Abstract
Severe injury disrupts normal immune regulation causing a transient hyperinflammatory reaction and suppressed adaptive immune function. This report addresses the potential contribution of dendritic cells (DC) to changes in adaptive immune function after injury by specifically measuring injury-induced changes in splenic DC numbers and subsets, cell-surface markers, TLR responses, and APC function. Using a mouse burn injury model, we found that injury did not markedly alter the relative percentage of lymphoid, myeloid, or plasmacytoid DC in the spleens of burn-injured mice. Moreover, we did not observe a significant reduction in cell-surface expression of several major costimulatory molecules, CD40, CD80, CD86, programmed death 1 ligand, ICOS ligand, and B7-H3, on DC. Instead, we observed increased cell-surface expression of CD86 at 1 day after injury with no significant changes in costimulatory molecule expression at 7 days after injury, suggesting that burn injury causes an early activation of DC. In addition, injury did not suppress DC reactivity to TLR2, TLR4, or TLR9 agonists. Most important, DC prepared from injured mice were able to present peptide antigen to naïve OTII TCR transgenic CD4+ T cells as efficiently and effectively as DC from sham-injured mice. We also found that CD4 T cells stimulated with antigen presented by DC from sham or burn mice showed similar levels of IL-2, IFN-γ, IL-10, and IL-13 production. Taken together, these findings support the conclusion that DC do not acquire a suppressive phenotype following severe injury in mice.
Keywords: Toll-like receptors, costimulatory molecules, Th1, Th2, CD4 T cells
INTRODUCTION
Severe injury upsets normal immune function predisposing the injured host to bacterial, viral, and fungal infections [1,2,3,4]. The findings from observational studies performed using blood samples from burn or trauma patients have documented that the development of suppressed immunity after severe injury correlates with changes in T cell phenotypes [5,6,7]. One well-characterized change is the observation that CD4 T cells display suppressed Th1 function following injury [8,9,10]. This and other associated clinical observations support the hypothesis that major injury induces a change in the immune system leading to suppressed adaptive immune system function. Injury studies performed in mice indicate that mice undergo similar changes in adaptive immune system function after injury [11,12,13,14]. In particular, it has been shown that mice that have been immunized at the time of injury with a T cell-dependent antigen develop a marked suppression of Th1-type antibody isotype formation and antigen-specific Th1 cytokine production [12, 15].
Given that antigen presentation is the first step in the initiation of CD4 T cell-mediated responses, these phenotypic changes in T cell responses suggest the possibility that injury may disrupt or alter APC function. APC sample their microenvironment, process foreign or self-antigens, and present these processed antigens on their cell surface. Cell types that express cell-surface MHCII have the ability to present antigen to CD4 T cells. These include dendritic cells (DC), macrophages, and B cells. Among APC types, DC have been shown to be relatively more effective at presenting antigens to CD4 T cells than macrophages and B cells [16, 17]. DC are also the primary APC population to present antigens to CD4 T cells following s.c. immunization, the route of antigen delivery used to show suppressed Th1-type reactivity after injury [18, 19]. There are three major DC subsets. In mice, all of these DC subsets express the DC marker CD11c, but each subset has a distinctive cell-surface marker expression profile. Myeloid DC are CD8–, lymphoid DC are CD8+, and plasmacytoid DC express B220 [20, 21]. These DC subsets produce different types of cytokines when stimulated and can direct the differentiation of naïve CD4+ T cells into Th1, Th2, Th17, or regulatory T cell (Treg) subsets [22,23,24].
The diverse immune regulatory features displayed by DC suggested to us that injury might alter DC function significantly and that this in turn could be a principal mechanism for the induction of suppressed Th1-type and increased Th2-type CD4 T cell reactivity that is seen after injury. Using a mouse injury model, we asked whether injury changes the relative abundance of lymphoid, myeloid, and plasmacytoid DC in the spleens of mice. We also examined injury-related changes in the cell-surface expression of some costimulatory receptor molecules including CD40, CD80, CD86, programmed death 1 ligand (PD-L1), ICOS ligand (ICOSL), and B7-H3. DC reactivity to TLR stimuli, TLR2, TLR4, and TLR9, was also assessed. Lastly, we investigated the influence of injury on DC antigen-presenting activity by testing the relative abilities of DC purified from sham versus burn mice to present peptide antigen to naïve CD4 T cells. Our results indicate that burn injury does not suppress DC antigen-presenting function significantly. Moreover, injury induced a significant increase in the expression of several costimulatory molecules on DC. We also observed that DC from burn mice did not skew antigen-stimulated, naïve CD4 T cells toward Th1 or Th2 cytokine production profiles. Therefore, it appears that although injury does cause some changes in DC numbers and activation, it does not alter their antigen-presenting activity significantly or influence naïve CD4 T cell responses.
