Abstract
Surfactant protein D (SP-D) is an important effector of innate immunity. We have previously shown that SP-D accumulates at sites of acute bacterial infection and neutrophil infiltration, a setting associated with the release of reactive species such as peroxynitrite. Incubation of native SP-D or trimeric SP-D lectin domains (NCRDs) with peroxynitrite resulted in nitration and nondisulfide cross-linking. Modifications were blocked by peroxynitrite scavengers or pH inactivation of peroxynitrite, and mass spectroscopy confirmed nitration of conserved tyrosine residues within the C-terminal neck and lectin domains. Mutant NCRDs lacking one or more of the tyrosines allowed us to demonstrate preferential nitration of Tyr314 and the formation of Tyr228-dependent cross-links. Although there was no effect of peroxynitrite or tyrosine mutations on lectin activity, incubation of SP-D dodecamers or murine lavage with peroxynitrite decreased the SP-D-dependent aggregation of lipopolysaccharide-coated beads, supporting our hypothesis that defective aggregation results from abnormal cross-linking. We also observed nitration, cross-linking of SP-D, and a significant decrease in SP-D-dependent aggregating activity in the lavage of mice acutely exposed to nitrogen dioxide. Thus, modification of SP-D by reactive oxygen-nitrogen species could contribute to alterations in the structure and function of SP-D at sites of inflammation in vivo.—Matalon, S., Shrestha, K., Kirk, M., Waldheuser, S., McDonald, B., Smith, K., Gao, Z., Belaaouaj, A., Crouch, E. C. Modification of surfactant protein D by reactive oxygen-nitrogen intermediates is accompanied by loss of aggregating activity, in vitro and in vivo.
Keywords: collectin, covalent cross-linking, free radicals, nitrogen dioxide, peroxynitrite
Pulmonary surfactant protein D (SP-D) (1) plays important roles in innate immunity and pulmonary homeostasis, with diverse contributions to microbial clearance, inflammatory and immune regulation, and the metabolism of surfactant (1,2,3). Although SP-D was originally isolated from the lung, it is widely expressed at other mucosal surfaces, where it could play similar roles as an effector of innate immunity (4).
Each SP-D molecule consists of one or more trimeric subunits, each with a short N-terminal peptide, collagen domain, trimeric coiled-coil neck domain, and trimeric C-terminal lectin domain (5, 6). The N-terminal domain normally mediates the covalent association of subunits, while the trimeric lectin domains mediate interactions with microbial cell wall glycoconjugates, organic particulate antigens, nucleic acids, apoptotic cells, and specific cellular receptors. Trimeric arrays of carbohydrate recognition domains (NCRDs) are necessary and sufficient for binding to a variety of ligands; however, higher order oligomerization of trimeric subunits is required for aggregation and bridging interactions between bacteria, virions, and other particulate ligands.
Acute and chronic active inflammation is accompanied by the generation of various reactive oxygen-nitrogen intermediates by inflammatory cells (7,8,9). These include superoxide (O2·−) hydrogen peroxide (H2O2), nitrogen dioxide (NO2), dinitrogen trioxide (N2O3), and peroxynitrite (ONOO−), as well as hypochlorous acid (HOCl) and nitryl chloride (NO2Cl). Although such species can contribute to antimicrobial host defenses, they can also damage host cells and tissues. For example, nitrated and oxidized proteins accumulate in the plasma and alveolar spaces of patients with inflammatory diseases (10,11,12,13). Previous studies have shown that surfactant protein A (SP-A), a related collagenous lectin, is nitrated and oxidized in vitro by reactive species generated by endotoxin-stimulated, rat alveolar macrophages (14). Nitrated SP-A loses its mannose binding activity and ability to enhance the adherence of Pneumocystis to alveolar macrophages in vitro (15, 16), and SP-A isolated from the lungs of lambs exposed to high concentrations of inhaled nitric oxide is defective in lipid aggregation (17). Nitrated SP-A was also shown to accumulate in the lavage of patients with ALI (18), providing the first direct evidence of injury-induced nitration of a specific protein in the human alveoli in vivo.
Decreased levels of lavage SP-D are observed in a variety of clinical settings characterized by acute or chronic inflammation. For example, SP-D is decreased in cystic fibrosis (19), in patients with severe manifestations of the adult respiratory distress syndrome (20), and in association with cigarette smoking (21). The mechanisms responsible for these changes are poorly understood. Previous reports indicate that neutrophil-mediated clearance of SP-D is increased during the first few hours following instillation of gram-negative lipopolysaccharides (22) and that SP-D is degraded by neutrophil serine proteases, both in vitro and in vivo (23,24,25). However, there is also evidence that SP-D can be oxidized in vitro with loss of bacterial aggregating activity (26).
We previously observed that SP-D accumulates at sites of acute bacterial pneumonia, codistributing with bacteria, neutrophils, and secreted neutrophil serine proteases (23). Given that granule exocytosis and bacterial phagocytosis are accompanied by the generation of reactive oxygen-nitrogen species, we examined the hypothesis that these mediators can modify SP-D structure and function via posttranslational modifications including nitration or oxidation. In particular, we examined the structural consequences of exposing native SP-D dodecamers and trimeric human SP-D neck + CRD domains to either purified ONOO− or 3-morpholinosydnonimine (SIN-1, an ONOO− generator) and used site-directed mutagenesis to elucidate specific sites of nitration and cross-linking. In addition, we assessed the presence of nitrated SP-D and implemented an SP-D-specific aggregation assay to determine the existence of functionally significant modifications in the bronchoalveolar lavage (BAL) of mice exposed to nitrogen dioxide, an oxidant gas known to cause extensive nitration of lung tissue.
MATERIALS AND METHODS
ONOO− was synthesized from sodium nitrite and H2O2, as described previously (27) and treated with manganese dioxide to remove H2O2. Working stocks were prepared in 1 or 0.01 N NaOH (pH 12) immediately prior to each assay, and the ONOO− concentration was determined spectrophotometrically at 302 nm (εM=1670 M−1cm−1). The monoclonal antibody to nitrotyrosine was initially generated and characterized by Dr. Joseph Beckman and is now produced at University of Alabama. The rabbit anti-mouse SP-D (AB3434) was purchased from Chemicon (Temecula, CA, USA). Except where indicated, cysteine, sodium nitrite, and all other chemicals were reagent grade and purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-morpholinosydnomine (SIN-1) was purchased from Calbiochem (La Jolla, CA, USA). Stock solutions of SIN-1 (100 mM) were stored at −20°C in 10 mM phosphate buffer, pH 5.5. SIN-1 is stable at pH 5.5; however, at physiological pH it decomposes to the stable compound SIN-1c and in the process releases both O2·− and NO, which may combine to form ONOO− (28). Escherichia coli J5 LPS (Rc chemotype) was obtained from List Biological Laboratories (Campbell, CA, USA). Fluoresbrite™ YG Polystyrene Microspheres, 2.5% (w/v), 1 μm diameter, were obtained from Polysciences (Warrington, PA, USA). Phosphatidylinositol was obtained from Avanti Polar Lipids (Alabaster, AL, USA). Fatty acid-free BSA with low immunoglobulin and low endotoxin content was from Equitech-Bio (Kerrville, TX, USA).
Recombinant SP-Ds
Recombinant human SP-D (RhSP-D) and recombinant rat SP-D (RrSP-D) were expressed in CHO-K1 cells, as described previously (29). SP-D was isolated by maltosyl-agarose chromatography, and dodecamers were purified by gel filtration chromatography on A-15 M agarose.
Wild-type trimeric human neck + CRD fusion proteins were isolated from inclusion bodies, refolded, and purified by sequential nickel affinity and gel filtration chromatography (30). Although intact fusion proteins were used for some studies, for most experiments the N-terminal tags were removed by enterokinase (EK) cleavage, followed by purification of the trimeric neck + CRD domains (30). The EK-cleaved protein contains a short N-terminal segment of vector-derived sequence, AMADIGS, which lacks potential sites of nitration or carbonylation. In recent experiments NCRDs were expressed without the tags and purified by maltosyl-agarose chromatography and gel filtration; the tagless NCRD lacks vector sequence but contains an N-terminal initiator methionine. Appropriate folding of the CRD was recently confirmed by crystallographic analysis of the EK cleaved (31) and tagless NCRDs (32). The recombinant NCRDs are fully active as lectins (30).
