Abstract
The ability to prospectively isolate adult neural stem cells and their progeny is crucial to study their biology and therapeutic potential. Stem cells in adult mammalian neurogenic niches are a subset of astrocytes. A major limitation in the field has been the inability to distinguish stem cell astrocytes from niche astrocytes. Here, we show that epidermal growth factor receptor (EGFR)-positive subventricular-zone (SVZ) astrocytes are activated stem cells that are eliminated by antimitotic treatment. We developed a simple strategy to simultaneously purify cells at different stages of the adult SVZ stem cell lineage by using FACS. This method combines the use of fluorescent EGF ligand, CD24, and GFP expression in GFAP::GFP transgenic mice and allows the simultaneous purification of activated stem cell astrocytes (GFP+EGFR+CD24−), niche astrocytes (GFP+EGFR−CD24−), transit amplifying cells (GFP−EGFR+CD24−), and neuroblasts (GFP−EGFR−CD24low). One in three EGFR+ astrocytes gives rise to neurospheres in vitro, a 20-fold enrichment over unsorted cells. Importantly, these cells constitute the neurosphere-forming population among SVZ astrocytes. This approach will be of great utility for future functional and molecular studies of the SVZ stem cell lineage.
Keywords: adult neurogenesis, FACS, astrocyte, lineage, differentiation
Neural stem cells present in specialized niches in the adult mammalian brain continuously generate new neurons that are functionally integrated into neural circuits. Adult neurogenesis occurs in 2 regions of the mammalian brain, the subventricular-zone (SVZ), which lies adjacent to the lateral ventricles and generates olfactory bulb interneurons, and the subgranular zone (SGZ) in the hippocampal formation (1). The stem cells in both neurogenic regions are a subset of astrocytes (2–7). The identification of astrocytes as stem cells raises the intriguing possibility that astrocytes, or a subset thereof, throughout the brain may have latent stem cell potential. Defining the differences between stem cell astrocytes and other brain astrocytes is key to eventually harnessing them for brain repair.
The complexity of adult stem cell niches and rare number of resident stem cells limit the study of their functional properties and molecular regulation. Fluorescence-activated cell sorting (FACS) has been an invaluable tool for the study of, among others, hematopoietic, muscle, and epithelial stem cells (8). Adult neural stem cells and their progeny have been difficult to purify using FACS due to the lack of markers that allow separation of cells at the different stages in the lineage. Approaches used to date, including Sox1::GFP (9), Nestin::GFP (10), CD15 (11), CD133 (12), Dlx2::LacZ (13), and fluorescently complexed EGF ligand (14), have constituted important steps forward in the enrichment and isolation of neurosphere-forming cells from the adult SVZ with up to 25% of isolated CD15+ cells generating neurospheres (11). However, most of the markers used in these studies are common to several stages in the lineage and have yielded mixed populations of neural progenitor cells. Cell separation based on cell size and binding of the lectin peanut agglutinin (PNA) and CD24 (15) was described as a very efficient method for the enrichment of neurosphere-forming cells (80%) but isolated only a fraction of the total stem cell population of the SVZ. Other strategies, such as the side population purification method, in which exclusion of the fluorescent dye Hoechst 33342 is used to prospectively isolate stem cells, are not selective for neural stem cells (16, 17). A major limitation in the adult neural stem cell field has been the lack of markers that distinguish between stem cell astrocytes and niche astrocytes, both of which express glial fibrillary acidic protein (GFAP) (3, 18). Moreover, being able to simultaneously isolate multiple cell types within adult neural stem cell lineages would greatly facilitate comparison of their functional properties and molecular signatures.
