Abstract
Junctional adhesion molecule (JAM)-C is an Ig superfamily protein, which is involved in the regulation of various inflammatory and vascular events such as transendothelial leukocyte migration. JAM-C is expressed highly on the surface of endothelial cells and platelets, whereas expression in T lymphocytes is not well studied. To investigate the specific gene regulation of JAM-C in T lymphocytes, we determined JAM-C expression in quiescent and activated human T cells. Treatment with the polyclonal T cell activator PHA increased surface and total JAM-C expression in T cells time- and dose-dependently, as determined by flow cytometry and immunoblot analysis. In contrast, no up-regulation of JAM-A in activated T cells was detectable. The highest level of JAM-C up-regulation by PHA was observed in CD3+forkhead box P3+ and CD4+CD25high T cells. Moreover, TCR activation with combined anti-CD3 and anti-CD28 stimulation induced JAM-C expression in T cells. JAM-C induction occurred at the mRNA level, suggesting a transcriptional regulatory mechanism of JAM-C expression. Accordingly, we studied the regulation of the human JAM-C gene promoter in transiently transfected T cells. Luciferase activity of a JAM-C promoter gene construct with three potential consensus sites for the transcription factor NFAT was induced markedly in activated T cells. Finally, pretreatment with two pharmacological inhibitors of calcineurin, cyclosporin A, and FK-506, but not with MAPK inhibitors, blocked JAM-C induction in activated T cells. In summary, JAM-C is up-regulated in activated human T lymphocytes via a transcriptional mechanism, suggesting a potential role of JAM-C in T cell functions.
Keywords: T lymphocytes, gene regulation, cell activation
INTRODUCTION
Junctional adhesion molecules (JAMs) belong to the Ig superfamily of proteins consisting of two extracellular Ig-like domains. JAMs are expressed predominantly in tight junctions of endothelial and epithelial cells [1,2,3] and can interact with postsynaptic density-95, discs large, zonula occludens-1 (PDZ) domain-containing molecules via their PDZ domain-binding motif located at the C terminus. Besides their propensity to interact in a homophilic manner through a conserved motif in the extracellular domain 2 [4, 5], JAMs are also engaged in heterophilic interactions as counter-receptors for leukocyte integrins: JAM-A binds to LFA-1, JAM-B associates with VLA-4, and JAM-C interacts with macrophage antigen-1 (Mac-1) [6, 7]. The third member of the JAM family, JAM-C, exhibits a cell-specific distribution and is expressed mainly on endothelial cells, platelets, and a subpopulation of B cells [8, 9]. By contrast, JAM-A is expressed on different blood cells, including platelets, neutrophils, monocytes, and lymphocytes [6].
Accumulating evidence has indicated that JAM-C plays an important role in transendothelial and transepithelial migration of neutrophils during the inflammatory response by the regulation of cell–cell contacts via homophilic and heterophilic interactions [10,11,12,13]. The mechanisms of how JAM-C expression is regulated in various cell types, however, are not well-understood. Recently, we and others [14, 15] have found a rapid increase of JAM-C surface expression on human endothelial cells after stimulation with the vasoactive compounds vascular endothelial growth factor (VEGF) and histamine, which has been shown to be mediated via translocation from intracellular storage pools of JAM-C. In contrast, slower kinetics of JAM-C gene up-regulation has been reported in endothelial cells by treatment with oxidized low-density lipoproteins [16]. Independently, Arrate and co-workers [17] have observed that resting human peripheral T lymphocytes did not express JAM-C and that upon pharmacological stimulation, JAM-C was up-regulated on the surface of T cells. The induction of JAM-C expression on T cells and the underlying regulatory mechanisms, however, have not been addressed in further detail.
Here, we studied the regulation of JAM-C in human T cells. It is demonstrated that JAM-C gene expression is induced in activated human T cells via a transcriptional mechanism that is mediated by a calcineurin-dependent signaling pathway.
MATERIALS AND METHODS
mAb
mAb against human JAM-C (clone Gi11) has been produced and characterized in our laboratory [10]. mAb specific for human JAM-A (clone F11) was purchased from AbD Serotec (Oxford, UK). mAb directed against GAPDH (clone 6C5) was from HyTest (Turku, Finland). mAb against human CD3 (clone HIT3a) and CD28 (clone CD28.2) were purchased from BD Biosciences (Heidelberg, Germany).
Isolation of peripheral T cells and cell culture
Peripheral blood lymphocytes were isolated from buffy coats by Ficoll-Paque density gradient (Pharmacia, Freiburg, Germany). Buffy coats were obtained from randomized, healthy, voluntary blood donors after giving their informed consent. T cells were purified from ∼2 × 108 peripheral blood lymphocytes by the use of immunomagnetic microbeads [Pan T cell isolation kit II (human), Miltenyi Biotec, Bergisch-Gladbach, German] on an automated separator (AutoMACS, Miltenyi Biotec), as recommended by the manufacturer. Aliquots of 4–10 × 106-purified T cells were cultured in flat-bottom plates (BD Biosciences) in RPMI media (Invitrogen, Karlsruhe, Germany), supplemented with 1% glutamine (Invitrogen), 100 U/ml penicillin (Invitrogen), 100 μg/ml streptomycin (Invitrogen), and 10% FBS (Biochrom, Berlin, Germany). Cells were then stimulated with PHA (Sigma, Deisenhofen, Germany) or mAb against CD3 and CD28 at various times and concentrations. Different immunosuppressants, cyclosporin A (Sigma), FK-506 (Sigma), and MAPK inhibitors PD98059 (Calbiochem, Schwalbach, Germany), SP600125 (Biomol, Hamburg, Germany), and SB202190 (Calbiochem) specific for ERK, JNK, and p38 MAPK, respectively, were added to cell cultures as indicated.
