Abstract
Sepsis, a leading cause of death worldwide, involves concomitant expression of an overzealous inflammatory response and inefficient bacterial clearance. Macrophage function is pivotal to the development of these two aspects during sepsis; however, the mechanisms underlying these changes remain unclear. Here we report that the PD-1:PD-L pathway appears to be a determining factor of the outcome of sepsis, regulating the delicate balance between effectiveness and damage by the antimicrobial immune response. To this end we observed that PD-1−/− mice were markedly protected from the lethality of sepsis, accompanied by a decreased bacterial burden and suppressed inflammatory cytokine response. To the extent that this is a macrophage-specific aspect of the effects of PD-1, we found the following: first, peritoneal macrophages expressed significantly higher levels of PD-1 during sepsis, which was associated with their development of cellular dysfunction; second, when peritoneal macrophages were depleted (using clodronate liposomes) from PD-1−/− mice, the animals' bactericidal capacity was lowered, their inflammatory cytokine levels were elevated, and protection from septic lethality was diminished; and third, blood monocytes from both septic mice and patients with septic shock shared markedly increased PD-1 levels. Together, these data suggest that PD-1 may not only be a dysfunctional marker/effector of macrophages/monocytes, but may also be a potential therapeutic target for designing measures to modulate the innate immune response, thereby preventing the detrimental effects of sepsis.
Keywords: cosignaling molecule, inflammation, innate immunity, PD-1, sepsis
An ideal inflammatory response should eliminate invading microorganisms while causing minimal damage to tissues, organs, or other host systems. Sepsis represents a complex clinical syndrome that is thought to develop when the initial host response against infection becomes inappropriately amplified, then dysregulated, thus becoming a harmful host response (1–3). This dysregulated host response is characterized by sustained infection and an uncontrolled systemic inflammatory response, which results in tissue damage and, ultimately, multisystem organ failure (MSOF), the clinical hallmark of sepsis and direct cause of mortality (4). The innate immune system is the host's first line of defense against infection and responds in a dynamic fashion during sepsis, ranging from an initial overwhelming inflammatory immune response to the later development of a generalized immune suppressive/anergic state, which is characterized by the dysfunction of effector immune cells (5, 6). Macrophages are critical effector cells contributing to the altered innate immune response against infection, as they are the most efficient pathogen scavengers and the predominant source of inflammatory cytokines (7, 8). Severe sepsis has been shown to be associated with progressing macrophage dysfunction (9, 10). However, the mechanisms underlying the decline in macrophage functional capacity remain unclear. Antigen-independent signals provided by pathways from B7:CD28 family, whether stimulatory or inhibitory, are critical to a balanced immune response (11). Distinguishing it from other members of the CD28 family, programmed death 1 (PDF-1) is widely expressed in tissues and organs and participates in a larger spectrum of immune responses. Studies have shown that PD-1 is inducibly expressed on CD4+ T cells, CD8+ T cells, NK T cells, B cells, and monocytes upon activation and plays critical roles in the regulation of autoimmunity, tumor immunity, viral/parasite immunity, transplantation immunity, allergy, and immune privilege (12–16). However, the role of PD-1 in bacterial infection has not been elucidated.
Here we examine the roles of PD-1 in severe bacterial infection, using a model of polymicrobial sepsis. Surprisingly, the results indicate that PD-1 plays a critical role in exacerbating the inflammatory response during sepsis, during which macrophage functions associated with bacterial clearance are also suppressed. Furthermore, PD-1 appears to mediate these effects by contributing to the deterioration of monocyte/macrophage function, thereby providing a potential novel therapeutic target.
Results and Discussion
PD-1−/− Mice Were Less Susceptible to CLP-Induced Lethality than WT Mice, and Displayed Reduced Organ Damage, Lower Levels of Inflammatory Cytokines, and Decreased Bacterial Burden.
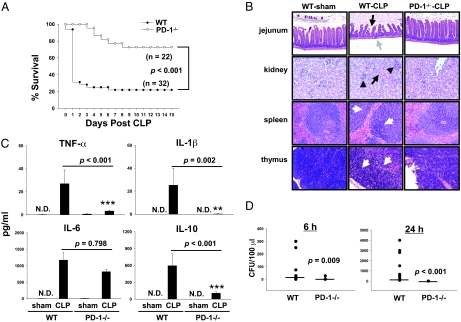
To determine the role of PD-1 in a murine model of experimental sepsis, both WT C57BL/6 (PD-1+/+) and PD-1−/− mice were subjected to cecal ligation and puncture (CLP) surgery and mortality was monitored. We observed that, in male WT mice, CLP caused mortality close to 80% (25 of 32 mice) at 7 days post-CLP (Fig. 1A). However, male PD-1−/− mice were significantly protected against CLP-induced lethality. None of the PD-1−/− mice died until 4 days post-CLP, at which time 72% of the WT mice (23 of 32 mice) were dead. At day 7 post-CLP, 77% of the PD-1−/− mice survived (17 of 22 mice) compared with 22% of the WT mice (7 of 32 mice). These data demonstrate that PD-1 deficiency can efficiently protect mice from early death in sepsis.
