Abstract
T cells differentiate into functionally distinct effector subsets in response to pathogen encounter. Cells of the innate immune system direct this process; CD1d-restricted invariant natural killer T (iNKT) cells, for example, can either promote or inhibit Th1 and Th2 responses. Recently, a new subset of CD4+ T helper cells, called Th17, was identified that is implicated in mucosal immunity and autoimmune disorders. To investigate the influence of iNKT cells on the differentiation of naïve T cells we used an adoptive transfer model of traceable antigen-specific CD4+ T cells. Transferred naïve CD25−CD62L+ CD4+ T cells were primed by antigen immunization of the recipient mice, permitting their expansion and Th17 differentiation. This study establishes that in vivo activation of iNKT cells during T-cell priming impedes the commitment of naïve T cells to the Th17 lineage. In vivo cytokine neutralization experiments revealed a role for IL-4, IL-10, and IFN-γ in the iNKT-cell-mediated regulation of T-cell lineage development. Moreover, by comparing IL-17 production by antigen-experienced T cells from unmanipulated wild-type mice and iNKT-cell-deficient mice, we demonstrate an enhanced Th17 response in mice lacking iNKT cells. This invigorated Th17 response reverts to physiological levels when iNKT cells are introduced into Jα18−/− mice by adoptive transfer, indicating that iNKT cells control the Th17 compartment at steady state. We conclude that iNKT cells play an important role in limiting development of the Th17 lineage and suggest that iNKT cells provide a natural barrier against Th17 responses.
Keywords: autoimmune encephalomyelitis, immune regulation, iNKT cells, multiple sclerosis
To respond to diverse microbial infections, T cells differentiate into functionally distinct subsets that secrete unique combinations of cytokines (1). Recently, a novel subset of T helper (Th) cells was identified—called Th17 cells—that produce IL-17A, IL-17F, and IL-22 (2). Th17 cells protect the host against extracellular pathogens encountered at mucosal surfaces, but they also play a detrimental role in experimental models of multiple sclerosis, as well as in human inflammatory bowel disease and psoriasis (2). In mice, cytokines TGF-β and IL-6 initiate the differentiation of T cells into the Th17 lineage by inducing expression of a transcription factor called retinoic acid receptor-related orphan nuclear receptor (ROR)-γt and of the IL-23 receptor (3–6). IL-21 and IL-23 further support the differentiation of Th17 cells, permitting IL-22 expression (6–10). In addition to providing autocrine support to the cells that produce them, some lineage-specific cytokines can impede the development of other Th lineages. For example, IL-21 secretion by Th17 cells inhibits Th1 cytokines (11), whereas the reciprocal regulation of Th1 and Th2 responses is mediated by IL-4 and IFN-γ (12), both of which antagonize Th17 differentiation (13, 14). Upon pathogen encounter, innate immune cells critically influence Th differentiation. For example, triggering of Toll-like receptors (TLRs) on dendritic cells (DCs) drives IL-12 production, which favors Th1 differentiation. Similarly, upon engagement of their receptors dectin-1 or nucleotide oligomerization domain 2 (Nod2), DCs produce IL-6 and IL-23, which orientate T cells to the Th17 lineage (15–17). Other cells of the innate immune system that influence Th differentiation include invariant natural killer T cells (iNKT), a CD1d-restricted T-cell population that expresses an invariant T-cell receptor α-chain by using a Vα14-Jα18 rearrangement in mice and a Vα24-Jα18 rearrangement in humans. Like conventional T cells, iNKT cells comprise distinct functional subsets. Notably, NK1.1pos iNKT cells produce IL-4 and IFN-γ, whereas NK1.1neg iNKT cells produce IL-17 and IL-21 (18–21). The iNKT cell response is elicited by the presentation of pathogen-derived or endogenous glycolipid antigens by CD1d-expressing antigen-presenting cells (APCs) (22). iNKT cells can support and sustain Th1 responses by activating NK cells, which secrete IFN-γ, thus facilitating DC maturation and IL-12 production (22). Under different inflammatory conditions, iNKT cell activation favors Th2 differentiation by producing IL-4, thereby conferring clinical benefit in animal models of organ-specific autoimmune diseases (23–27).
The ability of iNKT cells to regulate the differentiation of conventional Th1 and Th2 cells raises the question of whether they might also influence the Th17 lineage. On one hand, the NK1.1neg iNKT cell subset that produces IL-17 and IL-21 might support and sustain Th17 responses (18–21); on the other hand, the clinical benefit provided by the activation or enrichment of iNKT cells in Th17-driven models of organ-specific autoimmune diseases suggests that iNKT cells might control pathogenic Th17 responses (23–26, 28, 29). This study investigated the influence of iNKT cells on the Th17 response. We found that activation of iNKT cells in vivo impedes commitment of naïve CD4+ T cells to the Th17 lineage. Moreover, when comparing the Th17 compartment between wild-type and iNKT-cell-deficient mice, we found that antigen (Ag)-experienced CD4+ T cells produce more IL-17 when they are isolated from iNKT-cell-deficient mice (Jα18−/−). This invigorated Th17 response in the Jα18−/− mice can be corrected by the adoptive transfer of iNKT cells. Consequently, our data reveal that iNKT cells control the Th17 lineage in healthy, unmanipulated mice and illustrate the importance of iNKT cells both in regulating Th17 lineage development and in the persistence of the Th17 population.
