Abstract
Mast cell development is an important component of atopic and chronic inflammatory diseases such as asthma, multiple sclerosis, rheumatoid arthritis, and atherosclerosis. In this study, we found that IL-4 and IL-10 were produced constitutively in cultures of developing mast cells, correlating with mast cell purity. Deletion of either gene increased mast cell numbers and FcεRI expression during culture in IL-3 + stem cell factor (SCF). By adding exogenous IL-4 and IL-10 to bone marrow (BM) cultures containing IL-3 + SCF, we found that IL-4 + IL-10 suppressed mast cell development through mechanisms not used by either cytokine alone. IL-4 + IL-10 elicited a rapid cell death coincidental with reduced Kit receptor expression and signaling and enhanced mitochondrial damage and caspase activation. IL-4 or IL-10 costimulation, unlike either cytokine alone, altered mast cell ontogeny to yield predominantly macrophages in cultures that typically produce mast cells. This effect was observed consistently with unseparated BM cells, purified mouse BM stem cells, and erythrocyte-depleted human umbilical cord blood cells. These experiments demonstrated a major role for Stat6 and Stat3, but not the Stat3-induced transcriptional repressor Ets variant gene 3. Genetic background was also a critical factor, as BALB/c-derived BM cells were completely resistant to IL-10-mediated killing and expressed lower levels of IL-10R. Collectively, these results support the theory that IL-4 and IL-10 function as endogenous regulators of mast cell progenitor development, consistent with a role in immune homeostasis. Loss of this homeostasis, perhaps via genetic polymorphism, could contribute to the etiology of mast cell-associated disease.
Keywords: ETV3, bone marrow, interleuken-10, interleuken-4, Stat3
INTRODUCTION
Mast cells are known as effectors of Th2-mediated and innate immunity. Dysregulation of this response contributes to inflammatory diseases including asthma and allergy (reviewed in refs. [1,2,3]). Although traditionally studied in terms of atopic disease, the known role of the mast cell is diverse and expanding to include processes such as tissue remodeling and tumor angiogenesis and chronic inflammatory diseases such as multiple sclerosis, rheumatoid arthritis, and atherosclerosis [4,5,6,7,8,9]. Mast cells originate from pluripotent hematopoietic stem cells in the bone marrow (BM) but differentiate and mature in connective and mucosal tissues, largely as a result of signaling via the Kit growth factor receptor [10]. Controlling this developmental pathway could be critical to mast cell homeostasis and by extension, the conditions yielding inflammatory disease.
Mast cell development and function can be controlled partly by Th2-derived cytokines such as IL-4 and IL-10 through direct and indirect mechanisms [1]. Indirectly, IL-4 and the related cytokine IL-13 are required for B cell IgE isotype switching, which subsequently up-regulates FcεRI expression on mature mast cells [11,12,13]. IL-4 can also signal mast cells directly through the IL-4Rα and common γ chain. Although IL-4 activates multiple signal transduction pathways, Stat6 is perhaps the most critical [14,15,16]. Specifically, it was found that the addition of IL-4 to developing mouse BM-derived mast cells (BMMC) resulted in Stat6-dependent apoptosis [17,18,19]. IL-4 also acts via Stat6 to suppress FcεRI expression and signaling on differentiated mouse mast cells [20].
The reported effects of IL-4 on human mast cell development are somewhat contradictory [21,22,23,24,25,26]. IL-4 addition to developing cord blood progenitors decreased the percentage of mast cells as well as FcεRI and Kit expression [23]. However, in fetal liver mast cells, IL-4 was found to inhibit proliferation while promoting maturation [24]. Lastly, IL-4 has been found to increase proliferation and FcεRI expression in human intestinal mast cells [25]. One explanation for this is that the effects of IL-4 are maturation-dependent, allowing positive and negative regulation. This supposition is supported by a recent study showing that the suppressive effects of IL-4 are more apparent at early stages of mast cell development [26].
Another Th2 cytokine that is known for regulating mast cells is IL-10. We noted that IL-10 suppressed survival of developing mast cells in BM cultures [27] and decreased FcεRI function on differentiated mouse mast cells [28, 29]. Although IL-10 has been shown to similarly suppress IgE signaling on differentiated human mast cells [30], it had no effect on survival of fetal liver progenitors [31].
As IL-4 and IL-10 would logically be available simultaneously during the Th2 response, we have investigated the effects of costimulation with these cytokines. This is important, as combinations of cytokines frequently have synergistic or unexpected effects on target cells. For example, we found that the combination of IL-4 + IL-10, but neither cytokine alone, induced apoptosis and cell-cycle arrest in differentiated mouse mast cells [17]. As mast cell differentiation status can alter cytokine responses, the current study explored the combined effects of IL-4 and IL-10 on mast cell development from mouse and human precursors.
Our results support the theory that IL-4 and IL-10 function as endogenous regulators of mouse and human mast cell homeostasis, produced by developing mast cells as an autocrine/paracrine signal that suppresses precursor survival. These effects are mediated in part by Stat3 and Stat6 and result in loss of Kit receptor expression and signaling with an associated apoptosis via the intrinsic mitochondrial pathway. Combined signaling by IL-4 + IL-10 yielded a qualitatively distinct response, with effects on development not observed with either cytokine alone. There was also a striking genetic component to this effect, as BALB/c mice are completely resistant to the effects of IL-10. Loss of mast cell homeostasis is a logical part of inflammatory disease etiology. Understanding this process is therefore of considerable clinical importance.
MATERIALS AND METHODS
Mouse BM cell culture
BM cells were extracted from the femurs of mice, including BALB/c, C57BL/6, and C57BL/6x129 and STAT6-, IL-4-, IL-10-, p53-, and Bax-deficient mice purchased from Jackson Laboratories (Bar Harbor, ME, USA). Note that BALB/c background mice were only used in the studies shown (see Fig. 8). H2K-Bcl-2 transgenic (Tg) BM cells were the kind gift of Jos Domen (Duke University, Durham, NC, USA). Stat3-flox/flox and Tie2-Cre mice were generously provided by Dr. Kiyoshi Takeda and Dr. Pandelakis Koni and were bred to yield fl/Δ Cre+ and Cre– littermates as described [32]. Ets variant gene 3 (ETV3)-deficient mice were provided by Dr. Peter Murray and have not yet been published. All BM cells were cultured as described previously [33] in media containing IL-3 (5 ng/ml) and stem cell factor (SCF; 50 ng/ml). Other cytokines were added at the following concentrations: IL-4 (10 ng/ml), IL-10 (20 ng/ml), IL-13 (50 ng/ml), and IL-22 (50 ng/ml; R&D Systems, Minneapolis, MN, USA). Cells in suspension were transferred to new plates with fresh medium every 4–7 days. All cultures were plated and transferred under identical conditions, such that relative cell counts could be obtained.
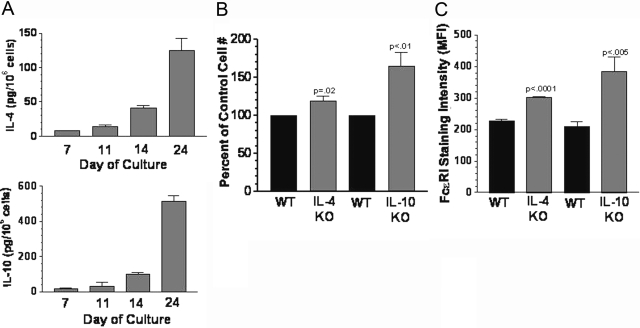
Fig. 1.
IL-4 and IL-10 are endogenous regulators of mast cell development. (A) Wild-type (WT) BM cells were cultured in IL-3 + SCF, and the concentration of IL-4 and IL-10 in the media was determined by ELISA at the indicated times. Cytokine concentrations were normalized to 1 × 106 cells/ml. Data shown are mean and sem from six to 12 samples/point. (B and C) BM cells from IL-4 knockout (KO) or IL-10 KO mice were cultured for 3 weeks in IL-3 + SCF, and viable cell numbers (B) or average FcεRI expression intensity (C) were determined by flow cytometry. Values shown are mean and sem from six to eight samples/point. All P values are for comparisons with matched littermate (WT) cells.