Here, we describe an approach to simultaneously isolate the different stages of the adult SVZ stem cell lineage and separate activated stem cell astrocytes from niche astrocytes. As SVZ cells progress from stem cell to neuron, they sequentially express different combinations of markers (Fig. 3A) (13, 18–21). GFAP+ SVZ stem cell astrocytes (type B cells) divide to generate rapidly dividing epidermal growth factor receptor (EGFR)-positive transit amplifying type C cells, which in turn divide to give rise to CD24+ neuroblasts (type A cells) that migrate to the olfactory bulb (3, 13, 19). A subpopulation of SVZ astrocytes expresses EGFR (13). We show here that these EGFR+ SVZ astrocytes correspond to activated stem cell astrocytes that are eliminated by antimitotic treatment and reappear with the first dividing cells that regenerate the SVZ. We therefore designed a strategy based on the differential expression of EGFR to prospectively purify activated stem cell astrocytes from other SVZ astrocytes as well as transit amplifying cells and neuroblasts from the adult SVZ stem cell niche, using a combination of CD24, fluorophore-complexed EGF ligand, and GFAP::GFP transgenic mice. Both activated stem cell astrocytes and transit amplifying type C cells generated neurospheres in vitro and represented a 20- and 7-fold enrichment, respectively, of neurosphere-forming cells over the unsorted SVZ. EGFR+ SVZ astrocytes represent all of the neurosphere-forming capacity in the astrocyte population of the SVZ. This approach will be of great use for the isolation of SVZ cell populations for future functional and molecular profiling studies.
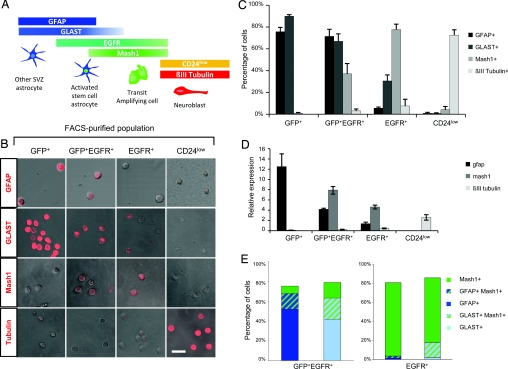
Fig. 3.
Purity and marker expression of FACS-purified SVZ populations. (A) Schema represents the SVZ stem cell lineage and the markers enriched at each cell stage. Activated stem cell astrocytes give rise to rapidly dividing transit amplifying cells that differentiate into migrating neuroblasts and eventually give rise to mature neurons of the olfactory bulb. A small percentage of SVZ stem cells differentiate into oligodendrocytes (not shown in the schema). Note that some markers are not specific to one cell type and persist into the following stage of the lineage. (B) Acute immunostaining of SVZ populations purified by FACS from the adult mouse brain. FACS-purified SVZ astrocytes (GFP+), activated stem cell astrocytes (GFP+EGFR+), transit amplifying cells (EGFR+), and neuroblasts (CD24low) were stained with antibodies against GFAP, GLAST, Mash1, and βIII-tubulin (TuJ1) to analyze the purity of each fraction. (Scale bar, 20 μm.) (C) Quantification of acute immunostaining. Data represent the mean percentage ± SEM of cells expressing the different markers in each of the FACS-sorted populations from 5 independent experiments. (D) Relative quantities of gfap, mash1, and βIII tubulin mRNA detected by qRT-PCR in each of the FACS-sorted populations. Data represent relative quantity normalized to gapdh. (E) Quantification of acute double immunostaining for GFAP/Mash1 (Left) and GLAST/Mash1 (Right) in the activated stem cell population (GFP+EGFR+) and transit amplifying (EGFR+) populations. Images of the acute immunostainings are shown in Fig. S4.
Results
EGFR+ SVZ Astrocytes Are Activated Stem Cells.
