Abstract
NK cells represent a critical component of the host innate immune response to viral infection and tumor transformation. Nevertheless, the fate of recently degranulated NK cells subsequent to a primary target cell interaction remains largely unexplored. Here, we investigated the long-term viability and killing potential of human NK cells following target cell lysis using live-sorting of CD107a-degranulated NK cells. We observed that sorted CD107a+ NK cells exhibited continued lytic potential against a wide variety of target cells, including tumor and virally infected target cells. CD107a-positive- and CD107a-negative-sorted NK cells displayed similar long-term viability, killing potential, and response to inflammatory cytokines such as IL-2, IL-15, and IFN-α. Interestingly, we observed that the CD107a signature is remarkably stable over time and that recently degranulated NK cells exhibit an amplification of CD107 expression immediately following a target cell interaction. Together, our data expand previous data showing that NK cells retain the capacity to kill multiple target cells in succession and reveal that NK viability, cytotoxicity, and response to inflammatory cytokines are not altered following a primary target cell interaction. Overall, our data argue for the strength of the NK cell compartment in the continuous surveillance of tumor and virally infected cells in the body and highlight the use of using CD107a expression as a stable marker for NK cytotoxicity.
Keywords: perforin/granzyme pathway, killing potential
NK cells represent a critical component of the host innate immune response to viral infection and tumor transformation. Through a complex balance of inhibitory and activating receptors, NK cells seek out and destroy damaged cells in the body that exhibit signs of stress or have down-regulated MHC Class I (MHC-I) proteins to escape CD8 cytotoxic T cell recognition. The interaction of NK inhibitory receptors with MHC-I proteins expressed on target cells induces inhibitory signals into the NK cell that limit cytotoxicity and cytokine production [1, 2]. Conversely, when MHC-I proteins are down-regulated during viral infection or tumor transformation, the lack of NK inhibitory receptor signaling renders the target cells susceptible to NK-mediated lysis. However, the full commitment of a NK cell to lyse a target also requires the interaction of specific NK-activating receptors with their cognate ligands on target cells [3, 4]. Examples of NK-activating receptors include the C-lectin-type receptor NKG2D, which recognizes stress-inducible proteins up-regulated on the surface of target cells; the FcγR (CD16), which interacts with the constant region of antibodies to mediate antibody-dependent cellular cytotoxicity (ADCC); and the Ig-like receptors (NKp30, NKp44, NKp46), which may interact directly with viral antigens [3,4,5,6]. NK activity is also influenced directly by soluble cytokines such as IL-2, IL-12, IL-15, IL-21, or IFN-α, which can augment NK lysis and enhance viability [7,8,9]. Overall, the balance between inhibitory and activating stimuli within a NK cell will determine the activation state, cytokine profile, and killing potential of NK cells toward a particular target.
Once triggered to kill, NK cells mediate lysis through two independent lytic mechanisms: the death receptor pathway of lysis and the perforin/granzyme mechanism of degranulation [6, 10, 11]. Strong activating stimuli such as MHC-I-devoid tumor targets or ADCC trigger rapid lysis (within 4 h) by NK cells through the perforin/granzyme pathway [6, 12, 13]. Currently, the impact of target cell lysis on the long-term viability and cytolytic potential of recently degranulated NK cells remains unknown. The recent discovery of a CD107a flow cytometric-based assay to detect cytolytic NK cells now allows for the separation and analysis of newly degranulated NK cells that expose CD107a during perforin/granzyme-mediated lysis [14, 15].
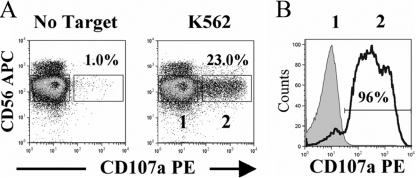
Here, we investigated the killing potential and viability of human NK cells following a primary degranulation event using live sorting of CD107a-degranulated NK cells. We enriched NK cells to 98% purity (see CD56 staining in Fig. 1A) from Ficoll-purified PBMCs via negative-selection magnetic beads according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). We then incubated freshly purified NK cells with MHC-I-devoid K562 tumor target cells for 3 h at a 4:1 E:T cell ratio in the presence of mouse anti-human CD107a antibody. This approach led to strong NK lysis of K562 target cells in a standard 3-h chromium lysis assay (data not shown) and the induction of CD107a degranulation on ∼20% of purified NK cells (Fig. 1A). Following staining with CD56 to separate NK cells from tumor target cells, we sorted CD56+/CD107+ double-positive NK cells (identified in Box 2) and CD56+/CD107a– single-positive NK cells (identified in Box 1). CD56+/CD107– NK cells that did not degranulate but were exposed to K562 cells were sorted to provide an internal control for the effect of coculture with lysed cells (Fig. 1A). CD107+ degranulated and CD107– nondegranulated NK populations were >95% pure after sorting, as shown in Figure 1B.