Site-directed substitutions of phenylalanine for tyrosine were performed at each of the 3 tyrosines of the human fusion protein, that is, at position 228 near the neck/CRD junction (Y228F) and at 306 (Y306F) and 314 (Y314F) within the human CRD; positions are numbered according to the mature protein. In addition, a double mutant (Y306F+ Y314F) and triple mutant (Y228F+Y306F+Y314F) were generated by sequential mutagenesis. The substitutions were performed using a QuikChange II XL Site-Directed Mutagenenesis Kit (200521; Stratagene, La Jolla, CA, USA) and the hSP-D neck + CRD DNA in pET-30a(+) as the template. All DNA sequences were verified by automated sequencing of the entire coding sequence of the fusion protein. Proteins were expressed as for the wild-type NCRDs. All proteins were recovered in similar amounts and bound to solid-phase mannan (see Results) (30).
Exposure of SP-D to ONOO−
SP-D preparations (native dodecamers, NCRD fusion proteins, and EK-cleaved or tagless NCRDs) were suspended in 10 mM HEPES (to minimize changes in pH following addition of ONOO−) with 10 mM CaCl2 (pH 7.4) and exposed to ONOO− (0.01-1 mM) at the indicated concentration. Because ONOO− has a half-life of less than 1 s at pH 7.4 (33), stock reagent (150 mM; pH 12) was added with agitation while still in sodium hydroxide. The pH was carefully monitored to ensure that reactions were performed at neutral pH. In initial control studies, cysteine (5 mM) or urate (1 mM), well-known scavengers of ONOO− (28), were added into some SP-D solutions prior to addition of ONOO−. For some experiments, ONOO− was inactivated by preincubation of the stock solution for several minutes at neutral pH. Previous reports indicate that ONOO− can interact with high concentrations of HEPES to produce reactive oxygen intermediates (34). However, the reactions are negligible or do not occur at the concentration of HEPES used for these studies.
For selected experiments, proteins were exposed to 3-morpholinosydnonimine (0.1-5 mM) as an ONOO− donor. Incubations were performed in 2 mM HEPES, pH 7.4, 25 mM NaHCO3 in a 5%CO2 atmosphere for 2 h at 37°C. ONOO− generation by SIN-1 was quantified by the oxidation dihydrorhodamine to rhodamine at 23°C (ε500 nm=78,000 M−1 cm−1), as described previously (28). The reaction mixtures were dispersed in 2 mM HEPES buffer (pH 7.4), which contained, in addition to SIN-1, diethylenetriamine penta-acetate acid (DTPC, 100 μM), dihydrorhodamine 123 (50 μM), and SP-D(2.5 μg).
SDS-PAGE and immunoblotting of ONOO−-treated SP-D
Full-length SP-D and trimeric human NCRDs were diluted as required in 150 mM NaCl, 5-10 mM CaCl2, 10 mM HEPES, pH 7.4, and incubated at the indicated concentration for 15 min at 21°C with ONOO− (0.1-1.5 mM). Incubations were also performed in the presence or absence of a scavenger, such as urate or cysteine (5 mM). Because ONOO− has a half-life of less than 1 s at pH 7.4, the reagent was added during vortexing.
Reaction products were resolved by SDS-PAGE (8 or 12% separating gel) with or without sulfhydryl reduction, using either 2-mercaptoethanol or dithiothreitol (DTT). Proteins were visualized by staining with the SYPRO Ruby Protein Gel Stain Stain (Invitrogen, Carlsbad, CA, USA) or Coomassie blue. For immunoblotting, protein gels were transferred to polyvinylidene fluoride (PVDF) membranes or nitrocellulose. For the detection of nitrotyrosine adducts, the membranes were sequentially incubated with a monoclonal antibody to nitrotyrosine (a generous gift of Dr. Joseph S. Beckman, Linus Pauling Institute, Corvallis, OR, USA) and HRP-conjugated secondary antibody. Immunoreactive species were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences, Piscataway, NJ, USA). Protein-associated carbonyl groups were detected using the OxyBlot kit (Chemicon) according to the manufacturer’s instructions. Briefly, treated and untreated proteins were derivatized with 2,4-dinitrophenylhydrazine (DNP), resolved by SDS-PAGE, and visualized by immunoblotting with anti-DNP as described previously (14).
Identification of nitrated amino acids by mass spectroscopy
Tandem mass spectral analyses were performed with a Q-Tof 2 mass spectrometer (Micromass, Manchester, UK) using electrospray ionization (27). In initial experiments, proteins were cleaved with trypsin, but some peptides were too large to be detected. Thus, for most studies, proteins were cleaved by incubating with the endoproteinase GluC (Staphylococcus aureus Protease V8; 1 ng Glu-C/50 ng protein; New England BioLabs, Ipswich, MA, USA) overnight at 25°C. This enzyme selectively cleaves peptide bonds after glutamic acid and at slower rate after aspartic acid, a process that allowed us to detect all tyrosine-containing peptides. The peptides were concentrated and desalted using ZipTips and then analyzed by LCMS/MS. The Q-Tof was operated in the automatic switching mode whereby multiple-charged ions are subjected to MS/MS if their intensities rise above 6 counts. The tandem mass spectra were processed with the MassLynx MaxEnt Software (Waters Corporation, Milford, MA, USA).
Bacterial pull-down assays
Maltose-sensitive binding of SP-D to E. coli J5 was used as a measure of lectin activity. SP-D is the major agglutinin for rough E. coli in rodent lavage (35), and E. coli J5 LPS is a well-characterized SP-D ligand (36). Pull-down assays allow direct visualization of binding, avoiding the confounding effects of modifications to epitopes or tags. The bacteria were harvested from agar plates, washed, quantified by measuring absorbance at an optical density of 600 nm, and diluted as required in 10 mM HEPES, 150 mM NaCl, pH 7.4 (HBS) containing 5 mM CaCl2 (HBS+C). Aliquots of EK-cleaved NCRD (8 μg) were preincubated for 15 min at room temperature (RT) with the indicated concentration of ONOO− (or an equivalent volume of NaOH), with or without prior inactivation of the ONOO− at neutral pH. The NCRDs were added to the bacterial suspension and incubated for an additional 15 min at 37°C in the presence or absence of competing maltose. The complexes were collected by microcentrifugation and washed with Ca2+-containing buffer, prior to transfer to a new tube. The original supernatant and the final pellet were dissolved in SDS sample buffer and examined by SDS-PAGE (12% acrylamide). Proteins were visualized by staining with Coomassie blue, and binding was assessed by densitometry within the linear range of protein staining. In the absence of maltose, NCRDs were nearly quantitatively removed from the supernatant and recovered in the pellet. In the presence of maltose, most of the protein was recovered in the supernatant.
Lipopolysaccharide (LPS) microsphere aggregation assay
Lyophilized LPS was resuspended in HBS to a final concentration of 2.0 mg/ml. The suspension was warmed 10 min at 37°C, shaken on a vortex mixer for 10 min, and warmed again for 30 min at 37°C to allow swelling. The suspension was vortexed vigorously immediately before dividing into aliquots to be frozen at −20°C.
Fluoresbrite™ YG polystyrene microspheres (200 μl of suspension, 4.5×1010 particles/ml) were washed 3 times in a microfuge tube with HBS. The beads were collected at 10,000 rpm, 5 min, 4°C, in a fixed-angle microcentrifuge and resuspended in 50 μl of HBS to give a final concentration of 1.8 × 1011 particles/ml. Fluorescent beads were not required for the current assays, and similar results were obtained with nonfluorescent beads. However, the yellow color facilitated washing procedures, and the standardized fluorescent beads are suitable for a variety of other assays.
For coupling, an aliquot of LPS was thawed, vigorously vortexed, and warmed to 37°C. The LPS was then added to an equal volume of beads, yielding a final LPS concentration of 1.0 mg/ml LPS. The LPS was adsorbed to the beads by mixing gently overnight on an oscillating agitator. The beads were washed 3 times using HBS as above, with a tube transfer after the second wash, and resuspended in 250 μl of HBS (3.6×1010 particles/ml, 9×109 total particles). The preparations were stable for at least 1 wk at 4°C.
Visual estimation of the extent of bead aggregation relative to a series of SP-D standards was highly reproducible and showed an obvious positive correlation. However, we wanted a more objective assay amenable to graphic representation. Sedimentation assays, as employed in our earlier studies of bacterial aggregation, are time consuming. They also require relatively large assay volumes, making them expensive in terms of purified protein and lavage. Because clumping of beads leads to intervening areas of clearing, we were able to develop a convenient and economical plate-based transmittance assay.
