Abstract
Senescence of endothelial cells increases with systemic aging and is thought to contribute to the development of atherosclerosis. Cell therapy with highly proliferative endothelial progenitor cells (EPCs) is an emerging therapeutic option to promote endothelial regeneration, but little is known about their senescence and their vulnerability to inflammatory stressors. We therefore studied the senescence of proliferative human EPCs and investigated the effects of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) on their senescence. Human EPCs had a significantly lower rate of senescence at baseline, compared with that of mature endothelial cells. However, EPCs up-regulated the expression of the senescence-associated cell cycle arrest protein p16INK4a and markedly increased measured senescence levels when exposed to chronic TNF-α treatment. Analysis of telomere length showed that the increases in senescence were not related to changes in telomere length. Inhibition of the p38 mitogen-activated protein kinase pathway blocked the induction of p16INK4a and cellular senescence. In conclusion, highly proliferative EPCs have a low rate of intrinsic senescence but are vulnerable to premature senescence induction by chronic proinflammatory stimulation. These findings will lead to a better understanding of physiological endothelial regeneration as well as to targeted therapies with the aim of promoting endothelial regeneration through endothelial progenitor cells.—Zhang, Y., Herbert, B.-S., Rajashekhar, G., Ingram, D. A., Yoder, M. C., Clauss, M., Rehman. J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-α via the p38 mitogen-activated protein kinase pathway.
Keywords: cytokines, aging, inflammation, vascular cells
The senescence of vascular cells is characterized by the loss of proliferation and regeneration potential and is associated with increasing systemic age as well as proatherogenic processes, thus suggesting that aging or senescence at a cellular level contributes to the link between systemic aging and the development of atherosclerosis (1). On the cellular level, two major types of aging or senescence have been described, which can act in concert during systemic aging: Replicative senescence via shortening of telomeres when cells reach a maximal number of cell divisions and stress-induced premature senescence when stressors such as inflammation or oxidative stress elicit a senescent cell phenotype (1).
Circulating endothelial progenitor cells (EPCs) may be able to regenerate senescent and apoptotic mature endothelial cells within the vessel wall, and the development of EPC-based therapies may allow for novel approaches to maintain endothelial health. Two major types of circulating EPCs have been identified (2,3,4). On the one hand, short-term culture of mononuclear cells yields a population of endothelial-like cells (“early EPCs”), which are highly angiogenic (4, 5) but minimally proliferate and are derived from monocytic/myeloid cells (6). The lack of proliferation is probably due to their high rate of intrinsic senescence of up to 50% (5, 7, 8), but their senescence can be reduced using pharmacological or genetic modifications (5, 7). On the other hand, long-term culture of mononuclear cells yields highly proliferative nonmyeloid EPCs (“late EPCs”), which are found in adult peripheral blood, but in substantially higher numbers in umbilical cord blood (9). Because of their high proliferative rate, such EPCs may be well suited for therapies targeting endothelial regeneration; however, little is known about their senescence. Because a better understanding of the senescence of proliferative EPCs may be a key step toward implementing EPC-based endothelial regeneration, we studied the senescence of proliferative EPCs and their vulnerability to inflammatory senescence induced by the proinflammatory cytokine tumor necrosis factor-α (TNF-α). We also focused on studying the p38 mitogen-activated protein kinase pathway and cyclin-dependent kinase inhibitor p16INK4a, which have both recently been shown to play major roles as mediators of the aging process in stem cells (10, 11).
MATERIALS AND METHODS
Isolation and culture of human umbilical cord blood EPCs
Highly proliferative EPCs were isolated from human umbilical cord blood as described previously (9, 12) in accordance with the rules of the Indiana University Institutional Review Board by isolating mononuclear cells over a Ficoll gradient and suspending the isolated mononuclear cells in endothelial basal medium (EBM-2; Lonza, Baltimore, MD, USA) supplemented with EGM-2-SingleQuots (Cambrex, Baltimore, MD, USA) and an additional 10% FBS (HyClone, Logan, UT, USA). This medium is specifically conducive to the growth of cord blood EPCs and is referred to as cEGM-2 (9, 12). Then, 5 × 106 mononuclear cells/cm2 were plated on collagen-coated tissue culture flasks (BD Biosciences, San Jose, CA, USA). After 1 day of culture, nonadherent cells were discarded, and the media were replaced daily for the next 2–4 wk. Subsequently, endothelial colonies appeared. The colonies were replated onto new plates, and highly proliferative endothelial cells grew out from these colonies, which then formed confluent monolayers, and have been kept in culture by us for at least 10–15 passages. To compare the degree of senescence between mature endothelial cells and cord blood EPCs, human aortic endothelial cells (HAECs) were obtained from Lonza and cultured according to the manufacturer’s instructions.