Results and Discussion
iNKT Cells Regulate IL-17 Production by CD4+ T Cells.
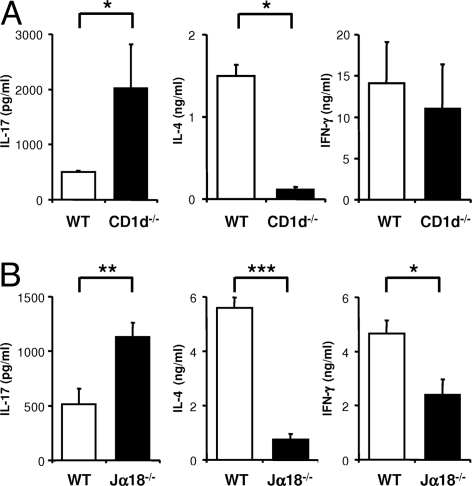
To evaluate the influence of iNKT cells on Th17 cells, we first compared the capacity of CD4+ T cells from wild-type and iNKT-cell-deficient (CD1d−/−, Jα18−/−) animals to produce IL-17. Splenic CD4+ T cells purified from unmanipulated C57BL/6 mice were stimulated for 3 days with plate-bound anti-CD3 and anti-CD28 mAbs in the presence of IL-23. Under such APC-free conditions, IL-23 promoted the expansion of preexisting IL-17-producing cells, but did not permit de novo Th17 differentiation of naïve CD4+ T cells (4, 6, 30). CD4+ T cells from the iNKT-cell-deficient CD1d−/− mice produced up to 3-fold more IL-17 than CD4+ T cells from wild-type mice (Fig. 1A), suggesting that a CD1d-dependent T-cell population controls the Th17 lineage. By contrast, the production of IL-4 was significantly reduced in CD4+ T-cell cultures from CD1d−/− mice, indicative of the absence of iNKT cells, which produce large amounts of IL-4 in response to TCR stimulation (31). To unequivocally assess the implication of CD1d-restricted iNKT cells we determined the cytokine production of stimulated CD4+ T cells from Jα18−/− mice that selectively lack iNKT cells. Similar to the phenotype observed with CD1d−/− mice, CD4+ T cells from Jα18−/− mice produced more IL-17 as compared with CD4+ T cells from wild-type mice (Fig. 1B), confirming that iNKT cells limit IL-17 production in vitro. Stimulated CD4+ T cells from Jα18−/− mice also produced less IL-4 than their wild-type counterparts (Fig. 1B) compatible with the absence of iNKT cells. A less pronounced reduction in IFN-γ secretion was equally observed. To assess if the impact of iNKT cells on the Th17 lineage extends to other mouse strains we compared the cytokine profile of stimulated CD4+ T cells from wild-type and Jα18−/− NOD mice, and observed a comparable inhibition of IL-17 secretion in the presence of iNKT cells (NOD: 864 ± 110 pg/ml vs. Jα18−/−: 2707 ± 379 pg/ml; P = 0.03). Interestingly, the CD4+ T-cell cultures from NOD mice produced more IL-17 than C57BL/6-derived cultures, potentially reflecting the autoimmune process developing in NOD mice.
Fig. 1.
iNKT cells negatively regulate IL-17 production by CD4+ T cells. Purified CD4+ T cells were stimulated for 72 h with plate-bound anti-CD3 and anti-CD28 mAbs and IL-23. (A) Release of IL-17 (Left), IL-4 (Middle), and IFN-γ (Right) by CD4+ T cells from (A) wild-type (WT) or CD1d−/− C57BL/6 mice or (B) wild-type (WT) or Jα18−/− C57BL/6 mice was assayed by ELISA. Each data point represents the mean ± SEM of 2 (A) or 3 (B) experiments.
Activating iNKT Cells in Vivo Inhibits Autoantigen-Specific Th1 and Th17 Responses.
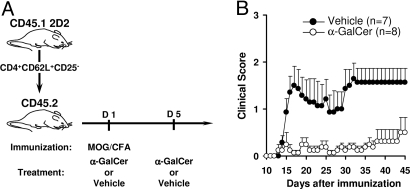
To investigate the influence of iNKT cells on the Th17 lineage in vivo, we used the mouse model of experimental autoimmune encephalomyelitis (EAE), in which immunization with a peptide fragment of myelin oligodendrocyte glycoprotein (MOG) induces myelin-specific Th1 and Th17 cells that synergistically contribute to the demyelinating lesions in the CNS (32, 33). In this model, activation of iNKT cells ameliorates the disease by reducing the intensity of the pathogenic T-cell response (24–26). To study the differentiation of autoreactive T cells at the single-cell level, we introduced a traceable population of naïve MOG35–55-specific CD4+ T cells into the mice before inducing EAE (Fig. 2A). These CD62L+CD25− autoreactive CD4+ T cells were purified from CD45.1 congenic, 2D2 transgenic mice expressing an I-Ab-restricted TCR (Vα3.2-Vβ11) specific for MOG35–55 on CD4+ T cells. These transferred MOG-specific T cells underwent an expansion of up to 3,700-fold, 9 days after immunization with MOG35–55 (Fig. S1). Analysis of the intracellular cytokine profile of the congenic (CD45.1+) MOG-specific T cells by FACS enabled us to quantify IFN-γ-producing Th1 and IL-17-producing Th17 cells (Fig. S1A). Both the Th1 and Th17 responses peaked 9 days after immunization in the spleen (Fig. S1B) and draining lymph nodes (LNs; data not shown), but the number of Th17 cells was significantly inferior to that of Th1 cells. These mice developed moderate clinical signs of EAE, on average, 17 days after immunization (Fig. 2).