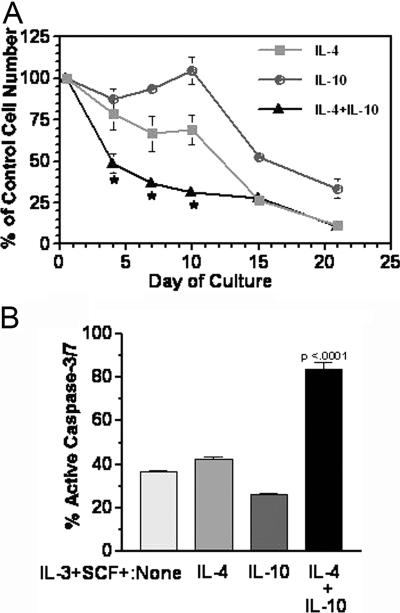
Fig. 2.
Costimulation with IL-4 + IL-10 suppresses survival of developing mast cells. (A) Mouse BM cells were cultured as described in IL-3 + SCF ± IL-4 and/or IL-10. At the indicated times, live cell numbers were determined by timed flow cytometry counting. Percent of control cultures (IL-3+SCF) was determined. Data shown are mean and sem of three to 12 samples/point. *, P < .05, by ANOVA when comparing IL-4 + IL-10 with IL-4 or IL-10. (B) Active caspases-3/7 was detected by flow cytometric analysis on Day 7 of culture as described. Data shown are four samples from two of five representative experiments. P value shown is for IL-4 + IL-10 compared with all samples.
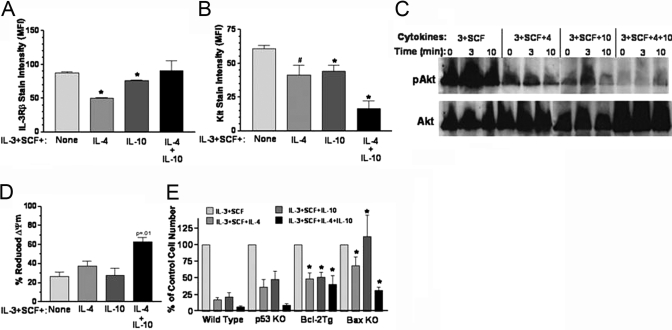
Fig. 3.
The effect of IL-4 and IL-10 on IL-3Rβ and Kit receptor expression. (A) Mouse BM cells were cultured in IL-3 + SCF ± IL-4 and/or IL-10 for 10 days, and IL-3Rβ expression was measured by flow cytometry staining. The average and sem of MFI measurements from five to six samples per point are shown. *, P < .001, when compared with cells cultured in IL-3 + SCF. (B) Cells were cultured as in A for 12–15 days, and Kit receptor expression was measured by flow cytometry. The average and sem of MFI measurements from five to six samples per point are shown. #, P < .025; *, P < .005, when compared with IL-3 + SCF control. IL-4 + IL-10 sample also has P < .005 when compared with IL-4 or IL-10 alone. (C) Cells were cultured for 7 days as in A, washed, incubated without cytokines for 4 h, and then restimulated with the original culture conditions for the indicated times. Total cell lysates were examined by Western blotting for serine-pAkt, stripped, and reprobed to detect total Akt. Data shown are one of two representative experiments. (D) Loss of mitochondrial membrane potential (ΔΨm) was determined by Di(OC6)3 staining of BM cells cultured for 7–8 days. Data shown are mean and sem from four samples harvested in four separate experiments. P value shown is for IL-4 + IL-10 compared with all samples. (E) BM cells harvested from various targeted mouse strains were cultured in the indicated cytokines as described for Figure 2. Relative live cell numbers were determined by flow cytometric counting of cells stained for PI-DNA analysis on Day 21 of culture. Data shown are mean and sem from four to six samples per point. *, P < .05, when compared with WT samples cultured under identical conditions.
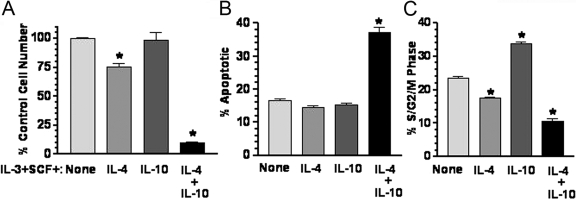
Fig. 4.
IL-4 + IL-10 suppresses survival of hematopoietic stem cells. Kit+/lineage– BM cells were prepared as described in Materials and Methods and cultured in IL-3 + SCF ± IL-4 and/or IL-10 for 21 days. Viable cell number (A), percent apoptotic cells (B), and the percentage of cells in proliferative phases of the cell cycle (S/G2/M) (C) were determined by PI-DNA staining of fixed cells after 3 weeks of culture. Data shown are mean and sem from four to five samples/point. *, P < .001, when compared with IL-3 + SCF control (“None”). Note that IL-4 + IL-10 also has P < .001 when compared with IL-4 in A.
Fig. 5.
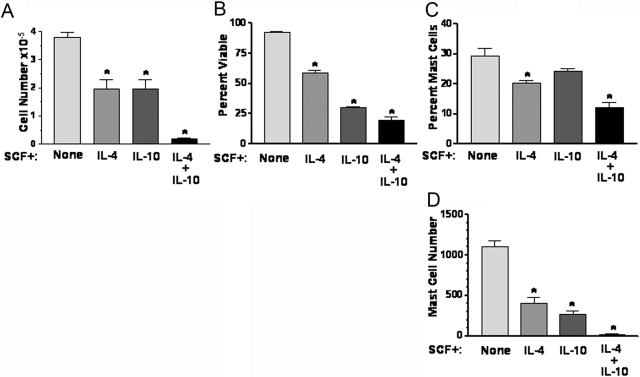
IL-4 + IL-10 suppresses mast cell development and survival in cultures of human umbilical cord blood cells. Erythrocyte-depleted human cord blood cells were cultured for 21 days in SCF-containing medium, with IL-4 and/or IL-10 added where indicated. On Day 21, cell number (A) and viability (B) were determined using trypan blue exclusion. Percent mast cells (C) and mast cell number (D) were determined using toluidine blue staining. Data shown are from triplicate samples harvested from one of two independent experiments that yielded similar results. *, P < .025, for all comparisons with control condition (“None”). Note that IL-4 + IL-10 also has P < .025 when compared with IL-4 or IL-10.
Fig. 6.
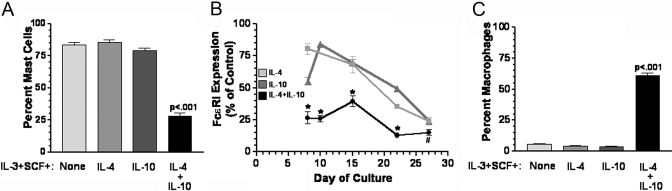
IL-4 + IL-10 suppresses mast cell development in mouse BM cultures. Mouse BM cells were cultured for 3 weeks in the indicated media. Percent mast cells (A) and the change in IgER staining on FcεRI+ cells (B) were determined using flow cytometry after staining for expression of FcεRI and Kit. (C) Percent macrophages were determined by flow cytometric analysis of CD11B and F4/80 expression. Data shown are mean and sem from three to 12 samples/point for A–C. P values were obtained via t-test in A and B and ANOVA in C. *, P < .01; #, P < .05, when compared with control condition.
Fig. 7.
