Abstract
Glucocorticoids, end products of the hypothalamic-pituitary-adrenal axis, influence functions of virtually all organs and tissues through the glucocorticoid receptor (GR). Circulating levels of glucocorticoids fluctuate naturally in a circadian fashion and regulate the transcriptional activity of GR in target tissues. The basic helix-loop-helix protein CLOCK, a histone acetyltransferase (HAT), and its heterodimer partner BMAL1 are self-oscillating transcription factors that generate circadian rhythms in both the central nervous system and periphery. We found that CLOCK/BMAL1 repressed GR-induced transcriptional activity in a HAT-activity- dependent fashion. In serum-shock-synchronized cells, transactivational activity of GR, accessed by mRNA expression of an endogenous-responsive gene, fluctuated spontaneously in a circadian fashion in reverse phase with CLOCK/BMAL1 mRNA expression. CLOCK and GR interacted with each other physically, and CLOCK suppressed binding of GR to its DNA recognition sequences by acetylating multiple lysine residues located in its hinge region. These findings indicate that CLOCK/BMAL1 functions as a reverse-phase negative regulator of glucocorticoid action in target tissues, possibly by antagonizing biological actions of diurnally fluctuating circulating glucocorticoids. Further, these results suggest that a peripheral target tissue circadian rhythm indirectly influences the functions of every organ and tissue inside the body through modulation of the ubiquitous and diverse actions of glucocorticoids.—Nader, N., Chrousos, G. P., Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications.
Keywords: brain-muscle-arnt-like protein 1, hypothalamic-pituitary-adrenal axis, GREs, GILZ, G6Pase, histone acetyltransferase
Glucocorticoids, steroid hormones secreted from the adrenal cortices, play crucial roles in the regulation of basal and stress-related homeostasis (1, 2). These hormones influence the functions of virtually all organs and tissues and are necessary for the maintenance of many important biological activities, such as the homeostasis of the central nervous system, the cardiovascular system, and the intermediary metabolism and the immune/inflammatory reaction, influencing mRNA expression of up to 20% of the expressed genome (1,2,3,4). The glucocorticoid receptor (GR), which belongs to the steroid/sterol/thyroid/retinoid/orphan nuclear receptor superfamily and functions as a hormone-activated transcription factor, mediates most of the known glucocorticoid actions (5, 6). Human GR, a modular protein, consists of three main domains, the N-terminal (NTD), the DNA-binding (DBD), and the ligand-binding (LBD) domains, the latter two connected with the hinge region, which spans from amino acid 481 to 520 (2, 6).
On hormone binding, GR translocates from the cytoplasm into the nucleus, where, among other actions, binds DNA at specific recognition sequences, the glucocorticoid response elements (GREs), located in the regulatory regions of glucocorticoid-responsive genes (6). Promoter-bound GR then modulates the transcription of downstream coding sequences by attracting numerous proteins and protein coactivator or corepressor complexes, such as the histone acetyltransferase (HAT) coactivator complex, which includes p160-type proteins, p300/CREB-binding protein (CBP), and p300/CBP-associated factor (p/CAF), as well as the SWI/SNF and vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein (DRIP/TRAP) chromatin-remodeling complexes, eventually influencing the activity of RNA polymerase II and its ancillary factors (6, 7).
Circulating levels of glucocorticoids are tightly regulated and fluctuate naturally in a circadian fashion, reaching their zenith in the early morning and their nadir in the late evening in diurnal animals, including humans (3, 8). Regulation of glucocorticoid secretion, including negative feedback glucocorticoid control, is exerted exclusively by the central components of the hypothalamic-pituitary-adrenal (HPA) axis (8, 9). The circadian activity of the HPA axis is generated by the hypothalamic suprachiasmatic nucleus (SCN), the master oscillator and generator of the circadian rhythm of the body (10, 11). Inputs from the SCN stimulate secretion of corticotropin-releasing hormone (CRH) and arginine-vasopressin from the paraventricular nucleus (PVN) of the hypothalamus, which, in turn, activate the corticotrophs of the anterior pituitary gland to release adrenocorticotropic hormone (ACTH) and ultimately augment the synthesis and secretion of glucocorticoids by the adrenal gland cortices (3, 8, 12, 13).
Circadian rhythms of both the central nervous system and peripheral tissues and organs are generated by the coordinated activation/inactivation of self-oscillating transcription factors (14, 15). Central among them are CLOCK and its heterodimer partner brain-muscle-arnt-like protein 1 (BMAL1), which belong to the basic helix-loop-helix (bHLH)-PER-ARNT-SIM (PAS) superfamily of transcription factors (14, 15). The CLOCK/BMAL1 heterodimer stimulates the transcription of other essential clock genes, such as the Period (PER1, PER2, and PER3) and Cryptochrome (CRY1 and CRY2) genes (14). PER and CRY proteins, then, suppress their own transcription by repressing the transcriptional activity of CLOCK/BMAL1, in essence forming a self-oscillating, negatively regulated feedback loop system (14). MOP4 [also called neuronal PAS domain protein 2 (NPAS2)] shares a high amino acid homology with CLOCK, forms heterodimers with BMAL1, and also participates in controlling the regulatory loop of circadian oscillator machinery (16,17,18,19,20). Interestingly, CLOCK also shares high amino acid and structural similarity with the activator of thyroid receptor (ACTR), a member of the p160-type nuclear receptor coactivator family with inherent HAT activity, and thus, like these proteins, has such enzymatic function, without which CLOCK/BMAL1 is unable to generate a circadian rhythm (21). CLOCK and MOP4 interact with the nuclear receptor family members retinoic acid receptor (RAR)-α and retinoic X receptor (RXR)-α, which negatively regulate CLOCK/BMAL1-mediated transcriptional activity of clock gene expression (22).
We demonstrate here that CLOCK/BMAL1 regulates glucocorticoid actions in peripheral tissues by directly interacting with and enzymatically targeting the GR. CLOCK/BMAL1 acetylates the GR at a cluster of lysine residues in its hinge region and represses GR-induced transcriptional activity by attenuating the association of GR to GREs. Our results suggest that the circadian rhythm indirectly influences the functions of numerous organs and tissues through modulation of the ubiquitous and diverse actions of glucocorticoids.