MATERIALS AND METHODS
Mice
Five-week-old C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in an accredited virus-antibody-free animal facility in accordance with the guidelines of National Institutes of Health (NIH; Bethesda, MD, USA) and the Harvard Medical School Standing Committee on Animal Research (Boston, MA, USA). The transgenic mouse strain OTII has a TCR transgenic specific for the 323–339 peptide of OVA (OVA323–339) and is bred and maintained in the C57BL/6 genetic background. These mice were used for our studies with kind permission from Drs. Francis Carbone and William Heath (Department of Microbiology and Immunology, The University of Melbourne, Australia, and the Division of Immunology, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) [25]. The OTII mice were typed by flow cytometry of tail blood samples using anti-Vα2 and -Vβ5.1 TCR-specific antibodies (BD PharMingen, San Diego, CA, USA). All mice used in these studies were acclimated for at least 1 week before being used for experiments at 6–9 weeks of age.
Reagents
The Escherichia coli LPS strain 0111:B4 used for these studies was purchased from Difco Laboratories (Detroit, MI, USA) and repurified to eliminate TLR2 reactivity as described by Hirschfeld et al. [26]. Synthetic bacterial lipopeptide {BLP; (S)-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH, trihydrochloride} was purchased from Bachem Bioscience (King of Prussia, PA, USA). The unmethylated CpG oligodeoxynucleotide (ODN) sequence 1826 (CpG ODN 1826; TCC ATG ACG TTC CTG ACG TT) with a phosphorothioate backbone was purchased from Coley Pharmaceutical Group (Wellesley, MA, USA). Fluorescently labeled antibodies for flow cytometry, including anti-CD11c, -CD4, -CD8, -B220, -CD40, -CD80, -CD86, -PD-L1, -ICOSL, and -B7-H3, as well as Fc block reagent, were purchased from BD Biosciences (San Diego, CA, USA). ELISA antibody pairs and kits for detecting IL-1β, TNF-α, IL-10, IL-12, IFN-γ, and IL-13 were purchased from R&D Systems (Minneapolis, MN, USA). IL-2 ELISA reagents were purchased from Caltag Laboratories (Burlingame, CA, USA). Culture medium (C5) was prepared by supplementing RPMI 1640 with 5% heat-inactivated FCS, 1 mM glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 10 mM HEPES, penicillin/streptomycin/fungiozone, and 2.5 × 10−5 β-ME, all purchased from Life Technologies (Grand Island, NY, USA).
Mouse injury model
The mouse burn injury protocol, approved by the NIH and the Harvard Medical School Standing Committee on Animal Research, was performed as described previously [27]. In brief, mice were anesthetized via i.p. injection of ketamine (175 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (20 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA). The dorsal fur was shaved, and the mouse was placed in an insulated plastic mold to expose 25% of its total body-surface area. The exposed dorsum was then exposed to 90°C water for 9 s. This treatment causes full-thickness injury to the skin and is, therefore, considered to be an anesthetic injury as a result of complete damage of the nerve cells at the injury site. The mortality of this injury protocol is routinely less than 5%. Sham (control) mice were treated exactly as burn mice, except they were exposed to isothermic water. Immediately after the procedure was performed, sham and burn mice were resuscitated by giving them an i.p. injection of 1 ml pyrogen-free normal saline.
Mouse splenic DC numbers and phenotype
At 1 or 7 days after sham or burn injury, spleens were harvested into RPMI 1640 without FBS. Spleens were crushed softly to disrupt the capsule and then incubated with Liberase CI enzyme (4 μg/ml; Roche, Indianapolis, IN, USA). After 8 min, the reaction was stopped by adding C5 medium. The Liberase canine islet (CI)-treated spleens were minced using sterile, fine-meshed stainless-steel screens to obtain single-cell suspensions. After two washes by centrifugation at 300 g for 10 min, cells were used for the DC purification procedure or flow cytometry. Injury-induced changes in DC subsets were determined by staining cells with APC-conjugated anti-CD11c antibody, FITC-conjugated anti-CD4+ antibody, Cy5-conjugated anti-CD8+ antibody, and PE-conjugated anti-B220+ antibody.
Mouse splenic DC (CD11c+) purification
MACS (Miltenyi Biotec, Auburn, CA, USA) CD11c MicroBeads for isolation of DC kits were used to prepare highly purified DC by positive selection from spleen cell suspensions following the manufacturer’s recommended protocols. This approach routinely yielded >85% pure CD11c+ cell populations, as judged by FACS analysis (data not shown).
In vitro DC TLR2, -4, or -9 agonist stimulation
Groups of sham- or burn-injured mice were killed at 1 or 7 days. Spleens were harvested, and DC were purified and then plated at a density of 2 × 105 cells/well of a 96-well, round-bottom, tissue-culture plate (Corning Costar, Corning, NY, USA) in the absence or presence of 1 mg/ml TLR agonists LPS, BLP, or CpG ODN 1826. After overnight incubation, supernatants were harvested and tested for IL-1β, TNF-α, IL-10, or IL-13 levels by ELISA. The cells were harvested, and costimulatory molecule expression levels were determined by flow cytometry.