Prior to each assay, a 96-well, flat-bottom, microtiter plate (3590; Corning, Corning, NY, USA) was blocked with 10 mM HEPES, 150 mM NaCl, 10 mM CaCl2, 1% BSA, pH 7.4, for 1 h at 23°C or overnight at 4°C, and washed 3 times with HBS + CaCl2. Aliquots of purified RrSP-D dodecamer were diluted by addition to blocked wells containing binding buffer (10 mM HEPES, 150 mM NaCl, 10 mM CaCl2, 0.05% Tween) and incubated in the plate for 30 min at RT to recalcify the protein. For lavage supernatants, HEPES, CaCl2, and Tween were added from concentrated stocks to the lavage samples in the blocked wells. Tween did not affect the stability of the beads and effectively eliminated self-aggregation of beads. Freshly washed, LPS-adsorbed beads in HBS were added to the wells of the plate (4 μl to yield 3.6×1010 particles/ml or 1.44×108 particles/well) and mixed by agitation. For some experiments, protein or lavage samples were preincubated with competing maltose or phosphatidylinositol, or with antisera. The plate was allowed to incubate at RT for 30 min and then gently tapped prior to analysis. Subsequently it was scanned on a flatbed scanner (UMAX PowerLook II, Techville, Inc., Dallas, TX, USA) to obtain a grayscale TIFF of the entire plate. Default autoexposure settings were used. The pixel density of a 6-mm-diameter circular area at the center of each well was measured using the Array Analysis mode of ImageQuant Total Lab software (Amersham Biosciences, Fairfield, CT, USA). The raw data were inverted to obtain a more intuitive graphic representation of the increase in aggregation, and the density of beads in the absence of SP-D was subtracted. The amount of SP-D in lavage (∼0.5 μg/ml or 1 μg/mouse) was estimated relative to parallel standards and confirmed by immunoblotting with anti-mouse SP-D. Although there was scatter attributed to differences in the size and distribution of aggregates, the dose response was suitably linear within the range of 0.2–2 μg/ml of purified SP-D with a correlation of r2 > 0.94. For some experiments images were also obtained using a Nikon TMS microscope (Nikon, Tokyo, Japan) equipped with a Zeiss digital camera (Carl Zeiss, Oberkochen, Germany) and OpenLab software (Improvision, Waltham, MA, USA).
Pull-down assay with LPS microspheres
LPS-coated polystyrene beads were prepared as described above. Aliquots of rat SP-D dodecamer (0.4 μg) or aliquots of lavage supernatant were diluted into HBS + CaCl2 (10 mM) in 1.5 ml microcentrifuge tubes. Samples were incubated with 1 mM ONOO− or pH-inactivated ONOO− for the aggregation assays. Samples were further incubated in the absence or presence of competing maltose (50 mM) for 30 min on ice. Freshly washed, LPS-adsorbed beads in HBS were then added to each tube to yield ∼1.44 × 108 beads/tube. The beads and protein were mixed by gentle agitation for 60 min at RT, and then collected by microcentrifugation at 10,000 rpm for 5 min at 4°C. Beads were washed 2 more times with the above buffer, but containing 0.05% Tween, then resuspended and transferred to a new microfuge tube. After one final spin, the beads resuspended in SDS sample buffer + DTT and boiled for 10 min at 100°C. Bound proteins were resolved by SDS-PAGE on a 10% minigel and visualized with Coomassie blue.
Exposure of mice to NO2 and collection of BAL
C57BL/6 mice were obtained from the Frederick Cancer Research and Development Center (National Cancer Institute, Frederick, MD, USA) and used at 8-12 wk of age (20-25 gm body weight). Mice were maintained in the animal barrier facility with 12 h light/dark cycle and provided with water and food ad libitum. Mouse procedures were performed at the University of Alabama and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Mice were exposed to NO2 (20 ppm for 4 h) in a hermitically sealed glass chamber (1500 ml). NO2 atmospheres were generated by blending high-concentration NO2 in N2 into a constant flow of humidified air (20% O2 and 80% N2) using mass flow controllers (Scott Specialty Gases, Houston, TX, USA) to obtain desired concentrations. Exposures were conducted under steady state, first-order conditions with respect to NO2 ([NO2]INFLOW≈20 ppm) at 25°C with a total flow of 550 ml/min to provide a chamber turnover every 3 min. The exposure system was thoroughly conditioned with ·NO2 prior to use in order to minimize background NO2 losses by reaction with chamber components. Control air exposures (no NO2) were conducted in a similar fashion. NO2 concentrations were continuously monitored with a chemiluminescent NO analyzer (Model 42; Thermo Environmental, Franklin, MA, USA) calibrated with NO, as described previously (37).
After a 4 h exposure, mice were returned to room air and euthanized with an intraperitoneal injection of pentobarbital (300 mg/kg). The thorax was opened, a trimmed, sterile 18-gauge intravenous catheter was inserted caudally into the lumen of the exposed trachea, and the lungs were lavaged with a total of 3 ml of 0.15 M NaCl given in 3 equally divided 1 ml aliquots. Recoveries of lavage fluid were identical from both experimental groups. The aliquots were pooled on ice, centrifuged immediately at 150 g for 10 min to remove cells, and the cell-free supernatant was temporarily stored at −20°C.
Detection of nitrotyrosine-modified proteins in lavage
An aliquot of cell-free lavage (45 μl) from each air- or NO2-exposed mouse was solubilized in SDS sample buffer, and proteins were resolved by SDS-PAGE in the absence or presence of sulfhydryl reduction. Proteins were electrophoretically transferred to a PVDF membrane that was blocked with PBS containing 0.05% Tween 20 and 5% (w/v) dry milk (PBSTM) for 1 h at RT, and then incubated overnight at 4°C with a monoclonal anti-nitrotyrosine antibody (1:1000) in PBSTM. After washing, immunoreactivity was detected with HRP-conjugated goat anti-mouse antibody (1:5000; Pierce, Rockford, IL, USA) in PBSTM using the ECL detection system. For some experiments, blots were stripped by washing with 2% SDS containing 100 mM 2-mercaptoethanol, prior to washing and incubation with antibody to SP-D. Where necessary, films were registered using a fluorescent ruler (RPI Corp., Mt. Prospect, IL, USA) and fluorescent protein standards.
Immunoprecipitation and detection of nitrated SP-D in lavage
Cell-free lavage from 2 mice each was pooled. Tris buffer, pH 7.4, was added to a final concentration of 50 mM. Protease inhibitor (Complete Mini; Roche Molecular Biochemicals, Indianapolis, IN, USA) and 1% (w/v) Triton X-100 were added. For some experiments, IgG was removed by twice passing the samples through a Protein A/G column (Pierce). SP-D was immunoprecipitated with 10 μl of rabbit anti-SP-D serum overnight at 4°C. Immunoprecipitates were captured with 100 μl of 50% Protein G beads at 4°C for 3 h. The beads were washed 4 times with the same buffer prior to solubilization with sample buffer in the absence or presence of 2-mercaptoethanol. Samples were resolved by SDS-PAGE, transferred to PVDF, and incubated with anti-nitrotyrosine antibody, and labeled proteins were detected with HRP-conjugate. The blots were then stripped as above, washed, and incubated with the polyclonal anti-SP-D antibody (1:2000). Immunoreactive proteins were visualized with HRP-conjugated Protein A (1:20,000) or goat anti-rabbit HRP conjugate and ECL detection. Despite preadsorption of IgG, residual mouse IgG was evident on most mouse anti-nitrotyrosine blots.
Nitrated recombinant rat SP-D dodecamers were used as positive controls. Rat SP-D (3 μg) in 100 μl HBS + CaCl2 (10 mM) was incubated with 1 mM ONOO− for 15 min at RT. The total volume was adjusted to 1 ml with 50 mM Tris-buffered saline (pH 7.4) containing 1% Triton X-100 prior to immunoprecipitation with anti-SP-D.
2-D gel analysis of nitrated proteins in lavage of NO2-exposed mice
Cell-free lavages from individual mice were placed in rehydration buffer (6 M urea, 2 M thiourea, 2% CHAPS, 0.5% lauryl maltoside, 1× biolyte ampholyte, and 30 mM DTT containing protease inhibitors as above). Immobilized pH gradient strips (pH 3-10) were rehydrated overnight at RT with the above buffer containing lavage samples. After isoelectric focusing (IEF) for 1 h at 2000 V, the strips were equilibrated with equilibration buffer (6 M urea; 2% SDS; 0.375 M Tris, pH 8.8; 20% glycerol; and 200 mg/ml dithiothreitol) for 15-20 min at RT. A 10% SDS-polyacrylamide gel was used for the second dimension. Proteins were electrophoretically transferred to a PVDF membrane, blocked, washed, and examined by Western as above. Some of the samples (from air- and NO2-exposed mice) were stained with SYPRO Ruby stain following the second-dimensional run.