Umbilical cord EPCs were treated with control medium or the proinflammatory cytokine TNF-α (recombinant human TNF-α) at the respective doses for up to 1 wk. Because cytokines such as TNF-α may undergo degradation when given over a prolonged period, cell culture medium and TNF-α were replaced daily if the treatment period extended to more than 24 h.
Staining for senescence-associated β-galactosidase
As described previously, senescence-associated β-galactosidase (SA-β-Gal) (13) can serve as a biomarker for cellular senescence. To assess such senescence in cord blood-derived EPCs, SA-β-Gal activity was measured using a standard senescence detection kit (Biovision, Mountain View, CA, USA) according to the manufacturer’s instructions. In brief, culture media were removed; the cells were then washed once with PBS and fixed with the fixation solution for 15 min at room temperature. After two additional washes with PBS, the staining solution containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactoside was added to each well. Cells were incubated at 37°C overnight and then observed under a microscope for development of blue color. The percentage of blue cells vs. total cells was measured by choosing 10 random microscopic fields.
Flow cytometry analysis
Surface marker staining of EPCs was performed with fluorescently conjugated antibodies (BD Biosciences) against the endothelial cell surface markers VE-cadherin and vascular endothelial growth factor receptor 2, as well as the monocyte/leukocyte markers CD14 and CD45 as described previously (6). Intracellular flow cytometry staining for p16INK4a was performed by initially adding cold 70–80% ethanol to the cell pellet and incubating the cells at −20°C for at least 2 h. Then cells were washed twice with 3–4 ml of staining buffer (PBS with 1% FBS) and centrifuged for 10 min at 200 g. Finally, directly conjugated anti-human antibody against p16INK4a (clone B56; BD Biosciences) was added to the cells. After incubation at room temperature in the dark for 20–30 min, cells were washed, resuspended in staining buffer, and analyzed on a FACSCalibur system (BD Biosciences).
Terminal restriction fragment (TRF) assay
Measurements of telomere lengths were performed as described previously (14,15,16). In brief, samples were lysed, and the proteins were digested in 10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 100 mM EDTA (pH 8.0), 1% Triton X-100, and 2 mg/ml proteinase K for 8 h at 55°C followed by inactivation of proteinase K for 30 min at 70°C and dialysis in 10 mM Tris-HCl (pH 7.5) and 1 mM EDTA (pH 8.0) at 4°C overnight. Genomic DNA (3 μg) was digested to completion with a multiple restriction enzyme mix (∼1 U/μg each of AluI, HaeIII, HinfI, MspI, and RsaI; Boehringer Mannheim, Mannheim, Germany). The digested DNA was separated on a 0.7% agarose gel in 0.5× TBE buffer (0.089 M Tris borate-0.002 M EDTA). The gel was denatured for 20 min in 0.5 M NaOH-1.5 M NaCl, rinsed with dH2O for 10 min, dried on Whatman 3MM paper under vacuum for 1 h at 55°C, and neutralized for 15 min in 1.5 M NaCl-0.5 M Tris-HCl (pH 8.0). The gel was probed with a 32P-labeled (C3TA2)4 telomere probe for 16 h at 42°C in 5× sodium chloride-sodium citrate (SSC) buffer, 5× Denhardt’s solution, 10 mM Na2HPO4, and 1 mM Na2H2P2O7 · 10H2O. The gel was then washed once with 2× SSC for 10 min and twice for 15 min each with 0.1× SSC at room temperature before exposure to a phosphor screen (PhosphorImager; Molecular Dynamics, Sunnyvale, CA, USA) overnight. The screen was visualized on a PhosphorImager using ImageQuant 5.2 analysis software (Molecular Dynamics). Mean TRF lengths were calculated as described previously using Telorun (14,15,16).