Costimulatory molecule expression on DC
At 1 or 7 days after sham or burn injury, purified DC cultured with TLR agonist or medium alone for 18 h were stained for costimulatory molecule expression using FITC-labeled anti-CD11c antibody to identify DC and PE-conjugated anti-CD40, -CD80, -CD86, -PD-L1, -ICOSL, or -B7-H3 antibodies. Cells were preincubated with FC-block reagent for 10 min prior to adding fluorescently labeled antibodies to prevent nonspecific staining via the FC receptor. PE-labeled, isotype-matched control antibodies were used to set unstained control levels. Costimulatory molecule expression levels on gated CD11c+ cells were judged by FACS analysis.
Purification of OTII TCR transgenic CD4 T cells and DC antigen-presentation cultures
Male OTII transgenic mice were killed, and their peripheral lymph nodes and spleens were harvested to prepare cell suspensions. CD4+ T cells were purified using MACS Mitenyi CD4 T cell isolation kits that purify CD4 T cells using a negative-selection approach. This purification protocol routinely yielded highly pure (>95% pure) CD4+T cell populations as judged by FACS analysis (data not shown). For mixing studies, purified CD4+ T cells (2×105 cells) were cultured with CD11c+ DC (5×104 cells) and OVA323–339 peptide (1 μg/ml) in wells of a 96-well, round-bottom plate in the absence or presence of TLR agonists (1 μg/ml). After 48 h incubation at 37°C in 5% CO2, supernatants were harvested and tested for IL-2, IFN-γ, IL-10, or IL-13 levels by ELISA.
Cytokine ELISA
ELISA, using antibody pairs specific for IL-1β, TNF-α, IL-10, IL-12, IL-2, IFN-γ, and IL-13, was used to measure cytokines in the supernatants from TLR-stimulated DC and OTII T cell cultures. They were performed using a conventional sandwich technique following the manufacturer’s protocol (R&D Systems). Serial dilutions of recombinant cytokine standards and unknown samples were added to individual wells in triplicate, and upon completion of the ELISA protocol, an ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA) and its accompanying computer software program SOFTmax PRO, Version 3.1.2, were used to analyze the results.
Statistical analysis
The results presented in the study were analyzed by two-way ANOVA and Tukey multiple comparison tests using Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA). P < 0.05 was considered significant.
RESULTS
The effect of injury on DC subset numbers
DC can be classified into lymphoid, myeloid, and plasmacytoid subsets based on their cell-surface marker profiles [28, 29]. To determine relative changes in DC subsets after injury, spleen cell suspensions were prepared from mice at Days 1 and 7 after sham or burn injury and were then stained with CD11c antibody to identify DC and anti-CD8, anti-CD4, or anti-B220 antibody to identify lymphoid (CD8+), myeloid (CD4+), or plasmacytoid (B220+) DC subsets. As shown in Figure 1, burn injury caused little change in DC percentages in the spleen at Day 1 or 7 after injury. The examination of time-dependent changes in DC subsets revealed that burn injury caused modest but significant reductions in the percentages of all three DC subsets at 1 day post-injury. By 7 days after injury, lymphoid DC percentages returned to sham levels, and plasmacytoid and myeloid DC percentages remained lower than those found in sham mice. Thus, injury induces a slight but significant reduction in DC subset percentages in the spleen.
Fig. 1.
Injury-induced changes in DC numbers and subsets. At Days 1 and 7 after sham or burn injury, spleen cells were harvested from mice and then stained with CD11c antibody to identify DC and anti-CD8, anti-CD4, or anti-B220 antibody to identify lymphoid (CD8+), myeloid (CD4+), or plasmacytoid (B220+) DC subsets. The data shown represent the mean ± sem of n = 7–11 mice from two experiments. *, P < 0.05, sham versus burn by ANOVA.
Injury-induced changes in DC costimulatory ligand expression
DC express costimulatory molecules on their cell surfaces that are necessary to activate and regulate T cell responses [30]. In these experiments, we wished to investigate whether burn injury might modulate cell-surface expression levels of several costimulatory molecules that are known to regulate CD4 T cell responses. These included CD40, CD80, CD86, PD-L1, ICOSL, and B7-H3. Spleen cells prepared from sham and burn mice at 1 or 7 days after injury were tested for relative changes in these cell-surface costimulatory molecules by flow cytometry. We observed that burn injury induced a significant increase in CD86 levels at 1 day after injury, and the expression levels of this costimulatory molecule returned to normal levels by 7 days after injury (Fig. 2). It is important to note that we did not detect a decrease in the expression of any of these costimulatory molecules on DC following injury. This result suggests that DC may acquire enhanced rather than suppressed APC function following injury.
Fig. 2.

Changes in costimulatory molecule expression on DC at 1 and 7 days after injury. Spleen cells were prepared from individual mice at Day 1 or 7 after burn injury. The cell suspensions were stained with FITC-labeled anti-CD11c antibody and counterstained with the indicated PE-labeled costimulatory molecule-specific antibodies. (A) Representative FACS plots showing costimulatory molecule-staining levels on gated CD11c+ cells; the numbers in the upper-right quadrants represent the percent-positive staining cells. (B) The plot shows the mean ± sem of n = 5–6 mice per group and is representative of three independent experiments. *, P < 0.05, sham versus burn by ANOVA.