MATERIALS AND METHODS
Plasmids and reagents
FLAG-mCLOCK, Myc-BMAL1, CMX-CLOCK, and CMX-MOP4 were kindly donated by Dr. P. Sassone-Corsi (University of California, Irvine, CA, USA) and Dr. Garret A. FitzGerald (University of Pennsylvania, Philadelphia, PA, USA; refs. 22, 23). pVP16-CLOCK, which expresses CLOCK fused with the VP16 activation domain (AD), was constructed by subcloning the coding sequence of the mouse CLOCK into pVP16 (Clontech, Palo Alto, CA, USA) in an inframe fashion. FLAG-CLOCK HAT Mut, which has replacements of its glycine, serine, and valine at amino acids 669, 670, and 672 with alanines, and thus, is defective in its HAT activity (21), was created by introducing the corresponding mutations into FLAG-CLOCK with a polymerase chain reaction (PCR)-assisted mutagenesis reaction. pRShGRα, pRSVerbA−1, and pR107 were all gifts from Dr. R. M. Evans (Salk Institute, La Jolla, CA, USA) (24). pRShGRα expresses the human GRα, while pRSVerbA−1 contains the thyroid hormone receptor cDNA in an inverse orientation and was used as a negative control for pRShGRα. pR107 expresses the human GRα under the control of the SP6 promoter in vitro. pM-GRα full-length (FL) (amino acids 1–777), NTD (1–427), DBD (410–490), and LBD (485–777), which express indicated portions of the human GRα fused with the GAL4 DBD, were constructed by subcloning the corresponding fragments of the human GRα cDNAs into pM (Clontech) in an inframe fashion. pGEX-4T3-GRα FL (amino acids 1–777), NTD (1–427), DBD (410–490), and LBD (485–777, which express the corresponding portions of the human GRα fused to the glutathione-S transferase (GST), were reported previously (25). pGEX-4T3-CLOCK FL (1–854), (1–370), and (371–854), which express the corresponding portions of mouse CLOCK fused with GST, were constructed by subcloning their cDNA fragments into pGEX-4T3 (GE Healthcare Bio-Sciences, Piscataway, NJ, USA). pRShGRαK480A, K492A, K494/495A, K480/494/495A, K492/494/495A, and K480/492/494/495A were created by introducing the corresponding mutations that replace the indicated lysines with alanines using a PCR-assisted mutagenesis reaction with pRShGRα as a template. pMMTV-luc, which expresses luciferase under the control of the glucocorticoid-responsive MMTV promoter, was a gift of Dr. G. L. Hager (National Cancer Institute, Bethesda, MD, USA; ref. 24). pRSV-RelA (p65) and pRSV-NF-κB I (p50), which, respectively, express p65 and p50 components of the nuclear factor-κB (NF-κB), were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program. (κB)3-Luc, which expresses luciferase under the control of three κB-responsive elements (REs), was reported previously (26). pGAL4-E1B-TK-Luc, which expresses luciferase under the control of four GAL4-REs, was a gift from Dr. J. H. Segars (NIH, Bethesda, MD, USA; ref. 27). pSG5 and pCMX, which are carrier plasmids for CLOCK, BMAL1, or MOP4, were purchased from Stratagene (La Jolla, CA, USA) and Promega (Madison, WI, USA), respectively, and were used as negative controls for these protein-expressing plasmids. pSV40-β-Gal was purchased from Promega. CLOCK, BMAL1, and control small-interfering RNAs (siRNAs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). CLOCK siRNA is the mixture of three siRNAs with different sequences, while BMAL1 siRNA is a single oligonucleotide.
Cell culture and reporter assays
The human colon cancer HCT116 cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum and 100 U/ml of penicillin and 100 μg/ml of streptomycin. The human cervical cancer HeLa and the human hepatoma HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium with the same supplements. HCT116 cells do not express endogenous GR, while HeLa and HepG2 cells have the endogenous, functional GR (28). For reporter assays, HCT116 and HeLa cells were transfected with Lipofectine (Invitrogen, Carlsbad, CA, USA) and FuGENE6 (Roche Applied Science, Indianapolis, IN, USA), respectively, with 0.1–0.5 μg/ml of indicated plasmids, together with 0.5 μg/ml of pMMTV-Luc and 0.1 μg/ml of pSV40-β-Gal in 12-well plates (28). Empty vectors were used to maintain the same amounts of transfected DNA. Dexamethasone (10−6 M) was added to the medium after 24 h of transfection, and the cells were incubated with this steroid for 24 h to allow expression of luciferase. They were then harvested and luciferase and β-galactosidase assays were performed as described previously (28).
SYBR Green-based real-time PCR
HeLa cells were transfected with the indicated plasmids by using the Nucleofector system (Amaxa, Cologne, Germany) with >80% transfection efficiency, as described previously (24, 29). Twenty-four hours after the transfection, cells were treated with 10−6 M of dexamethasone and their total RNAs were harvested after an additional 24 h. For the experiment addressing the circadian regulation of GR transcriptional activity, HeLa cells were transfected with control or CLOCK and BMAL1 siRNAs using the Nucleofector system. Eight hours after the transfection, cells were incubated with Dulbecco’s modified Eagle’s medium containing 50% horse serum (serum shock) for 4 h to synchronize their circadian rhythm (30). Cells were further incubated in the presence or absence of 10−6 M of dexamethasone for 6 h before each time point. Total RNA was purified every 6 h by up to 60 h after serum shock. We determined the time just after serum shock as time 0. After reverse transcription of mRNA into cDNA, real-time PCR was performed in triplicate using the SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) in a 7500 real-time PCR system (Applied Biosystems), as described previously (31). Primer pairs used for the reactions are shown in Table 1. Obtained threshold cycle (Ct) values of each gene were normalized for those of the acidic ribosomal phosphoprotein P0 (RPLP0), and their relative mRNA expression was demonstrated as fold induction over the baseline. The dissociation curves of primer pairs used showed a single peak, and samples after PCR reactions had a single expected DNA band in an agarose gel analysis (data not shown).
TABLE 1.
Primer pairs used in the SYBR Green-based real-time PCR
| Gene name | Primer sequence |
|---|---|
| CLOCK | |
| Forward | 5′-GAAGTTAGGGCTGAAAGAC-3′ |
| Reverse | 5′-GATCAAACCTTTCCAATGC-3′ |
| BMAL1 | |
| Forward | 5′-GACAAAGATGACCCTCATG-3′ |
| Reverse | 5′-CATGTTGGTACCAAAGAAG-3′ |
| PER1 | |
| Forward | 5′-CACTGGCCTGTGTCAAGC-3′ |
| Reverse | 5′-GTGTACTCAGACGTGATGTG-3′ |
| G6Pase | |
| Forward | 5′-TCATCTTGGTGTCCGTGATCG-3′ |
| Reverse | 5′-TTTATCAGGGGCACGGAAGTG-3′ |
| GILZ | |
| Forward | 5′-GATGTGGTTTCCGTTAAGC-3′ |
| Reverse | 5′-CTCTCTCACAGCATACATCAG-3′ |
| GR | |
| Forward | 5′-TGAAAATGGGTTGGTGCTTCTA-3′ |
| Reverse | 5′-GACAAGAATACTGGAGATTTG-3′ |
| RPLP0 | |
| Forward | 5′-GAGGACCTCACTGAGATTCG-3′ |
| Reverse | 5′-CTGGAAGAAGGAGGTCTTCTC-3′ |
Mammalian two-hybrid assays
HCT116 cells were transfected with 0.25 μg/ml of the indicated GAL4 DBD- and VP16 AD-fused protein-expressing plasmids together with 0.5 μg/ml of pGAL4-E1B-TK-Luc and 0.1 μg/ml of pSV40-β-Gal, using Lipofectin in 12-well plates. Empty vectors were used to maintain the same amounts of transfected DNA. Twenty-four hours after transfection, 10−6 M of dexamethasone was added to the medium. The cells were harvested after an additional 24 h, and luciferase and β-galactosidase assays were performed as described previously (24).