Fig. 2.
In vivo treatment with α-GalCer prevents EAE. (A) The 2D2 transfer model uses traceable MOG-specific naïve CD4+ T cells purified from CD45.1 congenic 2D2 mice. Twenty-four hours after transfer of 105 CD62L+CD25− CD4+ T cells, the recipient CD45.2 C57BL/6 mice were immunized with MOG35–55 in the presence either of α-GalCer or the PBS/DMSO solvent (vehicle). (B) The average clinical score from α-GalCer-treated mice (open circles, n = 8) and vehicle-treated controls (filled circles, n = 7) from 2 independent experiments is presented.
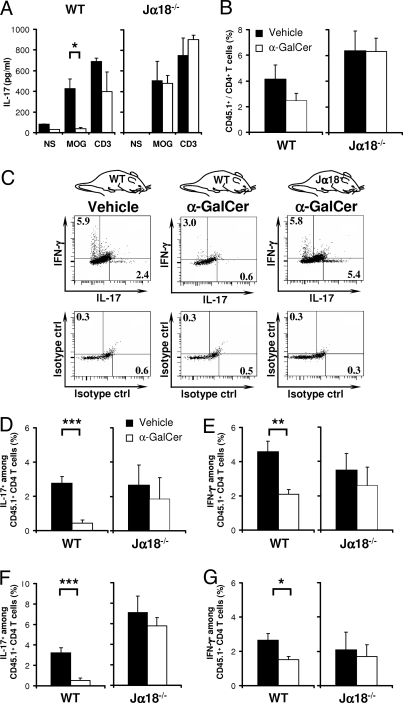
To validate the regulatory capacity of iNKT cells in this model, we treated immunized mice with 2 injections of α-GalCer (Fig. 2A), an exogenous glycolipid that closely resembles the microbial glycosphingolipids that are agonists of iNKT cells (34). These injections significantly reduced the severity of EAE when compared with the vehicle-injected control mice (Fig. 2B). In vitro recall responses to MOG35–55 revealed that α-GalCer injections inhibited the antigen-specific secretion of IL-17 (Fig. 3A), IFN-γ, and TNF-α (data not shown) by splenocytes. This was not due to loss of MOG-specific CD45.1+ CD4+ T cells, which were only slightly reduced after α-GalCer treatment (Fig. 3B). Instead, activation of iNKT cells with α-GalCer markedly reduced both the frequency (Fig. 3 C and D) and the absolute numbers (vehicle: 17 ± 6 × 103 vs. α-GalCer: 2 ± 1 × 103; P = 0.001) of Th17 cells as assessed by analysis of intracellular cytokines in the transferred CD45.1+ TCRVα3.2+ CD4+ T cells. A similar inhibition of Th17 differentiation was observed amongst endogenous CD45.1− CD4+ T cells (data not shown). Interestingly, although the activation of iNKT cells also reduced the frequency (Fig. 3E) and slightly reduced the absolute numbers (vehicle: 34 ± 12 × 103 vs. α-GalCer: 8 ± 2 × 103; P = 0.1) of Th1 cells, its impact on the Th1 response appeared more modest than on the Th17 response. This inhibition of the MOG-specific Th17 and Th1 responses is attributable to iNKT cells because α-GalCer treatment of Jα18−/− mice had no significant influence on the expansion and differentiation of autoreactive T cells (Fig. 3 B, D, and E). Similar results were obtained with cells from the draining LNs, where α-GalCer treatment influenced expansion only modestly (Fig. 4A), but blunted IFN-γ and IL-17 production by MOG-specific CD4+ T cells in an iNKT-cell-dependent manner (Fig. 3 F and G and Fig. S2). Taken together, our data show that activation of iNKT cells in vivo inhibits differentiation of naïve autoreactive CD4+ T cells into pathogenic Th1 and Th17 effector cells, and that the inhibitory effect is most pronounced on the Th17 lineage.
Fig. 3.