Fig. 1.
PD-1 deficiency protects mice from sepsis-induced lethality and displayed reduced organ damage, a less severe cytokine storm, and improved bacterial clearance. (A) PD-1−/− mice were resistant to CLP-treated as compared with WT mice. WT (n = 32) and PD-1−/− (n = 22) mice were subjected to CLP, and survival was monitored for 15 days. P < 0.001 by log-rank test. (B) PD-1−/− mice showed less histological evidence of tissue destruction than did WT mice during severe sepsis. PD-1−/− mice and WT were subjected to CLP surgery or sham control surgery. Mice were euthanized 40 hours after surgery. Tissues were stainined with hematoxylin and eosin. Original magnification, ×200 for jejunum and ×400 for kidney, spleen, and thymus. (Jejunum) Black arrow and gray arrow point to examples of villus shortening and mucosal wall thinning in WT septic mice, respectively; PD-1−/− septic mice do not show these pathologic conditions. (Kidney) Black arrow and arrowheads indicate acute tubular necrosis and congested glomeruli, respectively, in a kidney from a WT septic mouse. Kidney from PD-1−/− septic mice showed rare tubular necrosis and much less congestion. (Spleen and thymus) White arrows illustrate areas of kayrorrhectic, pycnotic, apoptotic cell bodies, which are absent in PD-1−/− septic mice. Data are representative of four to eight mice per group. (C) PD-1−/− mice showed significantly lower systemic levels of inflammatory cytokines at 24 hours post-CLP than did WT mice. Graphs depict data (mean ± SEM of 4–8 sham and 11–13 CLP) pooled from three independent experiments. N.D., not detectable. **P < 0.01 and *** P < 0.001, CLP-treated PD-1−/− mice vs. CLP-treated WT mice by Mann-Whitney test. (D) PD-1−/− mice had markedly reduced bacterial burden after CLP. Levels of aerobic bacteria were expressed as CFU per 100 μl. The graphs depict data pooled from two to three independent studies showing similar results (n = 9–11). Horizontal bar indicates median for each group. P value by Mann-Whitney test.
A hallmark of severe sepsis is the development of MSOF, which is believed to contribute to septic mortality (3–5). To determine the extent to which PD-1 deficiency can lessen the detrimental effects of the inflammatory response on various organ systems, we examined the general pathology of several organs after CLP. Some of the pathologic hallmarks of gut injury during sepsis are villus shortening, epithelial cell loss, mucosal cell sloughing, and mucosal wall thinning (17), which we observed here after CLP. Similarly, in the kidney, in response to CLP, evidence of tubular necrosis, elevated serum creatinine levels, and tubular damage can be seen (18) (Fig. S1). These changes in the jejunum and in the kidney were largely absent in septic PD-1−/− mice. Looking at two lymphoid organs, the spleen and thymus, we and others have seen not only marked disrupted tissue architecture and loss of cellularity but also substantial evidence of karyorrhectic/apoptotic cell bodies in septic WT mice (19). In contrast, septic PD-1−/− mouse splenic histology appeared relatively normal; and the thymus showed much fewer karyorrhectic/apoptitic cell bodies, although the loss of lymphocytes was only partially prevented (Fig. 1B). These results show that PD-1 deficiency reduces the pathological damage seen in multiple organs during severe sepsis, which likely improved the survival of PD-1−/− mice.