GST pull-down assays
35S-labeled human GRα and mouse CLOCK were generated by in vitro translation using pR107 and FLAG-mCLOCK as templates, respectively, and were tested for interaction with GST-fused CLOCK and GR proteins, immobilized on glutathione-Sepharose beads in the presence or absence of 10−6 M of dexamethasone in buffer containing 50 mM Tris-HCl pH 8.0, 50 mM NaCl, 1 mM EDTA, 0.1% Nonidet P-40, 10% glycerol, and 0.1 mg/ml BSA at 4°C for 90 min, as described previously (25). We found that 90 min of incubation was sufficient to examine the physical interaction between the translated proteins to GST-fused molecules (data not shown). After being vigorously washed with the buffer, proteins were eluted and separated on a 4–12% SDS-PAGE gel. Gels were fixed, treated with Enlighting (NEN Life Science Products, Boston, MA, USA), dried, and exposed to film.
Western blots to examine the acetylation of the GR
HCT116 cells were transfected with plasmids expressing GR lysine mutants and CLOCK-/BMAL1-expressing plasmids, and they were incubated in the presence or absence of 10−6 M of dexamethasone for 6 h. Cells were lysed in buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1 Tab/50 ml Complete tablet, and expressed GR was harvested by immunoprecipitation with anti-GR antibody (Santa Cruz Biotechnology), as described previously (28). Precipitated GR was separated in 4–20% SDS-PAGE gels, blotted on nitrocellulose membranes, and precipitated GR or GR acetylated with CLOCK/BMAL1 was detected using anti-GR antibody (Santa Cruz Biotechnology) or anti-acetylated lysine antibody (Millipore, Billerica, MA, USA), respectively.
To examine expression levels of GR mutants and GR fragments fused with GAL4 DBD, samples were lysed, run on 4–20% SDS-PAGE gels, and blotted to nitrocellulose membranes and GR-related proteins were visualized with anti-GR or anti-GAL4 DBD antibody (Santa Cruz Biotechnology), as previously reported (24). Expressed FLAG-CLOCK and Myc-BMAL1 were also visualized with anti-FLAG and anti-Myc antibodies (Santa Cruz Biotechnology), respectively.
In vitro binding assays for evaluation of the GR/GRE association
HeLa cells were transfected with CLOCK- or MOP4-expressing plasmid with BMAL1-expressing plasmid using Lipofectamine 2000 and were treated with 10−6 M of dexamethasone. After 2 h of incubation to examine binding of GR and GREs just after translocation of the GR into the nucleus, nuclear extracts were prepared using the nuclear extract kit (Active Motif, Carlsbad, CA, USA). In vitro binding assays for evaluating the association of GR to GREs were conducted by using the TRansAM GR Kit (Active Motif). Briefly, nuclear extracts were added to a 96-well ELISA plate, which was provided by the kit and had immobilized GRE oligonucleotides at the bottom of each well. Free oligonucleotide, which encoded wild-type or mutant GREs, was added to some reactions to monitor the specificity of the assay. The plate was then incubated first with anti-GR antibody and second with horseradish peroxidase-conjugated antibody, and binding activity of GR to GREs was estimated by measuring absorbance at 450 nm with a reference wavelength of 655 nm in the Victor 3 (Applied Biosystems).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed in HepG2 cells, as described previously (25, 31). Briefly, cells were transfected with plasmids expressing wild-type CLOCK or the CLOCK mutant defective in HAT activity, together with BMAL1-expressing plasmid using the Nucleofector system. The cells were exposed to either 10−6 M of dexamethasone or vehicle for 6 h and were subsequently fixed, DNA and bound proteins were cross-linked, and ChIP assays were performed by coprecipitating the DNA/protein complexes with anti-GR antibody or rabbit control IgG (Santa Cruz Biotechnology). We used 6 h of incubation with dexamethasone, as we found that this time point is sufficient to detect accumulation of proteins on GREs in our assay system (data not shown). The promoter region (−257 to −39) of the endogenous glucose-6-phosphatase (G6Pase) gene, which contains three functional GREs (32), and that (−1341 to −1209) of the endogenous glucocorticoid-induced leucine zipper protein (GILZ), which contains two tandem GREs (33), were amplified from the prepared DNA samples using the specific primer pairs (G6Pase: forward: 5′-CAGACCCTTGCACTGCCAAGAAGCATG-3′ and reverse: 5′-TATCCAGTATTCAGGTCAACCCAGCCC-3′; and GILZ: forward: 5′-CCTTAACTTCATCCAAACTG-3′ and reverse: 5′-CACCAGAAGGAGCAAGAG-3′) in the SYBR Green real-time PCR using the SYBR Green PCR master mix and a 7500 real-time PCR system. Obtained Ct values of ChIP samples were normalized for those of corresponding inputs, and their relative precipitation was expressed as fold precipitation above the baseline.
Statistical analyses
All experiments were performed in triplicate or quadruplicate and were repeated at least 3 times. Statistical analyses were carried out by unpaired Student’s t test with a two-tailed P value.
RESULTS
Overexpression of CLOCK/BMAL1 represses GR-induced transcriptional activity in HCT116 and HeLa cells
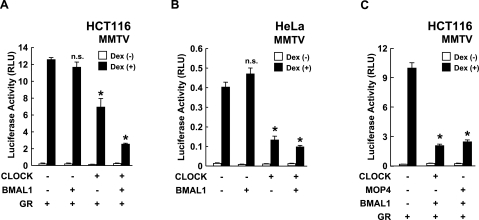
To the effect of CLOCK/BMAL1 on GR-mediated transcriptional activity, we first expressed CLOCK and/or BMAL1 in the human cell lines HCT116 and HeLa, along with the glucocorticoid-responsive pMMTV-Luc as a reporter (Fig. 1A, B). Overexpression of CLOCK and BMAL1 synergistically repressed GR-induced transcriptional activity in a dexamethasone-dependent fashion in HCT116 cells, while CLOCK, but not BMAL1, exerted a strong repressive effect on GR-mediated transactivation in HeLa cells. BMAL1 alone had no suppressive effect in either HCT116 or HeLa cells. These results indicate that the CLOCK/BMAL1 heterodimer has a negative effect on GR-induced transcriptional activity. Since these cells express endogenous CLOCK and BMAL1 (data not shown), it is likely that expressed CLOCK exerted its repressive effect by forming a heterodimer with endogenous BMAL1. Expressed BMAL1 failed to suppress GR-induced transcriptional activity, possibly due to sufficient amounts of endogenous BMAL1. Since MOP4 is a CLOCK homologue and interacts with some nuclear receptors (22), we examined the effect of this transcription factor on GR-induced transcriptional activity in HCT116 cells (Fig. 1C). As expected, MOP4/BMAL1 repressed dexamethasone-stimulated transcriptional activity of the MMTV promoter similarly to CLOCK/BMAL1.
Figure 1.
CLOCK/BMAL1 represses GR-induced transcriptional activity. A, B) CLOCK/BMAL1 suppresses GR-induced transcriptional activity in HCT116 and HeLa cells. HCT116 (A) and HeLa cells (B) were transfected with the indicated protein-expressing plasmids together with pMMTV-Luc and pSV40-β-Gal. C) MOP4/BMAL1 suppresses GR-induced transcriptional activity in HCT116 cells. HCT116 cells were transfected with the indicated protein-expressing plasmids together with pMMTV-Luc and pSV40-β-Gal. Bars represent mean ± se values (n=3) of luciferase activity normalized for β-galactosidase activity in the presence or absence of 10−6 M of dexamethasone (Dex). *P < 0.01 vs. control with dexamethasone. n.s., not significant.