Western blot analysis
To study the p38/MAPK phosphorylation levels, EPCs were serum starved for 4 h and then either treated with 10 ng/ml TNF-α (Sigma-Aldrich, St. Louis, MO) for up to 60 min or not treated. For immunoblot analysis, the whole-cell extracts from EPC populations were probed for the presence of phospho-p38 MAPK, stripped, and reprobed with total p38 MAPK antibody (both from Cell Signaling Technology Inc., Danvers, MA, USA) as per the standard procedure described earlier (17). Quantification of fold change in activated MAPK was analyzed by densitometry and normalized to total protein levels using ImageQuant 5.2. As a control, cell samples at 30 min of TNF-α stimulation were also obtained in the presence of the p38 MAPK inhibitor (10 μM SB203580) and showed the expected inhibition of p38/MAPK phosphorylation. To assess p16INK4a expression, whole-cell extracts from HAECs and EPCs at multiple passages were obtained and assayed as described previously by us (18) using a mouse-anti-human antibody against p16INK4a (G175-1239; BD Biosciences).
RESULTS
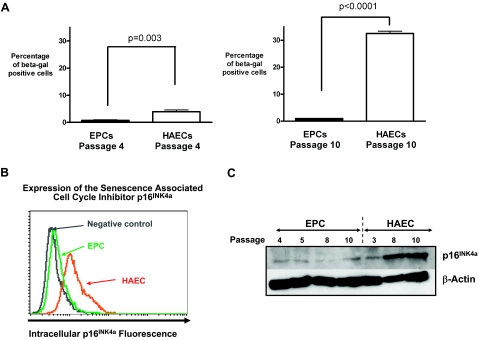
We confirmed that the isolated EPCs from human umbilical cord blood had a highly proliferative, nonmyeloid endothelial phenotype (Supplemental Fig. 1) (19), and we hypothesized that their high growth potential was in part due to a low level of intrinsic replicative senescence. We used the senescence biomarker SA-β-Gal to measure the degree of senescence in human proliferative EPCs and HAECs at similar passages (Fig. 1A). At any given passage studied by us, mature HAECs demonstrated significantly higher levels of senescence than EPCs. Remarkably, this difference between HAECs and EPCs became much more apparent over time as cells were kept in culture, because EPCs maintained their low state of senescence at higher passages, whereas HAECs showed a significant increase in SA-β-Gal-positive cells with passaging. At passage 4, EPCs contained 0.8 ± 0.2% SA-β-Gal-positive cells, whereas HAECs contained 3.9 ± 0.6% SA-β-Gal-positive cells (EPCs vs. HAECs: P=0.003; n=4). However, at passage 10, the proportion of SA-β-Gal-positive cells increased 8-fold in HAECs to 32.5 ± 0.8%, whereas EPC senescence remained nearly unchanged at 1.0 ± 0.1% (EPCs vs. HAECs: P<0.0001; n=4). To identify a potential mechanism for the observed difference in senescence, we investigated the expression of p16INK4a, which is a cyclin-dependent kinase inhibitor and known senescence-associated inducer of cell cycle arrest. Comparison of proliferative EPCs and HAECs showed that p16INK4a was expressed in HAECs but not in EPCs (Fig. 1B). To then identify whether the difference in HAEC and EPC expression of p16INK4a was dependent on the passage number of the cells, we performed Western blot analysis of the cells at various passages and were able to show that HAECs increased their p16INK4a expression with increasing passages in culture, whereas EPCs maintained their low level of p16INK4a expression even at passage numbers of 8 or higher (Fig. 1C). Because senescence has been associated with increased levels of oxidative stress and decreases in cellular nitric oxide (NO) (1), we also assessed NO and the reactive oxygen species (ROS) superoxide in low- and high-passage EPCs and HAECs. Interestingly, EPCs and HAECs did not show any significant change in cellular superoxide as they reached higher passages but did show decreases in cellular NO (Supplemental Fig. 2).
Figure 1.
