SUMMARY
The reservoir capacity of domestic cats and dogs for Trypanosoma cruzi infection and the host-feeding patterns of domestic Triatoma infestans were assessed longitudinally in 2 infested rural villages in north-western Argentina. A total of 86 dogs and 38 cats was repeatedly examined for T. cruzi infection by serology and/or xenodiagnosis. The composite prevalence of infection in dogs (60%), but not in cats, increased significantly with age and with the domiciliary density of infected T. infestans. Dogs and cats had similarly high forces of infection, prevalence of infectious hosts (41–42%), and infectiousness to bugs at a wide range of infected bug densities. The infectiousness to bugs of seropositive dogs declined significantly with increasing dog age and was highly aggregated. Individual dog infectiousness to bugs was significantly autocorrelated over time. Domestic T. infestans fed on dogs showed higher infection prevalence (49%) than those fed on cats (39%), humans (38%) or chickens (29%) among 1085 bugs examined. The basic reproduction number of T. cruzi in dogs was at least 8·2. Both cats and dogs are epidemiologically important sources of infection for bugs and householders, dogs nearly 3 times more than cats.
Keywords: surveillance, host-feeding, incidence, triatomine bugs, Chagas disease, Trypanosoma cruzi, Triatoma infestans, dogs, cats, infectiousness
INTRODUCTION
Chagas disease is a zoonosis caused by Trypanosoma cruzi and mainly transmitted by triatomine bugs. It is a major public health problem in Latin America, where approximately 11 million people may be infected with T. cruzi (Dias et al. 2002). Domestic dogs and cats, opossums and rodents play important roles in the ecology and epidemiology of Chagas disease. Dogs are involved in domestic transmission cycles of T. cruzi from southern USA (Beard et al. 2003) throughout the Americas (Minter, 1976). In the Southern Cone countries, where Triatoma infestans is the main or only domiciliary vector, dog and cat prevalence with T. cruzi were in the range of 8–50% and often exceeded those found in the respective human populations.
The significance of a host species as a reservoir of a vector-borne pathogen mainly depends on its prevalence of infection, the host’s capacity to infect bugs, and the rate of host-vector contact (Cohen and Gürtler, 2001). In rural northern Argentina, the prevalence of T. cruzi in dog populations determined through serodiagnosis and/or xenodiagnosis ranged from 23 to 34% (Mayer and Alcaraz, 1954; Lauricella et al. 1989) to 67–84% (Wisnivesky-Colli et al. 1985; Basombrío et al. 1993; Gürtler et al. 1993, 1996), and decreased rapidly after residual spraying with insecticides (Gürtler et al. 1990; Castañera et al. 1998). Dogs, humans and chickens usually are the most important bloodmeal sources for domestic T. infestans and other triatomines (Gürtler et al. 1997). The presence of infected dogs or cats in the household was significantly associated with the human prevalence and incidence of T. cruzi (Mott et al. 1978; Gürtler et al. 2005). The household is where transmission of T. cruzi between humans, dogs, cats and bugs is most intense. The evidence demonstrates the major role of dogs in the domestic transmission of T. cruzi.
The probability that an uninfected vector acquires the infection after a single bloodmeal from an infected host (hereafter referred to as infectiousness to bugs) can be measured by xenodiagnosis using laboratory-reared, uninfected bugs that later are examined individually for T. cruzi infection. The infectiousness of people seropositive for T. cruzi ranged from 2 to 3% to 26% and declined significantly with age (Maguire et al. 1982). In contrast, the mean infectiousness of naturally infected, seropositive dogs (45–57%) was loosely or not related to age and was positively associated with the domiciliary density of T. infestans infected with T. cruzi (Gürtler et al. 1992a, 1996). The latter may be taken as an index of the likelihood that household dogs would experience vector-mediated reinfections. This result was consistent with evidence showing that experimental reinfections enhanced parasitaemia in mice, monkeys and dogs infected with T. cruzi (Cabeza Meckert and Laguens, 1981; Riarte et al. 1995; Machado et al. 2001), but has not been verified in a longitudinal field study.
The epidemiological role of cats has not been studied as intensively and remains controversial (cf. Minter, 1976; Piesman et al. 1983; Mott et al. 1978; Wisnivesky-Colli et al. 1985; Gürtler et al. 1993; Catalá et al. 2004). Extensive xenodiagnosis surveys in Brazil reported 18–30% of infected cats in widely separated areas where the local vector was T. infestans, Panstrongylus megistus or Triatoma brasiliensis (e.g. Freitas, 1950; Alencar et al. 1974/1975; Mott et al. 1978). In north-western Argentina, the only surveys of T. cruzi based on sample sizes >30 cats revealed prevalences ranging from 18 to 39% as determined by xenodiagnosis (Mayer and Alcaraz, 1954; Gürtler et al. 1993). In spite of sometimes playing a less significant role as bloodmeal sources, infected cats contributed significantly to domestic T. cruzi infection after adjusting for the number of infected dogs in the household (Gürtler et al. 1993, 2005). At present, no prospective study has quantified the incidence of T. cruzi in dog and cat populations jointly with entomological data in a well-defined area of active transmission. As part of a larger project aimed at modelling the transmission dynamics of T. cruzi, here we describe the first longitudinal study of infection, seroreactivity and infectiousness in a rural dog population, provide data on cat infectiousness, and assess the relative contribution of domestic dogs and cats to bug infection by integrating host infection and infectiousness data with the host-feeding patterns of domestic bugs.
MATERIALS AND METHODS
Study area
The study was carried out in the rural villages of Trinidad and Mercedes (27°15′28′′S, 62°58′44′′W), located 9 km from the neighbouring village of Amamá in the Province of Santiago del Estero, Argentina. The study villages are in a semi-arid area covered with thorn hardwood forest, and comprised 35 houses with 171 inhabitants in 1988 (Gürtler et al. 1992b). Nearly all houses were built with mud-brick or mud-stick walls and thatched roofs, and had an extensive peridomestic area. Both villages are treated as a single unit because they were adjacent, had similar prevalence of domestic infestation with T. infestans (89–92%), and were not sprayed with insecticides by the National Chagas Disease Control Service until 1992, when the seroprevalence for T. cruzi in humans was 31% (Gürtler et al. 1998). Mean monthly temperatures corresponding to the survey months (September, December and March) were 18·3, 28·9, and 24·1 °C, respectively, at the closest meteorological station.
Study design
A baseline demographic, entomological and sero-parasitological survey was carried out in Trinidad and Mercedes during late September and early December 1988, respectively (Gürtler et al. 1992b, 1993).
Demographic survey
All houses were visited and family members censused within each house given a unique census number. The sleeping locations of every person, dog and cat at night were identified. A canine and feline census was carried out following a standard demographic questionnaire (World Health Organization, 1988). For each pet, the questionnaire asked name, sex, age (in completed months or years), size, colour and particular phenotypic features (all were mongrel pets), birthplace, name of the mother, age at which the pet entered the house, the pet’s main function, and reproductive data; a photograph was taken whenever possible. Householders were asked about mortality, its apparent cause and for information on the missing animals. Information was requested on movement of pets to a different home in or out of the study villages. All dogs and cats were re-censused in March and September 1989 following the same procedures. We present baseline demographic data for the entire dog population (86 dogs, including 11 goat-dogs, which sleep around goat-pens far from bedrooms), and infection or infectiousness data for household-associated dogs, which slept at night near bedroom areas, kitchens and store-rooms. Questionnaire data were used to reconstruct the cohort of pets present as of September 1988.
Entomological survey
A two-person team searched all bedroom areas, household goods and beds for triatomine bugs, totalling 4 person-h of search per house at the baseline survey. Searches for bugs were made before and after repeated spraying of walls and roofs with a dislodgant spray (0·2% tetramethrin, Icona, Buenos Aires, Argentina). Outbuildings and animal shelters were excluded from the survey, as they usually sustained very few bugs infected with T. cruzi. Domestic bug catches (total, 1180 bugs) and infection prevalence (42·4%) were very high. To reduce infestations and to calibrate the timed method, 2 days after timed collections 1 γ-hexachloro-cyclohexane (benzene hexachloride) fumigant tablet (Gammexane, Duperial, Buenos Aires, Argentina) was burned in each bedroom of 19 houses and 1 insecticide fumigant canister (Aguvac, Buenos Aires, Argentina) was applied in each of another 7 houses; a total of 1187 knocked-down bugs were collected 2–4 h after treatment and processed as before, but these bugs were not examined for infection. Householders reported that domestic infestations recovered fast. By 1992, 88% of the houses were found infested and the domiciliary density of infestations was linearly related to those recorded in 1988 (Cecere et al. 1998).
