Abstract
Protease-activated receptor-1 (PAR1) is a G-protein–coupled receptor uniquely activated by proteolysis. Thrombin, a coagulant protease, induces inflammatory responses and endothelial barrier permeability through the activation of PAR1. Activated protein C (APC), an anti-coagulant protease, also activates PAR1. However, unlike thrombin, APC elicits anti-inflammatory responses and protects against endothelial barrier dysfunction induced by thrombin. We found that thrombin and APC signaling were lost in PAR1-deficient endothelial cells, indicating that PAR1 is the major effector of protease signaling. To delineate the mechanism responsible for protease-selective signaling by PAR1, we examined the effect of APC and thrombin on the activation of RhoA and Rac1, small GTPases that differentially regulate endothelial barrier permeability. Thrombin caused robust RhoA signaling but not Rac1 activation, whereas APC stimulated a marked increase in Rac1 activation but not RhoA signaling, consistent with the opposing functions of these proteases on endothelial barrier integrity. Strikingly, APC signaling and endothelial barrier protection effects were abolished in cells lacking caveolin-1, whereas thrombin signaling remained intact. These findings suggest that compartmentalization of PAR1 in caveolae is critical for APC selective signaling to Rac1 activation and endothelial barrier protection. We further report that APC induces PAR1 phosphorylation and desensitizes endothelial cells to thrombin signaling but promotes limited receptor cleavage and negligible internalization and degradation even after prolonged APC exposure. Thus, APC selective signaling and endothelial barrier protective effects are mediated through compartmentalization of PAR1 in caveolae and a novel mechanism of PAR1 signal regulation.
Keywords: APC, endothelial, GPCR, Rac1, thrombin
The coagulant protease thrombin is generated in response to vascular injury and in thrombotic disease and drives fibrin deposition and platelet activation, which are critical for hemostasis and thrombosis (1). Thrombin also promotes pro-inflammatory responses and disrupts endothelial barrier permeability (2). Protease-activated receptor–1 (PAR1), a G-protein–coupled receptor (GPCR), is the predominant mediator of thrombin responses in cells. Thrombin activates PAR1 by cleaving the N-terminal domain, generating a new N terminus that binds intramolecularly to the receptor to trigger transmembrane signaling (3). Synthetic peptides that represent the newly formed N terminus of PAR1 can activate the receptor independent of thrombin and proteolytic cleavage. Interestingly, thrombin and peptide agonists differ in their capacity to promote endothelial barrier permeability and Ca2+ mobilization (4). These studies suggest that distinct cellular responses can be evoked by the same receptor when activated proteolytically by the tethered ligand versus untethered “free” synthetic peptide agonists. Similar phenomena have been reported for other GPCRs activated by different ligands and is a poorly understood process termed functional selectivity or biased agonism (5).
Activated protein C (APC), an anti-coagulant protease, displays cytoprotective and anti-inflammatory activities and has been shown clinically to reduce mortality of patients with severe sepsis (6). APC is generated on the endothelial cell surface via activation of protein C (PC) by the thrombin-thrombomodulin complex (7). APC bound to endothelial protein C receptor (EPCR) cleaves and inactivates factors Va and VIIa diminishing thrombin generation and induces cellular responses through the activation of PAR1 (8, 9). In contrast to thrombin, however, APC elicits anti-inflammatory responses and promotes endothelial barrier stabilization (10, 11). The mechanism by which APC exerts anti-inflammatory and cytoprotective signaling in endothelial cells is not fully understood.
Previous studies suggest that most endogenous PC is bound to EPCR on the endothelial cell surface and cleaved by the thrombin–thrombomodulin complex (12). The newly formed APC is then poised to signal via direct activation of PAR1. Thus, APC generation on the endothelial cell surface is linked mechanistically to PAR1 protective signaling. The cytoprotective and anti-inflammatory responses induced by APC also require the co-factor function of EPCR (9, 13). Interestingly, thrombomodulin, EPCR and PAR1 partition into lipid rafts and co-fractionate with caveolin-1, a structural protein essential for caveolae formation in endothelial cells (14), suggesting that these proteins reside at least partially in caveolar microdomains, a subtype of lipid rafts (15, 16). However, whether caveolae are required for APC activation of PAR1 signaling and endothelial barrier protective effects is not known.