Differences in cellular senescence between cord blood-derived EPCs and HAECs. A) Percentages of cells positive for SA-β-Gal in human cord blood-derived EPCs and mature HAECs. HAECs and EPCs are compared at a low passage (passage 4, P=0.003 by t test, n=4) as well as at a high passage (passage 10, P<0.0001 by t test, n=4). Quantification demonstrates a marked increase in the percentage of senescent cells among HAECs, and relatively minor increases in senescence among EPCs with passaging. B) Intracellular flow cytometric staining for the senescence-associated cell cycle inhibitor p16INK4a was performed. Cell numbers are depicted on the y axis and fluorescence intensity on a logarithmic scale is used as an indicator of expression on the x axis. At baseline, p16INK4a was expressed in HAECs (orange) and not in EPCs. The level of p16INK4a staining in EPCs (green) is the same as that of the negative isotype control (gray). C) Representative Western blot staining for the senescence-associated cell cycle inhibitor p16INK4a in HAEC and EPC samples demonstrates that p16INK4a expression markedly increases in HAECs as cells reach passages 8–10.
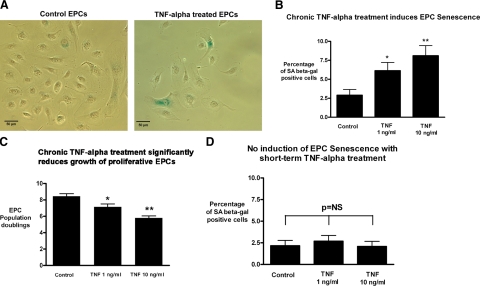
After observing the apparent resilience of proliferative EPCs to replicative senescence at baseline conditions, we investigated whether EPCs were vulnerable to senescence induced by chronic exposure to the proinflammatory cytokine TNF-α, because inflammatory stimulation is known to induce premature senescence in multiple tissues and cells. EPCs were chronically treated with TNF-α for 1 wk, and SA-β-gal staining was measured. Continuous treatment with TNF-α for 1 wk resulted in a dose-dependent increase in EPC senescence (Fig. 2A, B), which was nearly double the baseline EPC senescence (baseline rate: 2.9±0.8%) at the low dose of 1 ng/ml (6.1±1.1%, n=5, P<0.05 vs. baseline) and nearly triple the baseline rate at the higher dose of 10 ng/ml (8.1±1.3%, n=5, P<0.01 vs. baseline). Because the hallmark of cellular senescence is the inability to further proliferate, we investigated whether TNF-α treatment also reduced the growth of the highly proliferative EPCs. As shown in Fig. 2C, there was a dose-dependent drop in the EPC population doubling rate with TNF-α treatment that mirrored the observed increase in cell senescence measured by SA-β-Gal staining. Interestingly, short-term continuous treatment with TNF-α for 3 days did not induce EPC senescence (Fig. 2D). This result highlights the importance of the duration of proinflammatory stimulation in the activation of EPC senescence pathways.
Figure 2.
Chronic exposure to TNF-α increases the cellular senescence of cord blood-derived EPCs independent of changes in telomere length. EPCs were treated with varying doses of TNF-α for 1 week, and SA-β-Gal staining was measured. A) Representative phase-contrast images of control EPCs and cells treated with 10 ng/ml TNF-α. Scale bars = 50 μm. B) Degree of cell senescence was quantified as percentage of SA-β-Gal-positive cells. Continuous treatment with TNF-α for 1 wk resulted in a dose-dependent increase in EPC senescence, which was nearly double the baseline senescence rate at the low dose of 1 ng/ml (n=5, P<0.05) and was nearly triple the baseline EPC senescence rate at the higher dose of 10 ng/ml (n=5, P<0.01). To investigate whether TNF-α treatment also reduced growth of highly proliferative EPCs, cell numbers were assessed. C) Cell numbers were converted to cumulative population doublings. Treatment with TNF-α resulted in a marked, dose-dependent drop in EPC proliferation (P<0.05 at 1 ng/ml and P<0.01 at 10 ng/ml). D) Short-term treatment with TNF-α for only 3 days did not increase EPC senescence.