To isolate SVZ astrocytes, we used GFAP::GFP mice, in which the expression of GFP is under the control of the human GFAP promoter (22). GFP expression reliably reflects expression of GFAP within the SVZ, allowing the identification of SVZ astrocytes (23). Because a subpopulation of SVZ astrocytes expresses EGFR, we investigated whether a fluorescently complexed EGF ligand could be used to prospectively separate EGFR+ astrocytes from other SVZ astrocytes and isolate EGFR+ transit amplifying cells. We first confirmed that the fluorescently complexed EGF ligand binds to EGFR+ cells in vivo by injecting Alexa647-complexed EGF ligand (A647-EGF) stereotaxically into the lateral ventricles of GFAP::GFP mice. Thirty minutes later, whole-mount preparations of the SVZ were dissected, fixed, and immunostained for EGFR, GFP, or βIII tubulin to label EGFR-expressing cells, GFAP-expressing SVZ astrocytes, and neuroblasts, respectively. A647-EGF labeled EGFR-expressing cells in the SVZ (Fig. 1A). It also bound to a subpopulation of dim GFP+ cells (Fig. 1B) shown to express EGFR (23, 24). No βIII tubulin+ neuroblasts were labeled with the EGF ligand (Fig. 1C). The fluorescently complexed EGF ligand can therefore be used to distinguish cells expressing EGFR in vivo.
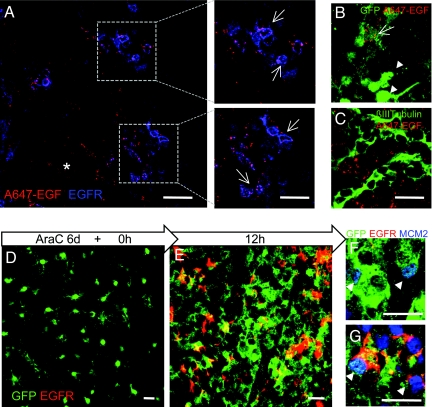
Fig. 1.
EGFR+ SVZ astrocytes are activated stem cells. (A–C) Validation of the use of EGF ligand to isolate EGFR-expressing SVZ cells. (A) In vivo binding of A647-EGF after intraventricular injection. Whole-mount preparation of the SVZ shows A647-EGF (red) bound to EGFR+ cells (blue). (Scale bar, 30 μm.) (Right) Higher-magnification view of boxes at Left. (Scale bar, 20 μm.) Note A647-EGF is also bound to noncellular extracellular matrix (asterisk). (B) Whole-mount preparation showing that A647-EGF binds to a subset of GFP dim cells (arrow) but not GFP bright cells (arrowhead) in GFAP::GFP mice. (C) Chains of neuroblasts (βIII tubulin, green) were not labeled by A647-EGF. (Scale bar for B, C, 20 μm.) (D–G) Analysis of SVZ astrocytes during regeneration at 0 (D) and 12 h (E) after AraC treatment with cytosine arabinofuranoside (AraC). (D) AraC treatment eliminates all GFP+EGFR+ cells, and only GFP+ (EGFR−) cells remain at 0 h. GFP+EGFR+ cells appear by 12 h (E). Both GFP+ (F) and GFP+EGFR+ (G) cells were labeled with anti-MCM2 (blue) at 12 h. GFP (green), EGFR (red). (Scale bars, 20 μm.)
The expression of EGFR by a subset of SVZ astrocytes raised the possibility that these may correspond to a population of activated stem cells. We therefore examined whether EGFR+ astrocytes were eliminated by the antimitotic drug cytosine β-d-arabinofuranoside (AraC), which kills actively dividing cells in the SVZ. When the brain is treated with this drug, a population of relatively quiescent astrocytes that survives AraC treatment rapidly regenerates the SVZ (3, 25). Mouse brains were infused for 6 days with AraC, and whole-mount preparations of the SVZ were analyzed at 0 and 12 h, when the first dividing astrocytes appear (3, 25). Whole-mount preparations were immunostained for GFP, EGFR, and minichromosome maintenance 2 (MCM2), which labels cells at the initiation of DNA replication (G1 to S phase; ref. 26). Immediately after AraC treatment (0 h time point), the only cells that remained were quiescent GFP+ EGFR-negative cells (Fig. 1D and Fig. S1A). At 12 h, GFP+EGFR+ cells were present (Fig. 1E), and many of them were MCM2 positive (Fig. 1G). Some GFP+EGFR− cells were also labeled with MCM2 at 12 h (Fig. 1F). Thus, GFP+EGFR+ astrocytes are activated stem cells that derive from a more quiescent stem cell astrocyte.
Prospective Isolation of SVZ Stem Cells and Their Progeny.
