Abstract
Bacterial DNA expressing unmethylated CpG motifs binds to TLR9, thereby stimulating a broadly protective, innate immune response. Although CpG-mediated signal transduction has been studied, the scope of TLR9-dependent gene expression is incompletely understood. To resolve these issues, mice were treated with immunostimulatory CpG oligonucleotides (ODN) and splenic mRNA levels monitored from 30 min through 3 days by microarray. Through the unique application of bioinformatic analysis to these experimental data, this study is the first to describe the complex regulatory networks responsible for TLR9-mediated gene expression. Current results are the first to establish that CpG-induced stimulation of the innate immune system proceeds in multiple waves over time, and gene up-regulation is mediated by a small number of temporally activated “major inducers” and “minor inducers”. An additional study of TNF knockout mice supports the conclusion that the regulatory networks identified by our bioinformatic analysis accurately identified CpG ODN-driven gene–gene interactions in vivo. Equally important, this work identifies the counter-regulatory mechanisms embedded within the signaling cascade that suppresses the proinflammatory response triggered in vivo by CpG DNA stimulation. Identifying these network interactions provides novel and global insights into the regulation of TLR9-mediated gene activation, improves our understanding of TLR-mediated host defense, and facilitates the development of interventions designed to optimize the nature and duration of the ensuing response.
Keywords: rodent, gene regulation, signal transduction, molecular biology
INTRODUCTION
Unmethylated CpG motifs present in bacterial DNA bind to TLR9, stimulating an innate immune response characterized by the production of proinflammatory cytokines, chemokines, and Igs [1,2,3,4,5,6,7]. The interaction of TLR9 with synthetic CpG-containing oligonucleotides (ODN) triggers a well-defined signaling cascade that proceeds via MyD88 [8,9,10,11,12,13] and results in a complex pattern of gene up-regulation. Microarray technology coupled with computational pathway analysis was harnessed to gain novel insights into the mechanisms underlying these broad changes in gene expression.
Previous attempts to clarify the effect of CpG stimulation on gene expression were performed on cell suspensions in vitro. Many of those studies analyzed cloned cell lines and thus could not identify changes attributable to cell–cell interactions [14, 15]. Other groups studied human PBMC or murine spleen cell cultures but were limited by the accumulation of culture-associated artifacts within 12–24 h [16, 17]. Thus, although there was evidence that regulatory networks influenced the pattern of TLR9-mediated gene expression and that the IFN-γ gene (IFNG) contributed to this process [17], little was known about the breadth or duration of CpG-dependent changes in gene expression under physiologic conditions.
To resolve these issues, mice were treated with immunostimulatory CpG ODN and splenic mRNA levels monitored from 30 min through 3 days by microarray. The spleen was selected for analysis on the basis of previous studies, establishing that this lymphoid organ accurately reflected the breadth of immunity induced by CpG ODN in vivo [1, 18,19,20,21,22]. It establishes that multiple waves of activation occur, regulated over time by a small number of stimulatory genes (referred to herein as “major inducers” and “minor inducer”). To establish the biological relevance of the results generated by this bioinformatic analysis, the effect of eliminating a major inducer—TNF—was evaluated in knockout (KO) mice. These studies further established the mechanisms by which CpG-activated genes are subsequently down-regulated. Results demonstrate that active suppression is key to the temporal decline in gene expression and that by targeting “inducers,” a small cohort of “suppressors” can extinguish entire networks.
MATERIALS AND METHODS
Oligodeoxynucleotides
Endotoxin-free phosphorothioate ODN were synthesized at the Center for Biologics Evaluation and Research core facility (U.S. Food and Drug Administration, Bethesda, MD, USA), as described previously [18]. ODN were dissolved in sterile water. Mice were injected i.p. with 400 μg equimolar mixture of CpG ODN 1555 (GCTAGACGTTAGCGT) and 1466 (TCAACGTTGA) or control ODN 1612 (GCTAGATGTTAGCGT) and 1471 (TCAAGCTTGA).
Mice and cell culture conditions
Two-month-old female BALB/c mice, C57BL/6, or C57BL/6 TNF KO mice were housed in the National Cancer Institute (NCI; Frederick, MD, USA)-specific pathogen-free animal facility. C57BL/6 TNF KO mice and C57BL/6 control mice were kindly provided by Dr. Rosalba Salcedo, NCI Frederick. All experiments were conducted under Animal Care and Use Committee-approved protocols. Spleens were surgically removed from mice under sterile conditions after 0.5, 1, 3, 9, 24, or 72 h, diced, and stored at –80°C in RNAlater (Qiagen, Valencia, CA, USA).