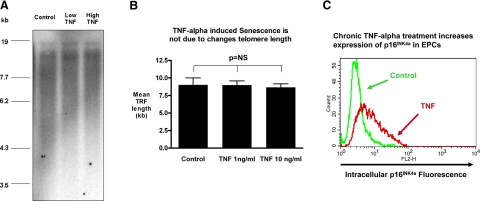
Cellular senescence can occur either via shortening of telomeres or by pathways independent of changes in telomere length (1). We therefore investigated whether the increases in EPC senescence with chronic TNF-α treatment were associated with reductions in telomere length. Analyses of TRF lengths on EPCs chronically treated with TNF-α did not show any difference in telomere lengths between control and TNF-α treatments (Figs. 3A, B), thus suggesting that the observed induction of senescence in proliferative EPCs was independent of changes in telomere lengths. We then measured the expression of the senescence-associated cell cycle inhibitor p16INK4a after continuous TNF-α exposure in EPCs by intracellular flow cytometry, because this cell cycle inhibitor is known to be activated by stress-induced, telomere-independent senescence pathways in stem cells (10, 11, 20). We observed a marked up-regulation of p16INK4a expression after the continuous TNF-α treatment, as is shown in a representative flow cytometry histogram in Fig. 3C. The increase in p16INK4a expression was primarily found in high-light forward scatter cells on flow cytometry, which is characteristic of large senescent cells (Supplemental Fig. 3). To evaluate whether this induction of senescence by TNF-α treatment was associated with a shift of the NO/ROS balance toward an unfavorable state, similar to the low NO state that we had observed in HAECs when they reached higher passages and senescence levels, we assessed NO and superoxide after chronic TNF-α treatment. Interestingly, TNF-α treatment resulted in marked increases of both cellular NO and cellular superoxide. However, the increase in NO appeared to be even more prominent than the increase in superoxide (Supplemental Fig. 4), and, thus, this cell state was very distinct from the decrease in NO that we had found in high-passage HAECs.
Figure 3.
Chronic exposure to TNF-α increases the cellular senescence of cord blood-derived EPCs independent of changes in telomere length. EPCs were chronically treated with TNF-α for 1 wk at the indicated concentrations, and cells were collected for analyses of TRF lengths. Genomic DNA was digested with restriction enzymes that do not cut telomeric sequences and analyzed via a modified Southern method. Results yield a smear that corresponds to the distribution of all telomere lengths within the population of cells collected. A) Representative gel. B) Statistical analysis of TRFs (n=3) showed no significant difference between control and TNF-α treatments. C) To assess whether TNF-α increased the expression of the senescence-associated cell cycle inhibitor p16INK4a intracellular flow cytometry was performed; representative histogram with a control-treated cell sample (green) and TNF-α-treated cell sample (red) is shown. Cell numbers are on the y axis; fluorescence intensity is used as a marker of expression on the x axis.
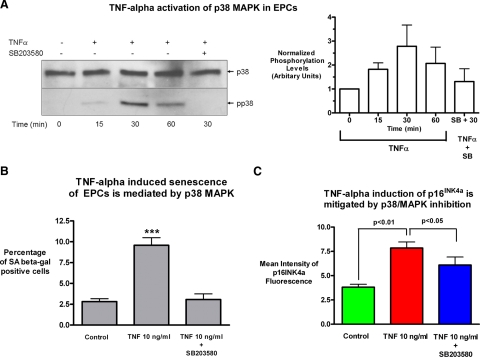
We then studied which pathway could mediate the effect of chronic TNF-α treatment on the observed nontelomere-dependent senescence in proliferative EPCs. We focused on the p38 MAPK pathway, because it is not only a key TNF-α signaling pathway but it has also recently been shown to be a major pathway regulating the self-renewal capacity of stem cells (10). We first demonstrated that the p38 MAPK pathway was activated by TNF-α stimulation, as evidenced by phosphorylation of p38, and that the p38 MAPK inhibitor SB203580 is able to block this activation at a concentration of 10 μM (Fig. 4A). Chronic treatment with TNF-α increased EPC senescence assessed by SA-β-Gal staining, with positive cells increasing from 2.8 ± 0.3 to 9.6 ± 0.9% (P<0.001), but addition of 10 μM SB203580 abrogated the effect of TNF-α and reduced the EPC senescence levels back to baseline levels of 3.1 ± 0.7% (Fig. 4B). Treatment with the p38 MAPK inhibitor also mitigated the induction of the senescence-associated, cell cycle arrest inducer p16INK4a by chronic TNF-α (Fig. 4C), although not quite down to baseline levels.