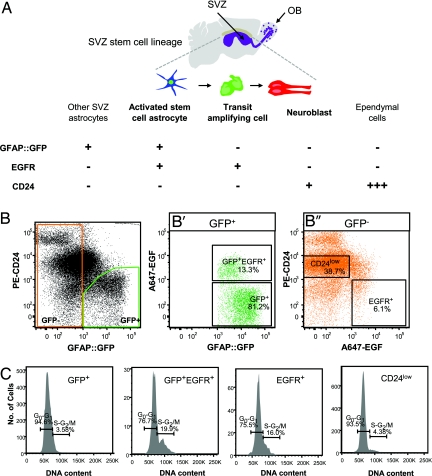
To prospectively isolate SVZ cells at each stage of the lineage, we combined GFP expression, A647-EGF binding, and CD24 immunostaining. The SVZs of GFAP::GFP mice were microdissected, dissociated to single cells, and incubated with A647-EGF and CD24 to label the different cell populations. All gates were set using controls (Fig. S2 A–C). SVZ astrocytes were separated based on GFP expression from GFP-negative SVZ cells (Fig. 2B). Within the GFP+ fraction, activated stem cell astrocytes (GFP+EGFR+CD24−, hereafter referred to as GFP+EGFR+) were easily distinguished from other SVZ astrocytes (GFP+EGFR−CD24−, hereafter called GFP+) by their differential expression of EGFR (Fig. 2B′). The GFP+EGFR+ cells were predominantly GFP dim and represented 13.3% of all GFP-positive cells. GFP+EGFR+ cells corresponded to on average 1.96%, and GFP+ cells 14.7%, of the total SVZ population. Intermediate transit amplifying type C cells (GFP−EGFR+CD24−, hereafter called EGFR+) and neuroblasts (GFP−EGFR−CD24low, hereafter called CD24low) were isolated from the GFP-negative cell fraction (Fig. 2B″) and represented 6.1% and 38.7% of total SVZ cells, respectively. Neuroblasts were easily distinguished from CD24high cells, which likely correspond in part to ependymal cells (19), by setting the fluorescence gates from 103 to 104 (Fig. S2C).
Fig. 2.
Prospective isolation of stem cells and their progeny from the adult SVZ. (A) Schema of a sagittal section of the adult mouse brain showing the lateral ventricle/SVZ (purple) and the SVZ stem cell lineage. Other SVZ astrocytes and ependymal cells lining the ventricles are also present in this region. Table shows the expression of markers used for the prospective isolation of each SVZ cell type. (B) FACS plots of the purification of each SVZ cell type from adult GFAP::GFP transgenic mice. GFAP-positive SVZ astrocytes were separated based on GFP expression from other GFP− SVZ cells. (B′) From the GFP+ pool, we isolated activated stem cell astrocytes (GFP+EGFR+CD24−) from other SVZ astrocytes (GFP+EGFR−CD24−) based on EGFR expression. The GFP+EGFR+ cell fraction largely corresponds to dim-GFP-expressing cells. (B″) From the GFP-negative cell fraction, we isolated transit amplifying cells (GFP−EGFR+CD24−) and neuroblasts (GFP−EGFR−CD24low). Percentages represented by each population are shown next to the gates and are averages of 10 independent experiments. (C) A DNA dye was used to separate the cells according to their cell-cycle status (G0/G1 phases from S/G2–M phases). Percentages represented by each population are shown next to the gates. See Fig. S2 for an example of a plot using Vybrant DyeCycle. All FACS data are presented using “logical” or biexponential display for 100,000 cells. Gates were determined by using control samples shown in Fig. S2.
Furthermore, by combining the above sorting parameters with labeling with Vybrant DyeCycle, a DNA dye, we were able to purify cells within each SVZ population according to their stage in the cell cycle (G0/G1 and S/G2–M phases) (Fig. 2C and Fig. S2D). As expected, greater fractions of the GFP+EGFR+ and EGFR+ populations were undergoing DNA synthesis and mitosis in vivo as compared with neuroblasts (CD24low) and other SVZ astrocytes (GFP+) (Fig. 2C). Therefore, comparing subpopulations of actively dividing and nondividing purified SVZ cells at the molecular level is possible (27).
