the formation of LTM (34). CREB influences memory consolidation in response to intracellular signals, such as elevations of cAMP and Ca2+, by activating the transcription of a set of down-stream genes (35, 36). We previously demonstrated that courtship LTM is impaired when a repressing isoform of Drosophila CREB (dCREB2-B) is expressed immediately before males are trained with a nonvirgin female for 7 h (20), providing evidence for the essential nature of the contribution of CREB to courtship LTM. To investigate the relationship between ecdysone signaling and CREB activity in courtship conditioning, we used a CRE-luciferase (luc) reporter, which makes it possible to monitor CREB activity by measuring the bioluminescence resulting from CREB-dependent luciferase expression (37). In the absence of 20E treatment, 7-h training resulted in a significant increase in luciferase activity (P = 0.008), but 5-h training did not (P = 0.47, Fig. 5). As shown in Fig. 1, a 7-h training, but not 5-h training, resulted in the formation of LTM. Thus, training-dependent CREB activation correlates with the formation of 5-day LTM. We also found that treating flies with 20E has a dramatic enhancing effect on CREB-dependent transcription, in both trained and naive males (Fig. 5). Importantly, when 20E was administered exogenously, not only 7-h training, but also 5-h training, led to a training-dependent increase in CREB activity (Fig. 5). Because 5-h training is sufficient to produce LTM in the presence of 20E (Fig. 1D), this experiment again indicates a correlation between a training-dependent increase in CREB activity and LTM. These results support the idea that courtship memory is converted to LTM through training-dependent activation of ecdysone signaling and CREB.
Fig. 5.
CREB-dependent gene expression is enhanced by training for courtship memory and 20E treatment. CREB-dependent luciferase activity in CRE-luc males is enhanced by 7-h training, but not by 5-h training. With 20E treatment, the luciferase activities were increased, and significant differences in the luciferase activity were observed between trained flies (both for 5 and 7 h), and the corresponding naive flies. P and N are shown. Bars represent mean ± SEM. □, trained; ■, naive. Student's t test was applied. *, P < 0.05; **, P < 0.01; NS, not significant.
Discussion
The objective of this study was to investigate whether the steroid molting hormone 20E regulates LTM formation in adult Drosophila. Here, we show that (i) training for courtship-memory leads to an elevation of 20E levels in adult Drosophila; (ii) administering exogenous 20E has either a positive or negative effect on courtship LTM, depending on the context; (iii) disrupting either ecdysone synthesis or function of the nuclear EcR results in defective LTM; (iv) functional ecdysone signaling in adult neurons during the training period is required for LTM; and (v) 20E induces CREB-mediated transcriptional activation. Together, these results indicate that the steroid molting hormone 20E has a novel, nondevelopmental role in the formation of long-lasting memory in adult insects.
The temporal profile of 20E titers during embryonic, larval, and pupal stages is essentially controlled by the genetically determined developmental program (9). As previously shown, environmental stimuli, such as high temperature and nutritional shortage, induce up-regulation of 20E levels in adult flies (14, 15). In this study, we have demonstrated that 20E levels are increased in male flies after they are paired with a mated female for 7 h, conditions under which a robust courtship LTM is generated. Ecdysone signaling activated by these environmental stimuli or social interactions may trigger specific molecular and cellular responses in adults, and lead to long-lasting changes in physiology and behavior.
In Drosophila, steroid hormone synthesis is known to occur primarily in 2 organs, the larval prothoracic gland and the adult female ovary (38–42). Ecdysteroids are present in adult males as well as females (11). It remains to be determined where ecdysteroids are produced other than in the female ovary, and how their synthesis is regulated in Drosophila adults. The last 4 sequential hydroxylations of their synthesis, which convert steroid precursors into 20E, are catalyzed by 4 cytochrome P450 enzymes encoded by “phantom”, “disembodied”, “shadow”, and “shade”, known collectively as the Halloween genes (43). The temporal changes in ecdysteroid levels during development are mainly attributed to transcriptional regulation of these genes (43). To understand the regulatory mechanisms for production of ecdysteroids in adult flies, it is important to examine where these enzymes are expressed, and how their expression and activity are regulated. Recent studies show that feeding the dopamine precursor L-DOPA to young Drosophila virilis females increases the dopamine (DA) content in the body, and subsequently results in a substantial increase in 20E levels (44, 45). Given that dopamine has been implicated in negatively reinforced memory, it is possible that this neurotransmitter acts as a mediator between environmental stimuli and an elevation of 20E level.