Our studies here reveal a critical role for caveolae in APC, but not thrombin, activation of PAR1 signaling and endothelial barrier protection. These findings demonstrate an essential role for caveolae in agonist selective signaling by PAR1. We further show that APC promotes protective effects in endothelial cells by desensitizing cells to thrombin signaling. Remarkably, however, APC causes limited PAR1 cleavage and negligible internalization and degradation, suggesting a novel mechanism for regulation of PAR1 signaling.
Results
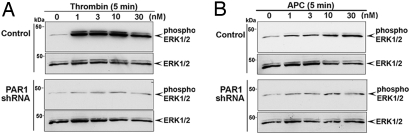
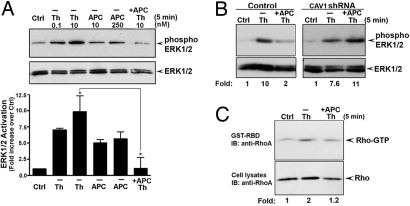
To determine whether PAR1 is essential for protease-selective signaling, we examined thrombin and APC signaling in human endothelial cells stably expressing a PAR1-specific shRNA (17). Endothelial cells expressing PAR1 shRNA displayed minimal PAR1 expression and signaling, whereas PAR2 signaling remained intact [supporting information (SI) Fig. S1], indicating loss of functional PAR1. In control cells, thrombin and APC induced a similar biphasic increase in extracellular signal–regulated kinase 1/2 (ERK1/2) activity (Fig. S2A). However, cells lacking PAR1 displayed minimal ERK1/2 activation in response to various concentrations of thrombin or APC compared with control cells (Fig. 1, S3, and S4). Thrombin-stimulated p38 MAP kinase activation was also lost in cells deficient in PAR1 expression (Fig. S2C). In contrast to thrombin, APC failed to stimulate p38 MAP kinase signaling in either control or PAR1-deficient cells (Fig. S2B). These findings suggest that PAR1, and not another receptor or factor, is critical for thrombin and APC signaling in endothelial cells.
Fig. 1.
PAR1 is essential for thrombin and APC signaling in endothelial cells. (A and B) Serum-deprived control and PAR1 shRNA-expressing EA.hy926 endothelial cells were incubated with thrombin or APC (0.5 U/ml hirudin) for 5 minutes at 37 °C. ERK1/2 activity was determined by immunoblotting and quantitated as described in Fig. S3. Data are representative of three independent experiments.
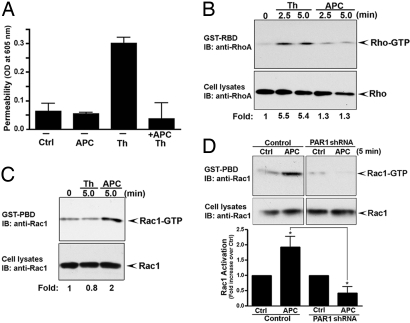
We next examined endothelial barrier permeability. Thrombin-stimulated endothelial barrier permeability was blocked in cells preincubated with APC (Fig. 2A). These findings are consistent with a role for APC in stabilization of endothelial cell–cell junctions and protection against endothelial barrier dysfunction induced by thrombin (10). We also evaluated the effect of thrombin and APC on the activation of endogenous RhoA and Rac1. Activation of RhoA promotes endothelial barrier dysfunction, whereas Rac1 signaling has been implicated in endothelial barrier stabilization (2). Thrombin induced RhoA activation but not Rac1 signaling (Figs. 2B and 2C). In contrast, APC stimulated Rac1 activation and minimal RhoA signaling (Figs. 2B and 2C). Thus, thrombin and APC have the capacity to elicit distinct signaling pathways and to differentially regulate endothelial barrier permeability. Moreover, APC-stimulated increase in Rac1 activation was lost in endothelial cells deficient in PAR1 expression (Fig. 2D). These studies demonstrate that endogenous PAR1 is essential for APC signaling and raise the question of how activation of the same receptor by two different proteases elicits distinct signaling responses in endothelial cells.
Fig. 2.