TLR-stimulated costimulatory molecule expression by DC is enhanced by burn injury
We next wanted to determine if TLR stimulation might change the expression levels or patterns of these same costimulatory molecules. In these experiments, DC were purified from the spleens of mice at Day 1 or 7 after sham or burn injury and were stimulated with optimal doses of the TLR2 agonist BLP, the TLR4 agonist, repurified E. coli LPS, or the TLR9 agonist unmethylated CpG ODN 1826. As shown in Figure 3, DC from burn as compared with sham mice showed a significant increase in CD86 expression in response to TLR2, TLR4, or TLR9 stimulation at 1 day after injury. In contrast, at 7 days after injury, DC expressed higher cell-surface PD-L1 levels when stimulated with BLP or LPS. However, no injury-induced difference in costimulatory molecule expression was found at Day 7 post-injury when DC were exposed to CpG DNA. These results demonstrate that DC retain their ability to respond to TLR2, TLR4, and TLR9 stimulation. Moreover, we demonstrate that DC show an early, preferential increase in CD86 expression and a later increase in PD-L1 expression when activated with TLR2 or TLR4 agonists.
Fig. 3.
TLR2-, TLR4-, and TLR9-induced effects on costimulatory molecule expression on DCs at 1 and 7 days after injury. Spleen cells were prepared from individual mice at Day 1 or 7 after burn injury. Purified CD11c+ cells were plated at 1 × 105 per well of a 96-well plate and stimulated with 1 μg/ml or BLP, LPS, or CpG ODN 1826. After 18 h incubation, cell suspensions were stained with FITC-labeled anti-CD11c antibody and counterstained with the indicated PE-labeled, anticostimulatory molecule antibodies. The data represent the mean ± sem of n = 3 mice per group of three independent experiments. *, P < 0.05, sham versus burn by ANOVA.
The influence of injury on TLR-stimulated DC cytokine production
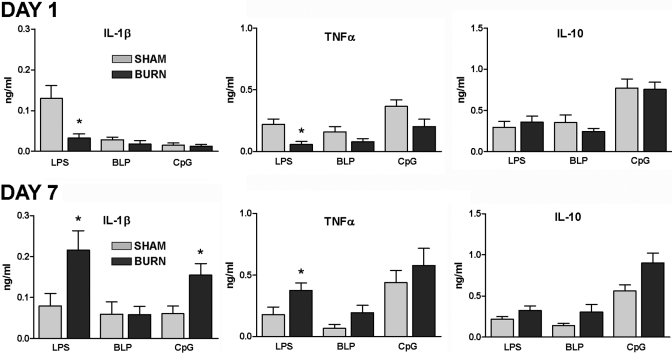
We next investigated the effect of injury on TLR-induced cytokine production by DC. Spleen DC were purified from sham or burn mice at 1 or 7 days after injury and exposed to the TLR agonists LPS, BLP, or CpG DNA. Supernatants from 2-day cultures were then tested for levels of IL-1β, TNF-α, and IL-10 by ELISA. The results shown in Figure 4 demonstrate that DC prepared from mice at 1 day after burn injury produced significantly lower levels of IL-1β and TNF-α in response to LPS than DC from sham mice. Stimulation with other TLR agonists, BLP and CpG DNA, did not show a similar reduction in IL-1β and TNF-α production. In contrast, at 7 days after injury, we observed significant increases in LPS- and CpG DNA-induced IL-1β and LPS-induced as well as BLP-induced TNF-α production by DC from burn as compared with sham mice. Interestingly, TLR-induced IL-10 production was not affected by burn injury. These results demonstrate that injury caused an early reduction in TLR4 reactivity but a later enhanced responsiveness to TLR4, TLR2, or TLR9 stimulation.
Fig. 4.
The influence of injury on TLR2-, TLR4-, and TLR9-stimulated cytokine production by DC, which were purified from the spleens of sham and burn mice at Day 1 or 7 after injury and plated at a density of 1 × 105 per well of a 96-well plate. After 48 h stimulation with 1 μg/ml or BLP, LPS, or CpG ODN 1826, supernatants were harvested and tested for cytokine production by ELISA. The data presented represent the mean ± sem of n = 3 mice per group of three independent experiments. *, P < 0.05, sham versus burn by ANOVA.
Injury effects on DC APC function
A primary function of DC is to initiate adaptive immune responses by presenting antigens to T cells. To test whether injury might alter DC antigen-presenting function, we used CD4 T cells prepared from OTII transgenic mice that express TCRs with specificity for the 323–339 peptide of OVA323–339 as a source of antigen-reactive CD4 T cells in an in vitro antigen-presentation assay. Using OVA323–339 peptide-induced cytokine production levels to judge APC efficiency, we found that DC from burn-injured mice were able to stimulate CD4 T cells as effectively as DC from sham-injured mice (Fig. 5). As shown, DC from Day 1 or 7 burn mice were able to induce similar levels of IL-2, IFN-γ, IL-10, and IL-13 production. This outcome indicates that burn injury does not suppress DC APC function. Moreover, these results suggest that DC from burn mice do not skew the cytokine production profiles of naïve CD4 T cells toward a Th1- or Th2-type response phenotype.