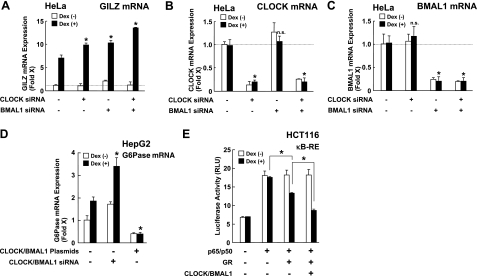
CLOCK and BMAL1 knockdown enhances mRNA expression of the endogenous glucocorticoid-responsive GILZ and G6Pase genes and influences the repressive effect of GR on NF-κB-induced transcriptional activity
We next knocked down endogenous CLOCK and BMAL1 by using their siRNAs. We examined mRNA expression of GILZ, a well-known glucocorticoid-responsive gene with multiple GREs in its promoter region, to monitor GR-induced transcriptional activity (34, 35; Fig. 2). Cotransfection of CLOCK and BMAL1 siRNAs synergistically enhanced dexamethasone-stimulated GILZ mRNA expression, while CLOCK or BMAL1 siRNA added alone moderately enhanced dexamethasone-mediated GILZ mRNA expression (Fig. 2A). These results indicate that both CLOCK and BMAL1 are necessary for repressing GR-induced transcriptional activity. CLOCK and BMAL1 siRNAs significantly suppressed mRNA expression of CLOCK and BMAL1, respectively (Fig. 2B, C). We also examined the influence of CLOCK/BMAL1 on another glucocorticoid-responsive gene, G6Pase (Fig. 2D). Knockdown of CLOCK/BMAL1 enhanced GR-induced mRNA expression of G6Pase mRNA, while overexpression of these proteins completely attenuated dexamethasone-induced stimulation of G6Pase mRNA expression. Interestingly, these manipulations of CLOCK/BMAL1 expression positively and negatively altered G6Pase mRNA expression in the absence of dexamethasone, indicating that CLOCK/BMAL1 directly influences G6Pase mRNA expression in addition to its effect through modulation of the GR activity.
Figure 2.
CLOCK/BMAL1 regulates both the transactivation and transrepression activities of the GR. A–C) CLOCK/BMAL1 knockdown enhances GR-induced transcriptional activity. HeLa cells were transfected with siRNAs for CLOCK and/or BMAL1 and were treated with 10−6 M of dexamethasone. Total RNA was harvested, and mRNA levels of glucocorticoid-responsive GILZ (A), CLOCK (B), and BMAL1 (C) were measured with SYBR-Green real-time PCR. Bars represent mean ± se values (n=3) of fold induction over baseline (control siRNA in the absence of dexamethasone) in the presence or absence of 10−6 M of dexamethasone. *P < 0.01 vs. control with dexamethasone. n.s., not significant. D) CLOCK/BMAL1 overexpression and knockdown respectively suppresses and enhances GR-induced transcriptional activity of the glucocorticoid-responsive G6Pase gene. HepG2 cells were transfected with CLOCK- and BMAL1-expressing plasmids or CLOCK and BMAL1 siRNAs and were treated with 10−6 M of dexamethasone. Total RNA was harvested, and mRNA levels of G6Pase were measured with SYBR-Green real-time PCR. Bars represent mean ± se values (n=4) of fold induction over baseline (with mock transfection in the absence of dexamethasone) in the presence or absence of 10−6 M of dexamethasone. *P < 0.01 vs. mock transfection with dexamethasone. E) CLOCK/BMAL1 enhances the repressive effect of GR on NF-κB-induced transcriptional activity. HCT116 cells were transfected with CLOCK- and BMAL1-expressing plasmids together with (κB)3-Luc and pSV40-β-Gal in the presence or absence of pRShGRα and/or pSRV-RelA (p65) and pRSV-NF-κB I (p50) transfection. Bars represent mean ± se values (n=3) of the luciferase activity normalized for β-galactosidase activity in the presence or absence of 10−6 M of dexamethasone. *P < 0.01.
We further examined the effect of CLOCK/BMAL1 on the transrepressive action of the GR (Fig. 2E). To do this, we transfected the plasmids expressing the NF-κB components p65 and p50 and examined the luciferase activity expressed from the κB-RE-driven reporter gene in HCT116 cells. Overexpression of p65/p50 stimulated this promoter activity, while dexamethasone suppressed it in a GR-transfection-dependent fashion. Overexpression of CLOCK/BMAL1 further suppressed p65/p50-induced transactivation of this promoter, indicating that CLOCK/BMAL1 enhances GR-induced transrepression of NF-κB-induced transcriptional activity.
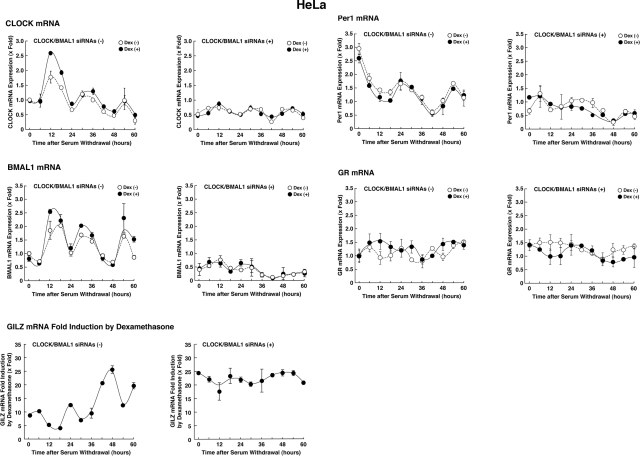
Transactivation activity of the GR fluctuates in a reverse-phase circadian fashion, mirroring the mRNA expression of CLOCK/BMAL1
We further examined the effect of CLOCK/BMAL1 on GR-induced transcriptional activity by comparing timed mRNA expression profiles of CLOCK/BMAL1, their responsive gene PER1, and GR-induced transcriptional activity time sequentially for up to 60 h after synchronizing cellular circadian rhythm by serum shock (Fig. 3). We evaluated GR-induced transcriptional activity by culturing the cells with 10−6 M of dexamethasone for 6 h before each time point and by measuring mRNA expression of the GILZ gene. mRNA expression of CLOCK and BMAL1 oscillated by ∼24 h cycles (Fig. 3, left panels). Dexamethasone-stimulated GR transcriptional activity estimated from fold induction of GILZ mRNA expression also fluctuated in a circadian fashion, mirroring the mRNA expression of CLOCK and BMAL1. PER1 mRNA expression was also reciprocal to that of CLOCK and BMAL1, consistent with a previous study (22). mRNA expression of GR fluctuated minimally, changing the expression levels to <50% variance throughout the experiment. The circadian rhythms of CLOCK, BMAL1, and PER1 mRNA expression and GR-transcriptional activity were completely abolished or greatly diminished by introducing CLOCK and BMAL1 siRNAs (Fig. 3, right panels). These results indicate that GR-induced transcriptional activity oscillates diurnally, possibly by a negative effect of CLOCK/BMAL1 on the GR.
Figure 3.