To determine whether the dim GFP expression in GFP+EGFR+ cells was due to lower levels of gfp transcription or perdurance of GFP protein, we performed quantitative real-time reverse transcription (qRT-PCR) for gfp mRNA (Fig. S1B). The gfp transcript was detectable in GFP+EGFR+ cells, although at lower levels than in GFP+ cells. In addition, we assessed which populations could bind A647-EGF in vitro after sorting. Both the GFP+EGFR+ and EGFR+ populations bound A647-EGF, but not the GFP+ and CD24low populations (Fig. S1C). We further analyzed the levels of EGFR expressed in each purified SVZ population by qRT-PCR. The levels of egfr were high in GFP+EGFR+ and EGFR+ cells and low to undetectable in other SVZ astrocytes (GFP+) and neuroblasts (CD24low) (Fig. S1D). Together, these results confirm that the use of fluorescent EGF ligand enriches for EGFR-expressing SVZ cells.
The cell purity of each FACS-purified SVZ population was analyzed for cell-type-specific markers by flow cytometry (Fig. S3), acute immunostaining, or both (Fig. 3). GFAP is a specific marker of SVZ astrocytes (18) and is not expressed in transit amplifying C cells and neuroblasts (Fig. 3A). The glutamate aspartate transporter (GLAST) is predominantly expressed in astrocytes, although it has recently been reported to persist into the transit amplifying stage (24), which we confirm below. Mash1, a basic helix–loop–helix transcription factor, is highly expressed in transit amplifying C cells but is also expressed in a subset of SVZ astrocytes and in some neuroblasts (20). Finally, βIII tubulin (TuJ1) is a specific marker of neuroblasts (18).
Flow cytometry for GFAP of the GFP+ and GFP+EGFR+ populations confirmed that both populations were greatly enriched for GFAP-expressing cells (84% and 87%, respectively) (Fig. S3). To analyze the composition of each purified population in more detail, we performed acute immunostaining. The vast majority of cells in both the GFP+ and GFP+EGFR+ populations expressed GFAP (76% of GFP+ cells and 72% of GFP+EGFR+ cells) and GLAST (90% of GFP+ cells and 70% of GFP+EGFR+ cells) (Fig. 3 B and C). The fraction of GFAP+ cells is higher when assessed by flow cytometry, likely due to the greater sensitivity of this method. GFP+ cells were negative for both Mash1 and βIII tubulin (Fig. 3 B and C). Both GFP+EGFR+ and EGFR+ populations contained Mash1-positive cells and very few βIII tubulin+ cells (Fig. 3 B and C). In contrast, the CD24low cells were predominantly βIII tubulin+, with a very small fraction expressing Mash1 (4%), and none was GFAP or GLAST positive (Fig. 3 B and C). The enrichment of the above markers in each purified SVZ population was further confirmed by qRT-PCR. The expression patterns for gfap, mash1, and βIII tubulin correlated with the expected profiles in each population (Fig. 3D).
Immunostaining of the GFP+EGFR+ and EGFR+ populations revealed that 37% and 77.8% of cells were Mash1 positive, respectively (Fig. 3 B and C), with Mash1 protein expressed at lower levels in GFP+EGFR+ cells than in EGFR+ cells. To better define these populations and establish whether the Mash1-positive cells coexpressed glial antigens in the GFP+EGFR+ pool, we performed double immunostaining for GFAP or GLAST and Mash1 in acutely plated cells (Fig. 3E and Fig. S4). The EGFR+ population was highly enriched for transit amplifying C cells, with 77% of the total population expressing only Mash1 and only 3.4% of all cells expressing GFAP (1.5% GFAP only and 2% GFAP+Mash1+). Interestingly, double staining for GLAST and Mash1 revealed that 16% were GLAST+Mash1+, confirming that GLAST continues to be expressed in early transit amplifying C cells, in cells that are negative for both GFAP and GFP, as recently described (24). In contrast, the GFP+EGFR+ population was less homogeneous and comprised 2 major populations, a GFAP+(or GLAST+) only pool and a Mash1+/GFAP+ (or Mash1+/GLAST+) population (Fig. 3E). In addition, there was a small population of Mash1-only cells that was negative for both GFAP and GLAST. These likely correspond to early transit amplifying C cells that still contain residual GFP expression. Of the Mash1-positive cells in the GFP+EGFR+ population, 68% coexpressed GFAP and therefore correspond to a subset of astrocytes in the SVZ.