Activation of iNKT cells by α-GalCer blocks the commitment of naïve MOG-specific T cells to the Th1 and Th17 lineages. By using the 2D2 transfer model described in Fig. 2, we assessed MOG-specific T-cell differentiation in immunized C57BL/6 (WT) and Jα18−/− recipient mice treated with α-GalCer or vehicle. (A) Nine days after immunization, we assessed the in vitro recall response by splenocytes from α-GalCer-treated recipients (white bars; WT, n = 4; Jα18−/−, n = 4) or vehicle-treated recipients (black bars; WT, n = 4; Jα18−/−, n = 4). IL-17 release was measured from unstimulated (NS), MOG35–55-stimulated (MOG; 100 μg/ml), and anti-CD3 mAb-stimulated (CD3) cultures after 72 h. (B) The number of CD45.1+TCRVα3.2+ CD4+ T cells was established in mice treated with α-GalCer (white bars; WT, n = 11; Jα18−/−, n = 4) or vehicle (black bars; WT, n = 11; Jα18−/−, n = 4). α-GalCer slightly reduced the proportion of 2D2 CD45.1+TCRVα3.2+ CD4+ T cells in the spleens of WT but not Jα18−/− mice. (C–G) The MOG-specific Th17 and Th1 differentiation was analyzed by establishing the intracellular IL-17 and IFN-γ production of CD45.1+TCRVα3.2+ CD4+ T cells from WT and Jα18−/− recipient mice treated with α-GalCer or vehicle. Th17 differentiation is indicated by the frequency of IL-17-producing 2D2 T cells in the spleen (C and D) and draining LNs (F). Th1 differentiation is indicated by the frequency of IFN-γ-producing 2D2 T cells in the spleen (C and E) and draining LNs (G). Intracellular cytokine analyses were also performed after antigen-specific restimulation with MOG35–55 and revealed a similar inhibition of Th1 and Th17 responses by α-GalCer treatment, although the number of cytokine-positive cells was generally lower. Cytokine secretion in (A) presents 1 representative experiment of 3 performed. Intracellular cytokine analyses in (B–G) present 3 independent experiments for the WT mice and 1 experiment for Jα18−/− mice.
Fig. 4.
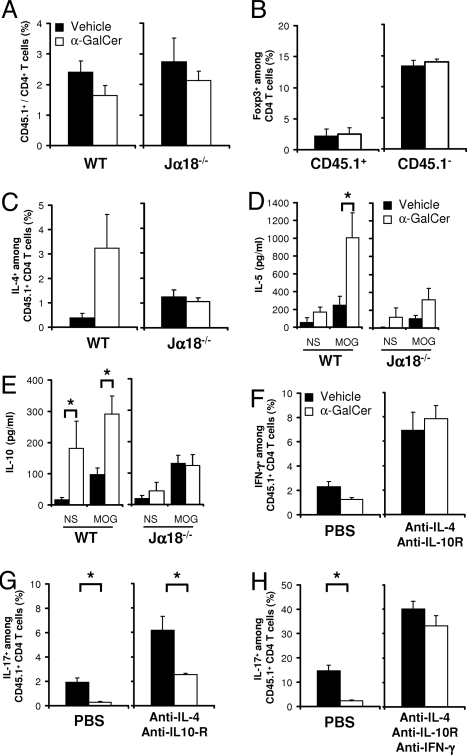
Activation of iNKT cells by α-GalCer regulates the Th1 and Th17 responses by immune deviation and IL-10 production. By using the 2D2 transfer model described in Fig. 2, we assessed MOG-specific T-cell differentiation in the draining LNs of immunized C57BL/6 (WT) and Jα18−/− mice. Nine days after immunization, the intracellular IFN-γ, IL-17, IL-4, and IL-10 expression by CD45.1+TCRVα3.2+ CD4+ T cells was assessed in α-GalCer-treated mice (white bars; WT, n = 11; Jα18−/−, n = 4) and vehicle-treated mice (black bars; WT, n = 11; Jα18−/−, n = 4). (A) The effect of α-GalCer on the proportion of 2D2 CD45.1+TCRVα3.2+ CD4+ T cells in the draining LNs of recipient mice. (B) The frequency of Foxp3-positive cells among 2D2 CD45.1+TCRVα3.2+ and endogenous CD45.1− CD4+ T cells. (C) The proportion of IL-4-positive cells among 2D2 CD45.1+TCRVα3.2+ CD4+ T cells increased upon α-GalCer treatment of WT, but not Jα18−/− mice, indicating an enhanced Th2 differentiation in the presence of iNKT cells but not in their absence. (D and E) The release of IL-5 (D) and IL-10 (E) during the in vitro recall response was assessed in α-GalCer-treated mice (white bars; WT, n = 4; Jα18−/−, n = 4) or vehicle-treated mice (black bars; WT, n = 4; KO, n = 4). Cytokine release in unstimulated (NS) and MOG35–55-stimulated (100 μg/ml) cultures was measured after 72 h by Luminex immunoassay. (F–H) The role of IL-4, IL-10, and IFN-γ in regulating T-cell differentiation was evaluated in vivo by injecting neutralizing mAbs. Th1 (F) and Th17 (G-H) commitment was assessed in mice treated with vehicle or α-GalCer that had received mAbs neutralizing IL-4 and IL-10R (F and G), or IL-4, IL-10R, and IFN-γ (H). The single-cell analysis presents 3 separate experiments for the WT mice (A and C) and a single experiment for the Jα18−/− mice (A and C). For the cytokine secretion (D and E), 1 representative experiment of 2 is shown. The cytokine neutralization data (F–H) are from 1 experiment involving 4 individual mice per group for each neutralization protocol.