Transactivation activity of the GR fluctuates in a circadian fashion, mirroring the mRNA expression of CLOCK/BMAL1. HeLa cells were transfected with CLOCK/BMAL1 siRNAs (right panels) or control siRNA (left panels) and exposed to serum shock for 4 h. Cells were incubated with 10−6 M of dexamethasone or vehicle for 6 h before each harvest. Total RNA was purified from cells every 6 h for up to 60 h. Circles indicate mean ± se values (n=3) of fold induction of indicated mRNAs over baseline (time 0 h) in the presence (solid circles) or absence (open circles) of 10−6 M of dexamethasone. Fold induction of GILZ mRNA expression was calculated by dividing values of mRNA expression obtained in the presence of dexamethasone by those obtained in the absence of the steroid.
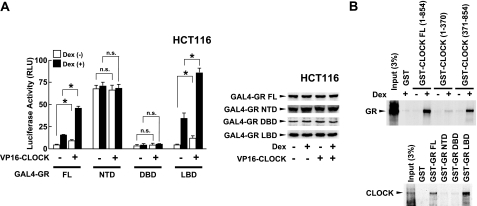
GR and CLOCK interact with each other in mammalian two-hybrid and GST pull-down assays
We next examined the potential interaction between CLOCK/BMAL1 and GR using a mammalian two-hybrid assay by using GAL4 DBD-fused GR subdomains and VP16 AD-fused full-length CLOCK in HCT116 cells (Fig. 4). GR full-length, NTD, DBD, and LBD, fused with GAL4 DBD, variously stimulated the basal transcriptional activity of the GAL4 RE-driven luciferase reporter gene. Expression of CLOCK fused with VP16 AD increased luciferase activity of full-length GR and GR LBD, and dexamethasone treatment strongly potentiated this effect (Fig. 4A, left panel). On the other hand, the CLOCK-VP16 fusion did not influence the transcriptional activity of GR NTD and DBD. Dexamethasone treatment and CLOCK/BMAL1 expression did not influence expression levels of GAL4 DBD-fused GR fragments (Fig. 4A, right panel). These results suggest that GR and CLOCK interact with each other through the GR LBD weakly and that the addition of dexamethasone strongly potentiates their interaction in this assay system. We further performed GST pull-down assays by using in vitro translated and radiolabeled GR and CLOCK, and GST-fused CLOCK and GR fragments (Fig. 4B). GR physically interacted with GST-fused full-length CLOCK and CLOCK (371–854) in a dexamethasone-dependent fashion but not with GST-CLOCK (1–370) (Fig. 4B, top panel), consistent with the previous study, which indicates that CLOCK binds RXRα with its domain enclosed by amino acids 370–509 in a ligand-dependent fashion (22). It is thus highly possible that CLOCK also uses this domain to interact with GR. Although GST-fused GR does not respond to glucocorticoids (25), we examined the interaction of GR and CLOCK by using in vitro translated and labeled CLOCK and GST-fused GR fragments. Consistent with our results in the mammalian two-hybrid assay, CLOCK interacted with the GST-fused full-length GR and GR LBD but not with GR NTD and DBD (Fig. 4B, bottom panel). Taken together, these results indicate that GR interacts with CLOCK at its C-terminal portion via LBD in a ligand-dependent fashion.
Figure 4.
GR interacts with CLOCK in a ligand-dependent fashion. A) GR interacts with CLOCK through its LBD in mammalian two-hybrid assays. Left panel: HeLa cells were transfected with plasmids expressing the GR fragments indicated fused with the GAL4 DBD and pVP16-CLOCK, which expresses CLOCK fused with VP16 AD, together with pGAL4-E1B-TK-Luc and pSV40-β-Gal. Bars represent mean ± se values (n=3) of luciferase activity normalized for β-galactosidase activity in the presence or absence of 10−6 M of dexamethasone. *P < 0.01. n.s., not significant. Right panel: Aliquots of the samples used in the experiment shown in the left panel were run of 4–20% SDS-PAGE gels and expression levels of GAL4 DBD-fused GRs were examined in Western blots using anti-GAL4-DBD antibody. B) GR interacts with CLOCK through its LBD in GST pull-down assays. In vitro translated and radiolabeled GR (top panel) and CLOCK (bottom panel) were incubated with GST or indicated GST-fused CLOCKs and GRs, respectively. For GR, 10−6 M of dexamethasone were added to the reaction; 3% of the labeled GR or CLOCK added to the binding reactions was loaded.
HAT activity is required for CLOCK to repress GR-induced transcriptional activity, and CLOCK acetylates multiple lysine residues of the GR
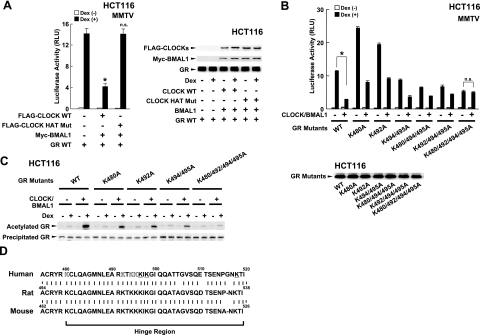
Since CLOCK has HAT activity (21), we examined the importance of this activity on CLOCK/BMAL1-mediated repression of GR-induced transcriptional activity (Fig. 5). Wild-type CLOCK repressed dexamethasone-stimulated GR transcriptional activity on the MMTV promoter in HCT116 cells, while the CLOCK mutant defective in HAT activity completely lost such an effect (Fig. 5A, left panel). Wild-type CLOCK and CLOCK HAT mutant, BMAL1, and GR were similarly expressed throughout the experiment (Fig. 5A, right panel). Therefore, we created plasmids expressing GRs whose lysine residues (lysines 480, 492, 494, 495, 496, 498, and 518) were replaced with alanines. Lysine 494 and 495 were previously shown to be acetylated (36), while residues closely located to lysines 494 and 495 were probably acetylated as well. It is known that the acetylation reaction usually occurs at multiple clustered lysine residues (37,38,39). CLOCK/BMAL1 strongly repressed the transcriptional activity of the wild-type GR, while its effect was gradually blunted by increasing the numbers of lysines replaced by alanines (Fig. 5B, top panel). We found that CLOCK/BMAL1 completely lost its repressive effect on the mutant GR, whose lysines located at amino acids 480, 492, 494, and 495 were all replaced with alanines. These GR lysine mutants were similarly expressed by their plasmids (Fig. 5B, bottom panel). We also found that GR lysines 496 and 518 did not contribute to the repression of GR transcriptional activity by CLOCK/BMAL1 (data not shown).
Figure 5.