Production of labeled cDNA
Total RNA was extracted from spleens using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) as specified by the manufacturer. Total RNA (20 μg) was reverse-transcribed using 3 μl 10× first-strand buffer (Stratagene, La Jolla, CA, USA), 2 μl (5 μg)-anchored oligo(dT) (Invitrogen), 150 U RT (StrataScript HC RT, Stratagene), 2 μl 20× aminoallyl-dUTP/dNTP mix, and 3 μl 0.1 M DTT in a final volume of 30 μl at 42°C for 90 min. A reference mouse RNA sample (Stratagene) was processed in parallel. Both cDNAs were purified using a MinElute PCR purification kit (Qiagen). cDNA (10 μl) was labeled with Cy5 (sample cDNA)- or Cy3 (universal reference cDNA)-reactive dyes (Amersham Biosciences, Piscataway, NJ, USA), diluted in 5 μl DMSO plus 1.7 μl 1 M NaHCO3 for 90 min in the dark. Labeled cDNA was purified using MinElute PCR purification kits (Qiagen).
ODN microarray hybridization
Murine ODN microarrays were produced by the NCI microarray facility (Gaithersburg, MD, USA). Cy3-labeled reference and Cy5-labeled sample cDNAs (10 μl each) were combined, denaturated by heating for 2 min at 98°C, and mixed with 36 μl hybridization solution at 42°C (Ambion, Austin, TX, USA). Microarrays were overlaid with this solution and hybridized for 18 h at 42°C using an actively mixing MAUI hybridization system (BioMicro Systems, Salt Lake City, UT, USA). Post-hybridization, the arrays were washed in 1× SSC/0.05% SDS and 0.1× SSC, centrifuged to remove remaining liquid with unbound cDNA, and dried. Arrays were scanned and intensity values generated using an Axon scanner and GenePix software 5.1 (Axon Instruments, Foster City, CA, USA). Data were up-loaded to the mAdb database [Microarray Database, a collaboration of the Center for Information Technology/BioInformatics and Molecular Analysis Section and NCI/Center for Cancer Research at the National Institutes of Health (NIH); http://nciarray.nci.nih.gov/] and formatted via the export function for use with Biometric Research Branch (BRB; NCI) ArrayTools.
Analysis of gene expression
Data from four independent experiments/time-point and four to six untreated controls were used for all statistical analyses. Reproducibility was established by comparing gene expression profiles among similarly treated mice from independent experiments in mAdb (referenced above). Expression analyses were performed using BRB ArrayTools. Data were background-corrected, flagged values were removed, spots in which both signals were <100 were filtered out, ratios were log base 2-transformed, and lowest intensity-dependent normalization was used to adjust for differences in labeling intensities of the Cy3 and Cy5 dyes [23]. Analysis was restricted to genes present on >70% of the arrays after filtering. In toto, 30,722 features were reproducibly tracked in all microarrays. The gene expression profile of all treatment groups was compared with that of the control groups. A P value cutoff of 0.00001 was used to identify genes whose expression was up-regulated significantly after CpG ODN stimulation when compared with controls. Data were evaluated using Ingenuity Pathway Analysis (IPA; Ingenuity Systems Inc., Redwood City, CA, USA). IPA maps each gene within a global molecular network developed from information contained in the Ingenuity Pathways Knowledge Base. Gene networks were generated algorithmically based on their connectivity in terms of expression, activation, transcription, and/or inhibition. A “network” in IPA is defined as a graphical representation of the molecular relationships between genes, which are represented as nodes, and the biological relationship between nodes is shown by a connecting line. All connections are supported by published data stored in the Ingenuity Pathways Knowledge Base and/or PubMed. IPA ranks all genes based on their connectivity, using a generalization of the concept of node degree, which measures the number of single genes to which a gene is connected {see https://analysis.ingenuity.com/pa/info/help/Ingenuity_Network_Algorithm_Whitepaper_ FINAL(2).pdf and ref. [24] for details}.
Statistical analysis
Genes that were expressed differentially in the treatment groups were identified using a random-variance t-test. The random-variance t-test is an improvement over the standard, separate t-test, as it permits sharing information among genes about within-class variation without assuming that all genes have the same variance [25]. Genes were considered statistically significant if their P value was <0.00001. The behavior of specific gene subsets was analyzed using the BRB Genelist Comparison Tool, which calculates the probability that the behavior of the selected genes differs from background. Differences between regulators in terms of number of genes regulated were established by χ2 analysis and Fisher exact test. Differences of fold-changes between groups of genes were established by t-test analysis (two-sided).
Accession codes
Microarray data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series Accession Number GSE11202.
RESULTS
Identification of the regulatory network underlying CpG-dependent gene expression in vivo
Normal BALB/c mice were injected i.p. with 400 μg of immunostimulatory CpG DNA. Changes in mRNA expression levels were monitored in the spleen by microarray at 0.5, 1, 3, 9, 24, and 72 h. A minimum of four animals/group was analyzed independently at each time-point and consistent changes in gene expression observed among similarly treated mice in independent experiments (R2=0.89±0.04). A stringency cutoff of P < 0.00001 was used to identify genes whose level of expression differed significantly from naive controls (n=6).