Flow cytometry analysis
Purified human T cells were analyzed for JAM-C surface expression by flow cytometry as described previously [10, 18]. Aliquots of 5 × 105 cells were labeled with Gi11-allophycocyanin and CD3-FITC (or CD4-FITC) and appropriate isotype control (BD PharMingen, San Diego, CA, USA) for 20 min at 4°C. For characterization of JAM-C+ cells, a variety of antibodies against lymphocyte surface was used (BD PharMingen) and subjected to the same staining protocol. After washing, labeled cells were measured on FACSCalibur™ (BD Bioscience).
Immunoblotting analysis
Purified human T cells were washed with 0.9% NaCl and lysed with 1% Triton X-100 (Sigma) in 50 mM Tris, 150 mM NaCl, pH 7.4, as described previously [19]. Cell lysate was centrifuged (13.000 g, 5 min at 4°C), and the protein concentration in the cell supernatant was determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). An aliquot of 20 μg protein was loaded onto a 12% SDS-polyacrylamide gel and was transferred onto a polyvinylidene difluoride membrane by electroblotting. Membranes were blocked with 10 mM Tris buffer, pH 7.5, containing 1% BSA (Serva, Germany) and 0.1% Tween (Sigma) for 1 h at room temperature. mAb against JAM-C, JAM-A, or GAPDH (as a control) were added at a concentration of 2 μg/ml for 12 h at 4°C. After washing, the membrane was incubated with peroxidase-labeled rabbit anti-mouse IgG (dilution 1:100,000) and visualized with the ECL plus chemiluminescence substrate system (GE Healthcare, Munich, Germany). The obtained chemiluminescent signals were scanned by videodensitometry and quantitated with Imagequant software [19].
RT-PCR
Total RNA from 10 × 106 nonstimulated and stimulated T cells was isolated with the RNEasy mini isolation kit (Qiagen, Duesseldorf, Germany) and was reverse-transcribed by the use of the Ready-To-Go kit (GE Healthcare). An aliquot of 1 μl cDNA was then amplified by PCR in 5 mM Tris buffer, pH 8.0, containing 50 mM KCl, 1.5 mM MgCl2, 0.125 mM each dNTP, 0.5 μM forward primer from position +160 to +175 (5′-ATG GCG CTG AGG CGG CCA-3′), 0.5 μM reverse primer from position +1092 to +1074 (5′-TCA GAT CAC AAA CGA TGA C-3′), and 0.5 μl AmpliTaq Gold polymerase (2.5 IU, Perkin Elmer, Wellesley, MA, USA) in a total volume of 25 μl. The JAM-C primers were numbered according to the published sequence (NM032801.3). Amplification was performed on a DNA thermal cycler PX2 (Thermo Electron Corp., Waltham, MA, USA) for 32 cycles. Each cycle consisted of denaturation at 95°C for 60 s, annealing at 59°C for 60 s, and extension at 72°C for 120 s. As a control, the GAPDH gene was applied under the same conditions using 0.125 μM each primer (R&D Systems, Wiesbaden, Germany). Different dilutions of PCR products were analyzed on a 1% agarose gel stained with ethidium bromide.
Determination of the transcription start site of the human JAM-C gene and cloning of the promoter 5′-flanking region and production of JAM-C promoter construct
The transcription start site of the JAM-C gene was determined by rapid amplification of cDNA ends (RACE) by using the SMART™ RACE amplification kit (Clontech, Palo Alto, CA, USA), as recommended by the manufacturer. Total RNA from HUVECs was used for first-strand cDNA synthesis by RT using the SMART II A™ oligonucleotide and gene-specific primer-1 from position +130 to +105 of the previously published cDNA sequence (5′-TAC AGC CCC TAT CAG GCA GCC CCT GA-3′) and gene-specific primer-2 from position +319 to +294 (5′-CAG TGT TTC TGC ACG ACC CGC CAA GTC-3′) [10]. The PCR products were cloned directly into the pGEM-T easy vector by the TA cloning strategy, according to the manufacturer’s recommendations, and DNA sequencing showed that the sequence for JAM-C ended with the base C at the most 5′ end (see Fig. 5). This C nucleotide was assigned as the start site of transcription and numbered +1. The human JAM-C promoter encompassing nucleotides +35 until –1282 (see Fig. 5) was prepared from genomic DNA of leukocytes by PCR using forward primer from position –1265 to –1282 (5′-GTC AGT GGG CAT AAG ACG-3′) and a reverse primer from position +35 to +20 (5′-CAT GTC GAG GGT TGC TGA GG-3′) relative to the transcription initiation site. The 1317-bp PCR product was cloned into the pGEM-T easy vector, and the identity of the JAM-C insert was confirmed by DNA sequencing using a Taq-FS dye terminator cycle sequencing kit and analyzed on ABI PRISM genetic analyzer 310 (Applied Biosystems, Foster City, CA, USA).