Thrombin and APC differentially regulate endothelial barrier permeability and Rho activation. (A) Confluent EA.hy926 cells were preincubated with or without 10 nM APC for 3 hours at 37 °C in medium containing 0.5 U/ml hirudin, and then treated with 10 nM APC or 10 nM thrombin (Th) for 20 minutes at 37 °C. Endothelial barrier permeability was as described in SI Methods (10). Data (mean ± SD, n = 3) are representative of three independent experiments. (B and C) Cells were incubated with or without 10 nM thrombin (Th) or 10 nM APC (0.5 U/ml hirudin) at 37 °C. Cells were lysed, and activated RhoA and Rac1 were detected by immunoblotting. Data are representative of three separate experiments. (D) Control and PAR1-deficient endothelial cells were incubated with or without 10 nM APC for 5 minutes at 37 °C, and activation of Rac1 was determined. Data (mean ± SE) are expressed as the fold-increase over untreated control and are the averages of three independent experiments. The difference between Rac1 activation induced by APC in control versus PAR1-deficient cells was significant (* P < 0.05).
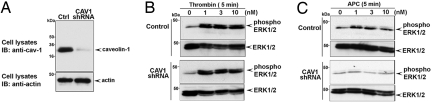
PAR1 and EPCR localize to lipid rafts and associate with caveolin-1 (15), but whether caveolae are essential for APC signaling and endothelial barrier protection has not been determined. To examine the role of caveolae in thrombin and APC signaling, we generated endothelial cells stably expressing a caveolin-1 shRNA to ablate caveolin-1 expression (Fig. 3A) (18). Importantly, the amount of cell surface PAR1 and EPCR was similar in control and caveolin-1–lacking cells (Fig. S5), suggesting that caveolae deficiency does not globally disrupt protein expression at the cell surface. Interestingly, thrombin activation of ERK1/2 was comparable in control and caveolin-1–deficient endothelial cells (Figs. 3B and S6), indicating that caveolae are not essential for thrombin signaling. Remarkably, however, activation of ERK1/2 by APC was lost in caveolin-1–deficient endothelial cells examined at early and late times (Figs. 3C, S6, and S7). These findings suggest that caveolae are critical for activation of PAR1 signaling by APC but not thrombin.
Fig. 3.
Caveolin-1 is critical for APC but not thrombin, activation of PAR1 signaling. (A) Control and caveolin-1 (CAV1) shRNA expressing EA.hy926 cell lysates were immunoblotted with an anti–caveolin-1 antibody. (B and C) Control and caveolin-1 (CAV1)–deficient cells were incubated with thrombin or APC for 5 minutes at 37 °C, and activation of ERK1/2 was examined by immunoblotting and quantitated as shown in Fig. S6. Data are representative of three independent experiments.
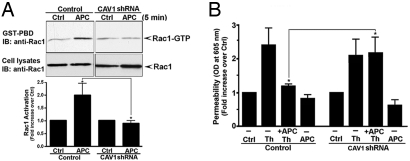
We next investigated the function of caveolae in APC-induced Rac1 activation and endothelial barrier protection. APC stimulated a marked increase in Rac1 activation in control cells that was virtually abolished in endothelial cells lacking caveolin-1 (Fig. 4A). These findings provide further evidence that caveolae are essential for APC activation of PAR1 signaling. Moreover, APC pretreatment failed to protect caveolin-1–deficient endothelial cells from thrombin-induced endothelial barrier permeability (Fig. 4B), consistent with loss of APC signaling in caveolin-1–defective cells. Thus, the compartmentalization of PAR1 in caveolae is essential for APC activation of PAR1 protective signaling in endothelial cells.
Fig. 4.
Caveolin-1 is essential for APC-induced Rac1 activation and endothelial barrier protection. (A) Control and caveolin-1 (CAV1) deficient cells were incubated with or without 10 nM APC at 37 °C, and Rac1 activation was determined. Data (mean ± SE) are expressed as fold-increase over control and are the averages of three different experiments. The difference between Rac1 activation induced by APC in control versus CAV-1 deficient cells was significant (* P < 0.05). (B) Control and CAV-1–deficient cells were treated with or without 10 nM APC for 3 hours at 37 °C and then incubated with 10 nM thrombin (Th) or 10 nM APC for 10 minutes at 37 °C, and permeability was monitored. Data (mean ± SE) are the averages of three independent experiments performed in triplicate. The difference between thrombin-induced permeability in control versus CAV-1–deficient cells was significant (* P < 0.05).