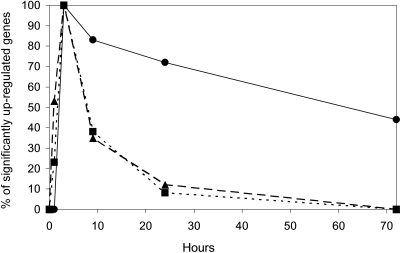
Changes in gene expression were present at 30 min, peaked at 3 h, and declined progressively thereafter (Fig. 1). These changes in mRNA expression were overwhelmingly CpG-specific, as <0.4% of these genes were stimulated in mice treated with control (non-CpG) ODN.
Fig. 1.
CpG ODN induce reproducible patterns of gene expression in vivo. BALB/c mice were injected i.p. with 400 μg CpG ODN. Gene expression in the spleen was monitored over time by microarray. Four biological replicates were analyzed independently at each time-point. Up-regulated genes were identified by comparison with untreated mice (n=6) using a stringency cut-off of P < 0.00001 and are displayed as percent of maximum gene expression. The behavior of genes up-regulated by IFNG (•), TNF (▴), or minor inducers (▪) is shown. Note that the expression of genes regulated by IFNG persists significantly longer than those regulated by TNF or minor inducers (P<.001).
IPA was used to identify the pattern of regulatory interactions underlying CpG-dependent gene activation in vivo. This widely used technique has been used successfully in other model systems [24, 26,27,28]). As described in Materials and Methods, IPA networks identify molecular relationships between genes, with lines identifying known gene–gene interactions. Analysis of the data from CpG-treated mice included all functional and regulatory interactions mapped in the IPA database in terms of expression, activation, transcription, and/or inhibition.
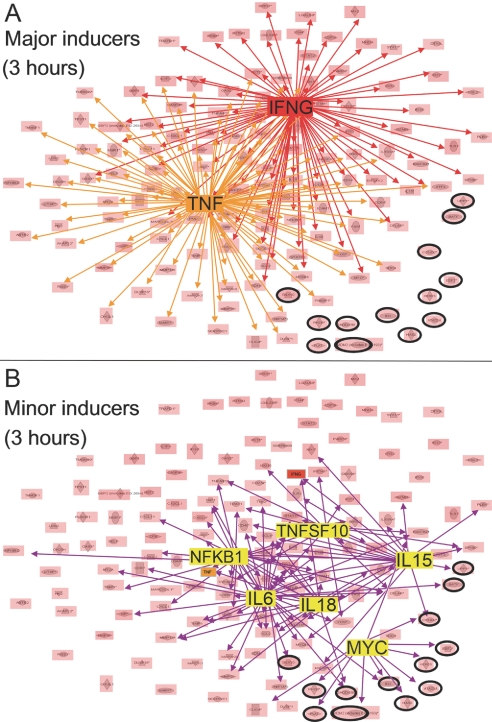
Initial analysis focused on the 3-h time-point when gene activation was maximal (Fig. 1). Results showed that 90% of all evaluable genes were under the regulatory control of TNFα (hereafter TNF) and/or IFNG (see red and orange cohorts, Fig. 2A; high-resolution, Supplemental Fig. S1 and Supplemental Table T1). Hereafter, regulatory elements such as TNF and IFNG, which individually impact the expression of ≥50% of all genes at a given time-point, will be referred to as major inducers.
Fig. 2.
Key regulatory networks activated by CpG ODN treatment (3 h time-point). All genes activated at 3 h (P<0.00001), whose regulatory interactions could be mapped by IPA, are shown. (A) Network analysis identifies two major inducers, each up-regulating >50% of all genes: IFNG (red network) and TNF (orange network). Those genes not regulated by IFNG or TNF are circled. (B) Network analysis also identifies minor inducers [IL6, NFKB1, IL15, TNF superfamily (TNFSF)10, MYC, and IL18, purple network] that regulate 10–50% of all genes, including those not regulated by IFNG or TNF.
Despite their importance, TNF and IFNG did not account for all of the gene activation observed at 3 h (see genes circled in black, Fig. 2). Network analysis identified an additional group of minor inducers, which individually controlled the expression of 10–25% of all genes, including those not stimulated by the major inducers (Fig. 2B). These minor inducers included IL6, NFKB1, IL15, TNFSF10, IL18, and MYC (Table 1 and Supplemental Table T1).
TABLE 1.