RESULTS
ONOO− causes nitration and cross-linking of full-length human SP-D
For our initial experiments, we sought to determine whether ONOO− caused oxidative modifications to SP-D. Purified human SP-D dodecamers were incubated with ONOO− (0-3 mM) for 15 min at 37°C. Because ONOO− is rapidly inactivated at physiological pH (T1/2<1 s at pH 7.4) (33), stock solutions of ONOO− were stored at −80°F at pH 12, and assayed immediately before use.
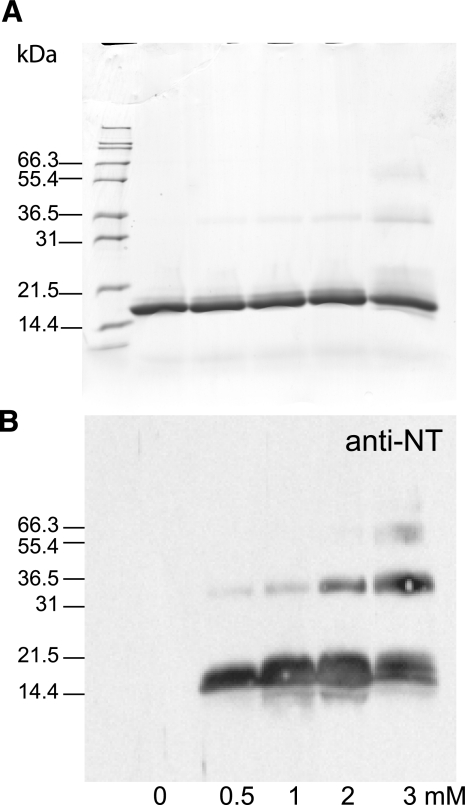
As shown in Fig. 1A, ONOO− caused dose-dependent nitration and cross-linking of SP-D, evident on both reduced and nonreduced gels. Comparable results were obtained for recombinant rat SP-D (data not shown). There was also no evidence of nitration or cross-linking when ONOO− was inactivated for 15 min at neutral pH prior to the incubation with SP-D, or when incubations were performed in the presence of a scavenger such as cysteine (Fig. 1B and data not shown).
Figure 1.
ONOO−-dependent nitration and cross-linking of human SP-D dodecamers. A) Dose dependence of modification. Human SP-D dodecamers were exposed to the indicated concentration of ONOO− (mM) prior to resolution of proteins by SDS-PAGE in the absence and presence of DTT. Proteins were immediately transferred to SDS sample buffer and resolved by SDS-PAGE (10% gel) in the absence and presence of DTT. Parallel gels were processed for protein staining with Coomassie blue (top panel) or transferred to nitrocellulose for immunoblotting with antinitrotyrosine (bottom panel). Note strong immunostaining of the ONOO− exposed protein and the appearance of nitrated, high molecular weight species consistent with covalent cross-linking of the modified protein. In other experiments, addition of cysteine (5 mM) prior to adding ONOO−, or preinactivation of the ONOO−at neutral pH, prevented nitration and cross-linking (not shown). SP-D trimers and monomers are indicated with arrows; positions of Mark 12 protein standards are also shown. Findings are representative of at least 7 independent experiments. With longer exposures, modification and nitration could be readily detected at ONOO− concentrations as low as 0.1 mM. B) pH inactivation of ONOO−. Native human SP-D dodecamers (100 μg/ml) were exposed to assay buffer, 1 mM ONOO−, or 1 mM pH-inactivated ONOO− for 15 min at RT. Gel was processed for immunoblotting with anti-nitrotyrosine. Both the posttransfer protein-stained gel (top panel) and Western blot (bottom panel) are shown. Mark 12 standards are shown between reduced and nonreduced samples; approximate sizes are indicated at right (kDa). Positions of Mark 12 standards shown with the blot were estimated based on the migration of internal prestained standards. Positions of unreduced SP-D trimers (arrows, left) and reduced monomers (arrows, right) are indicated. Minor band below the unreduced trimer corresponds to a small amount of dimer in the purified protein. Note ONOO− (+P)-dependent cross-linking and nitration that is not evident in the presence of pH-inactivated ONOO− (+iP). Presence of abnormal higher molecular weight forms, both with and without sulfhydryl reduction, indicates the formation of nondisulfide covalent cross-links.
ONOO− causes cross-linking and nitration of purified trimeric neck + CRDs
Because inhibition of aggregation could result from alterations in oligomeric structure and/or loss of carbohydrate binding activity, we examined the effects of ONOO− on trimeric human lectin domains. As shown in Fig. 2, ONOO− treatment resulted in the appearance of more slowly migrating species consistent with dimers and higher-order multimers. As for dodecamers, immunoblotting of NCRDs with antibodies to nitrotyrosine demonstrated strong labeling of both monomers and cross-linked species, indicating the formation of nitrotyrosine adducts within the recombinant protein (Fig. 2, bottom panel). Effects were blocked by the inclusion of scavengers of reactive nitrogen species, or prior inactivation of ONOO− at neutral pH, as illustrated for full-length SP-D (data not shown).
Figure 2.
ONOO−-dependent nitration and cross-linking of trimeric NCRDs. Tagless human NCRDs (50 μg/ml; total vol 20 μl) were exposed to ONOO− at the indicated concentration. Proteins (10 μg/lane) were then resolved by SDS-PAGE (12% gel) in the presence of DTT prior to protein staining (top panel) or immunoblotting with anti-nitrotyrosine (anti-NT; bottom panel). Sample without ONOO− (0 mM) contained same amount of NaOH as sample containing highest ONOO−concentration. Prior inactivation of ONOO− at neutral pH prevented nitration and cross-linking (data not shown). Findings are representative of at least 4 independent experiments performed by 3 different individuals.
Modification of SP-D by SIN-1
As shown in Fig. 3, SP-D nitration and cross-linking were also seen following exposure to SIN-1 (1 mM for 2 h). The rhodamine assays confirmed a time-dependent generation of ONOO− (28).
Figure 3.
ONOO−-dependent modification of SP-D by SIN-1. Human SP-D dodecamers (1 μg in 50 μl) were exposed to 1 mM SIN-1 or control buffer. Incubations were performed in a 5% CO2 atmosphere for 2 h at 37°C. Reaction products were resolved by SDS-PAGE with prior sulfhydryl reduction by DTT. Proteins were visualized by SYPRO staining (top panel) or by immunoblotting for nitrotyrosine (bottom panel). Positions of internal molecular weight standards are indicated at left. Position of reduced SP-D monomer (43 kDa, reduced) is indicated by arrow at right. Asterisks identify some of the most prominent cross-linked species. The 2 major species migrate near natural, unreduced, disulfide-cross-linked dimers and more slowly than unreduced, disulfide-cross-linked trimers. Note that control proteins sometimes contain small populations of nonreducible dimmers, as evident in the starting material.
Specific tyrosine residues are nitrated within the neck and CRD domains
In order to confirm nitration and identify the specific sites of modification, human SP-D dodecamers, intact fusion proteins, and trimeric NCRDs were exposed to ONOO− (1 mM for 15 min) prior to proteolytic fragmentation and mass spectral analysis as described in the Methods section. Using this approach, we identified peptides accounting for the entirety of the neck + CRD domain. As illustrated in Fig. 4, we identified 3 sites of nitration within the NCRD: Y228, Y306, and Y314. Full-length human SP-D was also nitrated at Y228 and Y306, but the peptide that contains Y314 was recovered in too small of a yield to allow an assessment of nitration. Human SP-D contains a fourth tyrosine residue, which is located in the amino-terminal peptide domain (Y6); however, the corresponding fragment was not identified.
Figure 4.