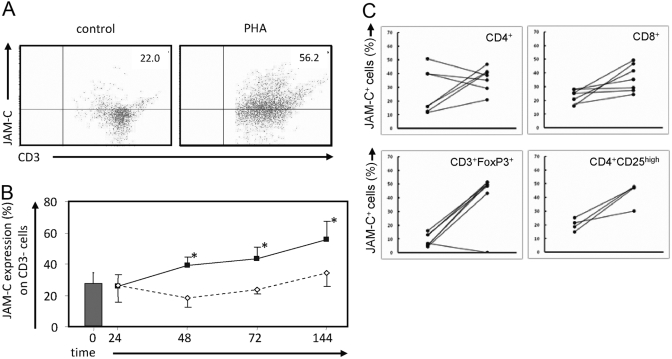
Fig. 1.
Up-regulation of JAM-C on the cell surface of PHA-activated human T lymphocytes. Human T cells were isolated as described in Materials and Methods and were cultured in the absence or presence of PHA (1 μg/ml) for 48 h. Lymphocytes were analyzed by flow cytometry with FITC-labeled anti-CD3 antibody and allophycocyanin-conjugated JAM-C antibody. Fluorescence-labeled mouse isotypes IgG2b and IgG1 were used as negative controls. (A) One representative from a total of nine independently performed experiments is shown. Numbers in the upper right indicate the percentage of JAM-C+ CD3+ cells. (B) Analysis of JAM-C expression on T cells shows a time dependency. *, P < 0.05, as determined by ANOVA; multiple comparisons were performed using the Bonferroni procedure. □, JAM-C expression under untreated conditions; ▪, JAM-C expression during PHA treatment. (C) Characterization of JAM-C-expressing cells before (left points) and after (right points) PHA stimulation. Isolated JAM-C+ CD8+ or CD4+ cells were stained for the markers indicated. The most pronounced increase in JAM-C expression is observed in cells staining positive for surface markers found on regulatory T cells (Tregs), i.e., CD3+forkhead box P3+ (FoxP3+) and CD4+CD25high. Changes in JAM-C expression (%) on indicated cell types are shown.
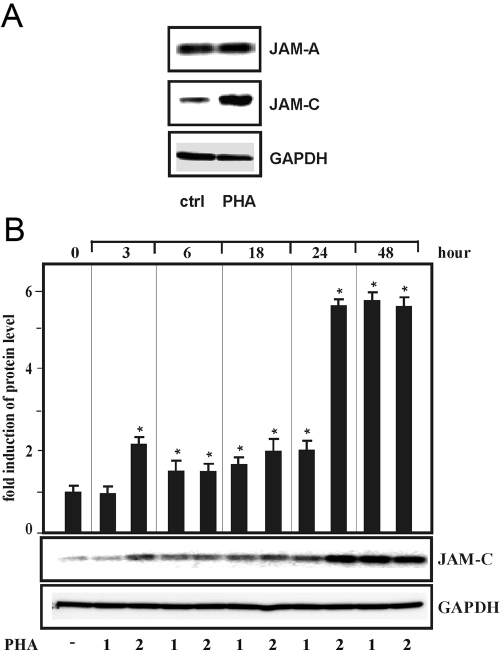
Fig. 2.
PHA-dependent induction of endogenous JAM-C gene expression in human T cells, which were isolated as described in Materials and Methods and were cultured (A) for 24 h in the presence of PHA (2 μg/ml). Total protein (20 μg) was subjected to Western blot analysis and probed sequentially with antibodies against human JAM-A, JAM-C, and GAPDH. The result of a representative Western blot is shown. (B) T cells were cultured with PHA at 1 and 2 μg/ml for the times indicated. Western blot analysis was performed as described above and a representative Western blot shown. Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry. Values ± se represent the fold induction of JAM-C protein levels normalized to GAPDH from at least three independent experiments. Statistics, Student’s t-test for paired values: *, significant differences, PHA versus control (ctrl), P ≤ 0.05.
Fig. 3.
Induction of JAM-C protein expression by antibodies against CD3 and CD28 in human T cells, which were treated for 18 h with PHA (2 μg/ml) and with mAb against CD3 and CD28 (1 μl/ml, respectively). Total protein (20 μg) was subjected to Western blot analysis and probed sequentially with antibodies against human JAM-C and GAPDH. A representative Western blot is shown in the upper panels. Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry. Values ± se represent the fold induction of JAM-C normalized to GAPDH from at least three independent experiments. Statistics, Student’s t-test for paired values: *, significant differences treatment versus control, P ≤ 0.05.