Kinetics of CpG-Mediated Gene Up-regulation
| Time (h) | Major inducers | Minor inducers |
|---|---|---|
| 0.5 | TNF, IL1B, IL1A | None |
| 1 | TNF, IL1B | IFNG, IL6, IL1A |
| 3 | TNF, IFNG | IL6, NFKB1, IL15, TNFSF10, IL18, MYC |
| 9 | TNF, IFNG | IL15, IL18 |
| 24 | IFNG | PARP9, STAT1, STAT2 |
| 72 | IFNG | STAT1, STAT2 |
IPA was used to identify major and minor inducers of gene activation (P<0.00001) from 0.5 to 72 h after CpG ODN treatment of BALB/c mice. Results reflect the analysis of four biological replicates analyzed independently at each time-point. Major inducers are defined as activating >50% of all genes up-regulated at a given time-point, whereas minor inducers regulate 10–50% of the corresponding network. PARP9, Poly(ADP-ribose polymerase 9.
Many of the genes up-regulated at 3 h were targeted by more than one major and/or minor inducer. These effects are quantified in Table 2, which compares the average expression level of genes regulated by a single major inducer (TNF) with those targeted by TNF plus additional inducers. Analysis of these interactions reveals a consistent pattern wherein the level of expression of genes targeted by multiple inducers is greater than those targeted by a single inducer, and major inducers have a greater effect on the magnitude of gene expression than minor inducers.
TABLE 2.
Effect of Multiple Inducers on the Level of Gene Expression
| Inducer | n | Avg-fold-increase in gene expression | P value |
|---|---|---|---|
| TNF alone | 18 | 4.0 ± 0.47 | |
| TNF plus minor inducer | 19 | 4.7 ± 0.71 | 0.4 |
| TNF plus major inducer | 19 | 6.2 ± 1.10 | 0.08 |
| TNF plus major and minor inducer | 35 | 6.7 ± 0.99 | 0.016 |
The network of genes up-regulated significantly at 3 h (P<0.00001) was analyzed by IPA. The fold-increase in mRNA expression of all genes regulated by TNF alone was compared with genes regulated by TNF plus various major and/or minor inducers (mean±sem). Genes targeted by multiple major and/or minor inducers showed higher levels of mRNA expression (P values vs. TNF alone).
Verification of the regulatory networks identified in vivo
To examine the reproducibility of the regulatory networks identified by IPA analysis of microarray data from CpG-treated BALB/c mice, an identical study was performed in C57BL/6 mice. As above, splenic mRNA levels were evaluated independently in four animals/group at 3 h. There was an 82% concordance rate between the genes up-regulated significantly in CpG-treated C57BL/6 (stringency cutoff P<0.0001) versus BALB/c mice treated identically. This concordance in gene expression differed significantly from random (P<10−7). In addition, bioinformatic analysis of the gene expression profile of CpG-stimulated C57BL/6 mice yielded a pattern of major and minor inducers, almost identical to that observed in CpG-treated BALB/c mice.
To clarify whether the regulatory networks identified by these analyses were indeed responsible for influencing gene expression and thus, of physiological relevance, this study was repeated using C57BL/6 mice lacking the TNF gene. TNF KO mice were selected for analysis, as TNF was identified as a major inducer of CpG-mediated gene expression 3 h after CpG administration in BALB/c and C57BL/6 mice (Fig. 2A, and data not shown). The number of genes up-regulated in TNF KO mice was 34% lower than in identically treated C57BL/6 mice (P<0.001). Of note, none of the genes up-regulated uniquely by TNF were induced by CpG treatment of TNF KO mice (0/12, P<0.0001). In contrast, the population of genes uniquely controlled by IFNG was up-regulated equivalently in CpG-treated C57BL/6 and TNF KO mice. These data support the conclusion that the regulatory networks identified by our bioinformatic analysis accurately identified CpG ODN-driven gene–gene interactions in vivo.
Temporal pattern of gene regulation mediated by TLR9 engagement
To better understand CpG-dependent changes in gene expression over time, the analysis was extended to data collected from 30 min through 72 h poststimulation. Using the same criteria described above, bioinformatic analysis showed that TNF, IL1B, and IL1A acted as major inducers of gene expression at 30 min and/or 1 h (Fig. 3, A and B, and Table 1). This group of major inducers behaved similarly: The network of genes they regulated were triggered rapidly but for only a short period (typically ≤1 day). This differed from the pattern of gene activation attributable to IFNG, which became a major inducer at 3 h post-CpG stimulation and remained the dominant source of stimulation thereafter (Fig. 2 and Table 1). Indeed, many of the genes triggered by IFNG remained active through 72 h, by which time, most genes regulated by other major inducers had waned (P<0.001; Fig. 1). Also of note, multiple major inducers contributed to the up-regulation of genes shortly after CpG treatment (85% at 30 min, 75% at 1 h, 44% at 3 h, 49% at 9 h), whereas IFNG was the sole major inducer of genes that remained active at 24–72 h (Figs. 2 and 3 and Table 1).
Fig. 3.
Key regulatory networks activated by CpG ODN treatment (30 and 60 min time-points). All genes up-regulated 3 h after CpG treatment are shown (modified from Fig. 2). Those up-regulated (P<0.00001) within (A) 30 min and (B) 1 h are highlighted in pink. Arrows identify the network involving major inducers (IL1A, IL1B, and TNF) responsible for gene up-regulation at these early time-points.