Mass spectroscopic analysis of ONOO−-modified dodecamers and NCRDs. Samples of purified human SP-D dodecamer or NCRDs were exposed to either ONOO− (1 mM for 15 min) or pH-inactivated ONOO−. Samples were digested with the endoproteinase Glu-C, which cuts after glutamic acid (E) or aspartic acid (D), allowing detection of all tyrosine (Y) residues within the neck and CRD domains. Resulting peptides were purified and analyzed by tandem mass spectral analyses as described in Materials and Methods. A) Mass chromatograms of control and NO2-modified fragments (Glu-C digests) of dodecamer. Bottom record indicates total ionic current generated from charged ions; x axis indicates elution times (s) through HPLC. Remaining records show m/z ratios of indicated control and nitrated fragments. Charge of each fragment is also shown (i.e., 2z=double protonated). Note shifts of m/z for nitrated fragments. Results are representative of 3 experiments. Calculations to estimate abundance of nitrated peaks are provided by MassLynx software. For ONOO−-treated proteins, 6-14% of recovered peptides containing tyrosine residues were nitrated. B) Representative spectra. MS/MS spectra of the GKFTYPTGE peptide (containing Y306) obtained by Glu-C treatment of enterokinase-cleaved human NCRD, following exposure of the protein to inactive (top panel) or active ONOO− (bottom panel). Both b and y fragments are shown. Tyrosine nitration is evident by the increase in m/z of y5 and b7 fragments by 45 m/z units. Glu-C control and nitrated fragments as well as m/z of various b and y fragments are shown. Results are representative of 3 experiments. C) Localization of modified sites within SP-D lectin domain. Sequences of the entire human SP-D neck and CRD are shown. Neck domain, as defined by exon structure, is underscored. Sites of nitration at 228, 306, and 314 (relative to mature protein) are indicated.
No dityrosine crosslinks were identified by mass spectroscopy of recovered peptides or fluorescence assays of nitrated proteins (data not shown); this was most likely due to the very low yield of dityrosine. We attempted to detect dityrosine in cross-linked components using monoclonal antibodies; however, the antibodies were not specific under the conditions of our blotting assays.
Although oxidation of Met295 was routinely observed for the nitrated samples, no other modifications were detected by mass spectroscopy of dodecamers or EK-cleaved NCRDs. Both control and ONOO−-treated NCRDs showed the expected intrachain disulfide bonds involving Cys261–Cys353 and Cys321–Cys345 by mass spectroscopy. No oxidation or nitrosylation of cysteine residues was observed.
Tyrosine mutants interfere with nitration and cross-linking
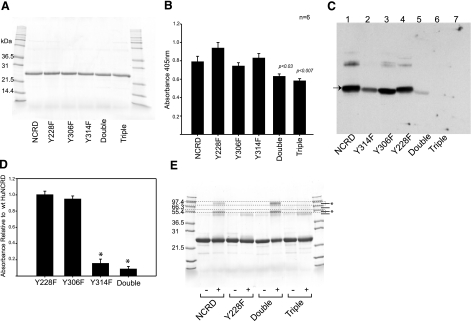
In order to assess the efficiency of modification at the 3 tyrosine residues and the contributions of tyrosine to the observed cross-linking, we expressed 5 tyrosine-deficient NCRDs. The substitution of phenylalanine removed the phenolic group, which is required for ONOO−-dependent tyrosine nitration or dityrosine formation, while preserving the aromatic ring, which might play important structural roles. All the mutant proteins were recovered as trimers, showed identical mobility on SDS-PAGE (Fig. 5A), and bound to solid-phase mannan (Fig. 5B). There was a small but significant decrease in mannan binding for the triple mutant, and possibly the double mutant.
Figure 5.
Tyrosine-deficient mutant NCRDs. Panel of tyrosine-deficient mutant NCRDs was generated by substituting phenylalanine (F) for tyrosine (Y) at positions 228, 306, and/or 314, as described in Materials and Methods. The 3 tyrosines are conserved in all known SP-Ds. There are no other tyrosines in the neck or lectin domain, and no tyrosine residues are found in the fusion tag. A) SDS-PAGE of mutant NCRDs. Equivalent amounts of purified mutant NCRD fusion protein (4 μg/lane) were reduced with DTT and resolved by SDS-PAGE on a 12% slab gel. Proteins were visualized by Coomassie blue staining. The 3 single-site mutants (Y228F, Y306F, and Y314F), a double mutant (Y306F+Y304F), and a triple mutant lacking tyrosine (Y228F+ Y306F+Y314F) are shown. B) Mannan binding assay. Carbohydrate binding activity was compared using a solid-phase mannan binding assay (30). Assays were performed in the absence and presence of maltose, and specific maltose-sensitive binding is shown; nonspecific binding was < 10%. Preliminary experiments demonstrated similar dose-dependent, saturable binding. Data are compiled from 6 independent experiments at a protein concentration of 20 μg/ml. Decrease in activity shown for double and triple mutants as compared to wild-type protein is small but statistically significant. C) Representative immunoblot showing nitration of mutants. Wild-type and mutant NCRD fusion proteins (8 μg) were exposed to ONOO− (1 mM) in a total reaction volume of 40 μl. Proteins (4 μg) were resolved by SDS-PAGE, and nitrated species were visualized by immunoblotting with anti-nitrotyrosine. Lane 1, wild-type NCRD; lane 2, Y314F; lane 3, Y306F; lane 4, Y228; lane 5, Y306F + Y314F; lane 6, triple mutant; lane 7, NCRD with inactivated ONOO− control. Arrow at left identifies the NCRD monomer. Differences in the distribution of larger cross-linked components are also evident; however, proportions of various forms cannot be reliably compared because of size-dependent differences in transfer efficiency. D) Summary of nitration data. Immunoblotting for nitrotyrosine was performed as for C. Nitration was quantified by densitometry; figure shows the mean ± se signal for the monomer band within the linear range of the film, normalized to the wild-type NCRD fusion protein. Data are derived from 5 independent experiments for Y314F, and 7 for other mutants. Signals for Y314F and double mutant were significantly decreased (∗). As illustrated in C, triple mutant showed no detectable signal, consistent with absence of tyrosine. E) ONOO−-dependent cross-linking of mutants. Wild-type and mutant NCRD fusion proteins were exposed to ONOO− as above, except the ONOO− concentration was increased to 3 mM to increase the extent of cross-linking. Proteins (6.5 μg) were reduced with DTT, resolved by SDS-PAGE, and visualized by staining with Coomassie blue. Regions containing major dimeric (*) and larger cross-linked species (*) are identified in dashed boxes. Note that Y228F shows an obvious decrease in both of these cross-linked components, while Y306F + Y314F shows the same pattern as wild type. Note also that the triple mutant shows minor residual cross-linked species consistent with tyrosine-independent modifications. Findings are representative of 3 experiments.
Equivalent amounts of the proteins were exposed to ONOO− and assayed for nitration by immunoblotting using films exposed within the linear range. As shown in Fig. 5C, D, Y228F and Y306F showed nitration at levels slightly lower than observed for the wild-type NCRD. By contrast, nitration was reduced for Y314F and Y306F + Y314F, with the residual nitration attributable to Y228. No nitration was observed following brief pH inactivation of ONOO− (data not shown). In addition, there was no detectable nitration of the triple mutant that lacks tyrosine (Fig. 5C), even on more prolonged exposures.
The fusion proteins were also examined by SDS-PAGE and protein staining to assess ONOO−-dependent cross-linking. Although cross-linking was detectable on nonoverloaded stained gels at 1 mM or lower, we used 3 mM ONOO− in an attempt to increase cross-linking and facilitate comparisons between mutants. As shown in Fig. 5E, ONOO− treatment of the hNCRD fusion protein gave at least 3 cross-linked species as resolved by SDS-PAGE in the presence of a reducing agent. The Y306F + Y314F double mutant showed the same distribution of cross-linked components as wild-type. However, these were greatly reduced for Y228F and the triple mutant, with only minor species migrating in the regions of components 2 and 3.
The cross-linked species are larger than shown in Fig. 2 because of the presence of the N-terminal tags. Nevertheless, the pattern of cross-linking is the same. Consistent with previous studies, the fusion proteins migrate with an apparent mass of 22-26 kDa following sulfhydryl reduction. Based on plots of mobility vs. log molecular weight, components migrating in the region of the first are 47-55 kDa, consistent with dimers, while components migrating in the region of the second are ∼87 kDa, most consistent with tetramers. As above, the results indicate that Y228 is involved in dimer and tetramer formation, while Y314 is the preferred site of nitration.
Murine lavage shows SP-D-dependent aggregation activity
SP-D dodecamers have the capacity to mediate bridging between microorganisms or other multivalent ligands. This can lead to lectin-dependent microbial aggregation (or agglutination), which can in turn modify responses of phagocytes and other host cells to the microbial challenge (38). For the current studies, we developed an aggregation assay using polystyrene beads with adsorbed purified ligand. The use of a defined particulate target offers convenience and reproducibility as compared to assays using live or killed organisms. Although there are many possible ligands, we selected rough E. coli J5 LPS (R3 chemotype) given its extensive characterization (36) and previous experience with the preparation of stable LPS-coated beads (39). Notably, previous studies have shown that SP-D is the major agglutinin for rough E. coli in rat lung lavage supernatant, and that the aggregating activity is selectively and efficiently removed by chromatography on maltosyl-agarose (35). This suggested that aggregation of rough E. coli LPS beads could provide a specific assay of SP-D activity in murine lavage, even in the presence of other carbohydrate binding proteins.