Fig. 5.

Injury-induced effect on DC APC activity. At 1 or 7 days after injury, DC were purified from the spleens of sham and burn mice and plated at a density of 5 × 104 per well of a 96-well plate. CD4 T cells purified from OTII TCR transgenic mice (2×105 cells) were cultured with DC and OVA323–339 peptide (1 μg/ml). After 48 h stimulation, supernatants were harvested and tested for cytokine production by ELISA. We did not detect any cytokine production when peptide was added to cultures of purified CD4 T cells from OTII mice in the absence of DC. The data represent the mean ± sem of four to six independent experiments. There was no significant difference between groups (P<0.05) as determined by ANOVA.
As TLR signaling is known to modulate DC APC function, we also wished to test the effect of TLR4, TLR2, or TLR9 stimulation on the antigen-presenting activity of DC. Purified DC from Day 1 or 7 sham and burn mice were cultured with OTII CD4 T cells, OVA323–339 peptide, and LPS, BLP, or CpG DNA. Culture supernatants were then tested for cytokines. We found that the production of antigen-induced IFN-γ, IL-10, and IL-13 was enhanced by LPS, BLP, or CpG stimulation as compared with cultures without addition of these TLR agonists (Figs. 6 and 5, respectively). However, we did not observe significant differences in antigen-induced cytokine production between sham and burn groups at Day 1 or 7 after injury. Taken together, these findings indicate that burn injury does not suppress or markedly alter the antigen-presenting function of DC and that DC respond to TLR agonist stimulation without markedly influencing the antigen-induced cytokine production by naïve CD4 T cells.
Fig. 6.
The influence of TLR stimulation on DC APC function. DC were purified from the spleens of sham and burn mice and plated at a density of 5 × 104 per well of a 96-well plate with CD4 T cells purified from OTII TCR transgenic mice (2×105 cells) and OVA323–339 peptide (1 μg/ml). LPS, BLP, or CpG ODN (1 μg/ml) were added to the cultures, and after 48 h incubation, supernatants were harvested to test for cytokine production by ELISA. The data represent the mean ± sem of five independent experiments. *, P < 0.05, for sham versus sham; **, P < 0.05, for burn versus burn between TLR agonists as determined by ANOVA. There was no significant difference between sham and burn groups (P<0.05) as determined by ANOVA.
DISCUSSION
These studies were initiated to determine if injury suppresses or alters the ability of DC to function as effective APCs. We were also interested in determining if injury might cause a loss or expansion of DC subsets or affect their capability to respond to pathogen-associated molecular patterns (PAMPs) [31]. As DC are among the most effective APC types, we reasoned that reduced DC numbers or APC function might explain, in part, the altered T cell-mediated immunity that occurs after injury. We also believed that DC would be the primary APC type responsible for directing the previously described production of Th2-type cytokines by CD4 T cells in injured mice [11, 12, 14]. On the other hand, we were aware of the possibility that injury might increase DC APC function or augment their reactivity to PAMP stimulation. This latter possible outcome is supported by the findings that injury primes the innate immune system for a more proinflammatory phenotype and the widely held impression that injury provides an activating signal to the immune system via danger signals [32,33,34].
The results reported here document that injury does not cause a marked reduction or increase in DC numbers or subsets. This observed stability in DC numbers, subsets, and APC function was not an expected result and suggests that DC are relatively resistant to the stress and tissue damage associated with burn injury in mice. Based on the results of a prior report examining the effect of burn injury on DC, we predicted that there may be a decrease in the percentage of CD8+ (lymphoid) DC [35]. In that study, it was shown that treating mice with fetal liver tyrosine kinase 3 ligand (FLT3L) improved immune function in mice, and that improved immune function was associated with an increase in CD8+ DC in the spleen. However, the experiments presented in that report did not examine specifically whether burn injury reduces CD8+ DC. Instead, they showed that FLT3L treatment caused a significant increase in CD8+ DC and that mice treated with FLT3L showed improved immune function. A subsequent study published recently by the same authors shows clearly that FLT3L has immune stimulatory activity beyond its effects on promoting the development of CD8+ DC in mice [36]. Another study that specifically examined the influence of trauma-hemorrhage (T-H) on splenic DC showed that T-H caused significant reductions in MHC class II expression, APC activity, and LPS-induced IL-12p40 and IL-12p70 production [37]. However, the T-H study examined the influence of injury on DC at 2 h after injury, and our studies examined much later post-injury time-points. Therefore, our data are not comparable with the T-H study, and it remains uncertain whether burn injury and T-H are dissimilar in their effects on DC phenotype and function. Thus, the present report contributes new information about the effects of injury on DC by demonstrating that burn injury does not markedly alter the expansion or deletion of DC or DC subsets.