The contribution of minor inducers was also examined at each time-point (Table 1). No minor inducers were detected at 30 min, but IL6 acted as a minor inducer 1 and 3 h after CpG administration. This was followed by TNFSF10, MYC, and NFKB1 at 3 h and IL15 and IL18 at 3–9 h. STAT1 and STAT2 modulated gene expression at 24 h and continued to influence gene activation through 72 h (Table 1). Thus, the minor inducers triggered within 3 h of CpG administration (IL6, TNFSF10, NFKB1, MYC, IL15, and IL18) behaved in a manner similar to the early major inducers (TNF, IL1B, and IL1A) in that they influenced gene expression for only a short period. By comparison, STAT1 and STAT2 behaved like IFNG, arising late but modulating mRNA expression for a prolonged period.
These findings suggest that CpG ODN administration triggers two distinct patterns of gene activation in the spleen: a rapid but brief period dominated by a group of early major and minor inducers, followed by a period of prolonged gene up-regulation mediated primarily by IFNG but supported by STAT1 and STAT2 (Figs. 1 and 2 and Table 1).
Down-regulation of CpG-induced gene expression
The number of genes up-regulated by CpG ODN treatment peaked at 3 h and then declined (Fig. 1). Bioinformatic analysis of genes whose expression decreased indicated that a small group of suppressors contributed to this effect. These suppressors included IL10, MYC, NF-κ light chain gene enhancer in B cells inhibitor, α (NFKBIA), suppressor of cytokine signaling 1 (SOCS1), SOCS3, IL1RN, and FOS (genes shown in dark blue boxes, Fig. 4A, and Supplemental Table T2). In analyzing the predicted activity of these suppressors, virtually every gene they targeted declined to background levels within 24 h (see circled genes, Fig. 4A). Indeed, ≈40% of all down-regulated genes were targeted directly by one or more suppressors, and these targets included the major inducers TNF, IL1A, and IL1B (Fig. 4A). Of importance, by inhibiting the expression of these major inducers, the suppressors had even greater (albeit indirect) effects on gene expression. For example, the suppressors IL1RN, NFKBIA, SOCS1, SOCS3, and IL10 targeted TNF, such that TNF mRNA levels declined 74% from 3 to 24 h after CpG treatment. As the expression of TNF fell, 76% of the genes up-regulated by TNF also declined to background (Fig. 4B).
Fig. 4.
Mechanisms underlying the down-regulation of genes 24 h after CpG ODN administration. All genes up-regulated 3 h after CpG treatment are shown (modified from Fig. 2). Genes that remain active through 24 h are highlighted in pink (P<0.00001), and those whose expression fell to background are in green. (A) Green arrows identify genes down-regulated by suppressors. Note that 33/35 of the genes targeted by suppressors (circled in green) are down-regulated and that five suppressors (SOCS1, SOCS3, IL1RN, NFKBIA, and IL10) target the major inducer TNF. (B) As TNF levels decline, the level of expression of most genes regulated by TNF (yellow arrows) also falls (green circles). (C) IFNG up-regulates 92% of all genes that remain active at 24 h (red arrows leading to red circles). Of note, 84% of the genes regulated by IFNG but targeted by a suppressor declined to background levels by 24 h (red arrows leading to green circles).
These findings suggest that two factors influence the time-related decline in gene expression: active suppression and loss of induction. These effects are additive: The level of expression of a gene declined 35–75% more when its major inducer (e.g., TNF) waned, and the gene was targeted by a suppressor (e.g., by MYC or IL10), than when affected only by the loss of its major inducer (data not shown). Similarly, suppressors down-regulated genes despite persistent activation by a major inducer. For example, 84% of the genes up-regulated by IFNG but targeted by a suppressor declined to background levels by 24 h (green circles, Fig. 4C), whereas 72% of the genes up-regulated by IFNG but not targeted by suppressors remained active (red circles, Fig. 4C).
DISCUSSION
The ability of CpG ODN to trigger a strong innate immune response is being harnessed therapeutically to reduce host susceptibility to infection and cancer [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Despite earlier studies about TLR9-mediated signal transduction [10,11,12, 44,45,46], the scope of CpG-induced gene expression is incompletely understood. This study uses bioinformatic network analysis of microarray data to identify the genes and regulatory networks triggered in vivo by CpG ODN.