Fig. 1.
Live cell sorting of CD107a-degranulated NK cells following target cell interaction. (A) Human NK cells were purified from PBMCs to 98% purity with NK-negative selection beads (Miltenyi Biotec) and incubated for 3 h at a 4:1 E:T cell ratio with K562 target cells in the presence of 0.2 μg mouse anti-human CD107a antibody (BD PharMingen, San Diego, CA, USA)/million cells. NK cells were then stained for 15 min with 0.2 μg mouse anti-human CD56 antibody (BD PharMingen)/million cells, and samples were run on a Dako Cytomation cell sorter (Dako Cytomation, Denmark). NK cells incubated without target cells for 3 h (No Target) were used as a control. CD107+/CD56+ degranulated NK cells in Box 2 were sorted along with CD107–/CD56+ control NK cells in Box 1. (B) Purity of CD107+ (black, empty histogram)- and CD107a– (gray, shaded histogram)-sorted NK cells from A is shown immediately following sorting. Results are representative of seven independent experiments.
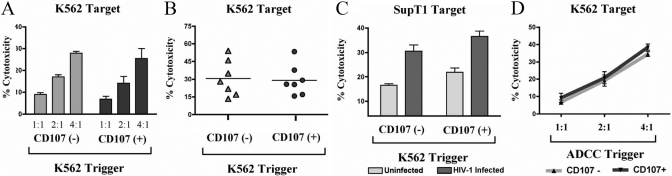
Prior to testing the cytotoxicity of sorted CD107+ and CD107– NK cells, we first investigated the levels of granzyme and perforin in CD107+ degranulated NK cells. As shown in Figure 2, we observed that despite having recently degranulated, sorted CD107+ NK cells retained comparable intracellular granzyme and perforin levels as CD107– control NK cells. Although a portion of the perforin and grazyme content was likely released into target cells during lysis, these results suggest that NK cells express a surplus of perforin and granzyme molecules, potentially enabling lysis of future targets. To investigate this possibility directly, we incubated purified NK cells with K562 target cells in two sequential cytolytic assays. In the first interaction, purified NK cells were incubated with K562 tumor target cells to induce CD107a degranulation for cell sorting as described in Figure 1A. Subsequently, CD107+ and CD107– sorted NK cells were incubated with chromium-labeled K562 targets cells in a standard 3-h chromium lysis assay at a 4:1 E:T ratio. We observed CD107+ NK cells possess a similar capacity to kill K562 tumor targets as control CD107– NK cells at every E:T cell ratio tested (Fig. 3A) and across multiple donors (Fig. 3B). We next tested if the capacity of NK cells to mediate repeated lysis was restricted to K562 tumor target cells or if it was a general feature of NK cells toward other targets. We incubated purified NK cells with K562 cells to induce degranulation as in Figure 1A and tested the ability of sorted CD107+ NK cells to lyse uninfected and HIV-1-infected SupT1 cells. Although HIV-1 infection of SupT1 cells increases the sensitivity of the cell line to NK-mediated lysis, uninfected SupT1 cells also possess intrinsic susceptibility to NK lysis. As shown in Figure 3C, sorted CD107+ NK cells possessed the same capacity to lyse uninfected SupT1 cells and HIV-1-infected SupT1 cells as sorted CD107– NK cells. Consistent with earlier data, these findings indicate that NK cells that have degranulated recently maintain continued lytic capacity against multiple, different target cells.
Fig. 2.
Intracellular granzyme and perforin staining of CD107+ and CD107– sorted NK cells. (A) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio in the presence of mouse anti-human CD107a antibody and sorted as in Figure 1A. CD107+ and CD107– sorted NK cells were then fixed, permeabilized, and stained with 0.2 μg mouse anti-human granzyme (BD PharMingen) or mouse anti-human perforin (BD PharMingen)/million cells. The percentage of NK cells staining positive for granzyme and perforin is shown for each histogram (in bold) along with the mean fluorescence intensity (upper-right corners). Results are representative of three independent experiments.