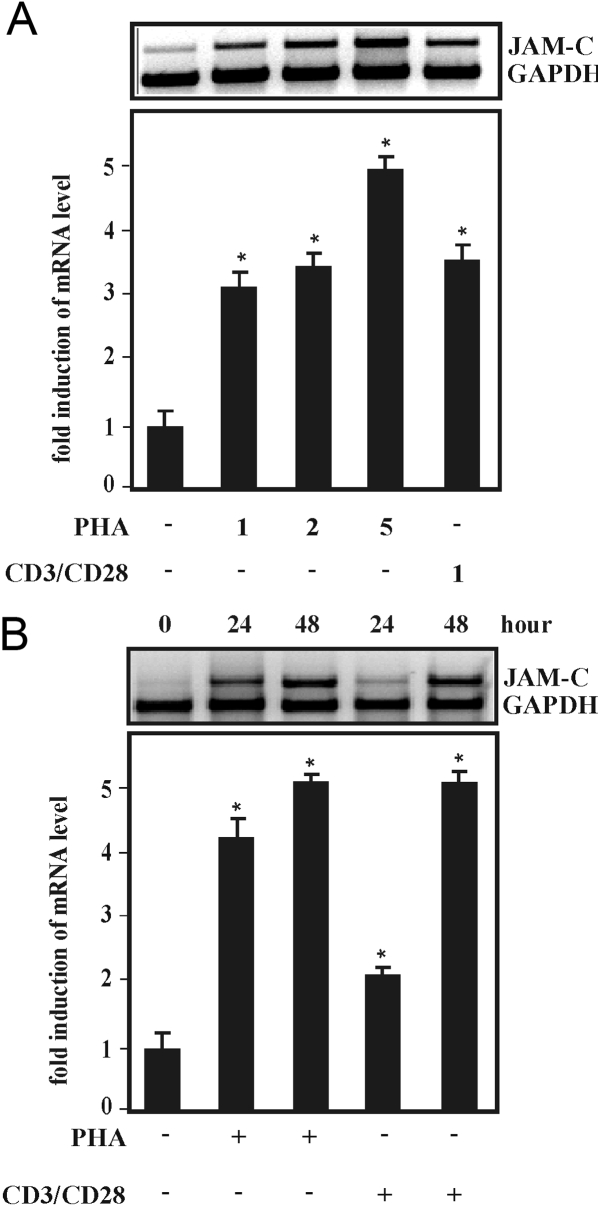
Fig. 4.
Induction of JAM-C mRNA expression by PHA and CD3 plus CD28 in human T cells, which were (A) treated with PHA at the indicated concentrations (μg/ml), mAb against CD3 plus CD28 (1 μg/ml), or control media for 18 h or (B) with PHA (2 μg/ml), mAb against CD3 plus CD28 (1 μl/ml), or control media for the times indicated. Total RNA was isolated, and 15 μg RNA was processed for RT-PCR as described in Materials and Methods. The obtained PCR products were analyzed on an agarose gel using GAPDH as internal standard. Autoradiograms from representative experiments are shown, respectively. Values ± se represent the fold induction of JAM-C mRNA normalized to GAPDH mRNA from at least three independent experiments. Statistics, Student’s t-test for paired values: *, significant difference treatment versus control.
Fig. 5.
Cloning of the human JAM-C gene promoter region and identification of potential NFAT sites. The sequence of the human JAM-C promoter is shown. Nucleotides are numbered relative to the transcription initiation site +1 and the GC box, and DNA sequences with homology to the consensus motif for NFAT, AP-1, and NF-κB are in bold type and underlined.
For the production of JAM-C promoter constructs, the JAM-C insert in the pGEM-T easy vector (see above) was reamplified by PCR using the same primers containing restriction sites of KpnI and XhoI endonuclease. The purified PCR product was then digested with these restriction enzymes (New England Biolabs, Frankfurt, Germany) and subcloned into the KpnI and XhoI cloning sites of the promoterless pGL3basic luciferase plasmid (Promega, Madison, WI, USA) to generate pJAM-1282. Subsequently, pJAM-1282 construct was sequenced for confirmation. Dr. James A. Goodrich (University of Colorado, Boulder, CO, USA) [20] provided the pGL3-NFAT3 plasmid. For the transfection study (see below), all plasmids were highly purified by the use of the EndoFree plasmid kits (Qiagen).
Transfection of human T cells and luciferase assay
Transfection of purified T lymphocytes was performed with human T cell Amaxa’s nucleofector method (Amaxa, Cologne, Germany). Freshly isolated. unstimulated T cells (4–10×106) were transfected with 1 μg reporter construct (pJAM-1282 or pGL3-NFAT3) and 0.2 μg Renilla luciferase vector (Promega) in the presence of 100 μl human T cell nucleofector solution for 20 min using the optimal program U-014 (Amaxa). Transfected cells were resuspended in 500 μl hepatoma tissue culture media (Amaxa), and after transfection, cells were cultured for 6 h in the presence of PHA, mAb against CD3 and CD28, or under control conditions. Cells were lysed, and luciferase activity was determined with the dual-luciferase reporter assay system (Promega) on Lumat LB9507 (Berthold Technologies, Bad Wildbach, Germany). The relative light units were correlated with the Renilla activity as described previously [21].