INKT Cell-Induced IL-4 and IL-10 Inhibit the Th1 Response, and IL-4, IL-10, and IFN-γ Are Required to Control the Th17 Response.
The therapeutic efficacy of α-GalCer in EAE depends on the production of anti-inflammatory cytokines IL-4 and IL-10 (24, 25). To assess whether α-GalCer promotes the commitment of CD4+ T cells to the IL-4-producing Th2 or IL-10-producing Tr1 lineages, we analyzed the intracellular cytokine-profile of the MOG-specific T-cell response. In addition, given the reciprocal relationship between Th17 and inducible regulatory T cells (3, 4, 35), we enumerated the frequency of Foxp3+ CD4+ T cells. No variation in the proportion of Foxp3+ CD4+ T cells was observed among congenic 2D2 T cells and in the endogenous CD4+ T-cell pool (Fig. 4B). Evidence of immune deviation was obtained, as the proportion of IL-4-producing congenic 2D2 CD4+ T cells (and endogenous Th2 cells; data not shown) augmented significantly in α-GalCer-treated wild-type mice when compared with the vehicle-treated mice (Fig. 4C and Fig. S2), and their restimulation in vitro is associated with elevated levels of the Th2-associated cytokine IL-5 (Fig. 4D). By contrast, α-GalCer treatment of Jα18−/− mice had no effect on the frequency of Th2 cells and their associated cytokines (Fig. 4 C and D), indicating that the Th2 bias seen in wild-type mice depended on iNKT cells. Interestingly, an enhanced production of IL-10 was observed in cultures from α-GalCer-treated mice (Fig. 4E) irrespective of MOG35–55 restimulation. In addition, no detectable increase in the frequency of IL-10-producing MOG-specific 2D2 T cells (Fig. S2) was observed, suggesting that iNKT cells can promote IL-10 production by non-T cells.
To assess whether IL-4 and IL-10 contributed to the inhibition of Th1 and Th17 differentiation by the in vivo α-GalCer treatment, we neutralized the effects of both IL-4 and IL-10 with mAbs injected i.p. Under these cytokine-blocking conditions, the proportion of Th1 2D2 cells tripled and the regulatory effect of iNKT cell activation by α-GalCer on Th1 cells was fully blocked (Fig. 4F and Fig. S3A). Concomitantly, the proportion of Th17 cells increased. Nevertheless, α-GalCer treatment still significantly limited the Th17 response (Fig. 4G and Fig. S3A). Given the capacity of iNKT cells to produce copious amounts of IFN-γ (22), the increase in IFN-γ-producing Th1 cells after IL-4 and IL-10R neutralization, and the capacity of IFN-γ to antagonize Th17 differentiation (13, 14), we asked whether IFN-γ could be an additional factor involved in the regulation of Th17 commitment by iNKT cells. Neutralizing IFN-γ in vivo doubled the proportion of Th17 2D2 CD4+ T cells in both PBS and α-GalCer-treated mice (Fig. S3B). Nevertheless, α-GalCer remained efficient in reducing the frequency of Th17 cells, indicating that IFN-γ is necessary but not sufficient for the iNKT-cell-mediated regulation of the Th17 response (Fig. S3A). Importantly, neutralizing IL-4, IL-10, and IFN-γ simultaneously tripled the proportion of Th17 cells and fully abrogated the capacity of α-GalCer to inhibit Th17 commitment (Fig. 4H and Fig. S3A). Blocking IL-4 + IL-10R and/or IFN-γ also prevented the EAE-protective effect of α-GalCer, rendering these mice susceptible to severe disease (Table S1). We conclude that iNKT-cell-induced IL-4 and IL-10 production mediates the inhibition of the Th1 response, whereas the regulation of Th17 commitment by iNKT cells requires IL-4, IL-10, and IFN-γ.
INKT Cells Regulate the Th17 Compartment at Steady State.
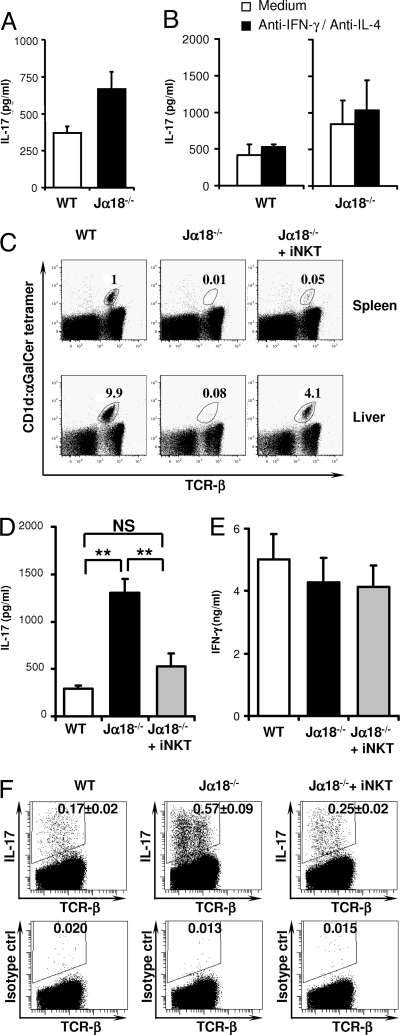
Having established that iNKT cells control Th17 differentiation, we aimed to assess whether this cellular mechanism is of importance in controlling the Th17 compartment under steady-state conditions in vivo. To this end, we compared IL-17 production by Ag-experienced CD4+ T cells from wild-type and Jα18−/− mice in vitro. Splenic CD62L− CD4+ T cells were purified by FACS sorting. In addition, using the CD1d-αGalCer tetramer we eliminated iNKT cells to exclude their potential in vitro effects. Polyclonal activation in vitro revealed a 2-fold enhancement of IL-17 production when the Ag-experienced T cells were isolated from Jα18−/− mice when compared with those isolated from wild-type mice (Fig. 5A). No influence on the IFN-γ response was observed (cells from wild-type mice produced 3,150 pg/ml IFN-γ, those from Jα18−/− mice produced 3,155 pg/ml IFN-γ). IL-17 production by Ag-experienced CD4+ T cells was not affected by neutralization of IL-4 and IFN-γ in vitro (Fig. 5B), consistent with the reported resistance of committed Th17 cells to IL-4- and IFN-γ-mediated regulation (13, 14). These findings suggest that iNKT cells control the Th17 compartment in vivo at steady state as they limit the IL-17-producing capacity of Ag-experienced CD4+ T cells.