Using a temperature-sensitive EcR allele and an RNAi that targets EcR, we have shown that courtship LTM is impaired by conditional suppression of EcR function during the training period. Also, we found that LTM was restored in the EcR temperature-sensitive mutants as long as they were maintained at the permissive temperature during the training period. These experiments demonstrate that ecdysone signaling through nuclear EcRs has an important role in the physiological processes that are necessary for the formation of LTM. How does ecdysone contribute to the formation of LTM? One possibility is that fully functional ecdysone signaling is required for effective sensory processing, and that the adverse effect of a 50% reduction in EcR expression on the learning process is due to severe sensory dysfunction. However, this possibility is not likely, because we found the courtship behavior of male flies with reduced EcR function to be qualitatively and quantitatively comparable with that of control males. Also, EcR/+ males exhibited a short-lasting courtship memory after 1-h training, which suggests that their sensory acuity and ability to acquire courtship memory are rather normal. Thus, we propose that ecdysone signaling operates in the CNS, and contributes to consolidation of the memories into a long-lasting form. The MB is considered to be the center of olfactory memory (46). The EcR RNAi experiments suggest that the MB is one of the brain structures required for the influence of ecdysone on the formation of courtship LTM. Also, our study using the CRE-luc reporter indicates that CREB, a key regulator of long-lasting modifications of the nervous system, is involved in ecdysone-dependent LTM formation.
Given that genetically programmed ecdysone signaling is known to control neuronal remodeling during development (47–49), it is interesting to speculate that certain experiences may recapture the ecdysone-mediated developmental processes in the adult brain and lead to structural and functional modifications to the nervous system that facilitate the formation of stable, LTM. The ability of ecdysone to remodel the nervous system is known not to be limited to developmental stages. For example, in the adult house cricket (Acheta domesticus) brain, ecdysone has been shown to inhibit proliferation of neuroblasts in the MBs and to trigger their differentiation into interneurons (50). Although there is no evidence of continued neurogenesis in the adult Drosophila brain, it is possible that ecdysone signaling induces significant changes in properties of existing neurons, resulting in structural and functional remodeling of neuronal circuits. A recent study has shown that the canonical ecdysteroid transcriptional cascade in the MB neurons of the adult worker honey bee (Apis mellifera L.) is initiated in response to activated ecdysone signaling, further suggesting the involvement of ecdysteroids in remodeling the adult nervous system (51).
Our findings in Drosophila indicate that regulation of memory by environmentally induced steroids could be ancient in origin, and widespread in species that have an ability to learn and remember. Thus, the molecular components and signaling pathways responsible for steroid-mediated memory regulation are likely to be shared, at least in part, by evolutionarily diverse animal species. This study has focused on the role of EcRs, nuclear hormone receptors that function through transcriptional regulation of their target genes, in the formation of LTM. Recently, a novel Drosophila G protein-coupled receptor (DmDopEcR) was found to be activated by ecdysteroids (52). Thus, it is also interesting to examine the possible involvement of rapid, nongenomic actions of ecdysone in regulation of memory. Considering the relatively simple nervous system of D. melanogaster, our extensive knowledge of the genetics of this organism, and the highly developed experimental tools available for its study, Drosophila should be an ideal model system to elucidate the molecular, cellular, and neural-circuit bases of memory regulation by steroid hormones.
Materials and Methods
Fly Strains.
The DTS-3 mutant, the wild-type Samarkand strain (22), EMS-induced EcR mutants (29), and their parental strain (cn bw) were described in ref. 16 and provided by A. F. Simon (University of California, Los Angeles, CA). P-element-induced EcR mutant alleles and their parental wild-type strain, iso5 (53), were obtained from the Drosophila Genetic Resource Center at Kyoto institute of technology and K. Ito (University of Tokyo, Tokyo, Japan), respectively. UAS-EcR RNAi transgenic flies were generated using the pWIZ plasmid (54). The GeneSwitch Gal4 driver strains, elav-GS-Gal4, and MB-GS-Gal4 were obtained from the Bloomington Stock Center. CRE-luc transgenic flies were provided by K. Iijima (Thomas Jefferson University, Philadelphia, PA) and J. C. P. Yin (University of Wisconsin, Madison, WI). Canton-S (CS) was used as the wild-type strain. Flies were reared at 25 °C and 65% humidity unless otherwise stated, on a conventional glucose-yeast-cornmeal agar medium.
Immunohistochemical Analysis.
Brains from 3-day-old adult flies were stained with mouse monoclonal antibodies DDA2.7 (1:20) or nc82 (1:10) (obtained from the Developmental Studies Hybridoma Bank at the University of Iowa). DDA2.7 and nc82 recognize the protein region common to all forms of the Drosophila EcR (55), and a presynaptic active zone protein, Bruchpilot (30), respectively. Alexa Fluor 488-conjugated anti-mouse IgG was used as secondary antibody (1:1,000; Molecular Probes). Images were acquired as a z-stack using a Zeiss LSM510 confocal microscope.