Sepsis is associated with high circulating cytokine levels and sustained infection, which contribute to development of MSOF, the direct cause of mortality (3–5). Studies have shown that the production of inflammatory cytokines and chemokines are augmented during CLP-induced sepsis and either pro-inflammatory or anti-inflammatory cytokines are associated with mortality when they are expressed at high levels (6, 20). Therefore, we chose to investigate the effect of PD-1 gene deficiency on systemic blood inflammatory cytokine/chemokine levels after CLP. The systemic cytokine profiles of sham- or CLP-treated WT and PD-1−/− mice were measured at 6 hours or 24 hours after surgery (Fig. 1C and Fig. S2A). Sham surgery did not cause a significant rise in cytokine production. The circulating levels of interleukin (IL)–6 and CCL2 at 6 hours were higher than those at 24 hours post-CLP, and the CCL2 levels in septic PD-1−/− mice were significantly lower than that in WT mice at both time points, however, the IL-6 levels in septic PD-1−/− mice were only significantly lower than that in WT mice at 6 hours, not at 24 hours post-CLP. Other pro-inflammatory cytokines (IL–1β and tumor necrosis factor [TNF]–α) and the anti-inflammatory cytokine (IL-10) reached their peak levels at 24 hours post-CLP, which were markedly higher in WT mice than in PD-1−/− mice, except TNF-α, which level in septic PD-1−/− mice was almost equivalent to the level in septic WT mice at 6 hours post-CLP. The TNF-α levels at 6 hours post-CLP suggests that TNF-α might play a protective role at the beginning of sepsis. Chemokine CXCL2 reached a higher level at 24 hours than at 6 hours post-CLP, and the levels were significantly lower in septic PD-1−/− mice than those in septic WT mice. Similar to the systemic blood cytokine profile, expression of local peritoneal lavage fluid inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-10) and chemokines (CXCL2 and CCL2) was attenuated in PD-1−/− mice when compared with those in WT mice (Fig. S2B), suggesting that the peritoneum may be an important contributor to circulating cytokine levels. Interferon (IFN)–γ and IL-12 were not detectable in the above samples. Overall, these results indicate that the inflammatory response to sepsis in PD-1 deficiency is less vigorous than that in WT mice, which may be one of the reasons for less severe organ failure and the decline in mortality in septic PD-1−/− mice.
In experimental sepsis, sustained/chronic infection is thought to be the cause as well as the result of overzealous inflammation (3, 4, 6). In this model, we found that at 6 hours post-CLP, WT mice had markedly higher systemic levels of bacteria than did PD-1−/− mice (median 12 colony forming units [CFU]/100 μl of WT samples vs. 0 CFU/100 μl of PD-1−/− samples; Fig. 1D Left). At 24 hours post-CLP, PD-1−/− mice displayed a profound capacity to clear bacteria, as none of the blood samples (from nine mice in three independent experiments) cultured developed any colonies, whereas WT mice had even higher systemic levels of bacteria than were seen at 6 hours post-CLP (median 110 CFU/100 μl of WT samples vs. 0 CFU/100 μl of PD-1−/− samples; Fig. 1D Right). Peritoneal lavage was performed at 4 hours and 24 hours after CLP so that the effects of PD-1 gene deficiency on the control of local site infection could be estimated. At 4 hours after CLP, it was evident that the bacterial colony development from the peritoneal lavage of PD-1−/− mice was significantly lower than that in WT mice (median 4.2 × 106 CFU/ml of WT vs. 1.3 × 106 CFU/ml of PD-1−/−), and this difference was even greater at 24 hours post-CLP (median 49 × 106 CFU/ml of WT vs. 4 × 106 CFU/ml of PD-1−/−) (Fig. S2C). Thus, PD-1−/− mice showed greatly improved capacity to clear bacteria, at both a local and systemic level.
Altogether, when compared with septic WT mice, septic PD-1−/− mice showed a less severe inflammatory response and a reduced bacterial burden, indicating that the balance between efficient pathogen clearance and a harmful overactive inflammatory response was affected by PD-1 gene expression, which in turn contributed to the development of MSOF and eventual death of these septic mice.
Macrophages Express Higher Level of PD-1 During Sepsis.