The mouse burn model used in these experiments was developed intentionally as a nonlethal injury model. The 25% total body-surface area burn injury causes <5% mortality, which allows us to study the physiological influence of injury on the immune system without bias toward survivors of the injury response. We find that increasing the burn size above 25% body-surface area causes a significant increase in mortality, suggesting that the injury is at a sufficiently high threshold to cause changes in immune function relevant to patients who survive severe injury. However, one limitation of this study is that the results are biased to a survivable injury response. Another limitation of this study is that we did not address whether lower or higher surface-area injury would cause a differential DC response. Nevertheless, as we did not observe suppressed DC function at the level of injury reported here, we would anticipate that less-severe injury would not be detrimental to DC function and that DC from mice that survive a more severe injury would likely resemble the DC prepared from mice that received a survivable 25% total body-surface area burn injury.
We next focused our studies on comparing APC function of DC prepared from post-injury Days 1 and 7 sham and burn mice. Using CD4 T cells from the OTII TCR transgenic mice as a source for naïve antigen-specific CD4 T cells, we show that burn injury does not suppress DC antigen-presenting activity as evidenced by the finding that highly purified DC from Day 1 or 7 sham and burn mice showed little or no difference in their ability to present a MHC class II-restricted peptide and activate CD4 T cells. We also documented that DC from burn mice did not skew the balance of Th1- or Th2-type cytokine production by antigen-stimulated naïve T cells. Again, this was not an anticipated outcome. Based on the literature, which supports the concept that DC are the principal APC driving Th1 or Th2 responses, we assumed that DC would contribute to the biased Th2-type T cell reactivity that occurs after injury [38, 39]. Our results support the idea that other innate or adaptive immune cell types play active roles in regulating the well-described changes in CD4 T cell activation and differentiation after injury. A shortcoming of this study is that we have not explored the possibility that other cells might act in concert with DC to skew or alter the antigen response of CD4 T cells in this experimental system. Our current research efforts are aimed at exploring this potential mechanism.
A number of studies have shown that PAMPs provide important activating signals for DC APC function [40,41,42]. Therefore, we wished to study the influence of injury on DC responses to TLR agonist stimulation. We examined TLR agonist effects on cytokine production, costimulatory molecule induction, and APC activity by DC. The results of these experiments suggest that injury does indeed alter the reactivity of DC to TLR4, TLR2, and TLR9 stimulation. At Day 1, DC from burn mice produced significantly less IL-1β and TNF-α in response to TLR4 but not TLR2 or TLR9 stimulation. This suggests that injury may have desensitized DC to TLR4 stimulation at this early time-point after injury in a manner similar to what has been reported for LPS tolerance [43]. Although speculative, endogenous TLR4 agonists such as high-mobility group box 1 protein, hyaluronic acid, or heat-shock proteins could be involved in desensitizing DC TLR4 responses after injury [44, 45]. In contrast, DC from Day 7 burn mice produced higher levels of the proinflammatory cytokines IL-1β and TNF-α when stimulated through the TLR4 pathway by LPS. Of note, burn injury had little or no effect on TLR2- or TLR9-induced cytokine production. This suggests that TLR4 represents the primary TLR pathway that is modulated by injury in DC. This increase in reactivity of DC to TLR4 stimulation at Day 7 after injury is consistent with other reports showing that burn injury primes innate immune cells, in particular, macrophages and monocytes, for high TLR4 responsiveness [32, 46,47,48].
DC from burn-injured mice showed increased expression of cell-surface CD86 at Day 1, but not Day 7, after burn injury. Yet, injury did not significantly change the expression levels of the other costimulatory molecules that were measured in these experiments. These included CD40, CD80, PD-L1, ICOS-L, and B7-H3. This finding suggests that injury provides an early activation signal to DC but that the response is limited to the CD86 activation pathway. By Day 7, DC from burn mice were indistinguishable from DC from sham mice with respect to costimulatory molecule-expression profiles. Treating DC with LPS, BLP, or CpG ODN caused a further increase in CD86 expression on DC from Day 1 burn mice and increased the overall expression of other costimulatory molecules on DC. As DC from burn mice as compared with sham mice showed similar responses to TLR stimulation, we conclude that burn injury does not impair the capacity of DC to respond to TLR activation. One exception to this was that we observed that LPS or BLP stimulation significantly enhanced PD-L1 expression on DC from Day 7 burn mice. Interestingly, PD-L1 is a costimulatory molecule that inhibits CD4 and CD8 T cell activation [49, 50]. The TLR-induced up-regulation of cell-surface PD-L1 may represent a negative-feedback response to help prevent excessive CD4 or CD8 T cell responses under conditions when TLRs are triggered following injury.