To determine how APC prevents thrombin from causing endothelial barrier dysfunction, we examined whether APC desensitizes cells to thrombin signaling. Thrombin caused robust ERK1/2 activation in naïve cells (Fig. 5A). By contrast, thrombin-stimulated ERK1/2 activation was markedly reduced in cells pretreated with APC (Fig. 5A). Interestingly, inhibition of thrombin-induced ERK1/2 activation by APC required caveolin-1 expression (Fig. 5B), consistent with a critical role for caveolae in APC signaling. Moreover, thrombin-induced RhoA activation and p38 kinase activation were considerably attenuated in cells pretreated with APC (Figs. 5C and S8), providing further evidence that APC desensitizes cells to thrombin signaling to promote endothelial barrier protection. Signaling by PAR2 agonist peptide, and UTP, an agonist for endogenous purinergic receptors, was unperturbed by APC pretreatment, indicating that endothelial cells are generally responsive to GPCR activation after APC pretreatment (data not shown).
Fig. 5.
APC desensitizes PAR1 to thrombin signaling. (A) EA.hy926 cells were preincubated with or without 10 nM APC for 1 hour at 37 °C and then stimulated with thrombin (Th) or APC at 37 °C; ERK1/2 activation was examined by immunoblotting. Data (mean ± SE) are expressed as the fold-increase over untreated control and are the averages of three separate experiments. The difference between thrombin-induced ERK1/2 activation in untreated versus APC-pretreated cells was significant (* P < 0.05). (B) Control and caveolin-1 (CAV1)–deficient cells were preincubated with 10 nM APC for 1 hour at 37 °C and then stimulated with or without 10 nM thrombin (Th) at 37 °C, and ERK1/2 activation was determined. (C) Cells were pretreated with or without 10 nM APC for 1 hour at 37 °C and then stimulated with or without 10 nM thrombin (Th) at 37 °C, and activated RhoA was examined. Similar findings were observed in two independent experiments.
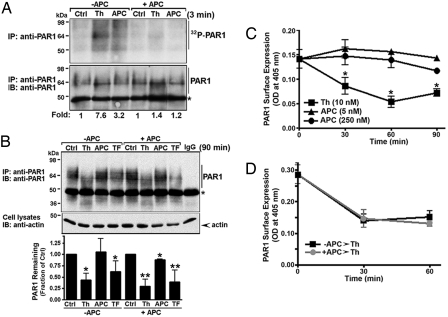
Agonist-induced PAR1 phosphorylation initiates receptor desensitization (19, 20). Thus, we determined whether APC promotes phosphorylation of endogenous PAR1 in endothelial cells. Phosphorylated PAR1 was detected after thrombin incubation and migrated as a broad band at ≈64 kDa (Fig. 6A) (21). Cells exposed to APC also showed an increase in PAR1 phosphorylation, which was detected as multiple high–molecular-weight species migrating at and above ≈64 kDa (Fig. 6A). Strikingly, in APC-pretreated cells, thrombin and APC failed to induce phosphorylation of PAR1 (Fig. 6A). These data suggest the APC regulates thrombin signaling at the level of the receptor to promote endothelial barrier protection.
Fig. 6.
APC stimulates PAR1 phosphorylation but not internalization or degradation. (A) EA.hy926 cells labeled with [32P]orthophosphate were preincubated with or without 10 nM APC for 3 hours at 37 °C and then stimulated with 10 nM Th or 10 nM APC for 3 minutes at 37 °C. Immunoprecipitated 32P-labeled PAR1 was detected as described (21). Similar results were observed in three independent experiments. (B) Cells pretreated with or without 10 nM APC for 3 hours at 37 °C, and then incubated with 10 nM thrombin (Th), 10 nM APC, or 100 μM TFLLRNPNDK for 90 minutes at 37 °C and the amount of PAR1 was determined as described (21). Asterisk indicates detection of the heavy and light chains of the immunoprecipitating antibodies. Data (mean ± SE) are expressed as the fraction of PAR1 protein remaining compared with untreated control and are the averages of three independent experiments. A significant difference (* P < 0.05 or ** P < 0.01) was detected between agonist-treated versus untreated controls in some cases. (C) Cells were incubated in the absence or presence of thrombin (Th) or APC for various times at 37 °C and the amount of PAR1 remaining on the cell surface was quantitated by enzyme-linked immunosorbent assay (ELISA). Data (mean ± SD, n = 3) are representative of three independent experiments. The difference between thrombin and untreated control at various times was significant (* P < 0.05). (D) Cells were preincubated with or without 10 nM APC for 3 hours at 37 °C, washed, and then treated with or without 10 nM thrombin (Th) for various times at 37 °C. Cells were fixed and processed, and the amount of PAR1 remaining on the cell surface was measured by ELISA. Data (mean ± SD, n = 3) are from one representative experiment.