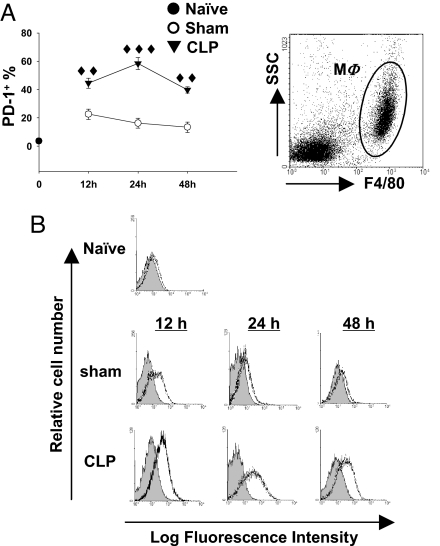
Macrophages are thought to be critical components in mediating both bacterial clearance and cytokine release in response to infectious insult (7, 8). However, although the PD-1:PD-L pathway is thought to be critical in T cell co-stimulatory signal regulation (12, 15, 16), its role in macrophage-mediated innate immunity is unknown. Therefore, we set out to test the hypothesis that PD-1 expression plays a role in sepsis-induced monocyte/macrophage dysfunction. To do this we initially investigated PD-1 expression on T cells, B cells, dendritic cells (DCs), and macrophages in response to sepsis by flow-cytometric analysis. Surprisingly, although it has been reported that thioglycolate-elicited peritoneal macrophages do not express PD-1 (21), we observed a significant up-regulation of PD-1 expression on peritoneal macrophages at 12, 24, and 48 hours post-CLP (Fig. 2 A and B). In contrast, the augmentation of PD-1 expression on splenic T cells and B cells during sepsis was modest and developed more slowly (i.e., was not evident until 24 hours post-CLP) than did the change in macrophage expression. Splenic DCs did not express PD-1 either before or after CLP surgery (data not shown). To further confirm that macrophages were indeed expressing PD-1, mRNA expression for PD-1 was detected in purified peritoneal macrophage from sham- or CLP-treated WT mice. Macrophages from CLP mice expressed consistently elevated levels of PD-1 compared with those seen in sham surgery–treated WT mice (Fig. S3). These results support the idea that sepsis induces a rapid up-regulation of PD-1 in tissue macrophages at both protein and genomic levels, suggesting that PD-1 expression on macrophages may alter their effects in the pathophysiology of sepsis.
Fig. 2.
Expression of PD-1 in macrophages is augmented by sepsis. (A) The percentage of PD-1+ macrophage increased during sepsis. PD-1 expression on peritoneal macrophages (gated as F4/80+) was determined by flow cytometry (n = 5–12 for sham-treated mice, n = 7–17 for CLP-treated mice pooled from two to three experiments). ♦♦, P < 0.01, ♦♦♦, P < 0.001 by Mann-Whitney test, CLP-treated WT mice vs. sham-treated WT mice. (B) Representative histograms of PD-1 expression on peritoneal macrophages. Peritoneal macrophages were gated as F4/80+ peritoneal leukocytes (Upper). PD-1 expression (black lines) was overlayed on isotype control (gray filled; Lower).
PD-1−/− Macrophages Were Resistant to Development of Sepsis-Induced Cellular Dysfunction.
Severe sepsis has also been shown to be closely associated with developing macrophage dysfunction, characterized by diminished bactericidal ability, decreased inflammatory cytokine production, and suppressed antigen-presenting function (9, 10, 22). In an attempt to determine whether the changes in PD-1 expression on macrophages were associated with the progression of their sepsis-induced dysfunction, macrophages were isolated from septic WT or PD-1−/− mice and their ex vivo cell functions were analyzed.
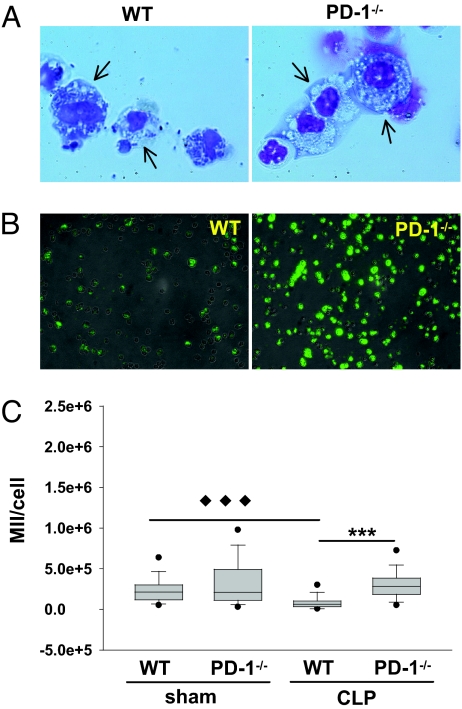
From a morphological perspective, we observed that macrophages from WT septic mice appeared to be more granular (vacuoles contain more opaque material), which was not seen in the septic PD-1−/− mouse cells (Fig. 3A), suggesting a difference in the degree of endogenous activation. In view of these data, we subsequently examined the phagocytic capacity of peritoneal macrophages from WT and PD-1−/− mice that had undergone sham or CLP surgery by using an in vitro assay system in which fluorescein-conjugated Escherichia coli were fed to macrophages in serum-free medium (23). Phagocytosis by septic WT macrophages was profoundly suppressed in comparison to that by WT macrophages from sham-treated mice; however, septic PD-1−/− macrophages displayed no such decline (Fig. 3 B and C). Importantly, this was evident regardless of whether the microbes were opsonized or not (Fig. S4).