The bugs collected were identified to species, counted by instar, and a sample of third instars and larger stages was microscopically examined for T. cruzi infection at a magnification of 400× within 10 days of capture. The estimated density of infected bugs in each house was calculated as a weighted average by multiplying instar-specific infection prevalences and bug captures. The individual bloodmeals of domestic T. infestans were identified by agar double-diffusion tests using 5 family-specific anti-sera (human, dog, cat, chicken, and goat-sheep) (Gürtler et al. 1997); of 1964 domestic T. infestans collected in Trinidad, Mercedes and Amamá from 1988 to 1992, 66% fed on humans, 45% on dogs, 27% on chickens, 11% on cats, and 2% on goat-sheep. The relation between bug infection with T. cruzi and type of host bloodmeal source in domestic T. infestans was analysed by the infective bloodmeal index defined by Zárate et al. (1980) as the percentage of T. cruzi infected bugs among bugs with bloodmeals (both unmixed and mixed) on a given host.
Sero-parasitological surveys
In the baseline survey, a blood sample was obtained by venipuncture from 59 dogs aged 2 months or older (79% of the registered population), but cats were not bled due to diffculties in handling them without using anaesthesia (Gürtler et al. 1993). Eleven goat-dogs were excluded from the survey because they were diffcult to handle and had little contact with domestic areas and people. To confirm T. cruzi infections parasitologically and measure infectiousness to bugs, 68 dogs and 28 cats (91% and 90% of the registered populations, respectively) were each exposed to 10 (puppies and small cats) or 20 (older animals) laboratory-reared T. infestans third instars placed in 2 wooden boxes for 25 min (Gürtler et al. 1996). The boxes were inspected after exposure to ensure that most of the insects had fully engorged, re-applied for another 10 min if needed, and then kept at ambient temperature without further feeding. The bugs were individually examined for T. cruzi infection 30 and 60 days later, and the numbers of exuviae and dead bugs in each box were recorded to indicate whether feeding had taken place. Of 3307 bugs used in dog xenodiagnosis, 22% were dead on first examination and 24% of the live bugs finally moulted; for cats, these figures were 775, 19% and 27%, respectively. Microscopists analysing the bugs were not aware of the identity or infection status of the pet on which the bugs had fed. Infectiousness to the vector was defined as the number of T. cruzi-positive bugs divided by the total number of bugs fed on a given host and examined for infection at least once, excluding those bugs that did not survive to the first examination. Xenodiagnosis bugs from a seropositive dog were not examined individually. Follow-up sero-parasitological surveys were conducted in March and September 1989, and additional samples of 9 and 7 seropositive dogs were taken in March 1990 and May 1993, respectively. ‘Infectious’ means having a positive xenodiagnosis. ‘Infectiousness’ referred both to whether a host had a positive xenodiagnosis or not, and to the proportion of xenodiagnosis bugs becoming infected after exposure to a given host (infectiousness to bugs).
Detection of antibodies to T. cruzi by the indirect haemagglutination test (IHA; Polychaco, Argentina), enzyme-linked immunosorbent assay (ELISA), and the immunofluorescent antibody tests (IFAT, in a subsample) was as described by Lauricella et al. (1998). Seroreactivity for T. cruzi of dog sera was demonstrated by ELISA readings of 0·2 or greater and IFAT and IHA titres of 1 : 16 or greater; sensitivity was estimated as 94%, 94% and 68%, whereas specificity was 96·2%, 100% and 100%, respectively. Each microtitre polystyrene plate had 2 positive and 1 negative control serum. Animals were defined as ‘seropositive’ when samples were reactive by at least 2 different serological tests (among ELISA, IHA or IFAT) in any one survey, and ‘infected’ when having positive xenodiagnosis and/or being seropositive to T. cruzi. Dogs with a positive xenodiagnosis or which were seropositive to T. cruzi or both were considered current cases of T. cruzi infection. Infected animals with lifetime residence (i.e. entering the household under 3 weeks of age) were considered autochthonous cases. For the purpose of our study, we defined a dog as a candidate to acquire an autochthonous T. cruzi infection if it was seronegative and/or xenodiagnosis-negative at baseline and had no travel history to other villages. Candidate dogs showing seroconversion as detected by any 2 methods or a positive xenodiagnosis were regarded as incident cases of T. cruzi.
Data analysis
The instantaneous per capita rate of conversion from negative to positive (λ) may be estimated retrospectively from age-specific dog seroprevalence or cat xenodiagnosis data using a catalytic model with recovery rate set to 0 (Muench, 1959), reflecting the absence of serorecovery or specific chemotherapy. This model assumes that the incidence of infection is constant over time and independent of age; individual hosts are homogeneously exposed; no time lag occurs between infection and infectiousness, and the association between age and prevalence is observed at equilibrium. λ was estimated using nonlinear least-squares procedures (Matlab 6.3, The MathWorks, Natick, MA), and the catalytic model λ=-ln(1-pa)/a, where pa is the proportion of seropositive or infected individuals within the age class whose midpoint is a.
The relationship between dog and cat T. cruzi infection or infectiousness (the binary response variables) and potential predictors was investigated using maximum likelihood logistic multiple regression analysis in Stata statistical software (Stata 9.0, StataCorp, College Station, Texas). Cluster effects on the probability of being infected or infecting bugs (due to household or subject, respectively) were allowed for in every model. The model included as predictors the numbers of T. infestans infected with T. cruzi collected at domiciliary sites per 4 person-hour at baseline (categorized in low- and high-risk levels, 0–2 and ≥9, as there were no households with 3–8 infected bugs and nearly half of the houses fell into each class), each individual dog or cat age (in months, either as a continuous variable or in 2 levels, when the sample size was small), sex, and host type (dog or cat). For models of the infectiousness to bugs, the proportion of xenodiagnosis bugs that moulted was included as a surrogate for effective bloodmeal size, which may differ between dogs and cats. Interaction terms were added stepwise and dropped from the final model if not significant. The probability used for nominal statistical significance was 5%. The Wald test examined the hypothesis that all regression coeffcients are 0.
To test for homogeneity of dog infectiousness to bugs when several seropositive dogs were not infectious, a Poisson test based on the observed count of infected bugs in each xenodiagnosis was not considered to be appropriate because the total number of bugs that were examined for infection varied among individual dogs, and because a true Poisson distribution has no finite upper limit (given by the maximum number of xenodiagnostic bugs applied to each dog). Instead, using Matlab we computed the binomial cumulative distribution function with the following parameters: m for the number of seropositive dogs that did not infect any bug; n for the total number of seropositive dogs examined by xenodiagnosis; q for the probability of a seropositive dog not infecting a xenodiagnosis bug in a single bloodmeal (q=1-p); and b for the minimum number of bugs examined for infection in each dog’s xenodiagnosis. The expression 1-binocdf(m-1,n,qb) provides a conservative test for the probability of observing m or more seropositive dogs that did not infect any bug, assuming as a null hypothesis that every seropositive dog has the same average probability p of infecting any single bug, and assuming independence among bugs and dogs.
RESULTS
Demographics
Dogs were almost 3 times as abundant as cats, with a human : dog ratio of 1·5 : 1 at baseline (Table 1). The median ages of dog and cat populations were 24 and 12 months, respectively, and both had a sex-ratio markedly biased toward males. Overall, 64% of dogs were native of the study villages, 18% came from nearby rural villages, and 18% from cities farther than 40 km; for cats these numbers were 76%, 20% and 4%, respectively. About 90% of dogs and cats entered the household under 2 weeks of age. House-dwellers reported that 59% of 71 household-associated dogs and 88% of 25 cats shared human sleeping areas. The September 1989 census registered 67 household-associated dogs and 27 cats, which included 60% of the dogs and 44% of the cats listed at baseline. Most of the missing animals died or disappeared, and very few were given away.
Table 1.
Demographic parameters of dog and cat populations (Trinidad and Mercedes, September 1988–1989.)
| Host | Number | % Houses owning pets (mean no./house, range) |
Median age (months) |
% Male |
% Resting in bedrooms |
% Re-censused after 1 year |
|---|---|---|---|---|---|---|
| Dogs | 86* | 97 (2·9, 0–6) | 24 (9–48)** | 81 | 59 | 60 |
| Cats | 31 | 71 (1·4, 0–5) | 12 (4–13) | 70 | 88 | 44 |
Includes 11 dogs used for herding goats.
First and third quartiles.