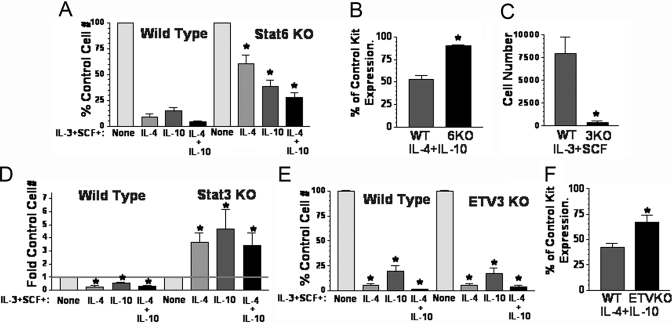
The role of Stat6, Stat3, and ETV3 in IL-4 + IL-10-mediated death of developing mast cells. (A) WT and Stat6-deficient (KO) BM cells were cultured for 3 weeks in the indicated conditions, and the change in cell number was determined by timed flow cytometry counting, comparing all values with the IL-3 + SCF control for the indicated genotype. Data are mean and sem from nine samples. *, P < .005, when comparing Stat6 KO with WT cells cultured in the same conditions. (B) WT and Stat6 KO cells were cultured as in A, and Kit expression was assessed on Day 21 by flow cytometry. Percent of Kit expression in IL-3 + SCF cultures was determined from three samples in one of two representative experiments. *, P < .001. (C) WT and Tie2-Cre Stat3 KO BM cells were cultured in IL-3 + SCF for 3 weeks. Data shown are live cell counts from timed flow cytometry analysis using PI-DNA staining. Data are mean and sem of nine samples. *, P < .002. (D) WT and Stat3 KO BM cells were cultured for 3 weeks as in A. Fold change in cell number was determined by timed flow cytometry counting, comparing all values with the IL-3 + SCF control for the indicated genotype. Data are mean and sem from six samples. *, P < .025, when comparing each culture condition with its IL-3 + SCF control group or when comparing WT with Stat3 KO cells. (E) WT and ETV3 KO BM cells were cultured and analyzed for survival as described in A. Data are mean and sem from nine to 10 samples. *, P < .0001, when comparing each culture condition with its IL-3 + SCF control group. (F) The effect of IL-4 + IL-10 on Kit receptor expression was determined using samples from D, harvested on Day 22. Percent of control Kit expression was derived by comparing cells cultured with IL-4 + IL-10 with control cultures from the same genotype. Data shown are from six to 12 samples/point. *, P < .002, when comparing WT and ETV3 KO samples.
Fig. 8.
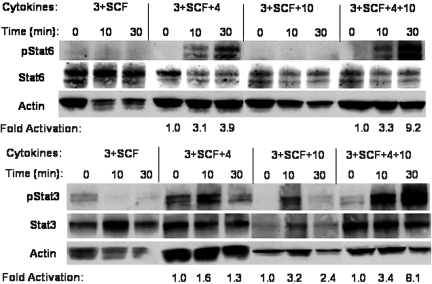
Hyperactivation of Stat6 and Stat3 by IL-4 + IL-10. BM cells were cultured for 7 days in the indicated conditions, washed, starved for 4 h, and restimulated with the identical cytokine mixture for 10–30 min. Cell lysates were subjected to Western blotting with antibodies specific for tyrosine pStat6 or pStat3 and then stripped and reprobed to detect Stat6, Stat3, or actin as indicated. Densitometry analysis was used to determine the fold activation of the tyrosine-phosphorylated band compared with the zero time-point sample for each group after normalizing to the actin-loading control. Data shown are representative of two experiments yielding similar results.
Human mast cell culture conditions
Human cord blood cells were cultured in media containing recombinant human SCF (100 ng/ml) as described previously [23].
Cell sorting
Isolation of c-Kit+/lineage– cells from murine BM was performed using a two-step procedure. BM cells were first depleted of lineage+ cells via magnetic cell sorting (MACS; Miltenyi Biotec, Auburn, CA, USA) and then subjected to flow cytometry-based cell sorting with anti-Kit to obtain Kit+/lineage–. Typical purity of these populations was >99%.
Flow cytometry staining
Sample (200 μl) was transferred to a 96-well v-bottom plate. Cells were washed twice with PBS containing 3% FBS and 0.1% sodium azide (FACS buffer). Cells were then incubated with 10 μl rat anti-mouse FcγRII/FcγRIII culture supernatant (clone 2.4G2) plus fluorescently labeled antibodies at 4°C for 45 min. The conjugated antibodies included FITC-coupled rat IgG isotype control, R-PE-coupled anti-rat IgG isotype control, FITC anti-mouse c-Kit, PE anti-mouse FcεRI, FITC anti-mouse F4/80, and PE anti-mouse CD11b (all from PharMingen, San Diego, CA, USA). All FITC-coupled antibodies were used at a final concentration of 10 μg/ml; all PE-coupled antibodies were used at a final concentration of 2 μg/ml. The samples were then washed twice with FACS buffer and analyzed for 30 s/sample (time resolution, 0.1 s) on a FACSCalibur (BD Biosciences, San Jose, CA, USA) to determine relative cell number and mean fluorescence intensity (MFI). Analysis was performed using CellQuest software (Becton Dickinson, San Diego, CA, USA). All measurements represented as percent of control were obtained by comparing the indicated sample with cells cultured in IL-3 + SCF, unless otherwise indicated. Active caspase and 3,3 - dihexyloxacarbocyanine iodide [Di(OC6)3] staining were performed as described previously [18].
Propidium iodide (PI) analysis of apoptosis
To detect apoptosis as determined by subdiploid DNA, a 200-μl sample was removed from cultures and washed twice with PBS. Samples were then fixed in an ethanol solution and stained with PI in the presence of RNase A. Each sample was analyzed for 45 s (time resolution, 0.1 s) using a Becton Dickinson FACScan with a forward-scatter/side-scatter gate set to exclude cellular debris. Total cell numbers and percent cells with subdiploid DNA content (apoptotic cells) were calculated with CellQuest software (BD Biosciences) by gating on those cells with PI fluorescence intensity less than the diploid peak in control cultures. This technique is distinct from PI exclusion, which only yields a live versus dead determination.
ELISA
Culture supernatants were harvested at the indicated times. IL-4 and IL-10 were detected by standard ELISA kits as described by the manufacturer (Peprotech, Rocky Hill, NJ, USA). The concentration of IL-4 or IL-10 was normalized to 1 × 106 cells/ml by accounting for the cellular density of each culture.
Western blot analysis
Western blotting was performed using 50 μg total cellular protein/sample. Protein was loaded and separated over a 10% polyacrylamide gel (Bio-Rad, Hercules, CA, USA). Proteins were transferred to nitrocellulose (Pall Corp., Ann Arbor, MI, USA) and blocked for 30 min in 5% nonfat dry milk (NFDM) in TBS-T. Blots were incubated in 5% NFDM/TBS-T with a 1:1000 dilution of antibodies against Akt, phospho (p)Akt, Stat5, pStat5, Stat6, pStat6, Stat3, pStat3, ERK, and pERK (Cell Signaling, Danvers, MA, USA) or with a 1:5000 dilution of β-actin overnight at 4°C with gentle rocking. Blots were washed five times for 10 min each in TBS-T, followed by incubation in 5% NFDM/TBS-T containing a 1:2000 dilution of HRP-linked anti-rabbit IgG (Cell Signaling) or HRP-linked rabbit anti-mouse IgG at 1:2000 (p38) or 1:10,000 (β-actin; Jackson ImmunoResearch, West Grove, PA, USA). Size estimates for proteins were obtained using molecular weight standards from Bio-Rad. Densitometry analysis was used to determined fold activation (Eagle Eye II, Stratagene, La Jolla, CA, USA).
RESULTS
IL-4 and IL-10 are endogenous regulators of mast cell development
We found recently that BM cells cultured in IL-3 + SCF contain detectable amounts of TNF and that loss of TNF precluded in vitro mast cell survival [34]. As IL-4 and IL-10 have been shown to alter mast cell development [17,18,19, 27], we assayed BM cultures for the presence of these cytokines. In fact, mouse BM cultures contained detectable amounts of IL-4 and IL-10, which increased in direct relation to mast cell purity (Fig. 1A). By Day 24, when these cultures were essentially purified mast cell populations, IL-10 was produced at levels of 500 pg/million cells.