In addition to phosphorylation, receptor internalization and lysosomal degradation also regulate PAR1 signaling (22). We therefore examined whether APC effects PAR1 trafficking. Thrombin induced rapid and robust PAR1 internalization (Fig. 6C). In contrast, APC failed to promote PAR1 internalization even at high concentrations (Fig. 6C), consistent with retention of PAR1 on the cell surface (23). Remarkably, however, thrombin stimulated comparable increases in PAR1 internalization in both untreated and APC treated cells (Fig. 6D), suggesting that APC exposure does not prevent thrombin-induced PAR1 internalization.
We further investigated whether thrombin promotes PAR1 degradation in cells exposed to APC. Thrombin caused a shift in mobility and a significant loss of PAR1 protein in control cells (Fig. 6B), consistent with thrombin cleavage and degradation of activated PAR1. Activation of PAR1 with the peptide agonist TFLLRNPDNK also decreased receptor protein without altering receptor mobility, as expected (Fig. 6B). To our surprise, prolonged exposure to APC failed to induce a significant change in PAR1 mobility or amount of receptor protein detected compared to control cells (Fig. 6B). Moreover, the extent of PAR1 degradation induced by thrombin and peptide agonist was comparable in APC-treated and untreated cells (Fig. 6B). Together these studies suggest that APC desensitizes cells to thrombin signaling by inducing PAR1 phosphorylation, but causes limited receptor cleavage and negligible internalization and degradation.
Discussion
In the present study, we define a novel function for caveolae in protease-selective signaling by PAR1. We show that endogenous PAR1 is required for thrombin and APC signaling in endothelial cells. We further demonstrate that caveolin-1 is essential for activation of PAR1 signaling by APC but not thrombin, indicating that caveolae are critical for protease-selective signaling by PAR1. Caveolae are also required for activation of PAR2 by tissue factor-factor VIIa but not the synthetic peptide agonist in transformed cells (24), consistent with a role for caveolae in protease-selective signaling. Moreover, a function for lipid rafts but not caveolae, in thrombin-induced cytoskeletal changes in endothelial cells has previously been reported (25). The cytoprotective and endothelial barrier protective effects of APC are also disrupted by methyl-β-cyclodextrin, a cholesterol-chelating agent, indicating a role for lipid rafts (16). However, the function of caveolae, a subtype of lipid rafts, in the regulation of APC signaling has not been previously examined. Our studies suggest that APC promotes protective effects by desensitizing endothelial cells to thrombin signaling. We found that APC stimulates PAR1 phosphorylation and inhibits thrombin signaling but causes limited receptor cleavage, and negligible internalization and degradation. Together these findings suggest that APC-mediated endothelial protective signaling requires compartmentalization of PAR1 in caveolae and a novel mechanism of PAR1 desensitization to control receptor signaling.
The molecular mechanism by which APC distinctly activates PAR1 signaling remains unclear. PAR1 is essential for APC signaling, but whether APC induces an active PAR1 conformation similar to thrombin is not known. Previous studies have shown that APC has the capacity to cleave PAR1, albeit with considerably less efficiency than thrombin (9, 26). However, whether APC cleavage of PAR1 is the only critical determinant that facilitates PAR1 activation of barrier protective signaling is not known. Thrombin cleaves the majority of PAR1, causing a shift in receptor mobility and induces receptor degradation, whereas the peptide agonist TFLLRNPNDK promotes PAR1 degradation but not cleavage and hence does not alter receptor mobility (Fig. 6). We show here that APC induces PAR1 signaling and phosphorylation but causes a minimal change in receptor mobility. However, signaling by catalytically inactive APC is markedly inhibited compared to fully active APC (Fig. S9), suggesting that activation of PAR1 by APC requires proteolytic activity. Interestingly, we also show that prolonged APC incubation does not prevent thrombin-induced PAR1 cleavage, internalization, or degradation. Thus, in endothelial cells exposed to APC, the majority of PAR1 is retained on the cell surface and susceptible to thrombin cleavage. Our findings raise the intriguing possibility that APC activates a subpopulation of PAR1 compartmentalized in caveolae and stabilizes an active receptor conformation that may be distinct from noncaveolar localized activated PAR1.