Dogs
At baseline, the composite prevalence of T. cruzi infection was 60·3% by serology and/or xenodiagnosis (number tested, n=68 dogs), and increased with age from 25% in dogs younger than 1 year to 92% in those aged 7 years or more (Fig. 1A). λ was 43·2 per 100 dog-years (with 95% confidence interval, CI=17·6–68·7%). The predicted proportions of infected dogs by age class did not depart significantly from the observed data (goodness-of-fit χ2=1·1, 3 D.F., P>0·5). Infection prevalence was slightly lower in males (57%, n=53) than in females (79%, n=14) (1 dog was not sexed). The age-specific seroprevalence of T. cruzi (mean, 64·4%; n=59) was indistinguishable from the infection prevalence. The only pup less than 6 months of age found to be seropositive for T. cruzi was xenodiagnosis-positive, thus excluding the likelihood of maternally derived antibodies yielding a false positive result. Concordance between ELISA and IHA was 91%; 2 of 3 dogs positive only by ELISA and 1 of 2 dogs positive only by IHA were xenodiagnosis-positive concurrently.
Fig. 1.
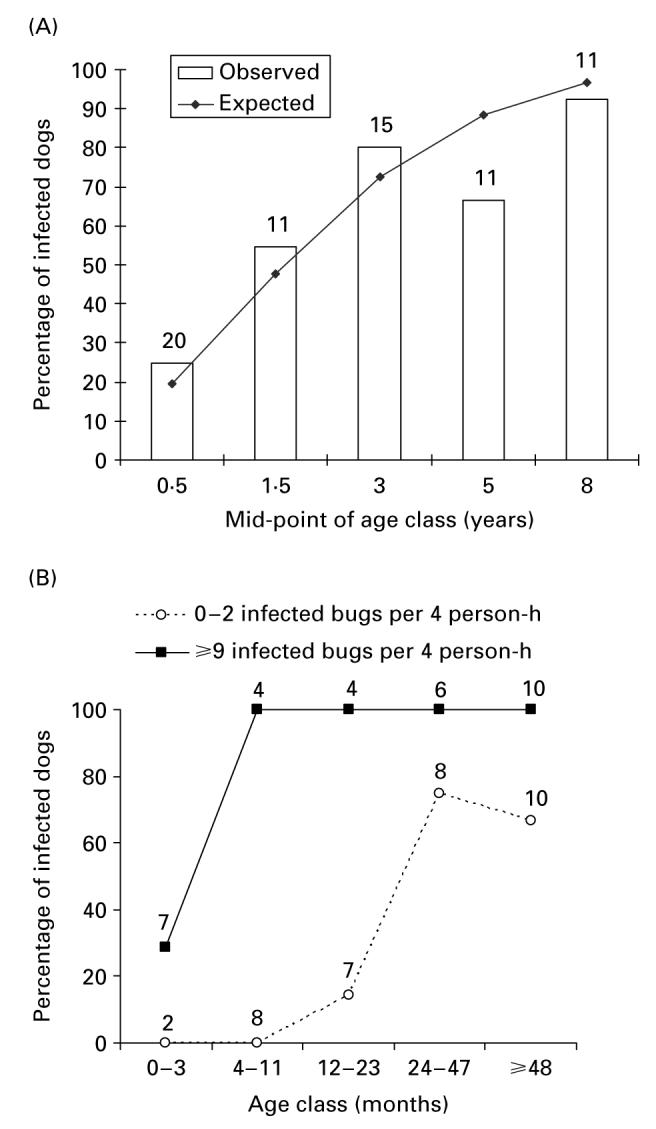
Age-specific prevalence of Trypanosoma cruzi infection in dogs, as determined through the composite results of serodiagnosis and xenodiagnosis (A), and relationship with the density of domestic T. infestans infected with T. cruzi (B). Trinidad and Mercedes, September and December 1988, respectively. The line in (A) is the fit of the catalytic model with constant force of infection over time and age. Numbers close to data points represent the numbers of dogs examined for infection.
The prevalence of T. cruzi infection in dogs at baseline increased significantly with the domiciliary density of T. infestans infected with T. cruzi (odds ratio, OR=19·7, 95% CI=5·4–72·1) as well as with per unit increase in age expressed in months (OR=1·05, 95% CI=1·01–1·09) but was not associated with sex (OR=0·41, 95% CI=0·09–2·01) based on multiple logistic regression analysis (Wald χ2=31·8, 3 D.F., P<0·0001) (Fig. 1B). At homes with 0–2 infected bugs per 4 person-hours, the age of the youngest infected dog was 12 months and prevalence reached 60% at 48 months of age or more. At homes with ≥9 infected bugs per 4 person-hours, all dogs aged 4 months or more were infected.
Of 36 dogs that were ELISA-positive at baseline, repeat serum samples were obtained from 23, 13 and 8 dogs on 2, 3 and 4 occasions, respectively. Only 2 ELISA-positive, old dogs at baseline turned seronegative (serorecovered) in the next survey and then disappeared. Two dogs seronegative for T. cruzi by all methods at baseline had a positive xenodiagnosis concurrently; both seroconverted and were xenodiagnosis-positive in the next survey, thus indicating they had been acute cases at baseline. Of 17 ELISA-negative dogs at baseline that were re-examined thereafter, evidence for seroconversion was obtained for 7 dogs and another seronegative dog was xenodiagnosis-positive concurrently. Thus 8 (47%) incident cases were detected within a year of followup. The incidence rate of T. cruzi (as determined by serological or parasitological conversion) among dogs from households with infected domestic bugs at baseline (4 conversions over 21 dog-months of exposure, or 228 cases per 100 dog-years) was 3 times greater than among dogs resident in houses with no infected domestic bugs at baseline (3 conversions over 48 dog-months, or 75 cases per 100 dog-years). Seven of 10 pups aged between 1 and 3·5 months (mean, 1·5 months) born during the follow-up were detected infected by T. cruzi on first examination.
At baseline, the percentage of T. cruzi-seropositive dogs that were infectious to bugs averaged 66% (n=38) and declined significantly with age (expressed in months) from 100% in dogs <1 year to 50% in dogs ≥7 years (OR=0·978, 95% CI=0·958–0·999) but not with sex (OR=2·68, 95% CI=0·74–9·63) (Fig. 2A). Similar trends observed in 1989 surveys were not statistically significant. A total of 536 (23·1%, n=2568) xenodiagnosis bugs applied to 85 dogs were found infected with T. cruzi during 1988–1989. Of 630 xenodiagnosis bugs applied to 37 seropositive dogs and examined for infection at baseline, 29·5% (95% CI=26·0–33·1%) were infected with T. cruzi; for March and September 1989 the percentages were similar: 34·8% (95% CI=30·7–38·9%; n=523) and 26.8% (95% CI=21·8–31·8%; n=299), respectively. The mean infectiousness to bugs decreased from 61·4% in seropositive dogs below 1 year of age to 13·8% in dogs aged ≥7 years (Fig. 2A). The variability of infectiousness estimates dropped markedly after 4 years of age in all surveys. Multiple logistic regression analysis showed that dog infectiousness to bugs adjusted for clustering on subject declined significantly with age (Fig. 2B) (OR=0·978, 95% CI=0·965–0·992), increased significantly with bug moulting proportions (OR=1·031, 95% CI= 1·004–1·058), and was not significantly modified by sex (OR=2·62, 95% CI=0·72–9·61) or infected bug density (OR=0·78, 95% CI=0·26–2·39) both at baseline (Wald χ2=19·58, 4 D.F., P<0·001) and in March 1989. These analyses reject the null hypothesis of no effects of dog age on infectiousness to bugs, and fail to reject the null hypothesis that infected bug density does not modify dog infectiousness. Infectiousness to bugs was not significantly related to antibody titres. Seven pups aged 2 months old examined only by xenodiagnosis infected 84% of 115 bugs in the March 1989 survey.
Fig. 2.