Activated SVZ Stem Cell Astrocytes Are Enriched in Neurosphere-Forming Cells.
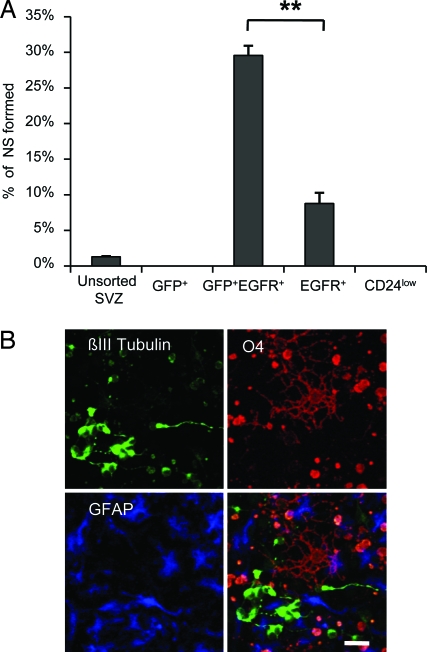
We then examined the stem cell properties of each purified population using the neurosphere assay, which assesses self-renewal and multipotency potential (28). Both GFP+EGFR+ and EGFR+ cells generated neurospheres in vitro and represented 20- and 7-fold enrichment, respectively, of neurosphere-forming cells over the unsorted SVZ (Fig. 4A). In contrast, the GFP+ and CD24low pools did not give rise to neurospheres. Approximately 1 in 3 GFP+EGFR+ cells and 1 in 10 EGFR+ cells gave rise to neurospheres (Fig. 4A). The identification of multiple neurosphere-forming populations in the SVZ lineage is consistent with previous reports (13). Primary neurospheres from both populations were generated in response to a variety of growth factors and gave rise to multipotent secondary and tertiary neurospheres (Table S1) that differentiated into neurons, astrocytes, and oligodendrocytes (Fig. 4B). Importantly, the fluorescently complexed EGF ligand used to isolate the cells was not sufficient by itself to induce neurosphere formation, because no neurospheres grew when cultured with platelet-derived growth factor (PDGF) alone (Table S1). GFP+EGFR+ and EGFR+ populations could also be cultured efficiently as adherent cultures and in clonal coculture assays (27). Thus, all neurosphere-forming activity among SVZ astrocytes is found in the activated EGFR+ astrocyte population.
Fig. 4.
Neurosphere formation by FACS-purified SVZ populations. (A) Histogram showing number of neurospheres (NS) formed per 100 plated cells of unsorted SVZ cells and purified SVZ populations in the presence of EGF and basic fibroblast growth factor. The same results were obtained with EGF only. GFP+EGFR+ SVZ astrocytes and EGFR+ transit amplifying cells were the only populations that generated neurospheres. Data represent the mean percentage of NS-forming cells in each population ± SEM from 3 independent experiments. **, P = 0.0033; two-tailed paired t test. (B) Both populations give rise to neurospheres that differentiate into neurons (βIII tubulin+, green), oligodendrocytes (O4+, red), and astrocytes (GFAP+, blue). (Scale bar, 20 μm.)