To determine the importance of endogenously produced IL-4 and IL-10, we examined cultures of IL-4- or IL-10-deficient BM cells to their littermate controls. As shown in Figure 1, B and C, loss of IL-4 resulted in a slight but consistent increase in mast cell numbers and FcεRI expression. In keeping with its higher concentration in these cultures, loss of IL-10 had a more striking effect, generating 64% more cells bearing 80% higher IgER levels. These results support the contention that IL-4 and IL-10 function as endogenous regulators of mast cell precursor homeostasis, limiting cell expansion and IgER expression.
Costimulation with IL-4 + IL-10 induces a rapid mitochondrial-mediated cell death in mast cell progenitors
To determine the effects of combined signaling by IL-4 and IL-10 on developing mast cells, we first cultured mouse BM in IL-3 + SCF, with and without IL-4 and/or IL-10 for 3 weeks. We have found previously that when added alone, IL-4 or IL-10 suppresses mast cell survival with a delayed time course of ∼2 weeks [27, 33]. In contrast, combined signaling with IL-4 + IL-10 strikingly inhibited cell survival, reducing live cell numbers by 50% within the first 4 days of culture (Fig. 2A). This effect was mimicked by the related cytokine IL-13, which also killed developing BM cells and synergized with IL-10 (data not shown). Cell death appeared to be caused at least in part by an apoptotic program selective for IL-4 + IL-10, as enhanced caspase-3/7 activation was detectable during the first 10 days of culture only when IL-4 + IL-10 was present (Fig. 2B).
One means by which IL-4 and IL-10 could kill developing mast cells is through suppression of growth factor signaling. We examined expression of IL-3Rβ and Kit receptor, as IL-3 and SCF are provided in the culture and support mast cell survival through a mitochondrial pathway [35, 36]. Kit is the most critical regulator of mast cell survival, as demonstrated by loss of mast cell development in Kit-deficient but not IL-3-deficient mice (reviewed in ref. [10]). It was interesting to note that the inhibitory effect of IL-4 + IL-10 correlated with a selective reduction in Kit receptor expression (Fig. 3).
The reduced Kit expression correlated with diminished survival signaling. BM cells cultured for 7 days in IL-4 and/or IL-10 were starved and restimulated with SCF and examined for phosphorylation of the survival protein Akt. Cells cultured previously in IL-4 or IL-10 showed reduced pAkt, and the most overt effect was seen when IL-4 + IL-10 was present (Fig. 3C). Akt is well known for supporting mitochondrial stability [37]. Accordingly, we found reduced ΔΨm in cultures containing IL-4 + IL-10 during the same period in which pAkt was suppressed (Fig. 3D). Furthermore, Bcl-2 overexpression or Bax deletion increased survival significantly. By comparison, p53 ablation did not enhance survival (Fig. 3E). These results are in keeping with a model in which IL-4 + IL-10 down-regulates the Kit receptor selectively, resulting in an accelerated, mitochondrial-dependent cell death that is not induced by IL-4 or IL-10 alone.
Recently Hu and co-workers [19] found that IL-4 induced mast cell apoptosis through a macrophage-dependent mechanism. To determine if macrophages or other accessory cells were required for cell death in our assays, purified Kit+/lineage– BM cells, a source of enriched hematopoietic stem cells, were cultured for 3 weeks with selection against adherent cells. In keeping with the earlier observation, IL-4 or IL-10 alone had little inhibitory effect in these cultures (Fig. 4). In striking contrast, IL-4 + IL-10 reduced live cell numbers by 90% in these cultures, concomitant with increased apoptosis and suppressed cell proliferation. These data support the contention that combined IL-4 + IL-10 signaling provides a qualitatively different stimulus to developing mast cells not conveyed by either cytokine alone.
IL-4 + IL-10 costimulation alters differentiation of mouse and human mast cell progenitors
To determine if our observations with murine cells conveyed to human mast cell development, we cultured erythrocyte-depleted human umbilical cord blood cells in SCF with or without IL-4 and/or IL-10 for 3 weeks. As shown in Figure 5, combined stimulation with IL-4 + IL-10 drastically reduced live cell numbers and overall viability. These results showed the consistency with which IL-4 and IL-10 suppress mast cell development from mouse or human progenitors.
There was an additional observation that drew our interest. The fraction of the population identified as mast cells was reduced by more than 50% after culture with IL-4 + IL-10, an effect not reproduced by IL-4 or IL-10 alone, although each cytokine suppressed the actual number of mast cells yielded from these cultures (Fig. 5, C and D). We next examined mouse BM cultures to confirm and extend this observation. As shown in Figure 6, IL-4 + IL-10 also reduced the mast cell fraction of these populations by more than half. In addition to this reduced proportion, the few mast cells that did develop in cultures containing IL-4 + IL-10 had greatly reduced expression of the high-affinity IgER, FcεRI (Fig. 6B). Concomitant with this reduction was a selective increase in macrophage development, an observation not noted with IL-4 or IL-10 alone (Fig. 6C). These data demonstrate that costimulation with IL-4 + IL-10 suppresses survival and differentiation pathways in developing mast cells derived from mouse or human precursors.
The contributions of Stat6, Stat3, and ETV3 to IL-4 + IL-10-mediated cell killing
IL-4 and IL-10 act through a variety of cell signaling pathways but are most dependent on Stat family transcription factors for their actions [14,15,16, 38]. We and others [19, 33] have found that IL-4-mediated mast cell apoptosis requires Stat6 and that IL-10-mediated killing of developing macrophages requires Stat3 [27]. However, the contribution of these transcription factors to IL-4 + IL-10 progenitor effects is unknown. Recently, IL-10 has been shown to induce the expression of the Ets family transcriptional repressor ETV3 through a Stat3-dependent mechanism [39]. As the reduction in Kit expression could be a result of transcriptional repression, we also examined the role of ETV3 in this system. BM from Stat6-, Stat3-, or ETV3-deficient mice were cultured in IL-3 + SCF with or without IL-4 and/or IL-10 for 3 weeks and assessed for survival.
As shown in Figure 7A, Stat6 ablation greatly improved survival when IL-4 was added to these cultures. Of note, IL-10-mediated killing was also blunted somewhat in Stat6-deficient cultures. As IL-10 is not known to activate Stat6, this latter result could be a result of the increased, proliferative capacity of Stat6-deficient cells, as published previously [40]. Costimulation with IL-4 + IL-10 yielded more cell death than was observed with either cytokine alone when added to WT or Stat6-deficient BM. However, Stat6-deficient cultures did exhibit less death than their WT counterparts, with a 75% reduction in cell numbers compared with the 90% reduction found in WT cultures. Kit expression was also partially rescued, as Stat6-deficient BMMC had near-normal Kit surface levels in cultures containing IL-4 + IL-10 (Fig. 7B). Therefore the effects of IL-4 + IL-10 were partly Stat6-dependent.
Stat3 deletion caused a near-complete blockade of mast cell development in response to IL-3 + SCF, yielding less than 5% of the cells generated in littermate cultures (Fig. 7C). Stat3 ablation also reversed the effects of IL-4 and/or IL-10 completely, with all combinations conveying enhanced survival rather than death (Fig. 7D). IL-4 and IL-10 also possess mast cell co-mitogen activity [41, 42]. It seems plausible that this function was the dominant signal in the absence of Stat3, yielding increased survival in a setting where the majority of the cells was dying. This is the first observation that Stat3 is required for mast cell survival and also emphasizes the importance of Stat3 in IL-10 signaling.
Deleting the repressor ETV3 had no effect on cell killing by IL-4 and/or IL-10, indicating that this transcription factor was not required for the effects of IL-4 and IL-10 (Fig. 7E). However, we did note that the IL-4 + IL-10-mediated reduction in Kit expression was blunted in ETV3-deficient cells as compared with littermate controls (Fig. 7F). These data suggest that ETV3 may repress Kit expression partly but that loss of this repression is not sufficient to rescue cell survival. It is striking that the 33% reduction in Kit expression observed with IL-4 + IL-10-treated, ETV3-deficient cells still correlated with a nearly complete cell death in these cultures.