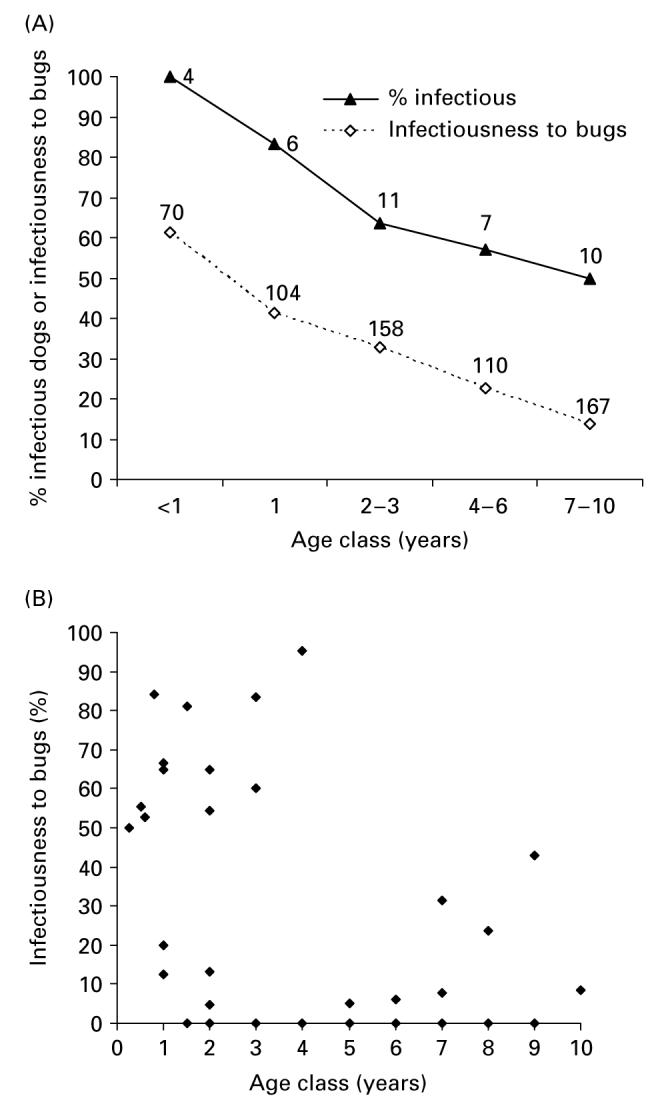
Age-specific prevalence of dogs seropositive for Trypanosoma cruzi and infectious to bugs (A) and percentage of xenodiagnosis bugs infected with T. cruzi after taking a single bloodmeal on seropositive dogs (infectiousness to bugs) (B). Trinidad and Mercedes, September and December 1988, respectively. Numbers close to data points in (A) represent the numbers of seropositive dogs examined by xenodiagnosis, or numbers of xenodiagnosis bugs examined for infection. Each symbol in (B) represents the percentage of bugs fed on a single dog.
Individual dogs varied very widely in infectiousness to bugs in all surveys (Fig. 3A). The distribution of infectiousness to bugs was always overdispersed. More than a third of the T. cruzi-seropositive dogs were either not infectious (35–42%) or infected less than 10% of the bugs fed on them (0–14%), whereas 29–39% of seropositive dogs infected >40% of the bugs. The probability that 13 or more of the 36 seropositive dogs examined would not infect any bug was very small (P<0·001) in 1988 (m=13, n=36, b=12, P=0·295, after excluding a xenodiagnostic test based on only 4 bugs). Similar results were obtained for March (m=12, n=31, b=11, P=0·348) and September 1989 (m=10, n=24, b=6, P=0·268), thus rejecting the null hypothesis (P<0·001) of homogeneity in seropositive dog infectiousness to bugs in a given survey.
Fig. 3.
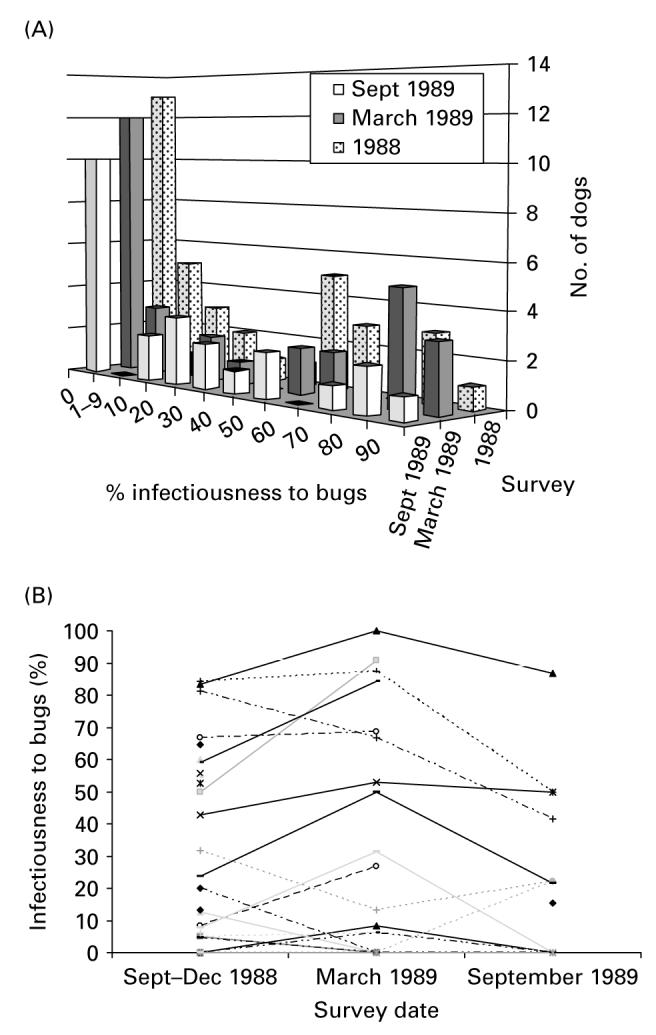
Frequency distributions of individual infectiousness to bugs of all dogs seropositive for Trypanosoma cruzi at 3 survey dates, September—December 1988, March 1989 and September 1989 (A); and individual infectiousness to bugs of dogs that were ever seropositive for T. cruzi and tested on 2 or 3 occasions (B). Trinidad and Mercedes.
Of 41 dogs that had been found infected with T. cruzi at baseline, repeat xenodiagnosis samples were obtained from 28, 17, 9 and 7 dogs in March and September 1989, March 1990 and May 1993, respectively. The individual infectiousness of seropositive dogs to bugs was positively and highly significantly correlated between any 2 surveys (Fig. 3B). R2 coeffcients were very high and ranged from 0·86 (1988 vs March 1989), 0·85 (1988 vs September 1989) to 0·83 (March vs September 1989). Even among the 7 infected dogs xenodiagnosed in 1988 and 1993 there was a highly significant correlation in infectiousness to bugs (R2=0·93), with data points clustered at low or high ends.
Cats and host comparisons
The percentage of all cats that were infectious at the baseline (39%, n=28) and follow-up surveys (40%, n=25) was very similar. The period prevalence of infectiousness over 1988–1989 in cats (42%, n=38, considering each individual subject only once, and if positive once, positive for the whole period) was similar to dogs’ at the baseline survey (41·2%, n=68). It increased with age (Fig. 4) but was not associated with sex (males, 46%; females, 40%). The force of infection λ was 38·6 per 100 cat-years (95% CI, 7·2–70·1%), also very similar to λ in dogs. Five of 6 xenodiagnosis-positive cats at baseline remained positive, and no parasitological conversion was detected among 9 negative cats re-examined in March 1989. However, 5 of the 10 cats censused and examined for the first time in March 1989 (mean age, 2 months) were xenodiagnosis-positive in spite of prior bug control actions and probably much lower bug infestations. None of 3 variables age, sex and density of infected bugs was a significant predictor of cat infectiousness. Three of 7 cats aged less than 12 months were found infectious in houses with no infected bugs. A female cat that was xenodiagnosis-negative twice had 1 of 3 kittens (2 weeks old) with a positive xenodiagnosis, while living in a house with only 2 infected bugs per 4 person-hour.
Fig. 4.
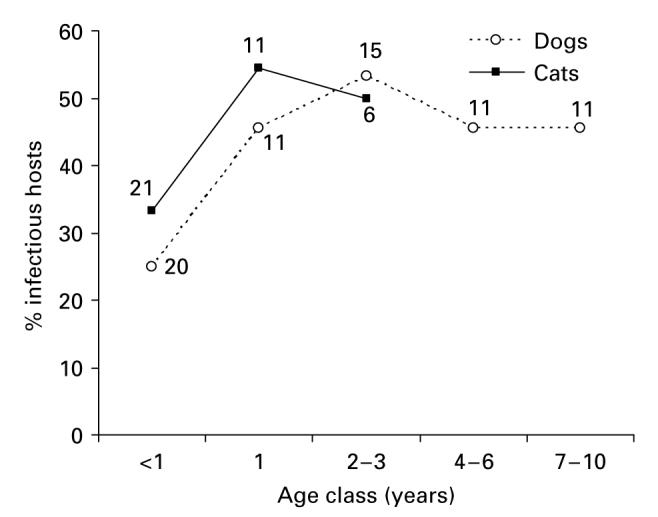
Age-specific prevalence of infectious hosts in the dog and cat populations, assessed by xenodiagnosis, regardless of serological data. Trinidad and Mercedes, September and December 1988, respectively. Numbers close to data points represent the numbers of hosts examined by xenodiagnosis.