Fig. 3.
Sepsis-induced macrophage dysfunction is associated with the expression of PD-1. (A) Septic mouse macrophages from WT mice showed significant impairment in bacterial clearance, whereas septic PD-1−/− macrophages did not. Peritoneal leukocytes were collected at 24 hours post-CLP, spun on slides, and stained with Giemsa staining solution. WT macrophages (arrows) displayed an exhausted phenotype in which bacteria accumulated within the cell plasma, whereas PD-1−/− septic mouse macrophages (arrows) exhibited an activated phenotype. Representative images from three independent experiments show similar results. Original magnification, × 200. (B and C) Phagocytosis assay of macrophages. (B) Representative images of septic peritoneal macrophages from WT or PD-1−/− mice after feeding with Fluorescein-conjugated E. coli. Light microscope images were overlayed with fluorescence images. (C) Quantitative analysis of phagocytosis. Approximately 150 cells from sham group (n = 2 of WT or PD-1−/− mice) or 300 cells from CLP group (n = 3 of WT or PD-1−/− mice) were calculated by transforming individual cells from image into mean integrated intensity (MII) of fluorescence per cell. Data are representative of a total of two experiments. Outliers are shown at 5th/95th percentiles. ◆◆◆ P < 0.001, CLP-treated WT mice vs. sham treated WT mice.*** P < 0.001, CLP-treated PD-1−/− mice vs. CLP-treated WT mice, by Mann-Whitney test.
Another characteristic of macrophage dysfunction observed as sepsis progresses is the reduction in their capacity to actively secrete inflammatory cytokines in response to bacterial stimuli; although, at the same time, cytokines in blood are reported to accumulate at high levels (9, 24). To investigate whether PD-1 gene deficiency also affects this property, we examined the cytokine productive capacity of septic peritoneal macrophages obtained from WT or PD-1−/− mice in response to lipoteichoic acid (LTA) or lipopolysaccharide (LPS) stimulation. As expected, septic WT mouse macrophages produced markedly lower levels of TNF-α, IL-6, and IL-1β as well as higher levels of IL-10 than macrophages from sham-treated mice, whereas PD-1−/− macrophages from CLP-treated mice exhibited a marked, albeit partial, restoration of the release of these inflammatory cytokines (Fig. S5).
With respect to T-cell immunomodulatory cytokines, septic PD-1−/− macrophages produced significantly higher levels of IFN-γ and IL-12 and substantially lower levels of IL-10 than did septic WT macrophages (Fig. S5). This suggests that PD-1 gene deficiency may also be beneficial in sustaining macrophage anti-pathogen capacity through regulatory potential that favors Th1 immunity.
Collectively, these results demonstrate that PD-1 deficiency appears to prevent the development of macrophage dysfunction on a number of levels during sepsis, which may contribute to the improvement in survival observed in PD-1−/− mice.
Peritoneal Macrophages Were Critical in Maintaining the Protective Effect Produced by PD-1 Gene Deficiency in Septic Mice.
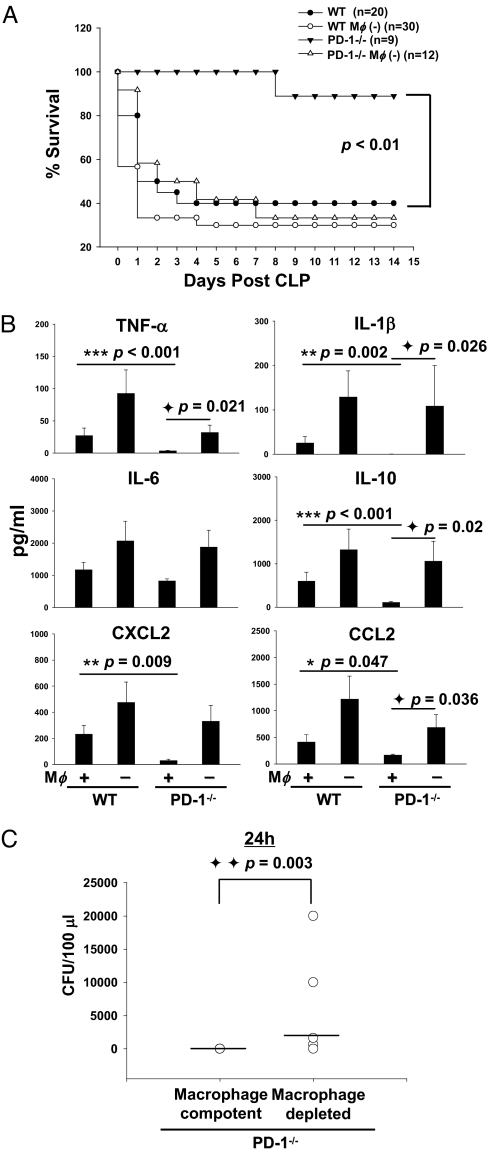
Because clodronate liposomes can deplete peritoneal macrophages but not spleen macrophages when injected into the peritoneum, this approach was used to evaluate the role of peritoneal macrophages in maintaining the survival benefit seen in PD-1−/− mice with severe sepsis. We determined that PD-1−/− macrophages are critical to maintaining the improved survival rate seen in PD-1−/− mice after CLP, because when peritoneal macrophages were depleted from PD-1−/− mice, they showed no increase in septic survival in comparison to WT mice (Fig. 4A).