RESULTS
Induction of JAM-C gene expression of human T lymphocytes by PHA
JAM-C expression has been shown previously to be up-regulated on the surface of peripheral human T lymphocytes after pharmacological activation with PMA and ionomycin [17]. To further investigate the mechanism(s) of how JAM-C expression is regulated in primary human T cells, we examined the expression of JAM-C in isolated resting T cells or in T cells that were treated with the polymeric carbohydrate-binding protein PHA, which is a potent polyclonal stimulator of resting T cells and causes activation, regardless of peptide-MHC specificity. In flow cytometry analysis, we observed that PHA treatment of T cells over 48 h led to a marked increase of JAM-C-expressing cells (Fig. 1). Whereas only 22.0% JAM-C+ cells were found in resting CD3+ cells, 56.2% JAM-C carrying CD3+cells were detectable after treatment with PHA, as demonstrated in a representative experiment (Fig. 1A). The increase of JAM-C expression on total CD3+ T lymphocytes was time (Fig. 1B)- and dose-dependent (data not shown). To further determine whether various subsets of T cells would express different levels of JAM-C after exposure to PHA, we identified T cell populations in JAM-C+ cells by labeling with different T cell markers. These studies indicated that CD3+FoxP3+ and CD4+CD25high cells exhibited the highest level of JAM-C induction after PHA stimulation (Fig. 1C).
To investigate JAM-C up-regulation in activated T cells in more detail, we analyzed the expression of the JAM-C gene in T cells by immunoblotting. As shown in Figure 2A, treatment with PHA increased JAM-C protein expression markedly, whereas JAM-A expression was not up-regulated after exposure to PHA. In addition, PHA-dependent induction of JAM-C was dose- and time-dependent with a maximum level of expression after 24 h (Fig. 2B).
To determine whether specific activation of the TCR would affect JAM-C expression, T cells were treated with mAb against CD3 and CD28. Treatment of T cells with both mAb led to a significant up-regulation of JAM-C expression levels, which was in a similar range to that elicited by PHA treatment (Fig. 3). Taken together, the data show that endogenous JAM-C gene expression is induced markedly in activated human T lymphocytes.
Induction of JAM-C mRNA expression in T cells
JAM-C expression has been shown recently to be up-regulated via induction of mRNA synthesis in synovial fibroblasts in a mouse model of experimental arthritis [22]. Moreover, the time-course of JAM-C expression after stimulation with PHA could suggest a transcriptional regulation in T lymphocytes. Therefore, we determined the level of JAM-C mRNA levels by semi-quantitative RT-PCR. JAM-C mRNA expression levels were up-regulated with increasing concentrations of PHA (Fig. 4A). In addition, treatment with CD3/CD28 mAb induced JAM-C mRNA levels to a similar magnitude in a time-dependent manner (Fig. 4B). These results suggest that JAM-C gene induction in activated T lymphocytes is mediated via a transcriptional mode of action.
Cloning of the human JAM-C gene promoter and identification of potential NFAT sites
To investigate a potential transcriptional mode of JAM-C gene induction in activated T cells, we cloned the promoter and 5′-flanking region of the human JAM-C gene and determined the transcriptional start site as detailed in Materials and Methods. The obtained 1317-bp DNA fragment was subcloned into pGEM-T, and the entire nucleotide sequence of this promoter region is shown in Figure 5. The transcription initiation site was localized 35 bp upstream of the translational start site. No TATA box was identified within the cloned proximal JAM-C promoter region, and a GC box was found at position –23 to –14 relative to the transcription start site. Putative cis-acting elements were localized by computer-assisted sequence comparison. Specifically, three potential consensus sequences (T/A)GGAAA were found, which could serve as binding sites for the transcription factor NFAT that mediates TCR-dependent activation signals in T cells (Fig. 5) [23]. In addition, a consensus DNA element with an 86% match for the classical AP-1 consensus sequence [24] and a DNA element, which exhibits an 80% match with the classical κB consensus site [25], were found in the proximal human JAM-C promoter region
Induction of JAM-C promoter activity in activated T cells
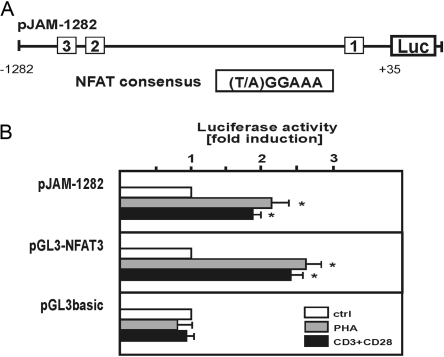
To evaluate the functional capacity of the cloned human JAM-C 5′-flanking promoter region, the luciferase reporter gene construct pJAM-1282 (Fig. 6A) was transiently transfected into T cells. Luciferase activity of this construct was determined under basal control conditions or upon activation by treatment with PHA or by that with mAb against CD3 and CD28 (Fig. 6B). For a comparison, the effect of these stimuli in human T cells was also determined for the pGL3-NFAT3 reporter gene construct, which contains three copies of the NFAT consensus sequence [20]. The level of induction that was conferred by the cloned human JAM-C promoter region to the luciferase reporter gene construct was in a similar range to that determined for the control construct pGL3-NFAT3 (Fig. 6B). By contrast, the empty reporter gene vector pGL3basic showed no regulation in response to these stimuli in transfected T cells. The results suggest that JAM-C gene expression is transcriptionally induced in activated T cells and that the transcription factor NFAT may be involved in this regulation.