The importance of Stat6 and Stat3 suggested the possibility that combined signaling by IL-4 + IL-10 altered the strength of the Stat6 or Stat3 signal, enhancing their death-promoting effects. To examine this, BM cells were cultured for 7 days in IL-3 + SCF alone or with IL-4 and/or IL-10. This is a time-point at which IL-4 + IL-10 demonstrated a strong suppression of cell survival (Fig. 2). These cells were then starved and restimulated with the identical conditions in which they had been cultured for up to 30 min. Cell lysates were examined for the extent of Stat6 and Stat3 tyrosine phosphorylation by Western blotting. As shown in Figure 8, combined stimulation with IL-4 + IL-10 enhanced Stat6 and Stat3 activation at least twofold compared with stimulation with either cytokine separately. These data, together with the demonstrated importance of Stat6 and Stat3 (Fig. 7), suggest that the cooperative effects of IL-4 + IL-10 are related to stronger downstream signaling.
Strain-dependent effects of IL-10-mediated cell death
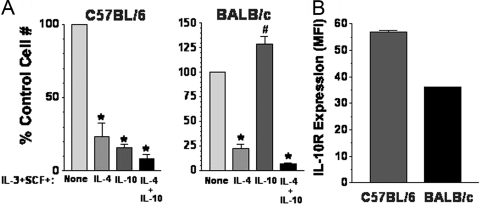
Mast cell responses resulting in allergy and asthma are partially a result of inherited traits [43]. Similarly, Yamashita and co-workers recently [44] found that the mast cell response to Lyn kinase deficiency was dependent on the genetic background of the mice. These issues encouraged an examination of IL-4 and IL-10 effects on mast cell development from different mouse strains. As shown in Figure 9A, we examined IL-4- and IL-10-mediated killing of BM cells harvested from Th1-prone C57BL/6 or Th2-prone BALB/c mice. In all cases, IL-4 or IL-4 + IL-10 greatly suppressed mast cell survival. However, the response to IL-10 was ablated completely in BALB/c mice; in fact, these cells expanded slightly better when IL-10 was present. Flow cytometry analysis revealed that mast cells from both strains of mice expressed surface IL-10R, but BALB/c expressed this receptor at 65% of C57BL/6 levels (Fig. 8B). This reduced expression could explain the difference in IL-10 responsiveness. As BALB/c mice are known for strong allergy- and asthma-associated Th2 responses [45], it is noteworthy that the suppressive effects of IL-10 appear to be lacking among mast cell progenitors from these mice.
Fig. 9.
Strain-dependent effects of IL-10-mediated suppression. BM cells were harvested from mice of the indicated strains and cultured for 3 weeks in IL-3 + SCF ± IL-4 and/or IL-10. (A) Live cell numbers were determined by timed flow cytometry counting and expressed as a percent of the IL-3 + SCF control group. Data are mean and sem from three to 12 samples/point. *, P < .005; #, P < .02, for all comparisons with control condition. (B) IL-10R surface expression was determined by flow cytometry. Data shown are mean and sem of MFI values from samples analyzed under identical conditions. n = 6; P < .001.
DISCUSSION
Mast cells appear to serve a protective role during parasitic and bacterial infections [1]. However, they are better known as a causative agent in immediate hypersensitivity responses, particularly, allergy and asthma. Work in several animal models has expanded this pathological role, demonstrating an importance for mast cells in atherosclerosis, inflammatory arthritis, and multiple sclerosis [5,6,7]. Loss of homeostasis is the foundation of chronic disease. Mast cell-associated pathology could certainly stem from loss of developmental controls, hence, mechanistic insight into this process is important.
Our understanding of mast cell homeostasis is growing more detailed but is far from complete. Recent work has identified a distinct mast cell progenitor [46, 47]. Prior work has clearly shown that the SCF-Kit receptor signaling cascade is the critical regulator of mast cell development and survival, working partly through Stat5 activation [1, 3, 48]. In terms of feedback regulation preventing chronic activation, several inhibitory molecules have been shown to suppress the mast cell response, including FcγRIIb [49] and leukocyte Ig-like receptor, subfamily B, member 4 [50].
Perhaps more enigmatic is the role of cytokines as feedback regulators in mast cell homeostasis. Several candidates are apparent, including IL-4, IL-10, and TGF-β1. These are known to enhance mast cell proliferation, survival, or development. However, true to the postulates of homeostasis, these same factors have also been shown to suppress mast cell proliferation, survival, or function in the proper conditions (reviewed in ref. [1]). The central issue separating these divergent functions may be time. Although the enhancing effects have generally been observed in short-term cultures, inhibitory actions are most often noted at time-points of 3 days or later [17,18,19, 23, 27, 29, 33, 51]. This supports the postulate that cytokines can frame an inflammatory “window”, during which, mast cell responses are protective, after which, the response is suppressed to preclude the initiation or maintenance of chronic disease.
Although cytokines are best known for acting in a paracrine manner, systemic cytokine signaling is possible. In support of this, serum IL-10 levels are elevated in patients suffering from septicemia [52, 53], a variety of cancers [54,55,56,57], chronic hepatitis, myocardial infarction, Grave’s disease, and systemic sclerosis [58,59,60,61]. In related studies using an animal model, Fred Finkelman’s group [62, 63] has shown that IL-4 concentrations exceeding 10 ng/ml are detected following anti-CD3 injection and that serum IL-4 concentrations remain elevated for more than 10 days during parasite infections. Each of these studies demonstrates that local inflammation can increase serum cytokine concentrations, possibly affecting cells at considerable distance from the site of origin. This is important to understanding mast cell homeostasis, as systemic cytokines could be affecting mast cells throughout their ontogeny.
Studies of disease-linked polymorphisms provide additional theoretical support for the notion that cytokines regulate mast cell biology. Allergy-related polymorphisms of the genes encoding IL-4, IL-10, IL-13, TGF, and the IL-4Rα chain have been noted [64,65,66,67,68,69,70,71,72,73,74,75]. One possibility is that these genetic changes prevent cytokine-mediated mast cell regulation, providing part of the etiological support for disease onset or maintenance.
Work from our lab [1] and others has shown the potential suppressive capacities of IL-4, IL-10, or TGF-β1. These studies have generally used exogenously added cytokines. The departure from this in the current work is important. We demonstrate that IL-4 and IL-10 are present in BM cultures and that their production increases with mast cell differentiation and is maximal when the cultures reach the stage of mast cell purity. This ability to produce IL-4 and IL-10 constitutively at a moderate level appears to have a regulatory role. IL-4-deficient mast cell cultures grew slightly but significantly better than their littermate counterparts and maintained higher FcεRI levels. Loss of IL-10 had a more impressive effect, generating 60% more mast cells that expressed FcεRI at 180% of littermate levels. Extrapolating these data to the in vivo environment is somewhat challenging. IL-4-deficient mice have increased peritoneal mast cell numbers, consistent with a suppressive role for IL-4 [76]. However, IL-4 is required for IgE synthesis [14,15,16], perhaps the strongest inducer of FcεRI expression, making in vivo studies of FcεRI baseline expression difficult to interpret. Likewise, IL-10-deficient mice develop autoimmunity that could confound in vivo interpretation of mast cell numbers. Further, we have found in initial experiments that peritoneal mast cells from IL-10-deificient mice have low FcεRI expression (our unpublished observations), which could be a result of an underappreciated role for IL-10 in IgE production [77].
The current study demonstrates that costimulation with IL-4 + IL-10 has a qualitatively distinct effect on mast cell progenitors compared with either cytokine alone. These effects included a more rapid onset of apoptotic death, greater suppression of Kit and FcεRI surface proteins, direct effects on hematopoietic precursors, and shifted developmental pathways to the macrophage lineage. As IL-4 and IL-10 are produced coincidentally during the Th2 response by T cells and mast cells [45, 78], costimulation by these cytokines is a logical and apparently powerful brake on mast cell ontogeny. In addition to its effects on mast cells, IL-4- and IL-10-mediated killing could be important to understanding BM suppression that is noted in some cancers. Related to this, T and B cell lymphomas are known to secrete IL-4 and IL-10 [79,80,81,82].