As shown in Fig. 6, purified SP-D dodecamers showed dose-dependent aggregation of the LPS beads. Aggregation, which was accompanied by an increase in transmittance, was blocked by competing maltose, the prototypical saccharide ligand (Fig. 6A). Aggregation was also blocked by EDTA or added phosphatidylinositol (data not shown). In addition, aggregating activity was blocked by antibody to mouse SP-D, but not control antibody (Fig. 6B).
Figure 6.
Inhibition of agglutinating activity. A) Aggregation of LPS beads by SP-D and murine lavage. Aggregation of E. coli J5 LPS beads by rat SP-D dodecamers (left) or lavage (right) was examined in the absence (black bars) or presence (white bars) of competing maltose as described in Materials and Methods. Relative pixel density indicated on the y axis. Inset: representative light micrograph of beads incubated with lavage in the absence and presence of maltose. Maltose largely prevents aggregation of the beads. SP-D dose response is a single assay. Lavage shows the mean ± se for at least 3 experiments. *P < 0.01 vs. control. Density shown for 2.5 μg/ml RrSP-D was near maximal for this type of assay. B) Inhibition of SP-D-dependent aggregation by anti-SP-D. A similar experiment was performed except that aggregation was examined in the absence (black bars) or presence of rabbit antiserum to mouse SP-D (white bars). Aggregation of beads by SP-D or lavage was largely blocked by antibody to SP-D, and there was no inhibition by an irrelevant control rabbit antiserum (Ctrl Ig, anti-laminin, gray bars). Inset: linear correlation between aggregating activity of dilution of unconcentrated murine lavage supernatant (0.25-0.5, abscissa) and density of the 43 kDa SP-D monomer in aliquots of the corresponding sample as assessed by immunoblot (ordinate). C) Effects of ONOO− on aggregation by lavage. Aggregation assays were performed as above except that SP-D or lavage was preincubated for 15 min with pH-inactivated ONOO− or 1 mM active ONOO−. Micrographs illustrate effects of ONOO− and inactivated ONOO− on bead ONOO− aggregation, as visualized by scanning of representative wells. For this experiment, rat SP-D dodecamers (RrSP-D, 1.25 μg/ml) were compared with 3-fold concentrated murine lavage, which contains a comparable amount of SP-D as assessed by immunoblotting. Preliminary studies confirmed that pH remained near neutral. Findings are representative of 3 independent experiments. D) Effects of ONOO− on lectin activity of purified and endogenous murine SP-D. Pull-down assay using LPS beads was performed as described in Materials and Methods. Bound SP-D was visualized by protein staining; specificity was confirmed using maltose as a competitor. Left panel (lanes 2-5): purified rat SP-D dodecamers (SP-D) were preincubated in the absence (−) or presence (+) of 1 mM ONOO− or with pH-inactivated ONOO− (iP) prior to incubation with beads in the absence or presence of 50 mM competing maltose. Right panel (lanes 7-10): unconcentrated murine lavage supernatant was similarly examined. Lanes 1 and 6 show protein standards. Findings are representative of 2 experiments.
As shown in Fig. 6A, murine lavage also caused efficient aggregation of the LPS beads. As for dodecamers, aggregation was largely blocked by maltose (Fig. 6A) and by anti-mouse SP-D (Fig. 6B). Using a dilution series of lavage supernatant, there was a direct linear relationship between the extent of aggregation and the amount of SP-D as estimated by immunoblotting (Fig. 6B, inset). Furthermore, immunoblotting and aggregation assays of lavage supernatant calibrated with purified native rat SP-D gave similar estimates of SP-D concentration (459±111 and 692±71 ng/ml; mean± se for 8 and 6 lavage samples, respectively). Together, the findings indicate that the J5 LPS bead aggregating activity of lavage is dependent on SP-D.
ONOO− inhibits the SP-D-dependent aggregating activity of murine lavage
As illustrated in Fig. 6C (bottom), 1 mM ONOO−, but not pH inactivated ONOO− (iP), greatly reduced the aggregating activity of lavage. Pull-down assays confirmed maltose-sensitive binding of murine lavage SP-D to the LPS beads (Fig. 6D, right). Consistent with the in vitro experiments with purified SP-D, there was no effect of ex vivo exposure to 1 mM ONOO− (+) or inactivated ONOO− on the binding of lavage SP-D to the LPS beads (Fig. 6D, right lanes), a process dependent on lectin activity. Thus, SP-D dependent aggregation by lavage is inhibited under conditions that do not appreciably alter lectin activity.
SP-D is nitrated and cross-linked by nitrogen dioxide in vivo
NO2 is widely recognized as a common environmental pollutant; however, formation can be triggered in the lung, for instance, via leukocyte peroxidase-catalyzed reaction of nitrite with hydrogen peroxide (9). NO2 is formed by the direct reaction of NO and O2 and the homolytic breakdown of ONOO−. NO2 is a potent nitrating agent. For these experiments C57BL/6 mice were acutely exposed to NO2 (10 or 20 ppm; 4 h), as described in the Methods section. Exposure resulted in lung injury as shown by physical signs (labored breathing with expiratory rales) during the latter part of the exposure. There was also an elevation of BAL protein (control: 132±3.4 μg/ml, n=5; NO2: 325±40.6 μg/ml, n=12; P<0.0003, Mann-Whitney test), indicating sufficient injury to cause high permeability edema. One advantage of the model is that effects occur rapidly and in an interval where alterations in SP-D production are less likely to confound interpretation.
In the first series of experiments, crude surfactant pellet, which was obtained by centrifugation of cell-free BAL at 14,000 g for 60 min, was resolved by SDS-PAGE after sulfhydryl reduction. Immunoblotting with a monoclonal anti-nitrotyrosine antibody revealed a nitrated species that comigrated with rat SP-D (43 kDa, reduced) in surfactant from NO2-exposed but not from air-exposed mice (data not shown). However, the majority of mouse SP-D accumulates in the lung in association with small rather than large lipid aggregates. Accordingly, subsequent studies used the cell-free lavage supernatant.
To assess the extent of nitration in the lavage supernatant and confirm specificity of the antibody to SP-D, supernatant from control or treated mice was subjected to 2-D IEF/SDS-PAGE prior to SYPRO staining and immunoblotting with anti-nitrotyrosine antibody. As shown in Fig. 7A (anti-NT, left panel), numerous nitrated species were observed. These included relatively acidic isoforms of ∼32-35 kDa, corresponding to SP-A. However, there were also more basic isoforms of ∼43 kDa, consistent with SP-D. The identity of the latter was confirmed by stripping the blots prior to reprobing with anti-SP-D (anti-SP-D, right panel). Superimposition of carefully registered, pseudocolored images confirmed extensive overlap of nitrotyrosine with the SP-D isoforms.
Figure 7.
Nitration and cross-linking of murine SP-D in vivo. A) Two-dimensional analysis of lavage. Mice were exposed to 10 ppm NO2, and aliquots of cell-free lavage were resolved by two-dimensional SDS-PAGE/IEF as described in Materials and Methods. Nitrated species were visualized by immunoblotting with anti-nitrotyrosine (anti-NT; left panel). Blot was then stripped and reprobed with anti-mouse SP-D (anti-SP-D; right panel). Components migrating in the expected position of SP-A (solid ellipse) and with the SP-D standard (dashed ellipse) are identified. Position of migration of reduced SP-D (43 kDa) is shown. Insert: in a separate experiment, superimposition of carefully registered, “pseudo-colored” images confirmed overlap (yellow) between SP-D isoforms (green) and nitrotyrosine (red). Higher molecular weight nitrated components were not specifically identified. No significant nitrated species were identified in aliquots of lavage from air-exposed controls (not shown). Appearance of the SP-D isoforms is typical and has been attributed to differences in sialylation (i.e., more heavily sialylated components are both slightly larger and more acidic). Comparable results were obtained in 3 experiments. B) Immunoprecipitation assay. Mice were exposed to 20 ppm NO2 or air. Aliquots of lavage supernatant were incubated with rabbit anti-mouse SP-D, and complexes were collected with Protein G beads. Complexes were solubilized in SDS sample buffer, and proteins were resolved by SDS-PAGE in the absence of sulfhydryl reduction prior to immunoblotting with mouse anti-NT (left panel). Following visualization of nitrated components, blots were stripped and reprobed with anti-SP-D (right panel). Lavage of NO2-treated mice contained a nitrated, immunoreactive species (bottom dashed box) that comigrated with nitrated recombinant rat SP-D (N-rSP-D). Higher molecular weight nitrated species were also identified (top dashed box). Loadings of nitrated rat SP-D standard were optimized to facilitate comparisons of electrophoretic mobility; higher loadings showed more obvious cross-linked components migrating near the position of aggregates formed in vivo. Note the presence of nitrated species (arrow, left) that does not react with anti-SP-D. The protein, which migrates more slowly than immunoglobulin heavy chains, was nonspecifically bound to the immune complex and/or beads. The increased intensity of this band in NO2-exposed animals was a reproducible finding.