Fig. 4.
Macrophage is an important mediator of the protective effects of PD-1 gene deficiency in septic mice. (A) Macrophages are pivotal in maintaining the protective effect of PD-1 gene deficiency in mice from the lethality of sepsis. WT and PD-1−/− mice were treated with clodronate liposomes or PBS (200 μl/mouse, i.p.) 48 hours before being subjected to CLP. The survival rate was followed for 15 days after surgery. Graph represents survival data pooled from two experiments. (B) Peritoneal macrophages are critical for regulating the cytokine response seen in PD-1−/− mice during sepsis. Concentrations of cytokines in plasma (sham, n = 4; CLP, n = 12–16) were measured by ELISA. * P < 0.05, ** P < 0.01, and *** P < 0.001, CLP-treated PD-1−/− mice vs. CLP-treated WT mice; ◆ P < 0.05, macrophage depleted PD-1−/− mice vs. macrophage intact PD-1−/− mice by Mann-Whitney test. Graphs depict data (mean ± SEM) pooled from two to three independent experiments. (C) Macrophages play a role in the improved bacterial clearance seen in PD-1−/− mice during sepsis. At 24 hours after CLP, macrophage-competent mice and macrophage-depleted mice were bled, spread on TSA plates, and incubated for 24–48 hours at 37 °C. Levels of bacteria were expressed as CFU per 100 μl. Horizontal bar indicates median for each group. (n = 5–9) P value by Mann-Whitney test.
To determine the extent to which PD-1−/− macrophages are critical in reducing the severity of the inflammatory cytokine storm seen during sepsis, we examined plasma and peritoneal lavage fluid cytokine profiles of clodronate liposomes or PBS-treated septic WT and PD-1−/− mice at 24 hours after surgery. Macrophage depletion caused a more severe systemic and local cytokine response in both WT mice and PD-1−/− mice (Fig. 4 B and C). PD-1−/− mice displayed a remarkable reduction in systemic and local cytokine levels in comparison with WT mice post-CLP; however, if PD-1−/− mice were depleted of macrophages, systemic levels of inflammatory cytokines (TNF-α, IL-1β, and IL-10) and chemokine CCL2 were markedly elevated. IL-6 and CXCL2 were also up-regulated, although the increase seen in macrophage depleted PD-1−/− mice compared with PD-1−/− mice was not statistically significant. The systemic levels of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-10) and chemokines (CXCL2 and CCL2) in macrophage depleted PD-1−/− mice were even higher than the cytokine level in septic WT mice. Moreover, although the cytokine level in macrophage-depleted WT mice was higher than that of macrophage depleted PD-1−/− mice, the difference was no longer significant (Fig. 4B). Macrophage depletion also affected cytokine levels in the peritoneum; inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-10), and chemokines (CXCL2 and CCL2) displayed a pattern similar to that seen in the serum (Fig. S6).
In parallel to cytokine production, peritoneal macrophage–depleted PD-1−/− mice exhibited an increased bacterial burden in blood in comparison with PD-1−/− mice (Fig. 4C). Although PD-1−/− mice could clear bacteria very efficiently (i.e., to the extent that no colonies developed in 100 μl of blood 24 hours post-CLP), after PD-1−/− mice had been treated with clodronate liposomes i.p., the bactericidal capacity of PD-1−/− mice was markedly reduced. This result demonstrates that the enhanced bactericidal potency in PD-1−/− mice was at least partially dependent on the presence of peritoneal macrophages. In combination with the results of the systemic blood and local peritoneal cytokine pattern, the change in systemic bacteria counts indicates that PD-1−/− macrophages are pivotal to maintaining a competent and balanced inflammatory response to septic challenge, culminating in improved survival.