CLOCK suppresses GR-induced transcriptional activity by acetylating multiple lysines located in its hinge region. A) CLOCK/BMAL1 suppresses GR-induced transcriptional activity in an inherent HAT domain-dependent fashion in HCT116 cells. Left panel: HCT116 cells were transfected with the indicated protein-expressing plasmids together with pMMTV-Luc and pSV40-β-Gal. *P < 0.01 vs. control with dexamethasone. n.s., not significant. Right panel: Aliquots of the samples used in the experiment shown in the left panel were run of 4–20% SDS-PAGE gels and expression levels of FLAG-CLOCK, Myc-BMAL1, and GR were examined in Western blots using anti-FLAG, anti-Myc, and anti-GR antibodies, respectively. B) Replacement of GR lysines with alanines attenuates CLOCK/BMAL1-mediated suppression of GR-induced transcriptional activity in HCT116 cells. Top panel: HCT116 cells were transfected with the plasmids expressing the GR lysine mutants indicated together with pMMTV-Luc and pSV40-β-Gal in the presence or absence of CLOCK- and BMAL1-expressing plasmids. *P < 0.01. Bottom panel: HCT116 cells were transfected with the plasmids expressing the GR lysine mutants indicated, and samples were run on a 4–20% SDS-PAGE gel. Expressed GR proteins were examined in Western blots using anti-GR antibody. C) CLOCK/BMAL1 acetylates multiple GR lysine residues in a dexamethasone-dependent fashion in HCT116 cells. HCT116 cells were transfected with the GR lysine mutant-expressing plasmids indicated in the presence of absence of CLOCK- and BMAL1-expressing plasmids treated with 10−6 M of dexamethasone, and cell lysates were harvested. GR was collected by immunoprecipitation with anti-GR antibody, and acetylated GR was detected with anti-acetylated lysine antibody in Western blots (top gel). Immunoprecipitated GR was visualized with anti-GR antibody (bottom gel). D) Lysine residues acetylated by CLOCK/BMAL1 are all preserved among human, rat, and mouse GR. Amino acid sequences of the human, rat, and mouse GR are aligned and compared. Outlined lysines are acetylated by CLOCK; underlined ones were tested for the possibility of acetylation/repression of transcription. Bars represent mean ± se values (n=3) of the luciferase activity normalized for β-galactosidase activity in the presence or absence of 10−6 M of dexamethasone (A, B).
We then examined acetylation of the GR by CLOCK/BMAL1 (Fig. 5C). Addition of dexamethasone weakly acetylated wild-type GR, while expression of CLOCK/BMAL1 strongly increased the acetylation. Replacement of lysine(s) 480, 492, or 494/495 with alanines reduced CLOCK/BMAL1-mediated acetylation of the GR, whereas mutations of all these lysines completely abolished the acetylation. Comparison of amino acid sequences of human, rat, and mouse GR revealed that all lysines acetylated by CLOCK/BMAL1 are preserved among these species (Fig. 5D). Taken together, these results indicate that CLOCK/BMAL1 acetylates lysines 480, 492, 494, and 495 of the GR and that acetylation of these residues is required for CLOCK/BMAL1 to repress GR-induced transcriptional activity. Since three out of four lysines acetylated by CLOCK/BMAL1 are included in the GR LBD fragments used to demonstrate the interaction with CLOCK, they may play a role in the physical interaction between GR LBD and CLOCK. Acetylation of these residues, however, does not appear to be necessary for the interaction, as GR physically interacted with CLOCK in the GST pull-down assay in the absence of acetylation.
CLOCK/BMAL1 attenuates binding of the GR to GREs in an inherent HAT-activity-dependent fashion
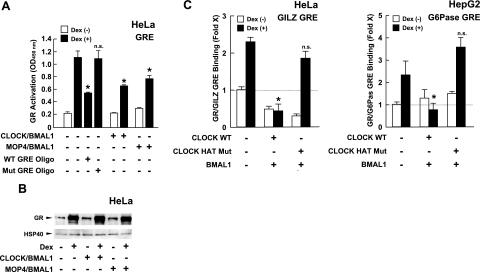
We examined the effect of CLOCK/BMAL1 on the binding of GR to sequences GREs in vitro and in vivo (Fig. 6). GR in the HeLa cell nuclear extracts bound GRE oligonucleotide immobilized on ELISA plates in a dexamethasone-dependent fashion, while wild, but not mutated, free GRE oligonucleotides competed with this binding (Fig. 6A). Transfection of CLOCK/BMAL1 or MOP4/BMAL1 attenuated the association of the purified GR to the immobilized GREs in a dexamethasone-dependent fashion. In the same nuclear extract samples, CLOCK/BMAL1 and MOP4/BMAL1 did not influence dexamethasone-induced accumulation of GR in the nucleus, indicating that they do not influence cytoplasmic to nuclear translocation of the GR (Fig. 6B). We further examined the influence of CLOCK/BMAL1 on the association of the GR to endogenous GREs in ChIP assays (Fig. 6C). Expression of wild-type CLOCK, along with BMAL1, strongly suppressed the association of the GR to GILZ and G6Pase GREs in a dexamethasone-dependent fashion, while expression of the mutant CLOCK defective in inherent HAT activity did not show an effect. Precipitation with the negative control IgG did not show any difference in precipitation of these GREs (data not shown). Taken together, these results indicate that CLOCK/BMAL1 represses GR-induced transcriptional activity by reducing association of the receptor to GREs through acetylation of multiple lysines of the GR.
Figure 6.
CLOCK/BMAL1 attenuates the association of the GR to GREs in an inherent HAT-domain-dependent fashion. A) CLOCK/BMAL1 and MOP4/BMAL1 suppress the association of the GR to GRE oligonucleotide in vitro. HeLa cells were transfected with CLOCK- or MOP4-expressing plasmids with BMAL1-expressing plasmid. Nuclear extracts were harvested from cells, and binding activity of GR to GREs was evaluated in the presence or absence of the wild-type or mutant GRE oligonucleotides (oligos) using the TRansAM GR kit (Active Motif). Bars represent mean ± se values (n=4) of absorbance at 450 nm in the presence or absence of 10−6 M of dexamethasone. *P < 0.01 vs. control with dexamethasone and without GRE oligos. n.s., not significant. B) CLOCK/BMAL1 and MOP4/BMAL1 do not influence dexamethasone-induced accumulation of GR in the nucleus. Aliquots of samples used in A, left panel, were run on 4–12% SDS-PAGE gels, and GR was visualized with anti-GR antibody. Control heat shock protein 40 (HSP40) was also blotted and visualized as a control for sample load. C) CLOCK/BMAL1 suppresses association of GR to GREs of GILZ and G6Pase genes in an inherent HAT-activity-dependent fashion in ChIP assays. HepG2 cells were transfected with plasmids expressing molecules indicated, and ChIP assays were performed using anti-GR antibody. Bars represent mean ± se values (n=3) of fold precipitation of GILZ GREs (left panel) or G6Pase GREs (right panel) compared with baseline (condition precipitated with control antibody in the absence of dexamethasone) determined by SYBR Green-based real-time PCR. *P < 0.01 vs. control with dexamethasone.
DISCUSSION
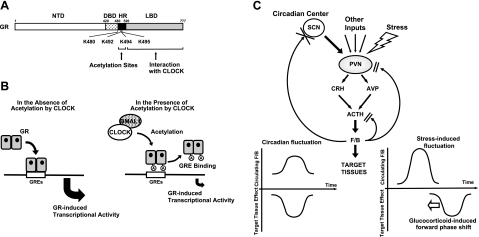
We demonstrated that the circadian rhythm-generating transcription factors CLOCK and BMAL1 repress GR-induced transcriptional activity (Fig. 7A, B). CLOCK/BMAL1 acetylated several lysine residues located as a cluster in the hinge region of the receptor, and this post-translational modification attenuated the binding of GR to GREs and its ability to influence glucocorticoid-responsive gene expression. In HeLa cells, whose circadian rhythm was synchronized with serum shock, mRNA expression of the examined CLOCK and BMAL1 mirrored the transcriptional activity of the GR in a reverse-phase fashion (Fig. 7C). These findings indicate that CLOCK/BMAL1 may function as a circadian negative regulator of glucocorticoid action in target tissues. SCN, the master circadian pacemaker of the organism in the CNS, has been shown to be spared from the GR-modifying CLOCK/BMAL1 effects, as this brain nucleus does not express functional GR and is not, therefore, a target of glucocorticoids (40). This suggests that it is peripherally, and in brain areas other than the SCN, that CLOCK/BMAL regulates the transactivational activity of the GR.