We examined whether dogs and cats over a similar age range (n=100) differed in their likelihood of being infectious, regardless of serological results (Fig. 4). Multiple logistic regression analysis showed no significant effects on infectiousness of host type (dog or cat) (OR=0·82, 95% CI=0·29–2·27) adjusted by age class (≤6 and 7–36 months) and infected bug density (two classes) at baseline (Wald χ2=10·6, 3 D.F., P<0·02).
A total of 171 (27·9%, n=768) xenodiagnosis bugs became infected with T. cruzi after a single bloodmeal on cats. In xenodiagnosis-positive cats the mean infectiousness to bugs over all surveys was 65·4% (95% CI=59·6–71·2%), but this varied significantly from 46·2% (95% CI=36·6–55·7%; n=104) in 1988 to 78·2% (95% CI=71·7–84·7%; n=156 bugs) in 1989, when the newly infected cats infected 93·1% of 58 bugs. Cat infectiousness over 1988–1989 adjusted for clustering on subject was not significantly related to age, sex, infected-bug density and bug moulting proportion (Wald χ2=9·5, 4 D.F., P<0·05). Among xenodiagnosis-positive cats and dogs, estimates of cat infectiousness to bugs adjusted for those factors were not significantly different from those in dogs (mean, 46·3%) (effects of host type, OR=1·50, 95% CI=0·72–3·12)(Wald χ2=22·6, 5 D.F., P<0·001).
Infectiousness and bloodmeal sources
The percentage of bugs with T. cruzi infection for identified bloodmeal sources (n=1647, including both unmixed and mixed bloodmeals) in 1085 domestic T. infestans (Fig. 5) showed that overall, bugs fed on dogs showed higher mean infective bloodmeal indices (49%) than those fed on cats (39%), humans (38%), and chickens (29%). Fewer bugs were fed on cats than on dogs, but the cat-fed bugs showed infective indices at least as high as on dog- and human-fed bugs in the 3 surveys. The lower infective indices observed in Amamá during the onset of domestic re-colonization in 1988–1989 were consistent with lower domestic bug infestations and lower infection prevalence in dogs (30%). The high infective bloodmeal indices of chicken-fed bugs (birds are refractory to T. cruzi), even in third or fourth instar nymphs with unmixed meals, pointed to the large host-feeding mobility of domestic T. infestans and high risk of infection.
Fig. 5.
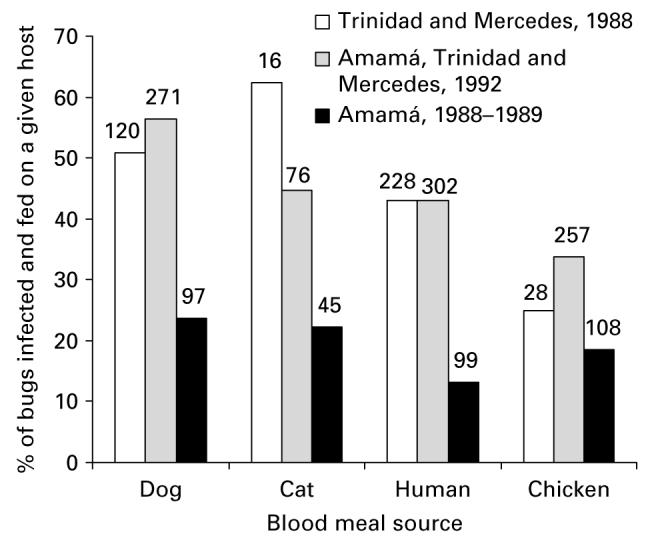
Association between Trypanosoma cruzi infection and host bloodmeal source in 1085 domestic T. infestans collected in Trinidad, Mercedes and Amamá from 1988 to 1992, which were examined for T. cruzi infection. Numbers on top of bars represent bloodmeals identified on each host blood source.
DISCUSSION
Both dogs and cats showed very high and similar prevalence and incidence of T. cruzi infection and infectiousness to bugs, much higher than the respective human population. A prospective estimate of the force of infection in children from the neighbouring village of Amamá yielded 4% per year (Gürtler et al. 2005). The prevalence and force of infection of dogs in Trinidad-Mercedes (not sprayed with insecticides previously) determined by early and late spring were lower (λ=43.2 per 100 dog-years) than those estimated in Amamá dogs by late summer 1992 (λ=72.7 per 100 dog-years), at the peak of domestic re-colonization by T. infestans following the 1985 insecticide spraying. This difference is most likely related to temperature-related increases in bug abundance and feeding frequency, which enhanced the intensity of transmission during the hot season. Moreover, despite a single application of knock-down insecticide tablets or fumigant canisters (which lack residual effects) and removal of large numbers of bugs, numerous new infections in very young dogs and cats were detected just 3 months later and the prevalence of T. cruzi remained constant. Like at least 1 child from Amamá, dog and cat cases occurred in houses with very low infected bug densities. This strengthens the view that the threshold domestic abundance of T. infestans below which transmission of T. cruzi to dogs and cats is unlikely was very low, if any threshold exists at all, and undetectable within the imprecision of vector sampling methods. Experimental studies showed that bloodmeal size of triatomine bugs correlated negatively with time to first defecation which, combined with other laboratory and field studies, was used to suggest that low-density bug populations with increased feeding success would pose the greatest risk of T. cruzi transmission (Kirk and Schofield, 1987). While the likelihood of transmission per feeding contact with the host may or may not increase at low bug densities, the cumulative probability of T. cruzi transmission over all feeding contacts between bugs and hosts during the relevant exposure period will increase with increasing densities of infected bugs (Fig. 1B). We conclude that dog and cat infection with T. cruzi is highly resilient despite vector control attempts that achieve only transient reductions in bug numbers.
One explanation of this pattern is that dogs and cats may contract T. cruzi infection by several routes (Minter, 1976). Vector-mediated transmission by contamination with bug faeces was the most likely avenue of transmission given the strong association between T. cruzi infection in dogs and the domiciliary density of infected bugs with large feeding frequencies on dogs. Frequent ingestion of bugs by some dogs was recorded experimentally (Reithinger et al. 2005), and jointly with licking of contaminated furs, both mechanisms may contribute to the observed prevalence in dogs and cats. In addition, vertical transmission is also implied by the occurrence of T. cruzi-infected pups born to seropositive female dogs in the absence of domestic infestations during the surveillance phase (Castañera et al. 1998). Ingestion or scavenging of wild mammals infected with T. cruzi were probably of less significance than vector-mediated or vertical transmission in the study area, although we have no direct evidence on ingestion or scavenging of wild mammals by dogs and cats.
Our study is the first to show that individual dog infectiousness to bugs was autocorrelated in time and aggregated at the population level. The seropositive dog population can be subdivided in groups showing an approximately constant pattern of low, intermediate, or high infectiousness to bugs over time. Some indication of this pattern was also suggested for adult people seropositive for T. cruzi (Cerisola et al. 1974).
Dog infectiousness to bugs was highly aggregated. About 33–45% of the seropositive dogs were not infectious, and about one-third infected >40% of bugs. Typical of host-parasite systems (Wilson et al. 2001), this aggregation was in part related to clinical and nutritional status, because the fraction of the local dog population that was malnourished and had low haematocrits included the most infectious hosts in another survey of Amamá dogs (Petersen et al. 2001). Dogs in the study area endured the joint effects of calorie- and protein-deficient diets, large tick infestations during the warm season, co-infection with other pathogens, and lack of any veterinary supervision and vaccination. Other factors, such as the size of the primary infectious dose and subsequent reinfections depending on type of T. cruzi strain and re-inoculum size (Machado et al. 2001; Riarte et al. 1995), may also underlie the aggregated infectiousness.