The consistent nature of the IL-4 and IL-10 response on mouse and human mast cell precursors warranted further investigation into the mechanisms involved. IL-4 + IL-10-induced death appeared to proceed partly via Kit suppression, which resulted in diminished activation of the survival protein Akt. The functional relevance of this inhibition was evidenced by subsequent mitochondrial damage and caspase activation. The effects of IL-4 were largely Stat6-dependent, in keeping with Stat6-mediated Kit inhibition on differentiated mast cells [83]. IL-4 + IL-10 costimulation elicited apoptosis in 75% of Stat6-deficient cells, but this was mostly a result of the Stat6-independent effects of IL-10. Enhanced Stat6 activation could partly explain IL-4 + IL-10 synergism. Stat6 was activated to a greater extent (at least twofold) by IL-4 + IL-10 compared with IL-4. Stat6-deficient cultures were also instructive, as they largely maintained Kit expression but still showed significant loss of viability. Hence, we conclude that loss of Kit suppression is not the only means by which IL-4 + IL-10 induces cell death.
Stat3 is known to control many aspects of IL-10 signaling in macrophages [38], and we were thus greatly interested in pursuing the role of Stat3. We found that like Stat6, Stat3 is hyperactivated by IL-4 + IL-10. However, the most striking effect obtained with Stat3-deficient BM cells was the near-total loss of mast cell development in vitro. As Stat3 is known to promote cell survival and proliferation and is linked to control of Bcl family proteins, loss of this transcription factor may elicit death by the mitochondrial pathway [84]. IL-4 and IL-10 have proliferative and survival effects on many cells, and it appeared that these positive effects were apparent when Stat3 was deleted, although these cultures still yielded few live cells. Stat3-deficient mice have a complex inflammatory disease and early lethality that has precluded detailed in vivo studies of mast cell survival thus far. However, with the advent of new Cre-expressing Tg mice, we hope to generate a mast cell-restricted, Stat3-deficient mouse. The newly described Stat3-induced transcriptional repressor, ETV3, appears to exert partial control over IL-4 + IL-10-mediated Kit suppression, but loss of ETV3-mediated suppression was not sufficient to rescue Kit expression or cell survival completely.
The effects of genetic background on cytokine responses were quite striking, as mast cell precursors from BALB/c mice were completely resistant to IL-10. As with IL-10 promoter polymorphisms noted in some atopic patients [67, 85,86,87], it seems plausible that changes in IL-10 production or responsiveness could support allergic disease etiology. This effect may be lineage-restricted. BALB/c macrophages and B cells are reported to express the IL-10R at levels equivalent to other strains of mice [88], but mast cells from BALB/c mice had reduced surface IL-10R levels compared with C57BL/6 mast cells. In support of this supposition, it was reported recently that Lyn kinase deficiency results in mast cell hyper-responsiveness on the 129 background but not on the C57BL/6 background, although both strains develop B cell-related, lupus-like symptoms when Lyn is deleted [44]. Likewise, it is possible that mast cell precursors could differ from other lineages in their IL-10 responsiveness.
Our data support the theory that IL-4 and IL-10 function as endogenous regulators of mast cell development and are produced by immature mast cells during their ontogeny as well as activated T cells or mast cells during an inflammatory response. The effects of costimulation by IL-4 + IL-10 are particularly powerful, suppressing mast cell development, perhaps in an effort to maintain homeostasis in the immune response. These data provide further evidence that changes in cytokine production or signaling, perhaps as a result of genetic polymorphism, can be predisposing factors supporting the etiology of mast cell-associated illness.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R01AI59638 and U191077435 to J. J. R. and K01AR053186 to C. O.) and The Jeffress Trust Foundation (J-833 to J. J. R.).
References
- Ryan J J, Kashyap M, Bailey D, Kennedy S, Speiran K, Brenzovich J, Barnstein B, Oskeritzian C, Gomez G. Mast cell homeostasis: a fundamental aspect of allergic disease. Crit Rev Immunol. 2007;27:15–32. doi: 10.1615/critrevimmunol.v27.i1.20. [DOI] [PubMed] [Google Scholar]
- Bradding P, Walls A F, Holgate S T. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Galli S J, Kalesnikoff J, Grimbaldeston M A, Piliponsky A M, Williams C M, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Ohsawa M, Ikura Y, Naruko T, Sugama Y, Suekane T, Kitabayashi C, Inoue T, Hino M, Ueda M. Mast cells in diffuse large B-cell lymphoma; their role in fibrosis. Histopathology. 2006;49:498–505. doi: 10.1111/j.1365-2559.2006.02534.x. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Riesel N, Soffer D, Abramsky O, Brenner T. Mast cell activity in experimental allergic encephalomyelitis. Mol Chem Neuropathol. 1991;15:173–184. doi: 10.1007/BF03159954. [DOI] [PubMed] [Google Scholar]
- Secor V H, Secor W E, Gutekunst C A, Brown M A. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie-Ryan M, Tanzola M B, Secor V H, Brown M A. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- Lee D M, Friend D S, Gurish M F, Benoist C, Mathis D, Brenner M B. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- Lindstedt K A, Mayranpaa M I, Kovanen P T. Mast cells in vulnerable atherosclerotic plaques—a view to a kill. J Cell Mol Med. 2007;11:739–758. doi: 10.1111/j.1582-4934.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Huff T F. Biology of mast cells. St. Louis, MO, USA: Mosby; AllergyPrinciples and Practices. 2003 [Google Scholar]
- Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- McKenzie G J, Emson C L, Bell S E, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie A N. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Kitaura J, Xiao W, Kawakami Y. IgE regulation of mast cell survival and function. Novartis Found Symp. 2005;271:100–107. [PubMed] [Google Scholar]
- Kaplan M H, Schindler U, Smiley S T, Grusby M J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Shimoda K, van Deursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A, Doherty P C, Grosveld G, Paul W E, Ihle J N. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signaling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Yeatman C F, II, Jacobs-Helber S M, Mirmonsef P, Gillespie S R, Bouton L A, Collins H A, Sawyer S T, Shelburne C P, Ryan J J. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med. 2000;192:1093–1103. doi: 10.1084/jem.192.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton L A, Ramirez C D, Bailey D P, Yeatman C F, Yue J, Wright H V, Domen J, Rosato R R, Grant S, Fischer-Stenger K, Ryan J J. Costimulation with interleukin-4 and interleukin-10 induces mast cell apoptosis and cell-cycle arrest: the role of p53 and the mitochondrion. Exp Hematol. 2004;32:1137–1145. doi: 10.1016/j.exphem.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hu Z Q, Zhao W H, Shimamura T, Galli S J. Interleukin-4-triggered, STAT6-dependent production of a factor that induces mouse mast cell apoptosis. Eur J Immunol. 2006;36:1275–1284. doi: 10.1002/eji.200526275. [DOI] [PubMed] [Google Scholar]
- Ryan J J, DeSimone S, Klisch G, Shelburne C, McReynolds L J, Han K, Kovacs R, Mirmonsef P, Huff T F. IL-4 inhibits mouse mast cell Fc εRI expression through a STAT6-dependent mechanism. J Immunol. 1998;161:6915–6923. [PubMed] [Google Scholar]
- Shiohara M, Koike K. Regulation of mast cell development. Chem Immunol Allergy. 2005;87:1–21. doi: 10.1159/000087566. [DOI] [PubMed] [Google Scholar]