Fig. 3.
Cytolytic capacity of CD107+ and CD107– sorted NK cells. (A) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107– and CD107+ sorted NK cells were then incubated with chromium-labeled K562 cells at multiple E:T cell ratios for 3 h, and a fraction of the supernatant was collected and tested in a γ-scintillation counter. Percentage cytotoxicity was calculated as described previously [16]. (B) Purified NK cells from seven individual donors were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107– and CD107+ sorted NK cells were then incubated with chromium-labeled K562 cells at a 4:1 E:T ratio for 3 h in a standard chromium lysis assay. (C) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107+ and CD107– sorted NK cells were then incubated with chromium-labeled, HIV-1-infected or -uninfected SupT1 cells at a 4:1 E:T ratio for 3 h. To generate HIV-1-infected SupT1 targets, SupT1 cells were infected with 150 ng HIV-1 × 4 tropic primary isolate (r) (TYBE) containing supernatant and centrifuged for 2 h at 1800 rpm in the presence of 8 μg/ml polybrene. HIV-1-infected SupT1 cells were incubated for 4 days until they were 95% p24-positive and then used as targets in chromium lysis assay. (D) Purified NK cells were incubated with antibody-coated CEM×174 targets for 3 h at a 4:1 E:T ratio and sorted as in Figure 1 A. CEM×174 cells were coated with 1 mg HIV-1 gp120 and incubated with a 1/1000 dilution of HIV-1-infected subject sera to induce ADCC. CD107+ (dark gray bar)- and CD107– (light gray bar)-sorted NK cells were then incubated with chromium-labeled K562 cells at a 4:1 E:T ratio for 3 h in a standard chromium cytotoxicity assay. Error bars represent the sd of the mean of quadruplicate values from each representative experiment.
We next tested if the type of target cell used to induce CD107a degranulation affected the capacity of sorted CD107+ cells to mediate lysis in a second target cell encounter. We triggered degranulation in purified NK cells with T-lymphoid SupT1 targets or antibody-coated CEM×174 cells (to mediate antibody-dependent cellular cytotoxicity, ADCC) and sorted CD107+ and CD107– cells as in Figure 1A. Although incubation of NK cells with SupT1 or antibody-coated CEM×174 cells triggered slightly different NK degranulation frequencies, CD107+ and CD107– NK cells from both target cell interactions were sorted to comparable intensity and purity as in Figure 1A (data not shown). Similar to our results showing that NK cells possessed the capacity to mediate repeated lysis against K562 cells in Figure 3A, we observed that sorted CD107+ NK cells retained comparable lytic potential as sorted CD107– NK cells, regardless of whether the trigger was a SupT1 tumor target cell (data not shown) or antibody-coated CEM×174 cells that triggered degranulation through ADCC (Fig. 3D).
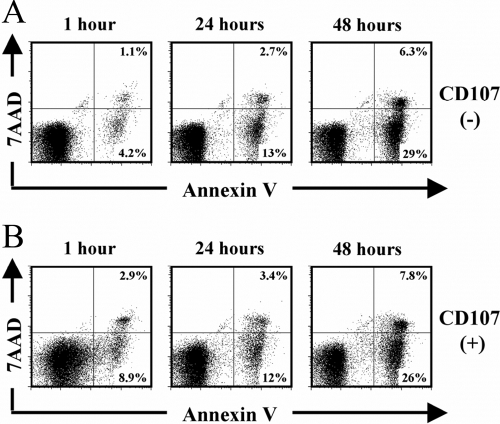
To investigate the kinetics of NK viability following degranulation, we incubated purified NK cells with K562 cells to induce degranulation as in Figure 1A and measured the apoptotic rate of CD107– and CD107+ sorted NK cells over time. Using 7-amino-actinomycin (7AAD) staining of cell death and Annexin V staining as a marker for early apoptosis, we observed that sorted CD107– and CD107+ NK cells maintained strong viability (>80%) in culture up to 24 h (Fig. 4, A and B). By 48 h however, we observed that >30% of sorted CD107+ degranulated and CD107– control NK cells began to undergo early apoptosis, indicating that NK cells have a finite lifespan in culture in the absence of exogenous cytokine stimulation (Fig. 4, A and B). By 96 h, >80% of sorted CD107+ degranulated and CD107– control NK cells exhibited apoptosis (data not shown). At all measured time-points, CD107+ degranulated NK cells exhibited comparable viability as CD107– NK cells, indicating that target cell-induced degranulation does not alter the subsequent viability of NK cells.