Figure 7.
Models of CLOCK actions on GR-induced transcriptional activity and regulatory loop between the circadian system and HPA axis in basal and stress conditions. A, B) Molecular actions of CLOCK/BMAL1 on GR modifying its transcriptional activity. CLOCK interacts with GR LBD and acetylates its hinge region lysine residue cluster (A), a post-translational modification that renders GR unable to fully interact with GREs and modify the transcription rate of glucocorticoid responsive genes (B). A, acetylation; HR, hinge region; K, lysine residue. C) Heuristic model of physiological implications of this study. PVN center of the HPA axis receives circadian inputs from the SCN and stress-related and other regulatory inputs from multiple brain sites. CRH and arginine-vasopressin (AVP) are secreted into the hypophysial portal system and transported to the anterior pituitary gland, where they synergistically stimulate ACTH secretion. ACTH released into systemic circulation stimulates the zona fasciculata of the adrenal cortices to secrete cortisol or corticosterone in primates and rodents, respectively. The master circadian pacemaker of the SCN thus produces a circadian rhythm of glucocorticoid concentrations in blood (left). At the same time, the SCN pacemaker synchronizes all peripheral tissue “slave” oscillators with resultant GR acetylation and generation of peripheral tissue resistance to glucocorticoids. Thus, peak glucocorticoid concentrations coincide with peak target tissue glucocorticoid resistance. In stress-related cortisol secretion, this protective feedback loop is decoupled (right). In this instance, glucocorticoids reset the peripheral CLOCK/BMAL1 system, phase-shifting the glucocorticoid resistance toward the circulating glucocorticoid peak. F/B, cortisol-corticosterone.
In vivo, the peripheral “slave” CLOCK/BMAL1 oscillators are normally synchronized with the central SCN master pacemaker through as yet largely unknown neural and neuroendocrine mechanisms (14, 16). This means that at baseline synchronized conditions, the peripheral oscillators form a local counterregulatory negative feedback loop on glucocorticoid-mediated effects of the HPA axis by creating an inverse-phase target tissue resistance to cortisol or corticosterone in primates and rodents, respectively (Fig. 7C). Since glucocorticoids have the ability to influence the phase of peripheral, rather than SCN, circadian rhythms, and since they mediate food restriction-induced shifts of circadian rhythms in peripheral tissues (40, 41), it is likely that peripheral CLOCK/BMAL1-mediated repression of GR-induced transcriptional activity modulates the effect of decoupling of the SCN from peripheral circadian rhythms by forward phase-shifting this activity. Such a decoupling occurs when glucocorticoids are acutely elevated in response to a stressor or when exogenous glucocorticoids are administered. Furthermore, these findings indicate the presence of a counterregulatory feedback loop on the peripheral circadian regulation of the cell cycle, granted that the glucocorticoid signaling pathway is actively involved in this regulation (42).
Glococorticoids influence the functions of virtually all organs and tissues and are necessary for the maintenance of many important biological activities (2, 3, 43, 44). By changing the responsiveness of peripheral tissues and non-SCN brain areas to endogenous and exogenous glucocorticoids, peripheral circadian rhythms potentially influence the biological functions of almost the entire organism. Whether disturbance of the circadian oscillators produces pathology through their effects on the glucocorticoid signaling pathway is still unknown. Mice heterozygous for a loss-of-function mutation in the CLOCK gene develop a Cushing syndrome-like clinical picture, including increased food intake, obesity, hyperglycemia, and hyperlipidemia, as well as adipocyte hypertrophy, suggesting such an involvement (1, 45, 46).
A circadian rhythm of the peripheral actions of glucocorticoids generated by peripheral oscillators may partly explain the measurable fluctuation in the expression of a large portion of all mammalian mRNA transcripts in various tissues, including ex vivo conditions (4, 47).
CLOCK/BMAL1 acetylated lysines 480, 492, 494, and 495, located in the hinge region of the GR and attenuated the association of the GR to GREs in vitro and in vivo. Acetylation plays a critical role in the regulation of transcription by targeting several different components of the transcription machinery beyond the GR. First, CLOCK itself and histone acetyltransferase coactivators acetylate specific lysine residues located in the N-terminal tail of chromatin-associated histones, promoting transcription by facilitating access of transcription factors, cofactors, and components of the transcription initiation complex to DNA (7, 21). Second, these histone acetyltransferases also acetylate transcriptional cofactors, ultimately changing their function. For example, p300/CBP acetylate ACTR and cause dissociation of this p160-type coactivator from nuclear receptors or other transcription factors (48). Third, acetylation of transcription factors, such as MyoD, p53, and GATA-1, by histone acetyltransferases also modulates the binding of these transcription factors to their specific binding elements in their responsive promoters (37, 39, 49, 50). CLOCK acetylates its heterodimer partner BMAL1, facilitates access of CRY1 to CLOCK/BMAL1, and represses their transcriptional effect on circadian genes (51).
Mutations in the hinge region of the thyroid hormone receptor change the affinity of its DBD to DNA sequences, while the length of the hinge region of nuclear receptors determines the numbers of nucleotides that space tandem responsive elements (52). In analogy, acetylation of lysine residues of the GR might alter functions of the GR hinge region, changing the binding affinity or even the specificity of the interaction of this receptor to GREs. We demonstrated that CLOCK/BMAL1 enhanced GR-mediated repression of NF-κB-induced transcriptional activity, while earlier studies (36) demonstrated that histone deacetylase 2 (HDAC2)-mediated deacetylation of the GR regulated negatively glucocorticoid-induced suppression of interleukin-1β-induced production of granulocyte/macrophage colony-stimulating factor. Since NF-κB is one of the most important mediators of inflammatory signals (53), circadian alterations in the acetylation of the GR could indirectly modulate the effect of glucocorticoids on inflammation through changing the inhibitory effect of ligand-activated GR on the activity of NF-κB. Indeed, normal immune functions have diurnal rhythmicity, while many inflammatory diseases have a diurnal pattern of clinical manifestations reflecting circulating levels of inflammatory cytokines secreted in a circadian fashion (54, 55). At pharmacological levels, the transactivation activity of glucocorticoids is well correlated with the side effects of these steroids, while the transrepressive activity is associated mostly with their beneficial anti-inflammatory activity (56). Since CLOCK may differentially regulate these two major class actions of glucocorticoids, administration of the steroids at a specific period of the circadian cycle might increase their pharmacological efficacy, while reducing their unwanted side effects.
Acknowledgments
This study was funded by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. We thank Drs. R. M. Evans (Salk Institute, La Jolla, CA, USA), G. L. Hager (National Cancer Institute, Bethesda, MD, USA), G. A. FitzGerald (University of Pennsylvania, Philadelphia, PA, USA), P. Sassone-Corsi (University of California, Irvine, CA, USA), and J. H. Segars (NIH, Bethesda, MD, USA) for providing plasmids and K. Zachman and Dr. T. Shibata for superb technical assistance.