Discussion
Here, we identify EGFR+ SVZ astrocytes as activated stem cells and developed a simple and powerful strategy to prospectively purify the different cell population in the SVZ neural stem cell lineage directly from the adult brain. By combining the use of a fluorescent ligand, a surface marker, and GFP expression in GFAP::GFP mice, we have been able to separate SVZ activated stem cell astrocytes from other SVZ astrocytes (that comprise dormant stem cell astrocytes and niche astrocytes) and purify transit amplifying cells and neuroblasts in a single FACS experiment. Even though previous approaches have been described to isolate adult neural stem cells using FACS (9–15), simultaneously isolating all of the cell types within an adult neural stem cell lineage or discerning between different neurosphere-forming populations in the SVZ has not yet been possible. This approach will greatly facilitate future molecular and functional studies of purified SVZ populations.
The use of fluorescently tagged ligands when appropriate antibodies are not available, either alone or in combination with other surface markers or genetically labeled cells, is a powerful and broadly applicable approach for the isolation of stem cell and progenitor populations and their lineages. Using EGF ligand allows the purification of EGFR+ cells in the SVZ. We show that using EGF ligand was not sufficient to cause neurosphere formation. However, until we can purify EGFR-expressing cells by other means, we cannot evaluate the effects of the ligand on downstream signaling.
EGFR+ astrocytes are actively dividing type B cells that are eliminated with AraC antimitotic treatment and reappear by 12 h of SVZ regeneration. These cells have a high capacity to give rise to neurospheres in vitro, undergo self-renewal, and are multipotent. This population comprises a mixed population of Mash1+ and Mash1− EGFR+ astrocytes. Defining markers to separate these 2 populations will allow their functional properties to be tested. Furthermore, our regeneration experiments reveal that only quiescent or slowly dividing GFP+ cells remain after AraC treatment. These cells do not give rise to neurospheres under standard culture conditions. Developing methods that allow the isolation of these quiescent stem cell astrocytes will be important. In the future, it will be interesting to compare the in vivo FACS-purified populations that we describe here to in vitro generated embryonic-stem-cell- and adult-derived neural stem cells (29, 30) as well as to define which stages of the lineage are labeled by CD133 (12) or CD15 (11). Indeed, as this study has revealed, additional approaches and markers will help to further define the subpopulations of cells that comprise the SVZ stem cell lineage.
Altogether, this simple purification strategy makes studying the cell populations of the adult SVZ neural stem cell lineage as they exist in vivo possible. Functional assays, such as gene overexpression and knockdown and transplantation, pharmacological screening, and molecular profiling, will be facilitated. As such, this isolation method provides an important step forward in the elucidation of the physiology and regulation of adult neural stem cells.
Materials and Methods
All animal care was in accordance with institutional guidelines.
FACS.
The SVZs from 2-month-old GFAP::GFP mice (The Jackson Laboratory), which express GFP under the control of the human GFAP promoter (22), or wild-type CD-1 mice (Charles River Laboratories) were dissected and dissociated as described in ref. 13. Both homo- and heterozygote GFAP::GFP mice were used in our studies and yielded the same results. The procedure was carried out on ice. Cells were simultaneously incubated for 30 min with phycoerythrin-conjugated rat anti-mCD24 (1:20; BD Pharmingen) and biotinylated EGF complexed with Alexa647–streptavidin (2 μg/mL; Molecular Probes). DAPI (1:500; Sigma) was added to the cells before sorting. In the cases where Vybrant DyeCycle Violet (1:1,000; Molecular Probes) was used, cells were incubated for 30 min at 37 °C before sorting. All of the different cell populations of cells were isolated in one single sort using a Becton Dickinson FACS Aria using 13 psi pressure and 100-μm nozzle aperture. Data were collected by using a linear digital signal process. Gates were set manually by using control samples (Fig. S2). Data were analyzed with FlowJo data analysis software and displayed using logical (biexponential) scaling (31).
In Vivo Injection of Fluorescent EGF Ligand into the Lateral Ventricles.
Fluorescently complexed EGF ligand (A647-EGF; Molecular Probes) was stereotaxically injected intraventricularly into both hemispheres of anesthetized 2-month-old GFAP::GFP males (coordinates relative to bregma: anterior, 0; lateral, ±1 mm; depth, ±2.6 mm). Over a period of 30 min, 0.5 μL of a 40 μg/mL solution in water of A647-EGF was injected into the lateral ventricles. Immediately thereafter, mice were perfused with saline, and whole-mount preparations were dissected, fixed, and processed for immunostaining (32).