How can activation of the same receptor by two different proteases elicit distinct cellular responses? If APC activates PAR1 through cleavage and unmasking of the tethered ligand like thrombin a similar active receptor conformation would be induced. However, the extent of PAR1 activation by APC would be different from thrombin, which efficiently cleaves the receptor (26). In this case, we would expect to observe quantitative, not qualitative, differences in signaling. Thrombin-activated PAR1 couples to Gαq, Gα12/13, and RhoA signaling, which induces endothelial barrier dysfunction (2). In contrast, we show that APC-activated PAR1 stimulates Rac1, but not RhoA, signaling and promotes endothelial barrier protection. Thus, the activation of PAR1 by APC may result in a distinct active receptor conformation that selectively couples to different signaling pathways. We further show that caveolin-1 is essential for APC but not thrombin activation of PAR1 signaling and endothelial barrier protective effects, suggesting that PAR1 localization to caveolae is critical for protease-selective signaling. Previous studies have shown that the APC co-factor EPCR, PAR1, Gαq and Gαi partition into lipid rafts and interact with caveolin-1 (15, 16, 27). The barrier protective signaling induced by APC is also blocked by pertussis toxin, suggesting a role for Gαi/o proteins in this process (15). Moreover, the binding of APC to EPCR facilitates efficient PAR1 cleavage in lipid rafts and endothelial barrier signaling, suggesting that caveolae localization may induce a distinct active receptor conformation that elicits barrier protective signaling (28). Thus, PAR1, EPCR, and Gαi/o proteins localize to caveolae and may exist as a preassembled complex poised to signal after PC binding to EPCR and generation of APC. In contrast, caveolin-1 is not essential for thrombin activation of PAR1 signaling (Figs. 3 and 4), indicating that caveolin-1 modulates PAR1 signaling only when selectively activated by APC and not by thrombin in endothelial cells.
Our studies provide new insight into the molecular mechanisms responsible for protease-selective signaling by PAR1. Evidence presented here suggests that compartmentalization of PAR1 in caveolae facilitates selective endothelial barrier–protective signaling. The molecular determinants that specify the targeting of PAR1 to caveolae are not known but may involve unique posttranslational modifications. The novel regulation of PAR1 signaling by APC is also critical for endothelial barrier protection. We previously showed that PAR1 trafficking is essential for the fidelity of thrombin signaling (22, 29). However, in contrast to thrombin, our findings here suggest that APC imparts a novel mechanism for regulation of PAR1 signaling that involves receptor phosphorylation but not internalization or degradation. Thus, in future pursuits it will be important to determine the mechanism(s) by which endothelial cells desensitize and resensitize to APC signaling.
Materials and Methods
Reagents.
See SI Methods.
Cells.
Human umbillical vein endothelial cell (HUVEC)–derived EA.hy926 cells were provided by C. Edgell (University of North Carolina, Chapel Hill, NC) and maintained as described (30). EA.hy926 cells expressing PAR1 and caveolin-1 shRNA were generated as described in SI Methods.
Permeability Assay.
See SI Methods.
RhoA and Rac1 Activity Assays.
See SI Methods.
Kinase Assays.
EA.hy926 cells plated at 0.7 × 105 cells per well in 24-well dishes and grown for 2 days were serum-starved overnight. After incubations, cell lysates were prepared, and ERK1/2 and p38 activity were determined as described elsewhere (17).
Phosphoinositide Hydrolysis.