To confirm nitration of SP-D, equivalent volumes of lavage supernatant were immunoprecipitated with anti-SP-D prior to resolution by SDS-PAGE in the absence or presence of sulfhydryl reduction. Immunoreactive, nitrated species were visualized by immunoblotting with mouse anti-nitrotyrosine antibody. To assist with localizing SP-D, the blots were stripped and reprobed with anti-SP-D. BAL from NO2-exposed mice, but not air-exposed controls, showed nitrated immunoreactive components that comigrated with nitrated rat SP-D (Fig. 7B, anti-NT). Notably, the treatment with 20 ppm NO2 resulted in the appearance of higher molecular weight cross-linked components of comparable size to those generated in vitro (Fig. 7B, anti-SP-D). For some animals, NO2 exposure was also associated with an obvious decrease in the intensity of normal SP-D trimer (e.g., Fig. 7B, anti-SP-D, lane 3). As illustrated in lane 1, control animals occasionally showed very low levels of nitrated SP-D.
NO2 exposure decreases the SP-D-dependent agglutinating activity of lavage
As shown in Fig. 8, exposure of mice to NO2 greatly decreased the ability of lavage supernatant to mediate the aggregation of E. coli J5 LPS beads. Immunoblots of unconcentrated lavage from the NO2-exposed animals showed no discernable decrease in immunoreactive SP-D (Fig. 8, inset). In fact, most, as illustrated here, showed a slight increase. Although the exposure is probably too short for increased SP-D production, SP-D is stored in the granules of bronchiolar cells and might be released in response to a toxic insult. In any case, nitration and cross-linking of SP-D in vivo were accompanied by a loss of SP-D-dependent aggregating activity, consistent with the ex vivo data for ONOO−.
Figure 8.
Decreased SP-D-dependent agglutinating activity of lavage from NO2-exposed mice. Mice were exposed to 20 ppm NO2 or air, and unconcentrated lavage was analyzed for SP-D-dependent agglutinating activity as described in Fig. 6. Data are shown for 4 air-exposed mice and 3 NO2-exposed mice. Corresponding control dose response with purified rat SP-D is shown at left. Blood-tinged lavage samples excluded from the analysis. Difference between control and NO2-exposed mice was reproducible and significant (P<0.007). Inset: aliquots of unconcentrated lavage supernatant from air- and NO2-exposed mice (10 μl) and purified rat SP-D dodecamer (5 ng) were resolved by SDS-PAGE in the presence of dithiothreitol and examined by immunoblotting with anti-SP-D. Despite the marked loss of SP-D-dependent aggregating activity, the amount of immunoreactive SP-D monomer (arrow, 43 kDa) was not decreased in lavage of NO2-exposed mice.
DISCUSSION
We have demonstrated that ONOO− can alter the structure and function of SP-D. ONOO− caused dose-dependent nitration and cross-linking of human SP-D with loss of aggregating activity in vitro, and inhibited the SP-D-dependent aggregating activity of murine lavage ex vivo. Specific nitration was confirmed by mass spectroscopy, and preferred sites of nitration and cross-linking were localized using a novel strategy of mutagenesis. Although most in vitro experiments used purified ONOO−, nitration and cross-linking also occurred with exposure to SIN-1, which provides prolonged exposure to lower concentrations of ONOO−, better mimicking the profile production by activated inflammatory cells. Furthermore, acute exposure of mice to NO2 in vivo was accompanied by nitration and cross-linking of SP-D with loss of its aggregating activity.
ONOO− and lectin activity
Because lectin activity is required for binding, as well as lectin-mediated aggregation of bound particles, damage to the CRD and/or abnormal protein cross-linking could theoretically account for a loss of aggregating activity following exposure to ONOO−. Examination of trimeric recombinant lectin domains confirmed dose-dependent nitration and cross-linking under the same treatment conditions used for the native protein. However, pull-down assays of ONOO−-treated dodecamers using E. coli J5 LPS beads showed no significant loss of binding activity. Preservation of carbohydrate binding activity is consistent with the absence of potential sites of nitration on the known binding surfaces of the CRD. Tyrosine 306 and 314 are located on the “back side” of the CRD, and Tyr228 is located near the neck/CRD junction. The findings are also consistent with the observation that the tyrosine double and triple mutants, which lack tyrosine in the lectin domain, showed no more than a small decrease in mannan binding activity.
As indicated in the Introduction, the related lung collectin, SP-A, can be nitrated within its CRD, and nitration is associated with loss of mannose and lipid binding activity (15, 40, 41). Nitrotyrosine was found at positions 164, 166, and 180, when numbered according to the mature protein (27). Although these sites of nitration do not involve the carbohydrate binding site, per se, crystallographic analysis indicates that Tyr164 is well exposed near the carbohydrate binding interface and contributes to a hydrophobic patch implicated in interactions with surfactant phospholipid (42). Notably, Tyr164 is replaced by alanine in SP-D.
Differential nitration of tyrosine
All 3 tyrosines within the NCRD were nitrated, as detected by mass spectroscopy. In addition, experiments using the panel of tyrosine-deficient NCRD mutants confirmed specific modification of tyrosine residues (i.e., no nitration of the tyrosine-free triple mutant) and demonstrated a strong preference for Tyr314. Given that the mutants are normally assembled and retain lectin activity, differences among protein in the extent of nitration and cross-linking are unlikely to reflect significant structural perturbations. However, we cannot entirely exclude context-dependent differences in the detection of specific nitrated residues with the anti-nitrotyrosine antibody. Although preferential nitration of Tyr314 was not evident from the mass spectroscopic analysis, the recovery of Glu-C peptides is not quantitative.
The differences in the efficiency of nitration revealed by the analysis of tyrosine mutants are consistent with known structure. Although the local environmental and conformational requirements for nitration are incompletely understood, solvent exposure has often been implicated (43). Notably, the phenolic group of 314 is partially solvent exposed near the posterior surface of the CRD. By contrast, Tyr306 shows low solvent exposure by crystallographic analysis. Nevertheless, modification of Tyr306 was identified in native SP-D, as well as the NCRDs, both by mass spectroscopy and immunoblotting of mutants. It could be significant that Tyr306 is flanked by a conserved proline (Pro307) and that phenolic group of the tyrosine side chain approximates the carboxylate group of Glu310. Nitrated residues are often found in close proximity to proline or glutamate residues (43). The mechanism of glutamate enhancement is not known; however, it has been suggested that nearby carboxylate groups can decrease the pKa of the tyrosine or participate in the formation of a nitronium-carboxylate intermediate (43).
Although Tyr228 is potentially buried within the coiled coil, crystallographic studies have demonstrated asymmetry of the 3 subunits of a trimer with differing solvent exposures of the side chain (44, 45). It has been suggested that this reflects an intrinsic flexibility of the CRD in relation to the neck. Thus, all 3 residues might have significant solvent exposure. Tyr228 occupies a key position within the unique C-terminal heptad repeat of the coiled-coil neck domain, 7 residues N-terminal to the proline that terminates the amphipathic helix. Thus, modification of this site might potentially influence the spatial distribution of the CRDs of a trimer or the stability of the trimer, thereby altering interactions with specific multivalent ligands as discussed below. Furthermore, it has been suggested that aromatic residues within the C-terminal heptad repeat of the coiled-coil trigger trimeric assembly (46). If confirmed, intracellular nitration of this conserved residue might alter trimerization, an event that is critical for the collagen helix formation and secretion, as well as the formation of functional trimeric lectin domains.
Human SP-D contains a fourth tyrosine (Tyr6) within the mature amino-terminal peptide. However, both rat and mouse SP-D lack tyrosine in the amino-peptide or collagenous domains and rat SP-D is efficiently cross-linked. Thus, the nitration and cross-linking of dodecamers observed in the NO2 injury model probably involve the C-terminal neck and/or lectin domains. However, contributions of other oxidative modifications cannot be excluded.