Fig. 4.
Kinetics of viability following sorting of CD107+ and CD107– NK cells. (A and B) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107– (A)- and CD107+ (B)-sorted NK cells were then incubated in complete media, and viability was tested at 1 h, 24 h, and 48 h postsort with 0.2 μg 7AAD and 50 μl Annexin V FITC/million cells. Results are representative of three independent experiments.
We next tested if sorted CD107+ cells maintained comparable long-term cytotoxic capacity as sorted CD107– NK cells, as we had with NK viability. As shown in Figure 5A, we found that sorted CD107+ NK cells exhibited an equivalent cytotoxic capacity as sorted CD107– NK cells at every time-point tested postsort. By using multiple E:T cell ratios, we confirmed that sorted CD107+ and CD107– NK cells possessed comparable lytic potential, even when effector cell numbers were limiting (Fig. 5A). Of note, we found no reduction in the cytotoxic capacity of sorted CD107+ or CD107– NK cells at 48 h, despite evidence of early apoptosis in both cell groups as shown in Figure 4. We interpret that this disconnect between viability and lytic potential may simply be a result of the increased sensitivity of using Annexin V staining as a marker of early apoptosis. Of note, sorted CD107+ NK cells retained their CD107 signature for the entirety of the 48-h incubation postsort, highlighting their fundamental difference from nondegranulated NK cells (Fig. 5B). Together, these data indicate that degranulated NK cells possess identical long-term cytolytic potential as CD107– control NK cells and bear a stable CD107 signature that can be followed over time.
Fig. 5.
Kinetics of NK cytotoxicity following sorting of CD107+ and CD107– sorted NK cells. (A) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107+ and CD107– sorted NK cells were then incubated in complete media, and cytotoxicity was tested at 1 h, 24 h, and 48 h postsort in a standard chromium lysis assay against K562 target cells for 3 h at multiple E:T cell ratios. Error bars represent the sd of the mean of quadruplicate values from each representative experiment. (B) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107+ (black, empty histograms)- and CD107– (gray, shaded histograms)-sorted NK cells were then incubated in complete media, and residual CD107a staining was tested at 1 h, 24 h, and 48 h postsort. Results are representative of three independent experiments.
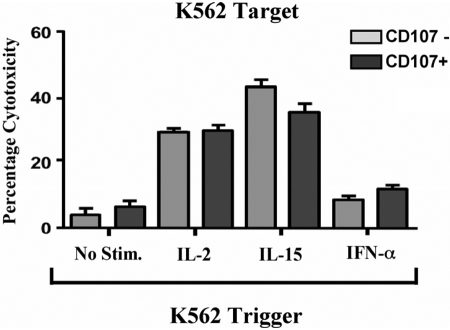
We next measured the response of CD107+ and CD107– NK cells to stimulation with various inflammatory cytokines. We incubated purified NK cells with K562 cells to induce CD107 degranulation as in Figure 1 and stimulated sorted CD107+ and CD107– NK cells for 18 h in the presence or absence of IL-2, IL-15, or IFN-α. We then measured the cytotoxic potential of cytokine-stimulated CD107+ and CD107– NK cells against K562 cells in a standard chromium lysis assay. As shown in Figure 6, sorted CD107+ and CD107– NK cells exhibited a comparable increase in cytotoxicity in response to IL-2, IL-15, and to a lesser extent, IFN-α. Overall, these data show that in addition to possessing comparable viability and cytotoxic potential, degranulated and nondegranulated NK cells maintain a similar capacity to respond to proinflammatory cytokines.
Fig. 6.
Response of CD107+ and CD107– sorted NK cells to cytokine stimulation. Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107+ and CD107– sorted NK cells were then incubated in complete media in the presence or absence of 100 u/ml IL-2, 100 ng/ml IL-15, or 1000 u/ml IFN-α for 18 h. NK cells were then tested in a standard chromium lysis assay against K562 target cells for 3 h at a 1:1 E:T cell ratio. Error bars represent the sd of the mean of quadruplicate values from each representative experiment.