Fig. 6.
Induction of JAM-C gene promoter activity in activated human T cells. (A) Localization of three NFAT consensus sites in the proximal promoter region of the human JAM-C gene is indicated. (B) Human T cells were transfected with luciferase (Luc) reporter gene constructs containing the proximal 1282 bp of the human JAM-C gene promoter region (pJAM-1282), three copies of the consensus sequence of NFAT (pGL3-NFAT3), or the control plasmid pGL3basic as described in Materials and Methods. Six hours after transfection, cells were treated for another 6 h in the absence or presence of PHA (2 μg/ml) or a combination of mAb against CD3 and CD28, as indicated. Cell extracts were assayed for luciferase activity, and the fold induction in each experiment relative to the control was determined. Values are means ± se from at least three independent experiments with duplicates of each point. Statistics, Student’s t-test for paired values: *, significant differences treatment versus control, P ≤ 0.05.
Calcineurin inhibitors block PHA-dependent induction of JAM-C gene expression
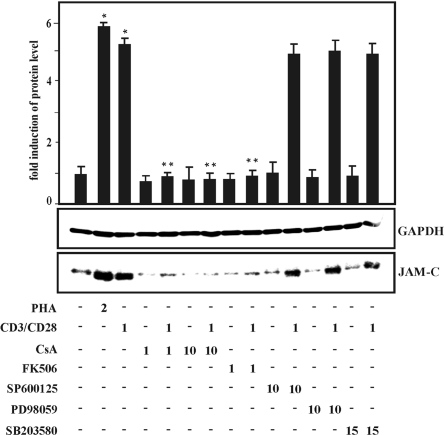
Regulation of gene expression via NFAT in activated T lymphocytes is mediated via the serine/threonine phosphatase calcineurin, which can be blocked specifically by pharmacological inhibitors such as cyclosporin A and FK-506, both of which are potent immunosuppressants [26]. We applied both inhibitors to identify the potential signaling pathways that could be involved in JAM-C gene induction of activated T cells. Cyclosporin A inhibited JAM-C induction by PHA in a dose-dependent manner, and similar results were obtained with FK-506 (Fig. 7). Moreover, an inhibitory effect of these compounds on PHA-dependent up-regulation of JAM-C surface expression was determined by flow cytometry analysis (data not shown). MAPK signaling pathways are involved in the regulation of gene expression in activated T cells [27]. Application of different, specific pharmacological inhibitors of the three major MAPK signaling cascades ERK, JNK, and p38 MAPK, however, did not result in a significant effect on PHA-induced JAM-C gene expression (Fig. 7).
Fig. 7.
Effect of calcineurin inhibitors and MAPK inhibitors on JAM-C induction in activated human T cells, which were cultured and pretreated for 1 h with cyclosporin A (CsA; 1 and 10 μM) or FK-506 (1 μM) or with MAPK inhibitors SP600125 (10 μM), PD98059 (10 μM), and SB203580 (15 μM), and cell culture was continued for 24 h in the absence or presence of PHA (2 μg/ml) or a combination of mAb against CD3 and CD28 (1 μg/ ml), as indicated. Total protein (20 μg) was analyzed by Western blot analysis with antibodies against human JAM-C and GAPDH. Similar results were obtained in three independent experiments. An autoradiogram of a representative experiment is shown. Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry. Values ± se represent the fold induction of JAM-C normalized to GAPDH from at least three independent experiments. Statistics, Student’s t-test for paired values: *, significant differences treatment versus control, **, treatment + CsA versus treatment or treatment + FK-506 versus treatment, P ≤ 0.05.
Taken together, the data suggest that the induction of JAM-C gene expression in activated T lymphocytes is mediated via stimulation of the calcium-dependent phosphatase calcineurin but not via MAPK signaling pathways.
DISCUSSION
In the present report, we demonstrate that JAM-C gene expression is transcriptionally induced during activation of T lymphocytes via a calcineurin-dependent signaling pathway.
Induction of JAM-C gene expression in activated T cells via a transcriptional mechanism
JAM-C has been shown previously to be highly expressed in a T cell line but not in resting human T cells [17]. Here, we demonstrate that gene expression of JAM-C is markedly induced in T cells by the lectin PHA, which activates T lymphocytes, independent of physical interaction of the TCR with MHC-presented peptides. The highest level of JAM-C surface up-regulation after PHA stimulation was observed for CD3+FoxP3+ and CD4+CD25high T cells (Fig. 1C), suggesting an important role of JAM-C in Tregs. JAM-C was also induced by treatment with mAb against CD3 and CD28, which is a well-established tool to trigger TCR signaling [27]. In contrast, gene expression of another JAM protein family member, JAM-A, was not induced in activated T cells, which is in agreement with previous findings by Williams and colleagues [28]. An increased JAM-C expression in activated T cells is in accordance with an earlier report by Arrate et al. [17], demonstrating JAM-C surface expression on T lymphocytes to be up-regulated by pharmacological activation with PMA and ionomycin. Our present data clearly show that the induction of JAM-C in activated T cells is transcriptionally regulated, as luciferase activity of a reporter gene construct with the human JAM-C gene promoter was up-regulated in activated T cells (Fig. 6). Similar to the present findings, a transcriptional mechanism of JAM-C gene induction has been shown recently in a study by Palmer et al. [22], in which JAM-C mRNA levels were reported to be increased markedly in fibroblasts of mice with an experimental arthritis. Transcriptional gene regulation has also been demonstrated for other cell adhesion molecules such as VCAM, ICAM-1, and E-cadherin, all of which are known to be induced by various proinflammatory stimuli. It is important to note, however, that JAM-C surface expression is up-regulated in human dermal micro-VE cells via a mechanism independent of transcription [14]. This mechanism involves the rapid and transient translocation of the protein from intracellular storage pools to the plasma membrane upon stimulation with VEGF and histamine.