Results indicate that a small group of major inducers is largely responsible for the patterned up-regulation of genes stimulated by CpG ODN administration. These major inducers included IL1A (30 min), IL1B (30–60 min), TNF (1–3 h), and IFNG (3–72 h; Table 1, Figs. 2 and 3, and Supplemental Table T1). Consistent with the known immunomodulatory properties of CpG ODN, the genes activated by these major inducers support the induction of a proinflammatory response [1, 47,48,49]. Major inducers worked in tandem to control early gene expression, with 85% of the genes up-regulated at 30 min and 75% at 1 h being targeted by two or more major inducers. The activation of TNF, IL1A, and IL1B triggered by CpG ODN was short-lived (Fig. 1), and the network of genes they controlled was activated for an equally brief period (Fig. 4B). In contrast, genes whose expression was regulated by IFNG generally remained active long-term (unless targeted for active suppression, Fig. 4, A and C). Thus, the persistent immune activation observed following CpG ODN treatment in vivo presumably reflects the ongoing effects of IFNG [32, 50]. A group of minor inducers was identified that influenced the pattern and magnitude of CpG-induced gene up-regulation. These minor inducers increased the level of expression of the genes they targeted (Table 2) and stimulated genes not targeted by major inducers (Fig. 2B).
Although much of the activation mediated by CpG ODN is short-lived [1, 18], previous studies failed to identify the global mechanism(s) underlying the waning of immune activity. As seen in Figure 1, CpG-dependent gene activation peaked at 3 h and declined progressively thereafter. Current findings identify the two key mechanisms responsible for this decline in gene expression. Suppressors down-regulated the expression of targeted genes directly within 24 h (Figs. 234 and Supplemental Table T2) and reduced the expression of major inducers (TNF, IL1A, and IL1B), thereby eroding the foundation upon which continued network stimulation relied (Fig. 4B, and data not shown).
Several groups previously examined the effect of CpG stimulation on mRNA levels in vitro [14,15,16]. Two groups analyzed the response of a murine macrophage cell line to various TLR ligands [14, 15]. Both observed that LPS altered gene expression more profoundly than CpG ODN and reported that several NF-κB-dependent genes were up-regulated following CpG treatment (e.g., CCL3, IL1RN, PG-endoperoxidase synthase 2, and TNF). Another group analyzed gene expression among human peripheral blood cells stimulated in vitro with CpG ODN for 3–24 h [16]. Consistent with current results, they identified a cluster of genes in the 6- to 24-h time-period, whose regulation was IFN-dependent and could be blocked by the addition of anti-IFN antibodies. It is formally possible that the changes in gene expression induced by CpG ODN in vivo might reflect secondary/indirect responses by cells that do not express TLR9, including cells outside the spleen. Although such a possibility cannot be excluded definitively, current findings are consistent with those from an earlier in vitro study of CpG-induced splenic gene expression. Fully, 85% of the genes shown to be up-regulated in vitro were up-regulated at corresponding time-points in vivo (P<0.0001) [17]. Moreover, current results confirm and extend considerably preliminary findings from the in vitro study concerning the existence of up-regulatory and supressive networks [17].
Unfortunately, earlier in vitro works shared a common limitation: They disturbed the microarchitecture of the spleen (recently shown to have a profound effect on cell–cell interactions [51]) and prevented the long-term analysis of gene expression (as spleen cells cultured for >12 h in vitro undergo significant alterations in mRNA expression that obscure the independent effects of CpG treatment; data not shown). Ex vivo analysis overcame this limitation by monitoring changes in gene expression over time under physiological conditions. Although this strategy could not identify the effect of CpG treatment on mRNA levels in individual cell types (as the spleen contains multiple TLR9-expressing cells), it provides the critical advantage of detecting changes in gene expression associated with complex cell–cell interactions in vivo over time.
Several lines of evidence support the quality of the microarray data and subsequent IPA analysis used in this study. First, a minimum of four animals/group were analyzed independently to generate each dataset, and consistent changes in gene expression were observed among similarly treated animals in independent experiments (R2=0.89±0.04). Second, a stringent cutoff of P < 0.00001 was used to insure that our analysis focused on genes, whose level of expression differed significantly from background. Third, as noted above, a majority of the genes that were up-regulated after 2–8 h of in vitro spleen cell stimulation were also up-regulated at early time-points in vivo. Fourth, those genes hypothesized to have suppressive activity in vitro (a finding that could not be confirmed as a result of technical limitations on the duration of that study) specifically down-regulated their targets in vivo [17]. Fifth, the same pattern of inducers and suppressors was identified in independent studies of two distinct strains of mice—BALB/c and C57BL/6. Finally, studies conducted in TNF KO mice confirmed bioinformatic network predictions by showing that genes controlled uniquely by TNF were up-regulated in C57BL/6 but not KO mice following CpG administration (P<0.0001). These findings support the conclusion that our bioinformatic analysis accurately identified key regulatory pathways responsible for CpG-dependent gene activation under physiologic conditions.
Based on these findings, we propose a model in which major and minor inducers determine the order and magnitude with which specific genes are up-regulated following TLR9 engagement in vivo (Supplemental Fig. S2). The duration of gene activation is determined by the persistence of the major inducer, whether the gene is regulated by a minor inducer, and whether the gene is targeted by a suppressor. The magnitude of gene expression is increased when acted upon by multiple inducers and decreased when targeted by multiple suppressive mechanisms.