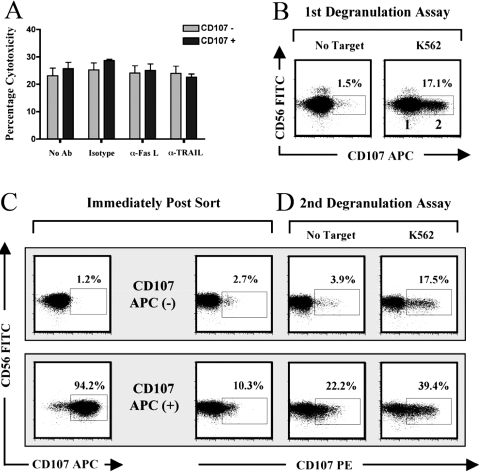
Lastly, we investigated the potential role of death receptor-mediated mechanisms of lysis in the continued lytic potential of CD107+ degranulated NK cells using neutralizing mAb to Fas ligand (FasL) and TRAIL. As shown in Figure 7A, blocking FasL and TRAIL had no measurable effect on lysis by CD107– or CD107+ NK cells. Although this result suggested that death receptors were not involved in the repeated lytic capacity of CD107+ NK cells, we also tested if NK cells could redegranulate in response to a second target cell interaction. Following a primary target cell interaction with K562 cells in the presence of an APC-labeled anti-CD107 mAb (Fig. 7B), we sorted NK cells as described in Figure 1A and incubated CD107 APC+ and CD107 APC– NK cells with K562 cells in a second degranulation assay in the presence of a PE-labeled CD107 mAb (Fig. 7D). Using this approach, we observed that sorted CD107+ NK cells could redegranulate following a secondary target cell interaction and that the frequency of CD107+ cells degranulating over background (39.4–22.2=17.2) was comparable with that observed with CD107– control NK cells (17.5–3.91=13.59). Interesting, the background of CD107 staining in the absence of target cells was distinctly higher among sorted CD107+ cells than the background in CD107– control cells, suggesting that residual CD107 remained on the cell surface (see Fig. 7C, second panel from left). CD107+ degranulated NK cells also exhibited spontaneous degranulation in the 3-h period following sorting in the absence of targets (Fig. 7, C and D, compare CD107+, middle two panels). Overall, these data indicate that NK possess the capacity to degranulate multiple times in response to target cells and suggest that the CD107 signature is not only stable but may also amplify over time.
Fig. 7.
Continued lysis of CD107-positive cells is mediated through repeated degranulation, not FasL or TRAIL-mediated cytotoxicity. (A) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio and sorted as in Figure 1A. CD107– and CD107+ sorted NK cells were then incubated in a standard chromium lysis assay against radioactively labeled K562 target cells for 3 h at a 4:1 E:T cell ratio in the presence of isotype control or blocking antibodies to FasL, TRAIL at 10 μg/ml. Error bars represent the sd of the mean of quadruplicate values from each representative experiment. (B) Purified NK cells were incubated with K562 target cells for 3 h at a 4:1 E:T ratio in the presence of 0.2 μg mouse anti-human CD107 APC antibodies and sorted as in Figure 1A. (C) CD107 APC– and CD107 APC+ sorted NK cells were stained for 15 min at 4°C with 0.2 μg mouse anti-human CD107 PE mAb. (D) CD107 APC– and CD107 APC+ sorted NK cells were incubated in a second degranulation assay with K562 target cells for 3 h at a 4:1 E:T ratio in the presence of 0.2 μg mouse anti-human CD107 PE mAb/million cells.
Using mathematical modeling of NK lysis over time, previous research suggested that NK cells possess the capacity to lyse multiple targets in succession [17]. Subsequent research from other groups showed that prolonged interaction (18 h) of NK cells with tumor target cells reduced NK viability and cytotoxic potential [18, 19]. However, technical limitations, including the inability to separate cytotoxic NK cells from the total NK population, limited interpretation of these early findings. Here, we used live cell sorting of CD107+ degranulated NK cells following a short-term (3 h), physiologically relevant target cell interaction to explore the long-term viability and lytic potential of NK cells following target cell lysis. We found that target cell-induced degranulation did not alter the granuole content (Fig. 2) or the viability (Fig. 4) of NK cells and that CD107+ sorted NK cells retained comparable long-term cytotoxic capacity against virally infected and tumor target cells compared with nondegranulated control NK cells (Figs. 3 and 5). Together, our results confirm previous data that NK cells possess the capacity to lyse multiple targets [17] and show further that degranulation does not alter the viability, lytic potential, or response of NK cells to cytokine stimulation. Our data also indicate that CD107-positive NK cells possess the capacity to redegranulate in subsequent lytic encounters and that the CD107a signature of degranulation is remarkably stable over time. These latter results suggest that the increase in constitutive NK CD107a degranulation observed during acute HIV-1 infection and in HIV-1-exposed, uninfected i.v. drug users may reflect recent in vivo NK lytic activity that can be captured ex vivo [20, 21]. Overall, our findings highlight the continuous activity of NK cells in surveillance of tumor and virally infected targets in the body and emphasize the usefulness of the CD107 assay system as a stable marker for NK lysis.