A primary objective of this study was to determine if burn injury might suppress the ability of DC to act as effective APCs for CD4 T cells. Our basic approach was to purify DC from the spleens of mice at an early time-point, Day 1, and at a time-point (Day 7) when mice show increased Th2-type cytokine production. These purified DC were then tested for their ability to present antigen (OVA323–339 peptide) to naïve OTII transgenic CD4 T cells. Under these experimental conditions, we found that DC from Day 1 or 7 sham and burn mice showed an equal ability to stimulate antigen-specific Th- and Th2-type cytokine production by CD4 T cells. Our original hypothesis was that we would find a significant difference in APC function between DC from sham and burn mice. Instead, we found that injury had no significant influence on the ability of DC to present antigen to naïve CD4 T cells and to stimulate cytokine production. We had also predicted that DC from burn mice would promote higher Th2-type cytokine production. In contrast, we did not find any statistically significant burn injury influence on antigen-induced IL-10 or IL-13 production. Although we attempted to measure IL-4 and IL-5 as additional Th2-type cytokines, they were not detected in our ELISA, suggesting that DC did not support the antigen-induced production of these cytokines by CD4 T cells. Thus, we conclude from these studies that DC do not play a significant role in suppressing antigen-specific Th1 responses or in skewing toward increased Th2 responses after injury. These findings also demonstrate that injury does not significantly change the capacity of DC to present antigen to CD4 T cells efficiently. As a whole, the outcome of these experiments indicates that burn injury does not affect the primary activation or differentiation of antigen-specific CD4 T cells by DC.
The final set of experiments tested whether TLR-stimulated DC from sham or burn mice influence the cytokine production profile of antigen-activated CD4 T cells. Based on the observation that burn mice respond strongly to TLR2 and TLR4 agonists and on the effects of TLR stimulation on CD86 expression, we anticipated that the addition of TLR agonists to DC cultures would cause increased T cell cytokine responses in the burn injury groups. Surprisingly, we found that adding TLR2, TLR4, or TLR9 agonists at optimal stimulatory concentrations to these T cell activation cultures did not induce major differences in antigen-induced cytokine production. However, TLR stimulation did cause detectable increases in IFN-γ (BLP) and IL-10 (CpG) production by antigen-stimulated, naïve CD4 T cells. These results indicate that injury does not markedly alter the influence of PAMPs on DC APC activity.
In summary, we report here for the first time that burn injury does not suppress the capacity of DC to function as effective APCs for naïve, antigen-specific CD4 T cell responses. Although injury did cause a minor loss of DC subsets and some changes in costimulatory molecule expression, these changes did not have a measurable negative or positive influence on their ability to activate antigen-specific CD4 T cells. These findings suggest that other APC types, including macrophages and B cells, may act in a dominant manner to skew antigen-specific CD4 T cell responses following injury. It is also possible that immune cell types such as Tregs or GR1+ myeloid suppressor macrophages, which have been shown to be induced or activated by burn injury, work in concert with APCs to suppress or alter CD4 T cell activation and differentiation [51,52,53]. Our future studies will seek to identify the cells responsible for altering CD4 T cell responses after severe injury. A better understanding of how injury influences the immune system will permit the design and development of approaches to help boost or control immune system function in critically injured patients.
Acknowledgments
This research work was supported by grant funding from NIH (GM35633 and GM57664) and by the Julian and Eunice Cohen and Brook Family Funds for Surgical Research. The authors thank the Department of Traumatology, Osaka University, Japan, for generously providing outstanding trainees to support our research efforts.
References
- Smith J W, Gamelli R L, Jones S B, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006;21:160–172. doi: 10.1177/0885066605284330. [DOI] [PubMed] [Google Scholar]
- Wilkinson R A, Fishman J A. Effect of thermal injury with Pseudomonas aeruginosa infection on pulmonary and systemic bacterial clearance. J Trauma. 1999;47:912–917. doi: 10.1097/00005373-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kobayashi H, Herndon D N, Pollard R B, Suzuki F. Burn-associated Candida albicans infection caused by CD30+ type 2 T cells. J Leukoc Biol. 1998;63:723–731. doi: 10.1002/jlb.63.6.723. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kobayashi M, Utsunomiya T, Herndon D N, Pollard R B, Suzuki F. Therapeutic protective effects of IL-12 combined with soluble IL-4 receptor against established infections of herpes simplex virus type 1 in thermally injured mice. J Immunol. 1999;162:7148–7154. [PubMed] [Google Scholar]
- Zedler S, Bone R C, Baue A E, von Donnersmarck G H, Faist E. T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med. 1999;27:66–72. doi: 10.1097/00003246-199901000-00028. [DOI] [PubMed] [Google Scholar]
- Murphy T, Paterson H, Rogers S, Mannick J A, Lederer J A. Use of intracellular cytokine staining and bacterial superantigen to document suppression of the adaptive immune system in injured patients. Ann Surg. 2003;238:401–410. doi: 10.1097/01.sla.0000086661.45300.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele M K, Chaudry I H. Surgical trauma and immunosuppression: pathophysiology and potential immunomodulatory approaches. Langenbecks Arch Surg. 2005;390:333–341. doi: 10.1007/s00423-005-0557-4. [DOI] [PubMed] [Google Scholar]
- Spolarics Z, Siddiqi M, Siegel J H, Garcia Z C, Stein D S, Denny T, Deitch E A. Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med. 2003;31:1722–1729. doi: 10.1097/01.CCM.0000063579.43470.AA. [DOI] [PubMed] [Google Scholar]