Figure 4.
Chronic TNF-α-induced senescence in EPCs is mediated by activation of the p38 MAPK pathway. A) To investigate whether the p38/MAPK pathway was involved in mediating TNF-α-induced EPC senescence, an immunoblot analysis with an anti-phospho-p38 MAPK antibody and a total p38 MAPK antibody was performed in EPCs treated with 10 ng/ml TNF-α at the indicated times after stimulation in the presence or absence of the specific p38 MAPK inhibitor SB203580 at a concentration of 10 μM. B) Quantification of fold change in activated MAPK was analyzed by densitometry and normalized to total protein levels. A representative Western blot and average densitometry with sem are shown (n=5). To assess whether p38 MAPK inhibition could prevent the induction of cellular senescence, cells were treated with TNF-α in the presence or absence of the specific inhibitor SB203580 (10 μM) for 1 wk, and senescence was quantified by SA-β Gal staining. Inhibition of the p38 MAPK pathway reduced the highly significant induction of cellular senescence by TNF-α in EPCs (P<0.001, n=4) down to the low baseline level. C) EPCs were also stained for the senescence-associated cell cycle inhibitor p16INK4a using intracellular flow cytometry. Measurement of mean fluorescence intensity showed the expected significant increase p16INK4a expression with chronic TNF-α treatment (P<0.01, control vs. treatment, n=3), while also showing a significant decrease of p16INK4a expression with p38 MAPK inhibition (TNF vs. TNF+10 μM SB203580, P<0.05, n=3).
DISCUSSION
We were able to demonstrate that in contrast to what has been reported for early or myeloid-monocytic EPCs (5, 7, 8), proliferative nonmyeloid EPCs have very low levels (<5%) of baseline senescence compared with mature endothelial cells of similar passages. We were also able to show that the senescence-associated cell cycle arrest inducer p16INK4a is expressed at higher levels in HAECs than in proliferative EPCs, which may partly explain the low level of senescence in proliferative EPCs. Although a previous study suggested that proliferative EPCs have lower activity of SA-β-Gal (21), potential cellular mediators of senescence such as the cell cycle arrest inducer p16INK4a were not investigated. We chose to investigate the senescence-associated marker p16INK4a because it has recently been shown to play a key role in regulating the aging process of hematopoietic stem cells (11) as well as mature endothelial cells (22), but little is known about its role in proliferative EPC senescence.
Our data suggest that proliferative EPCs do not express significant levels of p16INK4a at baseline compared with mature endothelial cells and may thus be of value as a marker to distinguish mature and progenitor vascular cells. Nevertheless, further work is still necessary to elucidate the exact mechanisms by which p16INK4a induces senescence in EPCs. Stem cells are also known to have low intrinsic replicative senescence levels, similar to what we observed in proliferative EPCs. However, stem cells remain vulnerable to stress-induced premature senescence, and a recent study showed that stem cell senescence can be activated via the p38 MAPK pathway, which induces p16INK4a (10). We therefore studied whether proliferative EPCs would also demonstrate a vulnerability to stress-induced senescence despite their low baseline senescence. We were able to show that prolonged inflammatory stimulation with TNF-α, but not short-term stimulation, was able to markedly increase the expression of p16INK4a and increase cellular senescence. The effect was independent of changes in telomere length, consistent with what has been previously observed in stress-induced premature senescence for other cell types (1). Interestingly, inhibition of the p38 MAPK pathway mitigated the induction of p16INK4a and senescence in proliferative nonmyeloid EPCs in our study. Because the p38 MAPK pathway also regulates the survival of early or myeloid-monocytic EPCs (23), it may be a shared survival/proliferation pathway in multiple EPC subtypes.