JAM-C induction via a calcineurin-dependent signaling pathway in activated T cells
Gene expression in activated T lymphocytes is regulated via a complex network of pathways comprising a variety of signaling molecules such as protein kinase C θ, phospholipase C-γ1, the protein serine/threonine phosphatase calcineurin, and MAPKs (for a review, see refs. [27, 29]). Our present data indicate that calcineurin plays a major role for the induction of JAM-C expression in activated T lymphocytes, as the pharmacological inhibitors cyclosporin A and FK-506 abrogated the PHA-dependent induction of JAM-C gene expression (Fig. 7). By contrast, pharmacological inhibition of the MAPKs ERK, JNK, and p38 MAPK did not alter the up-regulation of JAM-C in activated T cells (Fig. 7). A major regulatory role of calcineurin-dependent signaling for JAM-C gene induction is consistent with the fact that the luciferase activity of a reporter gene construct with the proximal promoter 5′-flanking region of the human JAM-C gene was induced in transiently transfected, activated T cells. The proximal sequence of the 5′-flanking sequence of the human JAM-C promoter contained three potential NFAT-binding sites with the 6-bp core sequence (T/A)GGAAA (Fig. 5) [23]. The level of luciferase induction for the pJAM-1282 reporter gene construct in activated T cells was in a similar range to that of a control gene construct with three copies of the classical NFAT site (Fig. 6) [20]. NFAT is the major NF of T cells, which regulates the inducible gene expression of a variety of genes such as IL-2 (for a review, see ref. [30]). Other transcription factors that regulate gene expression in activated T cells are AP-1 and NF-κB [27, 29]. Potential consensus sequences for the latter NFs have been identified in the human JAM-C gene promoter region, as indicated in Figure 5. Further studies are under way to characterize potential regulatory DNA elements, which could be involved in the JAM-C gene induction in activated T lymphocytes.
Physiological significance of JAM-C induction in activated T lymphocytes
The induction of JAM-C in activated T cells implies a role of JAM-C in T cell functions. The homophilic and heterophilic interactions of JAM-C may participate in the regulation of homotypic intercellular contacts between activated T cells and/or of heterotypic interactions of T cells with other cells of the immune system or endothelial cells. Specifically, JAM-C can undergo heterophilic interactions with the Mac-1 integrin [10], which is expressed on cells of the innate immunity such as macrophages or dendritic cells. Thus, a potential involvement of the JAM-C interaction with Mac-1 in the formation of the immune synapse between activated T cells and Mac-1 expressing APC can be envisioned. Furthermore, an increased surface density of JAM-C on activated T cells may be involved in the regulation of homotypic JAM-C interactions of T cells with the endothelium. In fact, JAM-C has been implicated in T cell transmigration through an endothelial cell monolayer, which has been attributed to homophilic interactions of JAM-C [31]. Thus, JAM-C may regulate the specific migration and retention of T cells at the site of inflammation [32, 33]. How JAM-C-dependent, adhesive interactions of T cells may interact with other adhesive events mediated by the LFA-1/ ICAM-1 and VLA-4/ VCAM-1 systems is an intriguing question that needs to be addressed experimentally in the future.
An increased expression of JAM-C may also be involved in intracellular signal transduction pathways of activated T cells. JAM-C has been shown previously to mediate cellular signaling events in micro-VE cells via regulating the activity of the small GTPase Rap1 and thereby, VE-cadherin-dependent cell–cell contacts [14]. Independently, another member of the JAM family, JAM-A, has been shown recently to play a critical role for an intact mode of cell–cell interaction of hepatic cells via modulation of VE-cadherin expression [34].
In conclusion, we have shown that JAM-C is induced transcriptionally in activated human T lymphocytes via a calcineurin-dependent pathway. As a result of the importance of T cell activation in a variety of pathophysiological situations, further studies about the mechanisms that are involved in the regulation of JAM-C gene expression are necessary. This understanding may help to develop novel, therapeutic strategies for the treatment of inflammatory disease and the prevention of organ transplant rejection.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft SFB 547 (S. I. and S. S.). We thank R. Böhnel, S. Werth, and A. Zeyer for excellent technical assistance. We also thank Dr. James A. Goodrich for the supply of the plasmid pGL3-NFAT3.