The 20E Quantification.
Total body ecdysteroids were extracted from 10 adult flies by using 500 μL of 70% methanol, and the levels of 20E were determined by enzyme immunoassay (ACE Enzyme Immunoassay; Cayman Chemical), using 20E EIA antiserum (Cayman Chemical). Calibration curves were generated using 20E obtained from Sigma.
Courtship Conditioning Assay.
The courtship conditioning assay was performed, and the data were analyzed as described previously (20), with some modifications (for details, see SI Materials and Methods). Briefly, training for short-term memory assays involved pairing a 3- to 5-day-old virgin male with the trainer female for 1 h. For LTM assays, a male was paired with the trainer female for 7 h. Trained males were tested with a freeze-killed virgin female as a courtship target. Memory tests for the short-term and LTM assays were performed 30 min and 5 days after training, respectively, in the courtship chamber. The courtship index was defined as the proportion of time spent in courtship behaviors (i.e., orientation, tapping, singing, licking, and attempted copulation) during a 10-min observation period. The index was assessed in a blinded fashion. The data were analyzed using the Mann–Whitney U test, and presented using box plots. All data, including outliers, shown as dots in the figures were analyzed by the statistical procedure.
Drug Treatment.
Flies carrying the RU486-inducible transgene were fed food containing 500 μM RU486 (Mifepristone; Sigma) or vehicle (ethanol; final concentration <2%) for 3 days before the experiment. For 20E treatment, flies were given food containing 1 mM 20E (Sigma), which was prepared from a 25 mM 20E stock solution in ethanol.
Luciferase Assay.
In vitro luciferase activity was measured as described (56), with some modifications. The head of a single fly carrying CRE-luc was homogenized at Zeitgeber time 14 with 50 μL of Glo lysis buffer (Promega), and analyzed using the Bright-Glo Luciferase Assay System according to the manufacturer's protocol (Promega). Luciferase activity was detected using a luminescence counter (Berthold Detection System), and normalized to total protein concentration.
For details of the materials and methods used, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank A. Simon (University of California, Los Angeles, CA), K. Ito (University of Tokyo, Tokyo, Japan), K. Iijima (Thomas Jefferson University, Philadelphia, PA), and J. C. P. Yin (University of Wisconsin, Madison, WI) for kindly providing fly strains. This work was supported by National Institutes of Health Grant MH62684 (to T.K.). H.I. was supported by an Uehara Memorial Foundation Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810213106/DCSupplemental.
References
- 1.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 2.Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 3.Parducz A, et al. Synaptic remodeling induced by gonadal hormones: Neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Cahill L, Prins B, Weber M, Mcgaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Memory–a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 6.Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann NY Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- 7.McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam Horm. 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- 9.Truman JW, Riddiford LM. Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- 10.Colombani J, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 11.Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev Biol. 1982;93:73–82. doi: 10.1016/0012-1606(82)90240-8. [DOI] [PubMed] [Google Scholar]
- 12.Buszczak M, et al. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- 13.Bender M, et al. Mutational dissection of the Drosophila ecdysone receptor (EcR) gene: EcR is required maternally For normal oogenesis and EcR-B isoforms are required for neuronal remodeling during metamorphosis. Dev Biol. 1998;198:221–227. [Google Scholar]
- 14.Rauschenbach IY, et al. Role of the ecdysteroid system in the regulation of Drosophila reproduction under environmental stress. Dokl Biol Sci. 2000;375:641–643. doi: 10.1023/a:1026610425973. [DOI] [PubMed] [Google Scholar]
- 15.Terashima J, Takaki K, Sakurai S, Bownes M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J Endocrinol. 2005;187:69–79. doi: 10.1677/joe.1.06220. [DOI] [PubMed] [Google Scholar]
- 16.Simon AF, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- 17.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 19.McBride SM, et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 20.Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci USA. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirashima A, Rauschenbach IY, Sukhanova MJ. Ecdysteroids in stress responsive and nonresponsive Drosophila virilis lines under stress conditions. Biosci Biotech Bioch. 2000;64:2657–2662. doi: 10.1271/bbb.64.2657. [DOI] [PubMed] [Google Scholar]