This report is the first to describe the complex regulatory networks responsible for controlling gene expression in mice stimulated via the ligand–receptor complex of CpG DNA with TLR9. It establishes that multiple waves of stimulation occur, mediated temporally by the activity of a small number of major and minor inducers. Current findings also establish the mechanisms by which CpG-activated genes are subsequently down-regulated. Results demonstrate that active suppression is key to this decline in gene expression and that by targeting inducers, these suppressors extinguish entire networks indirectly. Further defining these network interactions should improve our understanding of TLR-mediated host defense and facilitate the development of interventions designed to optimize the nature and duration of the ensuing response.
Supplementary Material
Acknowledgments
Analyses were performed using BRB ArrayTools developed by R. Simon and A. P. Lam. We thank R. Salcedo (NCI Frederick, NIH) for providing the C57BL/6 TNF KO mice and C57BL/6 control mice. Experiments were designed by S. K. and D. M. K., performed by S. K. and D. T., and analyzed by S. K. and D. M. K., and the manuscript was written by S. K. and D. M. K. The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the NCI at large. Hereby, we disclose that D. M. K. has patent interests involving CpG ODN.
References
- Klinman D M, Yi A, Beaucage S L, Conover J, Krieg A M. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNγ. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhang X, Tough D F, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey K J, Sweet M J, Hume D A. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2120. [PubMed] [Google Scholar]
- Ballas Z K, Rasmussen W L, Krieg A M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1847. [PubMed] [Google Scholar]
- Broide D, Schwarze J, Tighe H, Gifford T, Nguyen M D, Malek S, Van Uden J, Martin E, Gelfand E W, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-α-mediated shock. Eur J Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- Roman M, Martin-Orozco E, Goodman J S, Nguyen M, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Immunostimulatory DNA sequences function as T helper-1 promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Hacker H, Vabulas R M, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeroid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000;192:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Ulevitch R J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kirschning C J, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford G B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois C M, Klein D C, Monks B G, McKnight C J, Lamphier M S, Duprex W P, Espevik T, Golenbock D T. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- Gao J J, Diesl V, Wittmann T, Morrison D C, Ryan J L, Vogel S N, Follettie M T. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J Leukoc Biol. 2002;72:1234–1245. [PubMed] [Google Scholar]
- Schmitz F, Mages J, Heit A, Lang R, Wagner H. Transcriptional activation induced in macrophages by Toll-like receptor (TLR) ligands: from expression profiling to a model of TLR signaling. Eur J Immunol. 2004;34:2863–2873. doi: 10.1002/eji.200425228. [DOI] [PubMed] [Google Scholar]
- Kato A, Homma T, Batchelor J, Hashimoto N, Imai S, Wakiguchi H, Saito H, Matsumoto K. Interferon-α/β receptor-mediated selective induction of a gene cluster by CpG oligodeoxynucleotide 2006. BMC Immunol. 2003;4:8. doi: 10.1186/1471-2172-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaschik S, Gursel I, Klinman D M. CpG-mediated changes in gene expression in murine spleen cells identified by microarray analysis. Mol Immunol. 2007;44:1095–1104. doi: 10.1016/j.molimm.2006.07.283. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Takeshita F, Haddad D E, Ishii K J, Klinman D M. CpG oligodeoxynucleotides induce murine macrophages to up-regulate chemokine mRNA expression. Cell Immunol. 2000;206:101–106. doi: 10.1006/cimm.2000.1735. [DOI] [PubMed] [Google Scholar]
- Ishii K J, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A, Klinman D M. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J Exp Med. 2002;196:269–274. doi: 10.1084/jem.20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S, Elias F, Flo J. Immunostimulatory effects of plasmid DNA and synthetic oligodeoxynucleotides. Eur J Immunol. 2003;33:1382–1392. doi: 10.1002/eji.200323614. [DOI] [PubMed] [Google Scholar]
- Datta N, Mukherjee S, Das L, Das P K. Targeting of immunostimulatory DNA cures experimental visceral Leishmaniasis through nitric oxide up-regulation and T cell activation. Eur J Immunol. 2003;33:1508–1518. doi: 10.1002/eji.200323671. [DOI] [PubMed] [Google Scholar]
- Ito S, Ishii K J, Gursel M, Shirotra H, Ihata A, Klinman D M. CpG oligodeoxynucleotides enhance neonatal resistance to Listeria infection. J Immunol. 2005;174:777–782. doi: 10.4049/jimmunol.174.2.777. [DOI] [PubMed] [Google Scholar]