- Kaur D, Hollins F, Woodman L, Yang W, Monk P, May R, Bradding P, Brightling C E. Mast cells express IL-13R α 1: IL-13 promotes human lung mast cell proliferation and Fc ε RI expression. Allergy. 2006;61:1047–1053. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- Oskeritzian C A, Wang Z, Kochan J P, Grimes M, Du Z, Chang H W, Grant S, Schwartz L B. Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol. 1999;163:5105–5115. [PubMed] [Google Scholar]
- Lee E, Min H K, Oskeritzian C A, Kambe N, Schwartz L B, Wook Chang H. Recombinant human (rh) stem cell factor and rhIL-4 stimulate differentiation and proliferation of CD3+ cells from umbilical cord blood and CD3+ cells enhance FcεR1 expression on fetal liver-derived mast cells in the presence of rhIL-4. Cell Immunol. 2003;226:30–36. doi: 10.1016/j.cellimm.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Lorentz A, Wilke M, Sellge G, Worthmann H, Klempnauer J, Manns M P, Bischoff S C. IL-4-induced priming of human intestinal mast cells for enhanced survival and Th2 cytokine generation is reversible and associated with increased activity of ERK1/2 and c-Fos. J Immunol. 2005;174:6751–6756. doi: 10.4049/jimmunol.174.11.6751. [DOI] [PubMed] [Google Scholar]
- Thienemann F, Henz B M, Babina M. Regulation of mast cell characteristics by cytokines: divergent effects of interleukin-4 on immature mast cell lines versus mature human skin mast cells. Arch Dermatol Res. 2004;296:134–138. doi: 10.1007/s00403-004-0486-z. [DOI] [PubMed] [Google Scholar]
- Bailey D P, Kashyap M, Bouton L A, Murray P J, Ryan J J. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J Leukoc Biol. 2006;80:581–589. doi: 10.1189/jlb.0405201. [DOI] [PubMed] [Google Scholar]
- Kennedy Norton S, Barnstein B, Brenzovich J, Bailey D P, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle M R, Kepley C L, Murray P J, Ryan J J. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- Gillespie S R, DeMartino R R, Zhu J, Chong H J, Ramirez C, Shelburne C P, Bouton L A, Bailey D P, Gharse A, Mirmonsef P, Odom S, Gomez G, Rivera J, Fischer-Stenger K, Ryan J J. IL-10 inhibits Fc ε RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- Royer B, Varadaradjalou S, Saas P, Gabiot A C, Kantelip B, Feger F, Guillosson J J, Kantelip J P, Arock M. Autocrine regulation of cord blood-derived human mast cell activation by IL-10. J Allergy Clin Immunol. 2001;108:80–86. doi: 10.1067/mai.2001.115753. [DOI] [PubMed] [Google Scholar]
- Kambe M, Kambe N, Oskeritzian C A, Schechter N, Schwartz L B. IL-6 attenuates apoptosis, while neither IL-6 nor IL-10 affect the numbers or protease phenotype of fetal liver-derived human mast cells. Clin Exp Allergy. 2001;31:1077–1085. doi: 10.1046/j.1365-2222.2001.01126.x. [DOI] [PubMed] [Google Scholar]
- Panopoulos A D, Zhang L, Snow J W, Jones D M, Smith A M, El Kasmi K C, Liu F, Goldsmith M A, Link D C, Murray P J, Watowich S S. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D P, Kashyap M, Mirmonsef P, Bouton L A, Domen J, Zhu J, Dessypris E N, Ryan J J. Interleukin-4 elicits apoptosis of developing mast cells via a Stat6-dependent mitochondrial pathway. Exp Hematol. 2004;32:52–59. doi: 10.1016/j.exphem.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Wright H V, Bailey D, Kashyap M, Kepley C L, Drutskaya M S, Nedospasov S A, Ryan J J. IL-3-mediated TNF production is necessary for mast cell development. J Immunol. 2006;176:2114–2121. doi: 10.4049/jimmunol.176.4.2114. [DOI] [PubMed] [Google Scholar]
- Mekori Y A, Gilfillan A M, Akin C, Hartmann K, Metcalfe D D. Human mast cell apoptosis is regulated through Bcl-2 and Bcl-XL. J Clin Immunol. 2001;21:171–174. doi: 10.1023/a:1011083031272. [DOI] [PubMed] [Google Scholar]
- Bojes H K, Feng X, Kehrer J P, Cohen G M. Apoptosis in hematopoietic cells (FL5.12) caused by interleukin-3 withdrawal: relationship to caspase activity and the loss of glutathione. Cell Death Differ. 1999;6:61–70. doi: 10.1038/sj.cdd.4400452. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Tintignac L A, Zhuravleva E, Hemmings B A. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Lang R, Patel D, Morris J J, Rutschman R L, Murray P J. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- El Kasmi K C, Smith A M, Williams L, Neale G, Panopolous A, Watowich S S, Hacker H, Foxwell B M, Murray P J. Cutting edge: a transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007;179:7215–7219. doi: 10.4049/jimmunol.179.11.7215. [DOI] [PubMed] [Google Scholar]
- Kaplan M H, Daniel C, Schindler U, Grusby M J. Stat proteins control lymphocyte proliferation by regulating p27Kip1 expression. Mol Cell Biol. 1998;18:1996–2003. doi: 10.1128/mcb.18.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Nakahata T, Takagi M, Kobayashi T, Ishiguro A, Kikuchi T, Naganuma K, Koike K, Miyajima A, Arai K. Effects of interleukin-3 and interleukin-4 on the development of “connective tissue-type” mast cells: interleukin-3 supports their survival and interleukin-4 triggers and supports their proliferation synergistically with interleukin-3. Blood. 1990;75:421–427. [PubMed] [Google Scholar]
- Thompson-Snipes L, Dhar V, Bond M W, Mosmann T R, Moore K W, Rennick D M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan A M, Constant S, Bower M A, Ryan J J, Rivera J. Cutting edge: genetic variation influences Fc εRI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Rodewald H R, Dessing M, Dvorak A M, Galli S J. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- Chen C C, Grimbaldeston M A, Tsai M, Weissman I L, Galli S J. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne C, McCoy M, Piekorz R, Sexl V, Gillespie S, Bailey D, Gharse A, Mirmonsef P, Mann M N, Kashyap M, Wright H, Chong H, Bouton L, Ramirez C, Lantz C, Ryan J. Stat5: an essential regulator of mast cell biology. Mol Immunol. 2002;38:1187–1191. doi: 10.1016/s0161-5890(02)00061-5. [DOI] [PubMed] [Google Scholar]
- Daeron M, Malbec O, Latour S, Arock M, Fridman W H. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz H R. Inhibition of pathologic inflammation by leukocyte Ig-like receptor B4 and related inhibitory receptors. Immunol Rev. 2007;217:222–230. doi: 10.1111/j.1600-065X.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Hu Z Q, Zenda N, Shimamura T. Down-regulation by IL-4 and up-regulation by IFN-γ of mast cell induction from mouse spleen cells. J Immunol. 1996;156:3925–3931. [PubMed] [Google Scholar]
- Marchant A, Alegre M L, Hakim A, Pierard G, Marecaux G, Friedman G, De Groote D, Kahn R J, Vincent J L, Goldman M. Clinical and biological significance of interleukin-10 plasma levels in patients with septic shock. J Clin Immunol. 1995;15:266–273. doi: 10.1007/BF01540884. [DOI] [PubMed] [Google Scholar]
- Marchant A, Deviere J, Byl B, De Groote D, Vincent J L, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343:707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- O'Hara R J, Greenman J, MacDonald A W, Gaskell K M, Topping K P, Duthie G S, Kerin M J, Lee P W, Monson J R. Advanced colorectal cancer is associated with impaired interleukin 12 and enhanced interleukin 10 production. Clin Cancer Res. 1998;4:1943–1948. [PubMed] [Google Scholar]
- Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz M Z. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- Lech-Maranda E, Baseggio L, Bienvenu J, Charlot C, Berger F, Rigal D, Warzocha K, Coiffier B, Salles G. Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood. 2004;103:3529–3534. doi: 10.1182/blood-2003-06-1850. [DOI] [PubMed] [Google Scholar]