AraC Infusions.
A microosmotic pump filled with 2% AraC (Sigma) dissolved in saline was implanted onto the surface of the brain as described in ref. 25. After 6 days of AraC infusion, mice were killed immediately or after 12 h (n = 5 for each time point).
Neurosphere Assays.
Neurosphere assays were performed from FACS-purified SVZ populations as described in ref. 3. Cells were plated at a density of 0.3 cell per μL in the presence of combinations of basic fibroblast growth factor (10 ng/mL), PDGF-AA (20 ng/mL), or EGF (20 ng/mL). Primary neurospheres were counted 7 days after plating, and differentiation was performed as described in ref. 3.
Quantification and Statistical Analysis.
Images were taken under the 20× and 40× objectives of an inverted Axiovert 200 microscope (Zeiss). Cells were quantified blind using ImageJ software. A minimum of 20 randomly chosen fields were quantified from each population for purity analysis. In neurosphere formation assays, all neurospheres contained in each well were quantified in a minimum of 10 wells per population and condition. All experiments were done a minimum of 3 times. The mean ± SEM values are represented in the graphs. Statistical comparison of datasets was performed by two-tailed paired Student's t test. The differences are presented with their corresponding statistical significance or P value.
Supporting Information.
Details for immunostaining, in vitro EGF-binding assays, flow cytometry, RNA isolation, and qRT-PCR are included in the SI Methods.
Supplementary Material
Acknowledgments.
We especially thank Kristie Gordon of the Herbert Irving Comprehensive Cancer Center of Columbia University for assistance with FACS and flow cytometry. We thank the members of the Doetsch and Wichterle labs for critical discussions on the manuscript. We are grateful to J. Johnson for anti-Mash1 antibody. E.P. was supported by Grant T32 MH15174–29 and by a fellowship from the Spanish Ministerio de Educación y Ciencia. F.D. is a Packard Foundation Fellow and a Irma T. Hirschl Fellow. This work is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (Grant R01 NS053884) and is partially supported by the Jerry and Emily Spiegel Laboratory for Cell Replacement Therapies and the Anne and Bernard Spitzer Fund for Cell Replacement Therapy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810407106/DCSupplemental.
References
- 1.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 3.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 4.Garcia AD, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 5.Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laywell ED, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 8.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barraud P, et al. Isolation and characterization of neural precursor cells from the Sox1-GFP reporter mouse. Eur J Neurosci. 2005;22:1555–1569. doi: 10.1111/j.1460-9568.2005.04352.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi A, et al. Nestin-EGFP transgenic mice: Visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–273. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- 11.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 12.Corti S, et al. Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol. 2007;205:547–562. doi: 10.1016/j.expneurol.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Doetsch F, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 14.Ciccolini F, et al. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev Biol. 2005;284:112–125. doi: 10.1016/j.ydbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Rietze RL, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 16.Murayama A, et al. Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J Neurosci Res. 2002;69:837–847. doi: 10.1002/jnr.10339. [DOI] [PubMed] [Google Scholar]
- 17.Mouthon MA, et al. Neural stem cells from mouse forebrain are contained in a population distinct from the ‘side population’. J Neurochem. 2006;99:807–817. doi: 10.1111/j.1471-4159.2006.04118.x. [DOI] [PubMed] [Google Scholar]
- 18.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calaora V, et al. mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience. 1996;73:581–594. doi: 10.1016/0306-4522(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 20.Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- 22.Zhuo L, et al. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- 23.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2008;57:66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- 25.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiorano D, Lutzmann M, Mechali M. MCM proteins and DNA replication. Curr Opin Cell Biol. 2006;18:130–136. doi: 10.1016/j.ceb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the SVZ stem cell niche. Nat Neurosci. 2009 doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 29.Conti L, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheffler B, et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci USA. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzenberg LA, et al. Interpreting flow cytometry data: A guide for the perplexed. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 32.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.