The heterogeneous distribution of infectiousness to bugs implies that some seropositive dogs make a disproportionate contribution to domestic transmission, also observed in Leishmania-infected dogs and sandflies (Courtenay et al. 2002). This heterogeneity is expected to increase the basic reproductive number (Ro) of infection and the efforts required to eliminate the pathogen (Woolhouse et al. 1997). For several directly transmitted human diseases, however, the distribution of individual infectiousness around Ro was often highly skewed, with disease extinction more likely and outbreaks rarer but more explosive (Lloyd-Smith et al. 2005). For example, a dog became infected at 4 years of age, then sero-converted permanently and repeatedly infected 78–86% of third instar nymphs from 1988 to 1993, while surviving to 11 years of age. Highly infectious, long-lived dogs constitute ‘superspreaders’ for further transmission of T. cruzi, especially during recurrent house reinfestation after insecticide spraying. Conversely, some highly infectious pups and cats tended to die or disappear rapidly, but the small sample size precluded establishing whether their deaths were related to T. cruzi infection or not. Early observations (Mazza, 1934; Mazza and Jörg, 1936) documented both fatal cases and un-complicated acute infections with T. cruzi in mongrel dogs less than 3 months of age, and found no pathognomonic signs of clinical disease. In contrast, numerous fatal cases of hospitalized purebred dogs (mostly pups) were reported in USA (e.g. Williams et al. 1977). The occurrence of some seropositive dogs with high titres for different methods which were repeatedly negative by xenodiagnosis is unlikely to be accounted for by non-specific serological reactions, because of the simultaneous use of 2 serological tests with specificity >96%, and because no case of leishmaniasis has been detected in the study area (Lauricella et al. 1998). Furthermore, as some of these non-infectious dogs later displayed low infectiousness, we conclude that they were truly infected with T. cruzi.
The prevalence and infectiousness to bugs of T. cruzi-seropositive dogs declined significantly with increasing dog age as in another survey, and unlike others (Gürtler et al. 1992a, 1996; Lauricella et al. 1989). Age may be taken as a surrogate of time since primary infection, particularly because most dog infections in the study villages were acquired <1 year of age, and the latent period for parasitological and serological conversion to T. cruzi in dogs was experimentally estimated as 1 and 2 weeks, respectively (Lauricella et al. 1986; Machado et al. 2001). The variable age-infectiousness pattern across studies probably depends on sample size and the inclusion of recently infected pups and old chronic dogs, as these groups stretch out the distribution of infectiousness in opposite directions and may therefore render significant the regression analysis. In experimental dog infections, patent parasitaemia disappeared after a month and xenodiagnosis remained negative for 6–8 months after a single inoculation (Machado et al. 2001), and in nearly all dogs inoculated 5–12 years before (Araújo et al. 2002). In contrast, dogs inoculated with a more virulent parasite strain of T. cruzi showed long-lasting infectiousness (Lauricella et al. 1986). Also, unlike previous results (Gürtler et al. 1992a), dog infectiousness did not increase significantly with the domiciliary density of infected bugs. Similarly, in experimental dog infections repeated re-inoculations with 2 strains of T. cruzi differing in virulence enhanced infectiousness only slightly and transiently (Machado et al. 2001). Except for permanent seroconversion for T. cruzi (Castañera et al. 1998; Machado et al. 2001; Lauricella et al. 1986), natural and experimental dog infections with T. cruzi display different patterns. Such variations probably may be attributed to the complex interactions among host genetics and health status, parasite strain, infectious dose and epidemiologic context. Current evidence therefore does not show that exposure to vector-mediated reinfections enhances dog infectiousness to levels detectable by xenodiagnosis.
The prevalence and infectiousness of xenodiagnosis-positive cats and dogs were very high and not significantly different when adjusted for other factors. Cat infectiousness to bugs was age-independent, but lack of serological data hindered detecting the age-related decline observed in dogs and may also have underestimated the force of infection of cats to some extent. Both effects may not be particularly important since the cat population was very young. The large probability of bug infection after a single bloodmeal on a cat attested to cats’ reservoir capacity.
Both host populations were approximately stationary, had high annual turnover rates (40–56%), and were closely associated with human sleeping quarters. Because of their high loss rate and well-known nocturnal activity, cats likely have a shorter mean residence period and more variable exposure patterns to domestic bugs than dogs. Householders kept cats to combat rodents, and high cat birth rates allowed a steady inflow of kittens that contracted the infection as fast as dogs. Because cats were at least as infected and infectious as dogs, their per capita reservoir capacity was similar. The key issue and main focus of the long-standing controversy on the role of cats in transmission of T. cruzi infection is the magnitude of their contact rates with domestic bugs.
The published host-feeding patterns of domestic T. infestans show that it feeds frequently, though quite variably, on domestic cats (median, 11%; interquartile range, 4–15%) and dogs (median, 4%; interquartile range, 1–39%) (reviewed in Gürtler et al. 1997). Other triatomine species also showed significant frequencies of bloodmeals on cats (11–43%) exceeding those on dogs in at least 1 study for P. megistus, Rhodnius prolixus, T. brasiliensis and T. barberi. In household-based analyses, feedings on cats were positively related to the number of cats in bedroom areas during the hot season (and negatively related to the number of dogs), and households with human incident cases had consistently much higher percentages of cat-fed T. infestans than households without incident cases (Gürtler et al. 1997, 2005). Apparent inconsistencies between host-feeding results and the close association found between T. cruzi infection in children and dogs or cats in areas infested by P. megistus (Minter, 1976; Piesman et al. 1983; Mott et al. 1978) or T. infestans (Wisnivesky-Colli et al. 1985; Gürtler et al. 1993; Catalá et al. 2004) may be explained by heterogeneities in epidemiological context and methodology. Point surveys based on manual sampling of bugs (typically biased for large stages and particular collection sites) coupled with few bugs per house examined for bloodmeals may produce biased results and underestimate the actual contribution of a host. This problem is compounded by the high feeding mobility of domestic T. infestans shown by mixed bloodmeals. High infective bloodmeal indices for third-fourth instar nymphs fed only on chickens indicate that at an earlier instar the bugs had fed on some highly infectious source that was no longer detectable. Thus, biased vector sampling and presence of highly infectious sources may account for situations in which host-feeding patterns fail to pinpoint a significant reservoir host.
Infective bloodmeal indices clearly show that dogs ranked higher than cats and humans as the main domestic sources of T. cruzi in the study area. The evidence for dogs is consistent with observations elsewhere in Argentina (Wisnivesky-Colli et al. 1982) and Brazil (Barretto, 1968) but not in Chile (Schenone et al. 1985). In our study, the infective indices for cats showed large variations between surveys and households within a given survey. Most cat bloodmeals were mixed, thus indicating that repeated feedings on cats were much less likely than on other hosts, as expected from their more unstable exposure pattern. Although cats were much fewer than household-associated dogs (1 to 2·4), the evidence suggests that domestic cats contributed significantly to the domestic transmission of T. cruzi wherever cats occurred, depending on householders’ choices and individual animal behaviour. Molecular typing techniques of T. cruzi recently showed that dogs, cats and a large fraction of T. infestans from our study area shared the sublineage T. cruzi IIe (Marcet et al. 2006; Cardinal et al. unpublished results). Both domestic dogs and cats qualify as reservoir hosts (Cleaveland and Dye, 1995) because they can independently maintain the basic reproductive number Ro of T. cruzi above 1 and can act as a source of infection to other species, including humans. The presence of dogs and cats significantly increased Ro. Cats cannot be considered accidental reservoir hosts without impact on Ro.
A biologically sensible, operationally practical, quantitative way to measure the relative contributions of dogs, cats and humans to bug infection can be expressed in an index derived from the product of 3 numbers specific to that host population: (1) the fraction of bugs that have a bloodmeal from the particular host population (dogs, cats, humans); (2) the prevalence of infectious hosts in each host population; and (3) the infectiousness of each host population to an uninfected bug in a single bloodmeal. This index is related to one developed by Kilpatrick et al. (2005) to compare the importance of different mosquito species as vectors of West Nile virus. It attempts to measure how likely it is that each host population (dogs, cats, humans) contributes infection to a bug, and is intended for comparisons among host populations that are hosts of T. cruzi infection and sources of bug bloodmeals. The proposed index is imperfect because it does not take account of differences between different life stages of the bug in their likelihood of feeding on and becoming infected by each of the different host populations, or differences in bug feeding rate and bloodmeal size on each host species. This index ignores the indirect contributions of chickens to the increase of the bug population. For our study host populations, dogs and cats contributed respectively 13.9 and 4.8 times as much as humans to infection of domestic bugs (Table 2). The contribution index of dogs was 2·9 times that of cats, somewhat higher than the ratio between numbers of household-associated dogs and cats (2·4 : 1).
Table 2.