We found a decrease in NO levels but not superoxide levels, as assessed by flow cytometry, when both HAECs and EPCs reached high passages. Because only HAECs showed marked senescence at those passages, it did not appear that changes in NO levels were necessarily directly linked to senescence. Furthermore, the increase in cellular NO of EPCs by TNF-α also suggests that TNF-α-induced EPC senescence is not primarily due to a low NO state. Others have similarly found that TNF-α can increase NO in endothelial cells via enhanced inducible nitric oxide synthase activity (24), whereas TNF-α can also reduce the NO release by endothelial nitric oxide synthase activity (25). However, it is critical to remember that NO and ROS are able to act as signaling molecules and regulate numerous pathways (26). Therefore, future studies will be necessary to clearly define a potential modulatory role of NO/ROS and other signaling pathways in stress-induced senescence of EPCs.
The low baseline senescence of proliferative EPCs makes them candidate cells for therapies to enhance endothelial repair and regeneration. Cardiovascular cell therapies conducted in patients during the past 5 yr have demonstrated limited clinical success (27). Although there may be a multitude of reasons for this result, ranging from our lack of understanding of how regenerative cells function to interindividual differences among recipients (28), one reason may be that we have little knowledge of how transplanted cells interact with their pathophysiological milieu in the host. Understanding the senescence vulnerability to stimuli such as chronic TNF-α is necessary because transplanted EPCs may be exposed to inflammatory stimuli in the patient’s vascular milieu. In addition, identifying molecular switching pathways that control cell senescence in transplanted EPCs may also be necessary to limit the proliferation of EPCs after the targeted repair and regeneration process is completed to avoid uncontrolled growth of “malignant-like” EPCs, which are resistant to senescence.
In addition to improving EPC therapies with proliferative EPCs, we also believe that our findings point toward novel ways to understand the aging process within the vasculature. Colony-forming, highly proliferative EPCs not only are found as circulating cells in adult and umbilical cord blood but also exist within the vessel wall (12). It is therefore likely that endogenous vessel wall EPCs play an important role in ongoing repair processes as the vasculature ages and is exposed to stressors. Based on our findings, chronic inflammatory stimulation could prematurely result in EPC senescence and loss of self-renewal capacity, thus exhausting the repair potential of the blood vessel and contributing to the development of disease. One of the key genetic loci that has been recently identified as a risk factor for myocardial infarction is located on chromosome 9p21 in an area that encodes for p16INK4a (29), which was up-regulated after chronic TNF-α stimulation in our study. Future work will be necessary to understand the intriguing mechanisms underlying the senescence process in cardiovascular disease as well as whether EPCs derived from embryonic stem cells (30) show the same senescence vulnerability to inflammatory stimulation as seen in blood-derived EPCs. Ultimately, understanding the vulnerability of regenerative cardiovascular cells will be likely not only to contribute to our insight into the pathogenesis of atherosclerosis but also to help design effective regenerative therapies.
Supplementary Material
Acknowledgments
We thank Erin Gentry for her technical assistance. This work was supported in part by U.S. National Institutes of Health grant K08-HL080082 (principal investigator J.R.). B.-S.H. acknowledges support from the Indiana Genomics Initiative (INGEN) of Indiana University, supported in part by the Lilly Endowment. D.A.I. and M.C.Y own stock in EndGenitor Technologies, Inc. (Indianapolis, IN, USA). They have also acted as consultants to and served as officers or members of the board of EndGenitor Technologies, Inc., within the past 2 years.