- Yang Y H, Dudoit S, Luu P, Lin D M, Peng V, Ngai J, Speed T P. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano S E, Xiao W, Richards D R, Felciano R M, Baker H V, Cho R J, Chen R O, Brownstein B H, Cobb J P, Tschoeke S K, Miller-Graziano C, Moldawer L L, Mindrinos M N, Davis RW, Tompkins RG, Lowry S F, Inflamm and Host Response to Injury Large Scale Collab. Res. Program A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Wright G W, Simon R M. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19:2448–2455. doi: 10.1093/bioinformatics/btg345. [DOI] [PubMed] [Google Scholar]
- Shen J, Reis J, Morrison D C, Papasian C, Raghavakaimal S, Kolbert C, Qureshi A A, Vogel S N, Qureshi N. Key inflammatory signaling pathways are regulated by the proteasome. Shock. 2006;25:472–484. doi: 10.1097/01.shk.0000209554.46704.64. [DOI] [PubMed] [Google Scholar]
- Bowick G C, Fennewald S M, Elsom B L, Aronson J F, Luxon B A, Gorenstein D G, Herzog N K. Differential signaling networks induced by mild and lethal hemorrhagic fever virus infections. J Virol. 2006;80:10248–10252. doi: 10.1128/JVI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Srikanth R, Ahlfors H, Lahesmaa R, Rao K V. Capturing cell-fate decisions from the molecular signatures of a receptor-dependent signaling response. Mol Syst Biol. 2007;3:150. doi: 10.1038/msb4100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrsdorfer B, Weiner G J. CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy. Curr Opin Investig Drugs. 2003;4:686–690. [PubMed] [Google Scholar]
- Carpentier A F, Auf G, Delattre J Y. CpG-oligonucleotides for cancer immunotherapy: review of the literature and potential applications in malignant glioma. Front Biosci. 2003;8:e115–e127. doi: 10.2741/934. [DOI] [PubMed] [Google Scholar]
- Ashkar A A, Bauer S, Mitchell W J, Vieira J, Rosenthal K L. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J Virol. 2003;77:8948–8956. doi: 10.1128/JVI.77.16.8948-8956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A M, Homan L L, Yi A K, Harty J T. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–2434. [PubMed] [Google Scholar]
- Walker P S, Scharton-Kersten T, Krieg A M, Love-Homan L, Rowton E D, Udey M C, Vogel J C. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for Leishmaniasis via IL-12 and IFNγ dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine Leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]
- Krieg A M. Development of TLR9 agonists for cancer therapy 8. J Clin Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weighardt H, Feterowski C, Veit M, Rump M, Wagner H, Holzmann B. Increased resistance against acute polymicrobial sepsis in mice challenged with immunostimulatory CpG oligodeoxynucleotides is related to an enhanced innate effector cell response. J Immunol. 2000;165:4537–4543. doi: 10.4049/jimmunol.165.8.4537. [DOI] [PubMed] [Google Scholar]
- Dong L, Mori I, Hossain M J, Liu B, Kimura Y. An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J Gen Virol. 2003;84:1623–1628. doi: 10.1099/vir.0.19029-0. [DOI] [PubMed] [Google Scholar]
- Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdorfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- Horner A A, Raz E. Immunostimulatory sequence oligodeoxynucleotide-based vaccination and immunomodulation: two unique but complementary strategies for the treatment of allergic diseases. J Allergy Clin Immunol. 2002;110:706–712. doi: 10.1067/mai.2002.129122. [DOI] [PubMed] [Google Scholar]
- Halperin S A, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden J J. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21:2461–2467. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Leonard J P, Link B K, Emmanouilides C, Gregory S A, Weisdorf D, Andrey J, Hainsworth J, Sparano J A, Tsai D E, Horning S, Krieg A M, Weiner G J. Phase I trial of Toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- Jahrsdorfer B, Muhlenhoff L, Blackwell S E, Wagner M, Poeck H, Hartmann E, Jox R, Giese T, Emmerich B, Endres S, Weiner G J, Hartmann G. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res. 2005;11:1490–1499. doi: 10.1158/1078-0432.CCR-04-1890. [DOI] [PubMed] [Google Scholar]
- Hegele A, Dalpke A, Heeg K, Barth P, Varga Z, Hofmann R, Olbert P. Immunostimulatory CpG oligonucleotides reduce tumor burden after intravesical administration in an orthotopic murine bladder cancer model. Tumour Biol. 2005;26:274–280. doi: 10.1159/000087380. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Leifer C A, Gursel I, Ishii K, Takeshita S, Gursel M, Klinman D M. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto T, Katoaka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072–4076. [PubMed] [Google Scholar]
- Krieg A M, Yi A, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–548. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Halpern M D, Kurlander R J, Pisetsky D S. Bacterial DNA induces murine interferon-γ production by stimulation of IL-12 and tumor necrosis factor-α. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- Elkins K L, Rhinehart-Jones T R, Stibitz S, Conover J S, Klinman D M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–2298. [PubMed] [Google Scholar]
- Bajenoff M, Egen J G, Qi H, Huang A Y, Castellino F, Germain R N. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28:346–352. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.