PD-1 May Be a Marker of Monocyte Dysfunction During Sepsis.
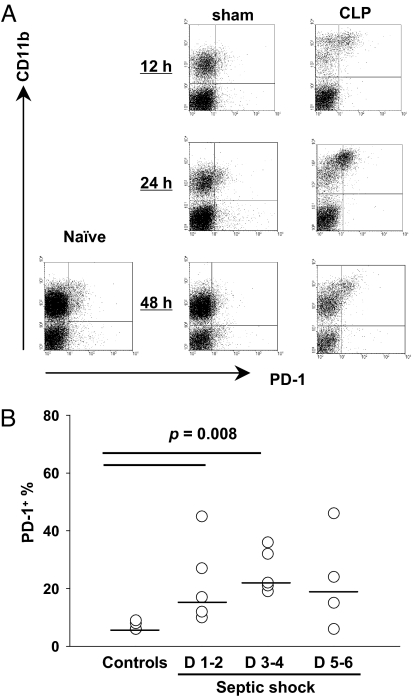
The above results show that in a murine model, PD-1 appears to be critical to the outcome of sepsis and contributes to its pathological effects, in part through its action in macrophages. Thus, we wondered to what degree PD-1 expression might serve as a potential marker of macrophage dysfunction. To assess this, PD-1 expression on circulating monocytes in septic mice was monitored. At 12 hours and 24 hours postsurgery, monocytes from septic mice expressed significantly higher levels of PD-1 than did monocytes from control mice (Fig. 5A and Fig. S7A). Importantly, we observed similar up-regulation in patients with septic shock. At days 1–4 after the onset of septic shock, the percentage of PD-1+ monocytes from septic shock patients was significantly higher than from healthy volunteers (median 17% of patients at days 1–2, 22% of patients at days 3–4 vs. 6% of healthy volunteers at either days 1–2 or days 3–4) (Fig. 5B and Fig. S7B). These results suggest that PD-1 may be a potential marker of tolerogenic monocyte/macrophage development during sepsis.
Fig. 5.
PD-1 may be a tolerance marker for circulating monocytes in sepsis. (A) PD-1 expression on circulating mouse blood monocytes was up-regulated after CLP. Representative data of PD-1 expression on mouse blood monocytes from naïve mice or mice subjected to sham or CLP surgery at indicated time points. (B) PD-1 expression on circulating monocytes was higher in patients with septic shock than in healthy volunteers. Horizontal bar indicates the median for each group. P value by Mann-Whitney test.
Our data show that besides the critical roles that PD-1 plays in viral infection (14–16), PD-1 also appears to be an important mediator in the pathophysiology of severe bacterial infection, like sepsis, because PD-1−/− mice are profoundly resistant to CLP-induced lethality. With respect to the nature of the mechanisms that contribute to this survival advantage, we found that PD-1−/− mice had a greater bactericidal capacity and produced a less severe inflammatory cytokine storm during the initial response to severe sepsis. These results demonstrate that PD-1 may be able to sway the outcome of bacterial infection by influencing the delicate balance between effective antimicrobial immune defense and immune-mediated tissue damage. Moreover, macrophages are protected from developing dysfunction during sepsis in PD-1−/− mice. Along with the up-regulation of PD-1 expression on WT macrophages during the progression of sepsis, these changes may in turn contribute to maintaining the balance between efficient pathogen clearance and inflammatory response, thus resulting in an improved outcome by attenuating the development of MSOF.