- 22.Holden JJ, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XII. The genetic and developmental effects of dominant lethals on chromosome 3. Genetics. 1973;73:445–458. doi: 10.1093/genetics/73.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holden JJA, et al. Analysis of molting and metamorphosis in the ecdysteroid-deficient mutant L(3)3dts of Drosophila-melanogaster. Dev Genet. 1986;6:153–162. [Google Scholar]
- 24.Walker VK, Watson KL, Holden JJ, Steel CGH. Vitellogenesis and fertility in Drosophila females with low ecdysteroid titres; the l(3)3DTS mutation. J Insect Physiol. 1987;33:137–142. [Google Scholar]
- 25.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 26.de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 27.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 29.Bender M, et al. Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell. 1997;91:777–788. doi: 10.1016/s0092-8674(00)80466-3. [DOI] [PubMed] [Google Scholar]
- 30.Wagh DA, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao ZM, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci USA. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 35.Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- 36.Pittenger C, Kandel E. A genetic switch for long-term memory. C R Acad Sci III. 1998;321:91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- 37.Belvin MP, Zhou H, Yin JC. The Drosophila dCREB2 gene affects the circadian clock. Neuron. 1999;22:777–787. doi: 10.1016/s0896-6273(00)80736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiriishi S, Rountree DB, Sakurai S, Gilbert LI. Prothoracic gland synthesis of 3-dehydroecdysone and its hemolymph 3 beta-reductase mediated conversion to ecdysone in representative insects. Experientia. 1990;46:716–721. doi: 10.1007/BF01939944. [DOI] [PubMed] [Google Scholar]
- 39.Dai JD, Gilbert LI. Metamorphosis of the corpus allatum and degeneration of the prothoracic glands during the larval-pupal-adult transformation of Drosophila melanogaster: A cytophysiological analysis of the ring gland. Dev Biol. 1991;144:309–326. doi: 10.1016/0012-1606(91)90424-2. [DOI] [PubMed] [Google Scholar]
- 40.Bownes M. The roles of juvenile hormone, ecdysone and the ovary in the control of Drosophila vitellogenesis. J Insect Physiol. 1989;35:115–117. [Google Scholar]
- 41.Warren JT, Bachmann JS, Dai JD, Gilbert LI. Differential incorporation of cholesterol and cholesterol derivatives into ecdysteroids by the larval ring glands and adult ovaries of Drosophila melanogaster: A putative explanation for the l(3)ecd(1) mutation. Insect Biochem Mol Biol. 1996;26:931–943. doi: 10.1016/s0965-1748(96)00059-8. [DOI] [PubMed] [Google Scholar]
- 42.Bownes M, Dubendorfer A, Smith T. Ecdysteroids in Adult Males and Females of Drosophila-Melanogaster. J Insect Physiol. 1984;30:823–830. [Google Scholar]
- 43.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 2006;34:1256–1260. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- 44.Rauschenbach I, et al. Dopamine affects the level of 20-hydroxyecdysone in Drosophila virilis females. Dokl Biol Sci. 2006;407:179–181. doi: 10.1134/s0012496606020190. [DOI] [PubMed] [Google Scholar]
- 45.Rauschenbach IY, et al. Dopamine and octopamine regulate 20-hydroxyecdysone level in vivo in Drosophila. Arch Insect Biochem Physiol. 2007;65:95–102. doi: 10.1002/arch.20183. [DOI] [PubMed] [Google Scholar]
- 46.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 47.Lee T, et al. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. doi: 10.1016/s0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 48.Zheng X, et al. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 49.Kraft R, Levine RB, Restifo LL. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J Neurosci. 1998;18:8886–8899. doi: 10.1523/JNEUROSCI.18-21-08886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cayre M, et al. Dual effect of ecdysone on adult cricket mushroom bodies. Eur J Neurosci. 2000;12:633–642. doi: 10.1046/j.1460-9568.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- 51.Velarde RA, Robinson GE, Fahrbach SE. Coordinated responses to developmental hormones in the Kenyon cells of the adult worker honey bee brain (Apis mellifera L. ) J Insect Physiol. 2009;55:59–69. doi: 10.1016/j.jinsphys.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava DP, et al. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshihara M, Ito K. Improved Gal4 screening kit for large-scale generation of enhancer-trap strains. Drosophila Inf Serv. 2000;83:199–202. [Google Scholar]
- 54.Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: Use of intron spacers. Methods. 2003;30:322–329. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 55.Talbot WS, Swyryd EA, Hogness DS. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell. 1993;73:1323–1337. doi: 10.1016/0092-8674(93)90359-x. [DOI] [PubMed] [Google Scholar]
- 56.Iijima-Ando K, et al. cAMP-response element-binding protein and heat-shock protein 70 additively suppress polyglutamine-mediated toxicity in Drosophila. Proc Natl Acad Sci USA. 2005;102:10261–10266. doi: 10.1073/pnas.0503937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.