Relative contribution of dogs, cats and humans to domestic Triatoma infestans infected with Trypanosoma cruzi as a function of host blood index and host infectiousness
(Data for the local human population were taken from Gürtler et al. (1996). Proportions of bugs fed on different hosts sum to more than 1 because some bugs fed on more than 1 host.)
| Host | Proportion of |
||||
|---|---|---|---|---|---|
| bugs fed on |
hosts infectious |
bugs infected after a single bloodmeal |
Contribution index (×100) |
Relative contribution |
|
| Dog | 0·446 | 0·420 | 0·463 | 8·7 | 13·9 |
| Cat | 0·112 | 0·412 | 0·654 | 3·0 | 4·8 |
| Human | 0·662 | 0·100 | 0·094 | 0·6 | 1·0 |
Our study has several implications for control and research. Identifying predictive correlates of high infectiousness and the highly infectious dogs or cats to treat, cull, or fit them with an insecticide collar that may repel or kill the bugs (Reithinger et al. 2005) would decrease dramatically the domestic transmission risk. Targeted control policies may outperform population-wide measures. Using a theoretical model for a vector-borne pathogen that induces very little disease-specific mortality relative to the disease-free mortality rate in dogs (Quinnell et al. 1997), the Ro of T. cruzi in dogs can be estimated as Ro=1+L/A, where L is the mean life expectancy of a dog and A is the average time for a dog to acquire infection. Using L=43 months (taken from Gürtler et al. 1990) and a conservative average estimate of A=6 months (suggested by 3 early or late summer age-prevalence curves in the Amamá area in the absence of control actions and the follow-up of newborn dogs in the current study), Ro is estimated to be 8·2 under the homogeneous mixing assumption. Therefore, the mean yearly coverage of a 100% effective control intervention (such as vaccination, culling, or application of dog collars) to ensure the elimination of T. cruzi can be estimated [from 100 (1-1/Ro)] to be 88%. Given the observed heterogeneities among houses and individual dogs (in infestation and infectiousness) and the existence of other reservoir hosts such as cats and humans, the actual Ro may be much higher than 8·2. Consequently more than 88% of the dog population would have to be covered by a 100% effective control intervention to reduce Ro<1 in dogs in the first intervention year. For cats, for which there is more uncertainty on the relevant demographic parameters, here approximated as L=24 months and A=6 months, Ro is estimated to be 5.
Infectiousness estimates serve as a yardstick to assess the impact of any future transmission-blocking vaccine (e.g. Basombrío et al. 1993). They also lend realism to mathematical models of T. cruzi transmission, most of which assume that human and dog infectiousness are host age-independent and homogeneous within each host population (Rabinovich and Himschoot, 1990; Cohen and Gürtler, 2001). Depending on the local particular structure of domestic transmission, dogs and cats may have to be treated separately in mathematical models of transmission. As cats have higher rates of population turnover than dogs, mass residual spraying with insecticides followed by sustained surveillance activities would clear T. cruzi from the cat population more rapidly than from the dog population. Past (Gürtler et al. 1997) and present data combined support the prediction that an increase in the number of domestic cats per household (while keeping constant their current exposure to bugs) would lead to more bloodmeals on cats and a greater contribution by cats to the domestic transmission of T. cruzi. High incidence of infection and infectiousness in dogs and cats combined with suitable exposure patterns to domestic bugs make them primary reservoir hosts and sources of T. cruzi infection for householders. Despite insuffcient evidence for a wide generalization, a prudent policy to recommend to householders of endemic rural areas is the permanent removal of dogs and cats as well as chickens from bedroom areas.
Acknowledgments
This work was funded by the University of Buenos Aires, Agencia Nacional de Promoción Científica y Técnica (Argentina), and by NIH Research Grant R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences (U.K. and R.E.G.). The participation of J.E.C. was also supported, in part, by U. S. National Science Foundation Grants DEB-9981552 and DMS-0443803. J.E.C. thanks Mr and Mrs William T. Golden for hospitality during this work. Accommodation and facilities at the study area were kindly provided by Mr Omar Citatti and Mrs María Moyano. We thank Diana Rubel, Nicolás Schweigmann and Rosario Petersen for their valuable cooperation, and Richard Reithinger and the ECLAT network for helpful comments. R.E.G. and M.C.C. are members of CONICET Researcher’s Career, Argentina.
REFERENCES
- Alencar JE, Almeida YM, Santos AR, Freitas LM. Epidemiology of Chagas’ disease in the state of Ceará, Brazil. IV. The role of dogs and cats as domestic reservoirs. Revista Brasileira de Malariología e Doenças Tropicales. 19741975;26/27:5–26. [PubMed] [Google Scholar]
- Araújo FMG, Bahia MT, Magalhães NM, Martins-Filho OA, Veloso VM, Carneiro CM, Tafuri WL, Lana M. Follow-up of experimental chronic Chagas’ disease in dogs: use of polymerase chain reaction (PCR) compared with parasitological and serological methods. Acta Tropica. 2002;81:21–31. doi: 10.1016/s0001-706x(01)00196-6. [DOI] [PubMed] [Google Scholar]
- Barretto MP. Estudos sôbre reservatórios e vectores silvestres do “Trypanosoma cruzi”. XXXI. Observações sôbre a associação entre reservatórios e vectores, com especial referência à região nordeste do Estado de Sao Paulo. Revista Brasileña Biología. 1968;28:481–494. [PubMed] [Google Scholar]
- Basombrío MA, Segura MA, Mora MC, Gomez L. Field trial of vaccination against American trypanosomiasis (Chagas’ disease) in dogs. American Journal of Tropical Medicine and Hygiene. 1993;49:143–151. doi: 10.4269/ajtmh.1993.49.143. [DOI] [PubMed] [Google Scholar]
- Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, Ramsey J, Wirtz RA, Robinson LE. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerging Infectious Diseases. 2003;9:103–105. doi: 10.3201/eid0901.020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckert P. Cabeza, Laguens RP. Chronic Chagas’ disease in the mouse. III. Absence of concomitant immunity after repeated infections. Medicina. 1981;41:543–548. [PubMed] [Google Scholar]
- Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-west Argentina. Annals of Tropical Medicine and Parasitology. 1998;92:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]
- Catalá SS, Crocco LB, Muñoz A, Morales G, Paulone I, Giraldez E, Candioti C, Ripol C. Entomological aspects of Chagas’ disease transmission in the domestic habitat, Argentina. Revista Saúde Pública. 2004;38:216–222. doi: 10.1590/s0034-89102004000200010. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. Factors limiting the domiciliary density of Triatoma infestans, vector of Chagas’ disease, in north-west Argentina: a longitudinal study. Bulletin of the World Health Organization. 1998;76:373–384. [PMC free article] [PubMed] [Google Scholar]
- Cerisola JA, Rohwedder R, Segura EL, Del Prado CE, Alvarez M, de Martini G. J. Wynne. El Xenodiagnóstico. Monografía. Ministerio de Bienestar Social, Secretaría de Estado de Salud Pública. 1974. pp. 1–127.