- Navarro-Zorraquino M, Lozano R, Deus J, Pastor C, Larrad L, Tejero E, Roman J, Palacios M J, Torcal J, Salinas J C. Determination of the immunoglobulin E postoperative variation as a measure of surgical injury. World J Surg. 2001;25:585–591. doi: 10.1007/s002680020089. [DOI] [PubMed] [Google Scholar]
- De A K, Kodys K M, Pellegrini J, Yeh B, Furse R K, Bankey P, Miller-Graziano C L. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52–66. doi: 10.1006/clim.2000.4879. [DOI] [PubMed] [Google Scholar]
- Mack V E, McCarter M D, Naama H A, Calvano S E, Daly J M. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303–1308. doi: 10.1001/archsurg.1996.01430240057007. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kavanagh E, Zang Y, Dolan S M, Kriynovich S J, Mannick J A, Lederer J A. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171:3983–3990. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- Kovacs E J, Duffner L A, Plackett T P. Immunosuppression after injury in aged mice is associated with a TH1-TH2 shift, which can be restored by estrogen treatment. Mech Ageing Dev. 2004;125:121–123. doi: 10.1016/j.mad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Zang Y, Dolan S M, Choileain N N, Kriynovich S J, Murphy T J, Sayles P, Mannick J A, Lederer J A. Burn injury initiates a shift in superantigen-induced T cell responses and host survival. J Immunol. 2004;172:4883–4892. doi: 10.4049/jimmunol.172.8.4883. [DOI] [PubMed] [Google Scholar]
- Kelly J L, O'Suilleabhain C B, Soberg C C, Mannick J A, Lederer J A. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39–45. doi: 10.1097/00024382-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Cassell D J, Schwartz R H. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med. 1994;180:1829–1840. doi: 10.1084/jem.180.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Ingulli E, Mondino A, Khoruts A, Jenkins M K. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M K, Khoruts A, Ingulli E, Mueller D L, McSorley S J, Reinhardt R L, Itano A, Pape K A. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- Randolph G J, Ochando J, Partida S N S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- Salomon B, Cohen J L, Masurier C, Klatzmann D. Three populations of mouse lymph node dendritic cells with different origins and dynamics. J Immunol. 1998;160:708–717. [PubMed] [Google Scholar]
- Manickasingham S P, Edwards A D, Schulz O, Reis e Sousa C. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur J Immunol. 2003;33:101–107. doi: 10.1002/immu.200390001. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu Y J, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M D, Baban B, Chandler P, Hou D Y, Singh N, Yagita H, Azuma M, Blazar B R, Mellor A L, Munn D H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden M J, Allison J, Heath W R, Carbone F R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Ni Choileain N, MacConmara M, Zang Y, Murphy T J, Mannick J A, Lederer J A. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–282. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Smith J L, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski C R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M E, Sharpe A H. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Paterson H M, Murphy T J, Purcell E J, Shelley O, Kriynovich S J, Lien E, Mannick J A, Lederer J A. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- Fan J, Kapus A, Marsden P A, Li Y H, Oreopoulos G, Marshall J C, Frantz S, Kelly R A, Medzhitov R, Rotstein O D. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–5259. doi: 10.4049/jimmunol.168.10.5252. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Toliver-Kinsky T E, Cui W, Murphey E D, Lin C, Sherwood E R. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J Immunol. 2008;180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Fujimi S, Lederer J A, Hubbard W J, Choudhry M A, Schwacha M G, Bland K I, Chaudry I H. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–4520. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- Liu Y J, Kadowaki N, Rissoan M C, Soumelis V. T cell activation and polarization by DC1 and DC2. Curr Top Microbiol Immunol. 2000;251:149–159. doi: 10.1007/978-3-642-57276-0_19. [DOI] [PubMed] [Google Scholar]
- Rissoan M C, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu Y J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Segal D M. Controlling the Toll road to dendritic cell polarization. J Leukoc Biol. 2004;75:721–730. doi: 10.1189/jlb.1003482. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Janeway C A, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Fan H, Cook J A. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- Beg A A. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- Bianchi M E. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Noel G, Guo X, Wang Q, Schwemberger S, Byrum D, Ogle C. Postburn monocytes are the major producers of TNF-α in the heterogeneous splenic macrophage population. Shock. 2007;27:312–319. doi: 10.1097/01.shk.0000239753.75088.5e. [DOI] [PubMed] [Google Scholar]
- Maung A A, Fujimi S, Miller M L, MacConmara M P, Mannick J A, Lederer J A. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- Schwacha M G. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- Keir M E, Liang S C, Guleria I, Latchman Y E, Qipo A, Albacker L A, Koulmanda M, Freeman G J, Sayegh M H, Sharpe A H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L, Fouser L A, Jussif J, Fitz L, Deng B, Wood C R, Collins M, Honjo T, Freeman G J, Carreno B M. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ni Choileain N, MacConmara M, Zang Y, Murphy T J, Mannick J A, Lederer J A. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- MacConmara M P, Maung A A, Fujimi S, McKenna A M, Delisle A, Lapchak P H, Rogers S, Lederer J A, Mannick J A. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J G, Guo X, Wells-Byrum D, Schwemberger S, Caldwell C C, Ogle C K. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23:115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]