References
- Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Peterson T E, Chatterjee S, Shah V, Vile R G, Simari R D. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- Yoder M C, Mead L E, Prater D, Krier T R, Mroueh K N, Li F, Krasich R, Temm C J, Prchal J T, Ingram D A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C H, Hur J, Park K W, Kim J H, Lee C S, Oh I Y, Kim T Y, Cho H J, Kang H J, Chae I H, Yang H K, Oh B H, Park Y B, Kim H S. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- Murasawa S, Llevadot J, Silver M, Isner J M, Losordo D W, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–1139. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell C M, March K L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- Assmus B, Urbich C, Aicher A, Hofmann W K, Haendeler J, Rossig L, Spyridopoulos I, Zeiher A M, Dimmeler S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–1055. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- Hill J M, Zalos G, Halcox J P J, Schenke W H, Waclawiw M A, Quyyumi A A, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Ingram D A, Mead L E, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz M J, Gilley D, Yoder M C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming H E, Saito Y, Waring M T, Dombkowski D M, Cheng T, DePinho R A, Sharpless N E, Scadden D T. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Ingram D A, Mead L E, Moore D B, Woodard W, Fenoglio A, Yoder M C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert B S, Hochreiter A E, Wright W E, Shay J W. Non-radioactive detection of telomerase activity using the Telomeric Repeat Amplification Protocol (TRAP) Nat Protoc. 2006;1:1583–1590. doi: 10.1038/nprot.2006.239. [DOI] [PubMed] [Google Scholar]
- Herbert B S, Shay J W, Wright W E. New York: John Wiley & Sons; Analysis of Telomeres and Telomerase. 2003 doi: 10.1002/0471143030.cb1806s20. [DOI] [PubMed] [Google Scholar]
- Ouellette M M, Liao M, Herbert B S, Johnson M, Holt S E, Liss H S, Shay J W, Wright W E. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- Rajashekhar G, Grow M, Willuweit A, Patterson C E, Clauss M. Divergent and convergent effects on gene expression and function in acute versus chronic endothelial activation. Physiol Genomics. 2007;31:104–113. doi: 10.1152/physiolgenomics.00157.2006. [DOI] [PubMed] [Google Scholar]
- Herbert B S, Wright W E, Shay J W. p16INK4a inactivation is not required to immortalize human mammary epithelial cells. Oncogene. 2002;21:7897–7900. doi: 10.1038/sj.onc.1205902. [DOI] [PubMed] [Google Scholar]
- Opitz C A, Rimmerman N, Zhang Y, Mead L E, Yoder M C, Ingram D A, Walker J M, Rehman J. Production of the endocannabinoids anandamide and 2-arachidonoylglycerol by endothelial progenitor cells. FEBS Lett. 2007;581:4927–4931. doi: 10.1016/j.febslet.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Blasco M A, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ha J M, Kim M R, Oh H K, Lee B H, Ahn H Y, Shin J C, Baek S H, Joe Y A. Outgrowing endothelial progenitor-derived cells display high sensitivity to angiogenesis modulators and delayed senescence. FEBS Lett. 2007;581:2663–2669. doi: 10.1016/j.febslet.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang X, Halicka D, Brodsky S, Avram A, Eskander J, Bloomgarden N A, Darzynkiewicz Z, Goligorsky M S. Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: permissive role of p53. Am J Physiol Heart Circ Physiol. 2006;290:H1575–H1586. doi: 10.1152/ajpheart.00364.2005. [DOI] [PubMed] [Google Scholar]
- Seeger F H, Haendeler J, Walter D H, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher A M, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gengaro P, Wang W, Wang X Q, Li C, Faubel S, Rivard C, Schrier R W. Role of NF-κB and PI 3-kinase/Akt in TNF-α-induced cytotoxicity in microvascular endothelial cells. Am J Physiol Renal Physiol. 2008;295:F932–F941. doi: 10.1152/ajprenal.00066.2008. [DOI] [PubMed] [Google Scholar]
- Yan G, You B, Chen S P, Liao J K, Sun J. Tumor necrosis factor-α downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-α1. Circ Res. 2008;103:591–597. doi: 10.1161/CIRCRESAHA.108.173963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger S W, Darley-Usmar V M, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif A, Bolli R, Tleyjeh I M, Montori V M, Perin E C, Hornung C A, Zuba-Surma E K, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- Rehman J. An inconvenient truth: recognizing individual differences in arteriogenesis. Circ Res. 2008;102:1146–1147. doi: 10.1161/CIRCRESAHA.108.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson D F, Magnusson K P, Andersen K, Levey A I, Backman V M, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper W C, Reilly M P, Granger C B, Austin H, Rader D J, Shah S H, Quyyumi A A, Gulcher J R, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Kupatt C, Horstkotte J, Vlastos G A, Pfosser A, Lebherz C, Semisch M, Thalgott M, Buttner K, Browarzyk C, Mages J, Hoffmann R, Deten A, Lamparter M, Muller F, Beck H, Buning H, Boekstegers P, Hatzopoulos A K. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.