References
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Mandell K J, Parkos C A. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kostrewa D, Brockhaus M, D'Arcy A, Dale G E, Nelboeck P, Schmid G, Mueller F, Bazzoni G, Dejana E, Bartfai T, Winkler F K, Hennig M. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 2001;20:4391–4398. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso S, Orlova V V, Song K, Sachs U J, Andrei-Selmer C L, Chavakis T. The homophilic binding of junctional adhesion molecule-C mediates tumor cell–endothelial cell interactions. J Biol Chem. 2005;280:36326–36333. doi: 10.1074/jbc.M505059200. [DOI] [PubMed] [Google Scholar]
- Muller W A. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Bradfield P F, Nourshargh S, Aurrand-Lions M, Imhof B A. JAM family and related proteins in leukocyte migration (Vestweber series) Arterioscler Thromb Vasc Biol. 2007;27:2104–2112. doi: 10.1161/ATVBAHA.107.147694. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof B A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- Ody C, Jungblut-Ruault S, Cossali D, Barnet M, Aurrand-Lions M, Imhof B A, Matthes T. Junctional adhesion molecule C (JAM-C) distinguishes CD27+ germinal center B lymphocytes from non-germinal center cells and constitutes a new diagnostic tool for B-cell malignancies. Leukemia. 2007;21:1285–1293. doi: 10.1038/sj.leu.2404689. [DOI] [PubMed] [Google Scholar]
- Santoso S, Sachs U J, Kroll H, Linder M, Ruf A, Preissner K T, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs U J, Nawroth P P, Preissner K T, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- Zen K, Babbin B A, Liu Y, Whelan J B, Nusrat A, Parkos C A. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926–3937. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert R J, Jones E Y, Kiefer F, Ruga P, Imhof B A, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and α(M)β2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova V V, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell–cell contacts. J Exp Med. 2006;203:2703–2714. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C, Hodivala-Dilke K M, Imhof B A, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–5710. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- Keiper T, Al-Fakhri N, Chavakis E, Athanasopoulos A N, Isermann B, Herzog S, Saffrich R, Hersemeyer K, Bohle R M, Haendeler J, Preissner K T, Santoso S, Chavakis T. The role of junctional adhesion molecule-C (JAM-C) in oxidized LDL-mediated leukocyte recruitment. FASEB J. 2005;19:2078–2080. doi: 10.1096/fj.05-4196fje. [DOI] [PubMed] [Google Scholar]
- Arrate M P, Rodriguez J M, Tran T M, Brock T A, Cunningham S A. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276:45826–45832. doi: 10.1074/jbc.M105972200. [DOI] [PubMed] [Google Scholar]
- Rubant S A, Ludwig R J, Diehl S, Hardt K, Kaufmann R, Pfeilschifter J M, Boehncke W H. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J Invest Dermatol. 2008;128:326–331. doi: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- Wijayanti N, Kietzmann T, Immenschuh S. Heme oxygenase-1 gene activation by the NAD(P)H oxidase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride via a protein kinase B, p38-dependent signaling pathway in monocytes. J Biol Chem. 2005;280:21820–21829. doi: 10.1074/jbc.M502943200. [DOI] [PubMed] [Google Scholar]
- Kim L J, Seto A G, Nguyen T N, Goodrich J A. Human Taf(II)130 is a coactivator for NFATp. Mol Cell Biol. 2001;21:3503–3513. doi: 10.1128/MCB.21.10.3503-3513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immenschuh S, Hinke V, Ohlmann A, Gifhorn-Katz S, Katz N, Jungermann K, Kietzmann T. Transcriptional activation of the haem oxygenase-1 gene by cGMP via a cAMP response element/activator protein-1 element in primary cultures of rat hepatocytes. Biochem J. 1998;334:141–146. doi: 10.1042/bj3340141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G, Busso N, Aurrand-Lions M, Talabot-Ayer D, Chobaz-Peclat V, Zimmerli C, Hammel P, Imhof B A, Gabay C. Expression and function of junctional adhesion molecule-C in human and experimental arthritis. Arthritis Res Ther. 2007;9:R65. doi: 10.1186/ar2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Chen L F, Greene W C. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez S, Redondo J M. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004;11:997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- Germain R N, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- Williams L A, Martin-Padura I, Dejana E, Hogg N, Simmons D L. Identification and characterization of human junctional adhesion molecule (JAM) Mol Immunol. 1999;36:1175–1188. doi: 10.1016/s0161-5890(99)00122-4. [DOI] [PubMed] [Google Scholar]
- Isakov N, Altman A. Protein kinase C(θ) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Johnson-Leger C A, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof B A. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- Von Andrian U H, Mackay C R. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Ludwig R J, Schultz J E, Boehncke W H, Podda M, Tandi C, Krombach F, Baatz H, Kaufmann R, von Andrian U H, Zollner T M. Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J Invest Dermatol. 2004;122:830–836. doi: 10.1111/j.0022-202X.2004.22318.x. [DOI] [PubMed] [Google Scholar]
- Konopka G, Tekiela J, Iverson M, Wells C, Duncan S A. Junctional adhesion molecule-A is critical for the formation of pseudocanaliculi and modulates E-cadherin expression in hepatic cells. J Biol Chem. 2007;282:28137–28148. doi: 10.1074/jbc.M703592200. [DOI] [PubMed] [Google Scholar]