Fig. 5.
iNKT cells inhibit IL-17 production by CD4+ T cells at steady state. (A) CD62L− α-GalCer-CD1d tetramerneg CD4+ T cells from wild-type (WT) or Jα18−/− C57BL/6 mice were sorted by FACS and stimulated with plate-bound anti-CD3 and anti-CD28 mAbs and IL-23. IL-17 levels were measured after 72 h. (B) As in (A) but in the presence or absence of neutralizing mAbs against IL-4 and IFN-γ. These mAbs failed to significantly enhance IL-17 production by Ag-experienced CD4+ T cells. Data are from 3 (A) or 2 (B) individual experiments using 2–4 pooled spleens per group. (C) Jα18−/− recipient mice received thymic iNKT cells (Jα18−/− + iNKT), and 3 weeks later the proportion of the CD1d:α-GalCer tetramer+ TCRβ+ (iNKT) cells (gated on mononuclear cells) in the spleen (Upper) and liver (Lower) of recipient mice was compared with that of the control wild-type (WT) and nonreconstituted Jα18−/− mice. A representative FACS plot is shown for each group. (D–G) Jα18−/− recipient mice received thymic iNKT cells as in (C). Three weeks later, MACS-purified splenic CD4+ T cells from the recipient and control mice were cultured with plate-bound anti-CD3 and anti-CD28 mAbs and IL-23. IL-17 (D) and IFN-γ (E) secretion were measured 72 h later in cultures derived from recipient (Jα18−/− + iNKT, n = 6) and control (WT, n = 6; Jα18−/−, n = 6) mice. The frequency of Th17 cells (F) was assessed under identical conditions in cultures derived from iNKT-reconstituted (Jα18−/− + iNKT, n = 2) and control (WT, n = 4; Jα18−/−, n = 6) mice. Data represent the mean ± SEM of n individual mice per group.
To investigate this novel function of iNKT cells in vivo, we reconstituted iNKT-deficient nonirradiated Jα18−/− mice with iNKT cells by adoptive transfer of 106 CD1d:α-GalCer tetramer-positive TCR-αβ thymocytes from wild-type mice. We assessed the impact of this iNKT cell reconstitution on the Th17 population after 21 days, a period that is probably insufficient for the Th17 compartment to be replenished by de novo activated naïve T cells at steady state. Thus, the data likely reflect the impact of the introduced iNKT cells on preexisting Ag-experienced cells. CD1d:α-GalCer tetramer staining revealed efficient grafting of wild-type iNKT cells in Jα18−/− mice as indicated by the 4% frequency of CD1d:α-GalCer tetramer-positive cells among liver mononuclear cells (Fig. 5C). Strikingly, this grafting of wild-type iNKT cells fully reversed the enhanced IL-17 production by CD4+ T cells from Jα18−/− mice (Fig. 5D), demonstrating that iNKT cells control the Th17 compartment at steady state. This effect was specific for the Th17 lineage because we observed no alteration in IFN-γ production by the Th1 compartment (Fig. 5E). No reciprocal induction of Foxp3+ regulatory T cells was observed after iNKT-cell reconstitution (data not shown). To address whether the transferred iNKT cells impacted the frequency of Th17 cells in vivo we enumerated the proportion of IL-17-producing CD4+ T cells using intracellular cytokine staining. The reconstitution of iNKT cells reduced the frequency of Th17 cells almost to the level observed in unmanipulated WT mice (Fig. 5F). These splenic CD4+ T-cell cultures from reconstituted mice express on average 0.05% of iNKT cells (Fig. 5C), making an in vitro effect by iNKT cells unlikely. As such, our findings reveal a previously undescribed function for iNKT cells in regulating the size of the Th17 pool in vivo at steady state.
Conclusions.