That said, it has been previously reported that thioglycolate-elicited peritoneal macrophages do not express PD-1 (21). However, we have shown here that macrophages and monocytes harvested from the peritoneum and blood from both sham-treated and CLP surgery–treated mice expressed PD-1 on their surface. This suggests that macrophage PD-1 expression may be induced by stimuli present during the septic inflammatory response in a fashion that would appear to be independent of the signals and processes used in these prior studies. To further confirm that PD-1 is specifically expressed by macrophages, similar changes in the mRNA expression of PD-1 could be seen in purified macrophages from sham- or CLP- treated mice. These results indicate that mice macrophages can express PD-1 not only when stimulated by inflammatory response encountered with the tissue injury associated with the sham or CLP protocol, but also are further amplified by inflammatory response against bacteria infection. Importantly, we have also found that blood monocyte PD-1 levels from both septic mice and septic shock patients are markedly increased, in keeping with a recent report that circulating leukocytes from traumatically injured patients expressed higher levels of PD-1 than those from controls (25). Taken together, these results demonstrate that the up-regulated PD-1 expression on macrophages/monocytes during sepsis associates closely with their functional decline, suggesting that PD-1 may be used as a marker of the developing of macrophages/monocytes dysfunction. These findings also extend our understanding of the function of PD-1, from its role as a tolerogenic marker and mediator of T-cell anergy (15, 16) to an immune-suppressive marker and effector of macrophage/monocyte anergy. Furthermore, our study identifies PD-1 as the first receptor of B7:CD28 family that can act directly on effector cells of innate immune system, revealing new ways in which B7:CD28 family members may regulate the immune response to sepsis and possibly other infectious/inflammatory conditions.
Until recently, apart from the administration of antibiotics, the treatment of sepsis and septic shock has largely been limited to supportive strategies (26). Because all of the clinical trials designed to neutralize single inflammatory cytokines have failed, some investigators have suggested that normalization of a number of components of the inflammatory cytokine storm through surface receptors in a “global” fashion might represent a potential alternative approach to improve overall prognosis (23). To this end, we believe that these data indicate that regulating innate immune cell (e.g., macrophage) function by modulation of surface receptors, such as PD-1, may be such a strategy for the treatment of sepsis.
Methods
Mice and Cecal Ligation and Puncture.
Cecal ligation and puncture (CLP) was used to induce acute septic peritonitis (27).
Patients.
Five patients, who were diagnosed with septic shock according to the criteria of the American College of Chest Physicians/Society of Critical Care Medicine, were enrolled in this study, along with 5 healthy volunteers. Cell phenotyping was assessed at 1–2, 3–4, and 5–6 days after the onset of shock (28).
Phagocytosis Assay.
Adherent macrophages were then co-cultured with Fluorescein-conjugated E. coli (Molecular Probes) in PBS at 37 °C for 1 hour and then were washed completely with phosphate-buffered saline (PBS) (23). The mean fluorescence integrated intensity (MII) per cell was calculated with MetaVue software (Meta Imaging Series 6.1, Universal Imaging Co.) and used as a measure of phagocytosis.
In Vivo Macrophage Depletion.
Macrophages were depleted by the pretreatment of mice with liposomes containing dichloromethylene bisphosphonate (clodronate liposomes) (29).
All Others.
Supplementary Material
Acknowledgments.
The authors acknowledge T. Honjo (Kyoto University Graduate School of Medicine, Kyoto) and M. Sykes (Massachusetts General Hospital, Transplantation Biology Research Center, Boston) for providing PD-1−/− mice. We also thank Virginia Hovanesian and Paul Monfils for technical assistance with the histologic specimens. The research was supported by National Instititutes of Health Grants R01s GM46354 (to A.A.), GM53209 (to A.A.), T32 GM65085 (to R.S.), and by funds from Hospices Civils de Lyon (to G.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.A.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809422106/DCSupplemental.
References
- 1.Angus DC, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 5.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 6.Bone RC. Immunologic dissonance: A continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 8.Underhill DM, Ozinsky A. Phagocytosis of microbes: Complexity in action. Annu Rev Immuno. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 9.Munoz C, et al. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: Mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl 1):S27–S38. [PubMed] [Google Scholar]
- 11.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 12.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okazaki T, Honjo T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 16.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 17.Coopersmith CM, et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. J Am Med Assoc. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, et al. Role of protein C in renal dysfunction after polymicrobial sepsis. J Am Soc Nephrol. 2007;18:860–867. doi: 10.1681/ASN.2006101167. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 20.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 22.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: A new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Does endotoxin play a major role in inducing the depression of macrophage function during polymicrobial sepsis? Arch Surg. 1995;130:1178–1184. doi: 10.1001/archsurg.1995.01430110036007. [DOI] [PubMed] [Google Scholar]
- 25.Laudanski K, et al. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci USA. 2006;103:15564–15569. doi: 10.1073/pnas.0607028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 27.Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor) Circ Shock. 1992;36:191–199. [PubMed] [Google Scholar]
- 28.Venet F, et al. Both percentage of gammadelta T lymphocytes and CD3 expression are reduced during septic shock. Crit Care Med. 2005;33:2836–2840. doi: 10.1097/01.ccm.0000189745.66585.ae. [DOI] [PubMed] [Google Scholar]
- 29.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide.”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.