EA.hy926 cells were plated at 6 × 104 cells per well in 24-well dishes and labeled overnight with 1 μCi/ml of myo-[3H]inositol (American Radiolabeled Chemicals, St. Louis, MO), treated with agonists; accumulated [3H]inositol phosphates (IPs) were measured as previously described (31).
Cell Surface Enzyme-Linked Immunosorbent Assay.
EA.hy926 cells were plated at 0.7 × 105 cells per well in 24-well culture dishes. After incubations, cells were fixed and processed as previously described (31). Cell surface PAR1 was detected using a rabbit polyclonal anti-PAR1 antibody (29, 32), and EPCR was detected using a monoclonal anti-EPCR JRK 1500 antibody provided by C. Esmon (Oklahoma Medical Research Foundation, Oklahoma City, OK).
PAR1 Phosphorylation and Degradation.
EA.hy926 cells plated at 5 × 105 cells per well in six-well dishes were grown overnight, and PAR1 degradation was determined as described elsewhere (21) and in SI Methods.
Data Analysis.
Data were analyzed using Prism 4.0 software (GraphPad), and statistical significance was determined using InStat 3.0 (GraphPad). Group comparisons were made using an unpaired t test.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health grant HL073328 (to J.T.) and an American Heart Association Established Investigator Award (to J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810687106/DCSupplemental.
References
- 1.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 2.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 3.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin JN, Shen L, Holinstat M, Brooks JD, DiBenedetto E. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 5.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 7.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor-1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 10.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine-1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 11.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR. Activated protein C mediates novel lung endothelial barrier enhancement. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 12.Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281:20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 13.Taylor FB, Stearns-Kurosawa DJ, Kurosawa S, Ferrell GL, Chang AC. The endothelial cell protein C receptors aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 14.Razani B, Engleman JA, Wang XB, Schubert W, Zhang XL. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 15.Bae J-S, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci USA. 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora P, Cuevas B, Russo A, Johnson GL, Trejo J. Persistent transactivation of EGFR and ErbB2/HER2 by protease-activated receptor-1 promotes breast carcinoma cell invasion. Oncogene. 2008;27:4434–4445. doi: 10.1038/onc.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuck S, Manninen A, Honsho M, Fullekrug J, Simons K. Generation of single and double knockdowns in polarized epithelial cells by retrovirus-mediated RNA interference. Proc Natl Acad Sci USA. 2004;101:4912–4917. doi: 10.1073/pnas.0401285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii K, Gerszten R, Zheng YW, Turck CW, Coughlin SR. Determinants of thrombin receptor cleavage: Receptor domains involved, specificity, and role of the P3 aspartate. J Biol Chem. 1995;270:16435–16440. doi: 10.1074/jbc.270.27.16435. [DOI] [PubMed] [Google Scholar]
- 20.Tiruppathi C, Yan W, Sandoval R, Naqvi T, Pronin AN. G protein-coupled receptor kinase-5 regulates thrombin-activated signaling in endothelial cells. Proc Natl Acad Sci USA. 2000;97:7440–7445. doi: 10.1073/pnas.97.13.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trejo J, Coughlin SR. The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J Biol Chem. 1999;274:2216–2224. doi: 10.1074/jbc.274.4.2216. [DOI] [PubMed] [Google Scholar]
- 22.Trejo J, Hammes SR, Coughlin SR. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci USA. 1998;95:13698–13702. doi: 10.1073/pnas.95.23.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111:2667–2673. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awasthi V, Mandal SK, Papanna V, Rao LVM, Pendurthi UR. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol. 2008;27:1447–1455. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlie-Klusacek ME, Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol. 2007;293:H366–H375. doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- 26.Ludeman MJ, Kataoka H, Srinivasas Y, Esmon NL, Esmon CT. PAR1 cleavage and signaling in response to activated protein C and thrombin. J BiolChem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 28.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor-1 in endothelial cells. J Thomb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 29.Paing MM, Johnston CA, Siderovski DP, Trejo J. Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization. Mol Cell Biol. 2006;28:3221–3242. doi: 10.1128/MCB.26.8.3231-3242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;12:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 32.Hung DT, Vu TK, Wheaton VI, Ishii K, Coughlin SR. Cloned platelet thrombin receptor is necessary for thrombin-induced platelet activation. J Clin Invest. 1992;89:1350–1353. doi: 10.1172/JCI115721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.