Th17 cells contribute to the host defense against extracellular pathogens, but excessive Th17 responses or those targeting self-antigens can provoke severe tissue injury (2, 36). Mechanisms controlling the Th17 subset might therefore be important to preserve the integrity of tissues. Our study reveals the importance of iNKT cells in regulating Th17 lineage commitment and persistence. We show that activation of iNKT cells with α-GalCer during priming of the CD4+ T-cell response prevents the differentiation of naïve CD4+ T cells toward the Th17 lineage without impairing T-cell expansion. The mechanism used by iNKT cells to abrogate Th17 commitment by naïve CD4+ T cells requires IL-4, IL-10, and IFN-γ. No reciprocal influence on Foxp3+ T cells was observed. After α-GalCer treatment IL-4 expression is likely initiated by iNKT cells and sustained by autoreactive Th2 cells that regulated the Th17 response. α-GalCer activation of iNKT cells and the ensuing NK response are expected sources of IFN-γ, with a moderate impact of IFN-γ by the conventional Th1 response due to its efficient inhibition by α-GalCer. Our data, therefore, suggest an important role for innate IFN-γ in regulating Th17 development. These mechanisms were effective in limiting CNS tissue damage in the paralytic mouse model of EAE, in which autoimmune Th1 and Th17 responses contribute to demyelinating lesions in the CNS (32, 33). This observation might also be of relevance for microbial infections where iNKT cells are activated by either pathogen-derived or endogenous glycolipid antigens presented in the hydrophobic groove of CD1d (37–39). It is conceivable that the activation of iNKT cells during infections might similarly influence T-cell differentiation and prevent the induction of an excessive Th17 response, thereby limiting secondary tissue damage.
Importantly, we show that iNKT cells also impact on the Th17 pool in vivo in unmanipulated mice. We reveal that iNKT cells restrain IL-17 production by CD4+ T cells in both C57BL/6 and autoimmune-prone NOD mice. Strikingly, the enhanced IL-17 production observed in iNKT-cell-deficient mice was fully reversed by the transfer of thymic iNKT cells. This was associated with the capacity of the transferred iNKT cells to reduce the frequency of Th17 cells in vivo, restoring a Th17 frequency similar to that observed in WT mice. Because no effect on IFN-γ production by antigen-experienced Th1 cells was observed, it is assumed that iNKT cells specifically control the persistence of Ag-experienced Th17 cells. Under steady-state conditions, iNKT cells are activated and functionally engage dendritic cells and B cells (40, 41), which is thought to be of importance to prepare the host for future pathogen encounter. Our findings suggest that, in addition, iNKT cells impact on Ag-experienced CD4+ T cells at steady state, implying a role for iNKT cells in shaping the T-cell response before pathogen exposure. Given the role of the Th17 cell subset in inflammatory tissue damage, its confinement by iNKT cells might provide a barrier that limits predisposition to inflammatory diseases and supports iNKT cells as a therapeutic target in Th17 mediated diseases (42).
Materials and Methods
Mice, EAE Induction, and in Vivo Treatments.
C57BL/6 mice (Charles River Laboratories), CD1d−/− mice (43), Jα18−/− mice (44), 2D2 MOG-TCR mice (45), and NOD mice were housed under specific pathogen-free conditions. All experimental protocols were approved by the local ethics committee and are in compliance with European Union guidelines. EAE was induced by s.c. immunization with 50 μg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK; Mimotopes) emulsified in CFA (Difco) and 2 i.v. injections of pertussis toxin (day 0, 200 ng; day 2, 400 ng; List Biological Laboratories). Disease severity was monitored daily (28). Where indicated, mice were treated with two 4-μg doses of α-GalCer (Kirin Brewery) on day 0 (emulsified in the MOG35–55 CFA mixture) and day 4 (i.p.) or with anti-IL-4 (11B11), anti-IL10R (1B1), or anti-IFN-γ (XMG1.2) mAbs injected i.p. at a dose of 0.5 mg/mouse on days −3, −2, 0, 2, 5, and 8 for 2D2 differentiation, or days −2, −1, 0, 3, (0.5 mg/mouse) and 5, 9, 13, 17, and 21 (0.15 mg/mouse) for EAE.
T-Cell Purification and Adoptive Transfer.
Splenic CD4+ T cells were isolated (>94% pure) by MACS positive selection (Miltenyi Biotec). Subsequently, conventional Ag-experienced CD4+ (CD1d:α-GalCer tetramerneg CD62L− CD4+) T cells were isolated by FACS (>97% pure). MOG-specific naïve CD62L+ CD25− CD4+ cells were purified from CD45.1 congenic 2D2 mice by MACS negative selection (Miltenyi Biotec) by using anti-CD8 (YTS169), anti-B220 (RA3–6B2), anti-Mac1 (CI:A3–1), and anti-CD25 (7D4) mAbs followed by positive selection with anti-CD62L-coated beads, providing >80% CD4+ T cells, of which 95% expressed the transgenic 2D2 TCR. For reconstitution studies, freshly isolated thymocytes were enriched for iNKT cells by CD8 depletion (Invitrogen Life Technology); 3%−5% of recovered thymocytes were CD1d:α-GalCer tetramer+ TCRβ+ iNKT cells. The equivalent of 1.0 × 106 iNKT cells was adoptively transferred i.v. into Jα18−/− mice.