- Hattori E, Okumoto K, Adachi T, Takeda T, Ito J, Sugahara K, Watanabe H, Saito K, Saito T, Togashi H, Kawata S. Possible contribution of circulating interleukin-10 (IL-10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol Res. 2003;27:309–314. doi: 10.1016/j.hepres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Tziakas D N, Chalikias G K, Hatzinikolaou H I, Parissis J T, Papadopoulos E D, Trypsianis G A, Papadopoulou E, Tentes I K, Karas S M, Hatseras D I. Anti-inflammatory cytokine profile in acute coronary syndromes: behavior of interleukin-10 in association with serum metalloproteinases and proinflammatory cytokines. Int J Cardiol. 2003;92:169–175. doi: 10.1016/s0167-5273(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Reiser M, Marousis C G, Nelson D R, Lauer G, Gonzalez-Peralta R P, Davis G L, Lau J Y. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. J Hepatol. 1997;26:471–478. doi: 10.1016/s0168-8278(97)80409-6. [DOI] [PubMed] [Google Scholar]
- Hussain M J, Peakman M, Gallati H, Lo S S, Hawa M, Viberti G C, Watkins P J, Leslie R D, Vergani D. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia. 1996;39:60–69. doi: 10.1007/BF00400414. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328–332. [PubMed] [Google Scholar]
- Finkelman F D, Morris S C. Development of an assay to measure in vivo cytokine production in the mouse. Int Immunol. 1999;11:1811–1818. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- Finkelman F D, Morris S C, Orekhova T, Mori M, Donaldson D, Reiner S L, Reilly N L, Schopf L, Urban J F., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- Hershey G K, Friedrich M F, Esswein L A, Thomas M L, Chatila T A. The association of atopy with a gain-of-function mutation in the α subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–1725. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- Hill M R, Cookson W O. A new variant of the β subunit of the high-affinity receptor for immunoglobulin E (Fc ε RI-β E237G): associations with measures of atopy and bronchial hyper-responsiveness. Hum Mol Genet. 1996;5:959–962. doi: 10.1093/hmg/5.7.959. [DOI] [PubMed] [Google Scholar]
- Hill M R, James A L, Faux J A, Ryan G, Hopkin J M, le Souef P, Musk A W, Cookson W O. Fc ε RI-β polymorphism and risk of atopy in a general population sample. BMJ. 1995;311:776–779. doi: 10.1136/bmj.311.7008.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs K, Negri J, Klinnert M, Rosenwasser L J, Borish L. Interleukin-10 and transforming growth factor-β promoter polymorphisms in allergies and asthma. Am J Respir Crit Care Med. 1998;158:1958–1962. doi: 10.1164/ajrccm.158.6.9804011. [DOI] [PubMed] [Google Scholar]
- Shirakawa I, Deichmann K A, Izuhara I, Mao I, Adra C N, Hopkin J M. Atopy and asthma: genetic variants of IL-4 and IL-13 signaling. Immunol Today. 2000;21:60–64. doi: 10.1016/s0167-5699(99)01492-9. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Li A, Dubowitz M, Dekker J W, Shaw A E, Faux J A, Ra C, Cookson W O, Hopkin J M. Association between atopy and variants of the β subunit of the high-affinity immunoglobulin E receptor. Nat Genet. 1994;7:125–129. doi: 10.1038/ng0694-125. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Hizawa N, Yamaguchi E, Kawakami Y. Association between a C+33T polymorphism in the IL-4 promoter region and total serum IgE levels. Clin Exp Allergy. 2000;30:1746–1749. doi: 10.1046/j.1365-2222.2000.00983.x. [DOI] [PubMed] [Google Scholar]
- Takabayashi A, Ihara K, Sasaki Y, Suzuki Y, Nishima S, Izuhara K, Hamasaki N, Hara T. Childhood atopic asthma: positive association with a polymorphism of IL-4 receptor α gene but not with that of IL-4 promoter or Fc ε receptor I β gene. Exp Clin Immunogenet. 2000;17:63–70. doi: 10.1159/000019125. [DOI] [PubMed] [Google Scholar]
- Arshad S H, Karmaus W, Kurukulaaratchy R, Sadeghnejad A, Huebner M, Ewart S. Polymorphisms in the interleukin 13 and GATA binding protein 3 genes and the development of eczema during childhood. Br J Dermatol. 2008;158:1315–1322. doi: 10.1111/j.1365-2133.2008.08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabesch M, Schedel M, Carr D, Woitsch B, Fritzsch C, Weiland S K, von Mutius E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006;117:269–274. doi: 10.1016/j.jaci.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Chien Y H, Hwu W L, Chiang B L. The genetics of atopic dermatitis. Clin Rev Allergy Immunol. 2007;33:178–190. doi: 10.1007/s12016-007-0041-8. [DOI] [PubMed] [Google Scholar]
- Rosa-Rosa L, Zimmermann N, Bernstein J A, Rothenberg M E, Khurana Hershey G K. The R576 IL-4 receptor α allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104:1008–1014. doi: 10.1016/s0091-6749(99)70082-5. [DOI] [PubMed] [Google Scholar]
- Chen X J, Lycke N, Enerback L. Surface and gene expression of immunoglobulin E receptors on mast cells and mast-cell numbers in interleukin-4-gene knockout mice. Immunology. 1999;96:544–550. doi: 10.1046/j.1365-2567.1999.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uejima Y, Takahashi K, Komoriya K, Kurozumi S, Ochs H D. Effect of interleukin-10 on anti-CD40- and interleukin-4-induced immunoglobulin E production by human lymphocytes. Int Arch Allergy Immunol. 1996;110:225–232. doi: 10.1159/000237291. [DOI] [PubMed] [Google Scholar]
- Burd P R, Thompson W C, Max E E, Mills F C. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki A, Ishida T, Ishii T, Komatsu H, Iida S, Ding J, Yonekura K, Takeuchi S, Takatsuka Y, Utsunomiya A, Ueda R. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: high interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int J Cancer. 2006;118:3054–3061. doi: 10.1002/ijc.21688. [DOI] [PubMed] [Google Scholar]
- Harwix S, Zachmann K, Neumann C. T-cell clones from early-stage cutaneous T-cell lymphoma show no polarized Th-1 or Th-2 cytokine profile. Arch Dermatol Res. 2000;292:1–8. doi: 10.1007/pl00007454. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Stapleton G, Dhar V, Pearce M, Schumacher J, Rugo H, Barbis D, Stall A, Cupp J, Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Saed G, Fivenson D P, Naidu Y, Nickoloff B J. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sezary syndrome expresses a Th2-type profile. J Invest Dermatol. 1994;103:29–33. doi: 10.1111/1523-1747.ep12388985. [DOI] [PubMed] [Google Scholar]
- Mirmonsef P, Shelburne C P, Fitzhugh Yeatman C, II, Chong H J, Ryan J J. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3′-kinase. J Immunol. 1999;163:2530–2539. [PubMed] [Google Scholar]
- Klampfer L. The role of signal transducers and activators of transcription in colon cancer. Front Biosci. 2008;13:2888–2899. doi: 10.2741/2893. [DOI] [PubMed] [Google Scholar]
- Hang L W, Hsia T C, Chen W C, Chen H Y, Tsai J J, Tsai F J. Interleukin-10 gene –627 allele variants, not interleukin-I β gene and receptor antagonist gene polymorphisms, are associated with atopic bronchial asthma. J Clin Lab Anal. 2003;17:168–173. doi: 10.1002/jcla.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi L, Fontaine C, Gougat C, Avinens O, Eliaou J F, Guglielmi P, Demoly P. IL-10 promoter and IL4-Rα gene SNPs are associated with immediate β-lactam allergy in atopic women. Allergy. 2006;61:921–927. doi: 10.1111/j.1398-9995.2006.01067.x. [DOI] [PubMed] [Google Scholar]
- Sohn M H, Song J S, Kim K W, Kim E S, Kim K E, Lee J M. Association of interleukin-10 gene promoter polymorphism in children with atopic dermatitis. J Pediatr. 2007;150:106–108. doi: 10.1016/j.jpeds.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Qi Z M, Wang J, Sun Z R, Ma F M, Zhang Q R, Hirose S, Jiang Y. Polymorphism of the mouse gene for the interleukin 10 receptor α chain (Il10ra) and its association with the autoimmune phenotype. Immunogenetics. 2005;57:697–702. doi: 10.1007/s00251-005-0036-7. [DOI] [PubMed] [Google Scholar]