References
- Chrousos G P, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos G P. Glucocorticoid effect on gene expression. Steckler T, Kalin N H, Reul J M H M, editors. Amsterdam, The Netherlands: Elsevier BV; Handbook on Stress and the Brain Part 1. 2004:295–312. [Google Scholar]
- Chrousos G P. Glucocorticoid therapy. Felig P, Frohman L A, editors. New York: McGraw-Hill; Endocrinology & Metabolism. 2001:609–632. [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea J J, Chrousos G P, Bornstein S R. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Kino T, De Martino M U, Charmandari E, Mirani M, Chrousos G P. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85:457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- Chrousos G P, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- Lonard D M, Lanz R B, O'Malley B W. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- Chrousos G P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Kino T, Chrousos G P. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004;40:137–155. doi: 10.1042/bse0400137. [DOI] [PubMed] [Google Scholar]
- Ralph M R, Foster R G, Davis F C, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Buijs R M, van Eden C G, Goncharuk V D, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Schibler U, Brown S A. Enlightening the adrenal gland. Cell Metab. 2005;2:278–281. doi: 10.1016/j.cmet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Green C B, Menaker M. Circadian rhythms. Clocks on the brain. Science. 2003;301:319–320. doi: 10.1126/science.1087824. [DOI] [PubMed] [Google Scholar]
- Dudley C A, Erbel-Sieler C, Estill S J, Reick M, Franken P, Pitts S, McKnight S L. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Hogenesch J B, Chan W K, Jackiw V H, Brown R C, Gu Y Z, Pray-Grant M, Perdew G H, Bradfield C A. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- Hogenesch J B, Gu Y Z, Jain S, Bradfield C A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y D, Barnard M, Tian H, Li X, Ring H Z, Francke U, Shelton J, Richardson J, Russell D W, McKnight S L. Molecular characterization of two mammalian bHLH-PAS domain proteins selectively expressed in the central nervous system. Proc Natl Acad Sci U S A. 1997;94:713–718. doi: 10.1073/pnas.94.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo S P, Rudic R D, Sehgal A, Chakravarti D, FitzGerald G A. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo J J, Sassone-Corsi P. Circadian clock control by SUMOylation of BMAL1. Science. 2005;309:1390–1394. doi: 10.1126/science.1110689. [DOI] [PubMed] [Google Scholar]
- Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos G P. G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino M U, Bhattachryya N, Alesci S, Ichijo T, Chrousos G P, Kino T. The glucocorticoid receptor and the orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II interact with and mutually affect each other’s transcriptional activities: implications for intermediary metabolism. Mol Endocrinol. 2004;18:820–833. doi: 10.1210/me.2003-0341. [DOI] [PubMed] [Google Scholar]
- Mirani M, Elenkov I, Volpi S, Hiroi N, Chrousos G P, Kino T. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J Immunol. 2002;169:6361–6368. doi: 10.4049/jimmunol.169.11.6361. [DOI] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos G P, Pavlakis G N. Human immunodeficiency virus type-1 accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76:9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Ichijo T, Amin N D, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng Y L, Garabedian M J, Kawasaki E, Pant H C, Chrousos G P. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21:1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- Ichijo T, Chrousos G P, Kino T. Activated glucocorticoid receptor interacts with the INHAT component Set/TAF-Iβ and releases it from a glucocorticoid-responsive gene promoter, relieving repression: implications for the pathogenesis of glucocorticoid resistance in acute undifferentiated leukemia with Set-Can translocation. Mol Cell Endocrinol. 2008;283:19–31. doi: 10.1016/j.mce.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Ichijo T, Voutetakis A, Cotrim A P, Bhattachryya N, Fujii M, Chrousos G P, Kino T. The Smad6-histone deacetylase 3 complex silences the transcriptional activity of the glucocorticoid receptor: potential clinical implications. J Biol Chem. 2005;280:42067–42077. doi: 10.1074/jbc.M509338200. [DOI] [PubMed] [Google Scholar]
- Vander Kooi B T, Onuma H, Oeser J K, Svitek C A, Allen S R, Vander Kooi C W, Chazin W J, O'Brien R M. The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol Endocrinol. 2005;19:3001–3022. doi: 10.1210/me.2004-0497. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat M L, David M, Biola-Vidamment A, Lecoeuche D, Zennaro M C, Bertoglio J, Pallardy M. GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood. 2004;104:215–223. doi: 10.1182/blood-2003-12-4295. [DOI] [PubMed] [Google Scholar]
- Blind R D, Garabedian M J. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit P, Russcher H, de Jong F H, Brinkmann A O, Lamberts S W, Koper J W. Differential regulation of synthetic glucocorticoids on gene expression levels of glucocorticoid-induced leucine zipper and interleukin-2. J Clin Endocrinol Metab. 2005;90:2994–3000. doi: 10.1210/jc.2004-2298. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes P J, Adcock I M. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Imhof A, Yang X J, Ogryzko V V, Nakatani Y, Wolffe A P, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown S A, Marcacci L, Tronche F, Kellendonk C, Reichardt H M, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeis T, Lahiri K, Nica G, Vallone D, Santoriello C, Neumann C J, Hammerschmidt M, Foulkes N S. Glucocorticoids play a key role in circadian cell cycle rhythms. PLoS Biol. 2007;5:e78. doi: 10.1371/journal.pbio.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G P. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Chrousos G P. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Chrousos G P. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24:S50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- van Rossum E F, Russcher H, Lamberts S W. Genetic polymorphisms and multifactorial diseases: facts and fallacies revealed by the glucocorticoid receptor gene. Trends Endocrinol Metab. 2005;16:445–450. doi: 10.1016/j.tem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch M P, Miller B H, Su A I, Schook A B, Straume M, Schultz P G, Kay S A, Takahashi J S, Hogenesch J B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin R J, Xie W, Wilpitz D, Evans R M. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Puri P L, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang J Y, Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- Luo J, Li M, Tang Y, Laszkowska M, Roeder R G, Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A. 2004;101:2259–2264. doi: 10.1073/pnas.0308762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- Nascimento A S, Dias S M, Nunes F M, Aparicio R, Ambrosio A L, Bleicher L, Figueira A C, Santos M A, de Oliveira Neto M, Fischer H, Togashi M, Craievich A F, Garratt R C, Baxter J D, Webb P, Polikarpov I. Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J Mol Biol. 2006;360:586–598. doi: 10.1016/j.jmb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Barnes P J, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Crofford L J, Kalogeras K T, Mastorakos G, Magiakou M A, Wells J, Kanik K S, Gold P W, Chrousos G P, Wilder R L. Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 1997;82:1279–1283. doi: 10.1210/jcem.82.4.3852. [DOI] [PubMed] [Google Scholar]
- Vgontzas A N, Papanicolaou D A, Bixler E O, Lotsikas A, Zachman K, Kales A, Prolo P, Wong M L, Licinio J, Gold P W, Hermida R C, Mastorakos G, Chrousos G P. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Rosen J, Miner J N. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005;26:452–464. doi: 10.1210/er.2005-0002. [DOI] [PubMed] [Google Scholar]