Intracellular and Intranuclear Cytokine Staining and Flow Cytometry.
Single-cell suspensions from LNs (0.5 × 106/well) or spleen (3 × 106/well) were stimulated for 4 h with PMA (0.5 μg/ml), ionomycin (1 μg/ml; Sigma), and GolgiPlug™ (BD Biosciences). After FcγR blockade (mAb 2.4G2), cells were stained with fluorescently labeled mAbs: anti-TCR Vα3.2 (RR3–16), anti-CD4 (RM4–5), anti-CD45.1 (A20), and anti-cytokine mAbs against IL-10 (JES5–16E3), IFN-γ (XMG1.2), IL-17 (TC11–18H10), IL-4 (BVD4–1D11), or IgG1 isotype control (A110–1) using a Cytofix/Cytoperm™ Plus Kit (BD Biosciences) or an intranuclear Foxp3 detection kit (eBiosciences). Labeled cells were analyzed with a LSRII flow cytometer (Becton Dickinson) using the BD FACS-Diva software. Cell sorting was performed using a FACSAria Flow Cytometer (Becton Dickinson).
In Vitro Cytokine Production.
2.0 × 105 CD4+ T cells or 0.5 × 105 Ag-experienced CD4+ T cells were cultured with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (10 μg/ml) mAbs (BD Biosciences) and IL-23 (10 ng/ml; R&D Systems). When indicated, anti-IFN-γ (10 μg/ml) and anti-IL-4 (10 μg/ml) mAbs (BD Biosciences) were added. For in vitro recall responses, single-cell suspensions of LN cells (0.5 × 106/well) or splenocytes (1 × 106/well) were cultured with the indicated stimuli. Supernatants were analyzed for IFN-γ, IL-17A, or IL-4 by Duoset ELISA (R&D Systems) or tested for 10 different cytokines (IFN-γ, TNF-α, CXCL10, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, and IL-17A) by using LINCOplex Multiplex immunoassays and Luminex instrumentation (Millipore).
Statistical Analyses.
Data are presented as means of n individual mice per group ± SEM, unless otherwise stated. The Mann-Whitney U test was used, except for cytokine production, which was analyzed using the unpaired Student t test. Kinetics of EAE development was analyzed using the log rank test; EAE incidence and mortality using a χ2 test; and severity using the Mann-Whitney U test. *, P < 0.05, **, P < 0.01, ***, P < 0.001.
Supplementary Material
Acknowledgments.
The authors thank Kirin Brewery for KRN7000 (α-GalCer); the NIH tetramer facility and M. Kronenberg and M. Leite-de-Moraes for the CD1d:α-GalCer tetramer; M. Taniguchi for the Jα18−/− mice; V. Kuchroo for the 2D2 mice; and L. Van Kaer for the CD1d−/− mice. We also thank A. Saoudi and D. Dunia for their feedback during the study, and A. Estival and A. Desquesnes (Genopole, IFR31) for the Luminex analyses. Financial support came from Institut National de la Santé et de la Recherche Médicale, Association pour la Recherche sur la Sclerose en Plaques, Agence Nationale de la Recherche (Grant ANR-06-MIME), European Union (Neuropromise Grant SHM-CT_2005–018637), the Midi-Pyrenees region, Centre National de la Recherche Scientifique, Chancellerie des Universités de Paris (Legs Poix), Ministère de la Recherche and the Vietnamese government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809317106/DCSupplemental.
References
- 1.Mosmann TR. Directional release of lymphokines from T cells. Immunol Today. 1988;9(10):306–307. doi: 10.1016/0167-5699(88)91323-0. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 7.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 11.Mehta DS, Wurster AL, Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004;202:84–95. doi: 10.1111/j.0105-2896.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 13.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27(4):660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Schnare M, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2(10):947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 17.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 18.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada M, et al. IL-21-induced Bepsilon cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med. 2006;203(13):2929–2937. doi: 10.1084/jem.20062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci USA. 2008;105(32):11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rachitskaya AV, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180(8):5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 24.Singh AK, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahng AW, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furlan R, et al. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35–55-induced EAE: Critical roles for administration route and IFN-gamma. Eur J Immunol. 2003;33(7):1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270(5243):1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 28.Mars LT, et al. Cutting edge: V alpha 14-J alpha 281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J Immunol. 2002;168(12):6007–6011. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- 29.Grajewski RS, et al. Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by a mechanism involving innate IFN-gamma production and dampening of the adaptive Th1 and Th17 responses. J Immunol. 2008;181(7):4791–4797. doi: 10.4049/jimmunol.181.7.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179(4):1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205(7):1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 35.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burchill MA, et al. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun. 2003;71(6):3437–3442. doi: 10.1128/IAI.71.6.3437-3442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 38.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 39.Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 40.Galli G, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197(8):1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent MS, et al. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3(12):1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 42.Mars LT, et al. Therapeutic manipulation of iNKT cells in autoimmunity: Modes of action and potential risks. Trends Immunol. 2004;25(9):471–476. doi: 10.1016/j.it.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Mendiratta SK, et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6(4):469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 44.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 45.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197(9):1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.