- Cleaveland S, Dye C. Maintenance of a microparasite infecting several host species: rabies in the Serengeti. Parasitology. 1995;111:S33–S47. doi: 10.1017/s0031182000075806. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American Trypanosomiasis. Science. 2001;29:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. Journal of Infectious Diseases. 2002;186:1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- Dias JCP, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America — A review. Memorias do Instituto Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Freitas JLP. Observações sobre xenodiagnósticos practicados em reservatórios domésticos e silvestres do Trypanosoma cruzi em uma localidade endémica da moléstia de Chagas no Estado de São Paulo. Hospital. 1950;38:521–529. [PubMed] [Google Scholar]
- Gürtler RE, Kravetz FO, Petersen RM, Lauricella MA, Wisnivesky-Colli C. The prevalence of Trypanosoma cruzi and the demography of dog populations after insecticidal spraying of houses: a predictive model. Annals of Tropical Medicine and Parasitology. 1990;84:313–323. doi: 10.1080/00034983.1990.11812475. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Petersen RM, Lauricella MA, Wisnivesky-Colli C. Infectivity to the vector Triatoma infestans of dogs infected with Trypanosoma cruzi in north-west Argentina. Annals of Tropical Medicine and Parasitology. 1992a;86:111–119. doi: 10.1080/00034983.1992.11812640. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Rubel DN, Schweigmann NJ. Determinants of the domiciliary density of Triatoma infestans, vector of Chagas disease. Medical and Veterinary Entomology. 1992b;6:75–83. doi: 10.1111/j.1365-2915.1992.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cécere MC, Petersen RM, Rubel DN, Schweigmann NJ. Chagas disease in north-west Argentina: association between Trypanosoma cruzi parasitaemia in dogs and cats and infection rates in domestic Triatoma infestans. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87:12–15. doi: 10.1016/0035-9203(93)90400-k. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Castañera MB, Canale D, Lauricella MA, Chuit R, Cohen JE, Segura EL. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. American Journal of Tropical Medicine and Hygiene. 1996;55:24–31. [PubMed] [Google Scholar]
- Gürtler RE, Cohen JE, Cecere MC, Chuit R. Shifting host choices of the vector of Chagas disease Triatoma infestans in relation to the availability of hosts in houses in north-west Argentina. Journal of Applied Ecology. 1997;34:699–715. [Google Scholar]
- Gürtler RE, Chuit R, Cecere MC, Castañera MB, Cohen JE, Segura EL. Household prevalence of seropositivity for Trypanosoma cruzi in three rural villages of northwest Argentina: environmental, demographic and entomologic associations. American Journal of Tropical Medicine and Hygiene. 1998;59:741–749. doi: 10.4269/ajtmh.1998.59.741. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Chuit R, Segura EL, Cohen JE. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. American Journal of Tropical Medicine and Hygiene. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerging Infectious Diseases. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk ML, Schofield CJ. Density-dependent timing of defaecation by Rhodnius prolixus, and its implications for the transmission of Trypanosoma cruzi. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:348–349. doi: 10.1016/0035-9203(87)90262-8. [DOI] [PubMed] [Google Scholar]
- Lauricella MA, Riarte AR, Lazzari JO, Barousse AP, Segura EL. Enfermedad de Chagas en perros experimentalmente infectados con Trypanosoma cruzi. Medicina. 1986;46:195–200. [PubMed] [Google Scholar]
- Lauricella MA, Sinagra AJ, Paulone I, Riarte AR, Segura EL. Natural Trypanosoma cruzi infection in dogs of endemic areas of the Argentine Republic. Revista do Instituto de Medicina Tropical de São Paulo. 1989;31:63–70. doi: 10.1590/s0036-46651989000200001. [DOI] [PubMed] [Google Scholar]
- Lauricella MA, Castañera MB, Gürtler RE, Segura EL. Immunodiagnosis of Trypanosoma cruzi (Chagas’ Disease) infection in naturally infected dogs. Memorias do Instituto Oswaldo Cruz. 1998;93:501–507. doi: 10.1590/s0074-02761998000400016. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature, London. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado EMM, Fernandes AJ, Murta SMF, Vitor RWA, Camilo DJ, Jr., Pinheiro SW, Lopes ER, Adad SJ, Romanha AJ, Dias J. C. Pinto. A study of experimental reinfection by Trypanosoma cruzi in dogs. American Journal of Tropical Medicine and Hygiene. 2001;65:958–965. doi: 10.4269/ajtmh.2001.65.958. [DOI] [PubMed] [Google Scholar]
- Maguire JH, Mott KE, Hoff R, Guimaraes A, Franca JT, Souza JAA, Ramos NB, Sherlock IA. A three-year follow-up study of infection with Trypanosoma cruzi and electrocardiographic abnormalities in a rural community in northeast Brazil. American Journal of Tropical Medicine and Hygiene. 1982;31:42–47. doi: 10.4269/ajtmh.1982.31.42. [DOI] [PubMed] [Google Scholar]
- Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gürtler RE, Schijman AG. PCR-based identification of Trypanosoma cruzi lineages in feces of triatomine bugs from rural northwestern Argentina. Parasitology. 2006;132:1–9. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer HF, Alcaraz I. Investigaciones sobre esquizotripanosis en perros y gatos de la zona suburbana de Resistencia. Anales Instituto de Medicina Regional de Tucumán. 1954;4:9–17. [Google Scholar]
- Mazza S. Difusión de la infección natural por S. cruzi en perros de la Provincia de Jujuy. Misión de Estudios de Patología Regional Argentina. 1934;17:23–28. [Google Scholar]
- Mazza S, Jörg ME. Infección natural mortal por S. cruzi en cachorro de perro “Pila” de Jujuy. 9na. Reunión de la Sociedad Argentina de Patología Regional del Norte; Mendoza. October 1–4, 1935.1936. pp. 365–411. [Google Scholar]
- Minter DM. New Approaches in American Trypanosomiasis Research. Washington DC: 1976. Effects on transmission to man of the presence of domestic animals in infected households; pp. 330–337. Pan American Health Organization Scientific Publication No. 318. [Google Scholar]
- Mott KE, Muniz TM, Lehman JS, Jr., Hoff R, Morrow RH, Jr., Oliveira TS, SherlocK IA, Draper CC. House construction, triatomine distribution and household distribution of seroreactivity to Trypanosoma cruzi in a rural community in northeast Brazil. American Journal of Tropical Medicine and Hygiene. 1978;27:1116–1122. doi: 10.4269/ajtmh.1978.27.1116. [DOI] [PubMed] [Google Scholar]
- Muench H. Catalytic Models in Epidemiology. Harvard University Press; Cambridge, MA, USA: 1959. [Google Scholar]
- Petersen RM, Gürtler RE, Cecere MC, Rubel DN, Hansen D, Lauricella MA, Carlomagno M. Association between nutritional indicators of dogs seroreactive for Trypanosoma cruzi in a rural area of northwestern Argentina. Parasitology Research. 2001;87:208–214. doi: 10.1007/s004360000324. [DOI] [PubMed] [Google Scholar]
- Piesman J, Sherlock IA, Christensen HA. Host availability limits population density of Panstrongylus megistus. American Journal of Tropical Medicine and Hygiene. 1983;32:1445–1450. doi: 10.4269/ajtmh.1983.32.1445. [DOI] [PubMed] [Google Scholar]
- Quinnell RJ, Courtenay O, Garcez L, Dye C. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology. 1997;115:143–156. doi: 10.1017/s0031182097001200. [DOI] [PubMed] [Google Scholar]
- Rabinovich JE, Himschoot P. A population-dynamics simulation model of the main vectors of Chagas’ disease transmission, Rhodnius prolixus and Triatoma infestans. Ecological Modelling. 1990;52:249–266. [Google Scholar]
- Reithinger R, Ceballos LA, Stariolo R, Davies CR, Gürtler RE. Chagas disease control: deltamethrin-treated collars reduce Triatoma infestans feeding success on dogs. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:502–508. doi: 10.1016/j.trstmh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Riarte A, Sinagra A, Lauricella M, Bolomo N, Moreno M, Cossio P, Arana R, Segura EL. Chronic experimental infection by Trypanosoma cruzi in Cebus apella monkeys. Memorias do Instituto Oswaldo Cruz. 1995;90:733–740. doi: 10.1590/s0074-02761995000600014. [DOI] [PubMed] [Google Scholar]
- Schenone H, Christensen HA, Vásquez AM, González C, Méndez E, Rojas A, Villarroel F. Fuentes de alimentación de triatomas domésticos y su implicancia epidemiológica en relación a enfermedad de Chagas en áreas rurales de siete regiones de Chile. Boletín Chileno de Parasitología. 1985;40:34–38. [PubMed] [Google Scholar]
- Williams GD, Adams L. Garry, Yaeger RG, McGrath RK, Read WK, Bilderback WR. Naturally occurring Trypanosomiasis (Chagas Disease) in dogs. Journal of the American Veterinary Medical Association. 1977;171:171–177. [PubMed] [Google Scholar]
- Wilson K, Bjørnstad ON, Dobson AP, Merler S, Poglayen G, Randolph SE, Read AF, Skoping A. Heterogeneities in macroparasite infections: patterns and processes. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP, editors. The Ecology of Wildlife Diseases. Oxford University Press; Oxford, UK: 2001. pp. 6–44. [Google Scholar]
- Wisnivesky-Colli C, Gürtler RE, Solarz ND, Salomón DO, Ruiz AM. Feeding patterns of Triatoma infestans (Hemiptera, Reduviidae) in relation to transmission of American Trypanosomiasis in Argentina. Journal of Medical Entomology. 1982;19:645–654. doi: 10.1093/jmedent/19.6.645. [DOI] [PubMed] [Google Scholar]
- Wisnivesky-Colli C, Gürtler RE, Solarz ND, Lauricella MA, Segura EL. Epidemiological role of humans, dogs and cats in the transmission of Trypanosoma cruzi in a central area of Argentina. Revista do Instituto de Medicina Tropical de São Paulo. 1985;27:346–352. doi: 10.1590/s0036-46651985000600009. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlewood JD, Garnett GP, Hagan P, Hii JLK, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proceedings of the National Academy of Sciences, USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Report of WHO consultation on dog ecology studies related to rabies control. Geneva: 1988. 22–25 February 1988. WHO/Rabies Research/88.25 (mimeographed document) [Google Scholar]
- Zárate LG, Zárate RJ, Tempelis CH, Goldsmith RS. The biology and behavior of Triatoma barberi (Hemiptera: Reduviidae) in Mexico. I. Blood meal sources and infection with Trypanosoma cruzi. Journal of Medical Entomology. 1980;17:103–116. doi: 10.1093/jmedent/17.2.103. [DOI] [PubMed] [Google Scholar]