Acknowledgments
We thank Jeffrey S. Faust, David E. Ambrose, and Daniel Hussey at The Wistar Institute Flow Cytometry Core Facility for their contribution in sorting all NK samples for this study. We also thank Dr. Farida Shaheen (Supervisor, Centers for AIDS Research Viral Core Facility, University of Pennsylvania, Philadelphia, PA) for the expansion and titering of all HIV-1 strains used in this study and Deborah Davis for her contribution as phlebotomist at The Wistar Institute. This study was supported by grants from the National Institutes of Health (AI51225, AI47760, U01AI065279, AI07632, AI068405), the Philadelphia Foundation, and funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health. The authors have no financial conflicts of interest.
References
- Long E O, Burshtyn D N, Clark W P, Peruzzi M, Rajagopalan S, Rojo S, Wagtmann N, Winter C C. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–144. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Lanier L L. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari M C, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- Bottino C, Biassoni R, Millo R, Moretta L, Moretta A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum Immunol. 2000;61:1–6. doi: 10.1016/s0198-8859(99)00162-7. [DOI] [PubMed] [Google Scholar]
- Djeu J Y, Jiang K, Wei S. A view to a kill: signals triggering cytotoxicity. Clin Cancer Res. 2002;8:636–640. [PubMed] [Google Scholar]
- Biron C A, Nguyen K B, Pien G C, Cousens L P, Salazar-Mather T P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4:S161–S167. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Wysocka M, D'Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante N M, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- Velthuis J H, de Bont H J, Medema J P, Kuppen P J, Mulder G J, Nagelkerke J F. Interleukin-2 activated NK cells do not use the CD95L- and TRAIL-pathways in the rapid induction of apoptosis of rat colon carcinoma CC531s cells. Immunobiology. 2003;207:115–127. doi: 10.1078/0171-2985-00226. [DOI] [PubMed] [Google Scholar]
- Mori S, Jewett A, Murakami-Mori K, Cavalcanti M, Bonavida B. The participation of the Fas-mediated cytotoxic pathway by natural killer cells is tumor-cell-dependent. Cancer Immunol Immunother. 1997;44:282–290. doi: 10.1007/s002620050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrberg M. The CD107 mobilization assay: viable isolation and immunotherapeutic potential of tumor-cytolytic NK cells. Leukemia. 2005;19:707–709. doi: 10.1038/sj.leu.2403705. [DOI] [PubMed] [Google Scholar]
- Derby E G, Reddy V, Nelson E L, Kopp W C, Baseler M W, Dawson J R, Malyguine A M. Correlation of human CD56+ cell cytotoxicity and IFN-γ production. Cytokine. 2001;13:85–90. doi: 10.1006/cyto.2000.0804. [DOI] [PubMed] [Google Scholar]
- Betts M R, Brenchley J M, Price D A, De Rosa S C, Douek D C, Roederer M, Koup R A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Alter G, Malenfant J M, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Tomescu C, Chehimi J, Maino V C, Montaner L J. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol. 2007;179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- Ullberg M, Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981;153:615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156:907–915. [PubMed] [Google Scholar]
- Cavalcanti M, Jewett A, Bonavida B. Irreversible cancer cell-induced functional anergy and apoptosis in resting and activated NK cells. Int J Oncol. 1999;14:361–366. doi: 10.3892/ijo.14.2.361. [DOI] [PubMed] [Google Scholar]
- Ravet S, Scott-Algara D, Bonnet E, Tran H K, Tran T, Nguyen N, Truong L X, Theodorou I, Barre-Sinoussi F, Pancino G, Paul P. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- Long B R, Ndhlovu L C, Oksenberg J R, Lanier L L, Hecht F M, Nixon D F, Barbour J D. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:4785–4792. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]