In this regard, recent studies have shown that cysteine residues in the N-peptide are susceptible to S-nitrosothiol formation with associated destabilization of SP-D multimers (47). It is important to note that peroxynitrite anions, per se, do not nitrosate cysteine, and nitrosation of cysteines is not expected in our in vitro experiments. However, N2O3, the product formed by the interaction of NO2 with O2, is an excellent nitrosating agent.
ONOO−-dependent cross-linking via Tyr228
Studies with the tyrosine mutants demonstrated that most of the ONOO−-dependent cross-linking of the NCRD is dependent on the presence of Tyr228. There was greatly reduced cross-linking of Y228F and the triple mutant, while cross-linking of Y306F + Y314F was even slightly increased as compared to wild type. In the absence of novel cross-links involving Tyr228, the data indicate the formation of dityrosine cross-links. Dimers (e.g., Fig. 5, arrow 2) could theoretically form via intra- or intertrimeric dityrosine cross-linking. However, the observed trimeric or quatrameric components (e.g., Fig. 5, arrow 3) could only form as a result of intertrimeric cross-linking. Although dimers could result from intratrimeric cross-linking of Y228, trimers or larger forms must include intertrimeric cross-links. Cross-linking between trimers would by necessity alter the spatial distribution of binding domains, thereby modifying bridging interactions required for aggregation.
It is not surprising that the major site of tyrosine-dependent cross-linking (Y228) differs from the major site of nitration (Y314). Tyrosine-dependent cross-linking and tyrosine nitration are fundamentally competitive reactions; nitrated residues do not participate in dityrosine formation. Although we have not yet confirmed the predicted dityrosine cross-link by mass spectroscopy, individual cross-linked species are generated in small yield, and many of cross-linked peptides are too large for spectroscopic analysis. Additional experiments using other proteases, combinations of proteolytic enzymes, and reduction and alkylation are planned.
Despite the importance of Tyr228-dependent cross-links, other types of cross-linking must also occur. This is indicated by the formation of minor cross-linked species following ONOO− treatment of Y228F and the triple mutant (Fig. 7). There were some mobility shifts of cross-linked components in the presence and absence of sulfhydryl reduction; however, these are readily attributed to the cross-linking of components stabilized by normal inter- and/or intrachain disulfide bonds. In other experiments, OxyBlot assays demonstrated ONOO− concentration-dependent carbonylation of SP-D dodecamers and tagless NCRDs, as well as the cross-linked forms of both proteins (data not shown). These oxidative modifications were evident at concentrations as low as 1 μM ONOO− and were blocked with scavengers. Thus, it is possible that some of the residual ONOO−-dependent modification results as a consequence of oxidative deamination of lysine or other residues. Although the N-terminal tags have no tyrosine, and similar cross-linked species were observed with tagless wild-type NCRDs, we cannot yet exclude the possibility that some of the residual cross-linking of the mutant fusion proteins involves the tags.
Potential mechanism of defective aggregation
Multimerization of trimeric SP-D subunits is important, if not critical, for aspects of SP-D function. The 4 arms of SP-D molecules are characteristically arrayed in pairs, suggesting that apposing pairs of trimeric lectin domains could be important for mediating effective bridging interactions between particles. No more than 2 chains of a 12-chain molecule would need to be cross-linked to cross-link trimeric subunits or molecules, and either type of cross-linking within the neck domain could alter the spatial organization of the lectin domains.
It is important to emphasize that aggregation is not a simple all-or-none or disorganized phenomenon. Our assays with LPS beads allow us to easily quantify defective formation of large aggregates, but are not sensitive to aggregation involving small numbers of beads. Although maltose can eliminate microaggregation (i.e., by blocking lectin activity), peroxynitrite treatment does not (Fig. 6C). Aggregates of multivalent lectins with multivalent carbohydrate ligands consist of highly organized cross-linked lattices, influenced by the protein valency and structure of the carbohydrate. It is possible that abnormal cross-linking of SP-D is associated with effects on lattice formation that preferentially interfere with the formation of large aggregates. In much the same way as small amounts of abnormally folded proteins can interfere with protein crystal formation, even small subpopulations of abnormal SP-D molecules could alter the ability of SP-D to mediate the formation of large aggregates. Notably, our published studies of SP-D proteolysis have shown that aggregation is a much more sensitive assay of degradation than simple ligand binding (23).
Nitration of SP-D in vivo
In the current studies, we confirmed nitration in vivo using a model of acute NO2 exposure. Inhaled nitrogen dioxide concentrations are highest at the level of the distal airways and alveolar ducts, major sites of SP-D production and accumulation. Presence of a variety of target molecules, as well as a variety of scavengers including thiols and urate, could result in differences between in vivo and in vitro nitration efficiency and ensuing modifications. In addition, the effective concentration in the peripheral lung is probably much lower than the delivered concentration. Given the short-term exposure, observed effects on cross-linking and aggregating activity could be predominantly by NO2 per se. However, high concentrations can also be locally generated at sites of cell injury and active inflammation. In this regard, myeloperoxidase (MPO)-deficient mice show decreased nitration of lavage proteins in a model of gram-negative bacterial pneumonia (48).
Previous studies have demonstrated specific degradation of SP-D by neutrophil-derived serine proteases (23, 24); more recently, oxidative damage has been observed in vitro (26). Given that these enzymes and MPO are packaged in the same granules and discharged together in the vicinity of SP-D at sites of acute inflammation, we hypothesize that the local balance between specific reactive species, antioxidants, active proteases, and protease inhibitors will determine the nature and extent of functional inactivation and/or depletion of SP-D in various inflammatory disorders. Although we have thus far focused on acute injuries involving neutrophils, SP-D is internalized by macrophages in conjunction with the clearance of LPS in vivo (49) and is synthesized by a variety of epithelial cells in the lung and other tissues. Both classes of cells could similarly contribute to modifications of SP-D structure and function through the elaboration of reactive nitrogen species and oxidants at sites of tissue injury and active inflammation.
Potential consequences of defective aggregation
Microbial aggregation is required for the efficient SP-D-dependent neutralization of specific pathogens, particularly respiratory viruses, such as influenza A virus (50). For example, SP-D dependent aggregation correlates with increased macrophage uptake and killing of Klebsiella pneumoniae in vitro (21). In addition, multimers, but not trimeric subunits, can inhibit adhesion of gram-negative organisms to lung epithelial cells, a likely critical event for colonization (51). There is also recent evidence suggesting collectin aggregates can augment the inflammatory responses of macrophages (52). If this is true for SP-D, abnormal aggregation of SP-D molecules as a consequence of nitration or oxidative modifications might elicit or augment inflammatory reactions in the lung, as well as impairments in host defense.
In summary, our studies show that exposure of SP-D to reactive oxygen-nitrogen intermediates result in posttranslational modifications, both in vitro and in vivo. These modifications are accompanied by functional alterations that could alter the host response to microbial challenge.
Acknowledgments
We thank Sharmin Sheikh and Bruce Linders for technical assistance, and Janet North for administrative support. We thank Dr. Edward Postlethwait and his lab for performing the nitrogen dioxide exposures. We also thank German Fierro Perez for work performed early in the course of this investigation. The studies were supported by U.S. National Institutes of Health grants HL-44015 and HL-29594 (E.C.C.) and UO1ES015676, HL075540, and HL311197 (S.M.).
References
- Kishore U, Greenhough T J, Waters P, Shrive A K, Ghai R, Kamran M F, Bernal A L, Reid K B, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Whitsett J A. Surfactant proteins in innate host defense of the lung. Biol Neonate. 2005;88:175–180. doi: 10.1159/000087580. [DOI] [PubMed] [Google Scholar]
- Wright J R. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D (SP-D) on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- Crouch E C. Surfactant protein D and pulmonary host defense. Respir Res. 2000;1:93–108. doi: 10.1186/rr19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore U, Reid K B. Structures and functions of mammalian collectins. Results Probl Cell Differ. 2001;33:225–248. doi: 10.1007/978-3-540-46410-5_12. [DOI] [PubMed] [Google Scholar]
- Eiserich J P, Cross C E, Jones A D, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid: a novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- Van der Vliet A, Eiserich J P, Halliwell B, Cross C E. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite: a potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gole M D, Souza J M, Choi I, Hertkorn C, Malcolm S, Foust R F, III, Finkel B, Lanken P N, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;278:L961–L967. doi: 10.1152/ajplung.2000.278.5.L961. [DOI] [PubMed] [Google Scholar]
- Cochrane C G, Spragg R, Revak S D. Pathogenesis of the adult respiratory distress syndrome: evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983;71:754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznajder J I, Fraiman A, Hall J B, Sanders W, Schmidt G, Crawford G, Nahum A, Factor P, Wood L D. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. Chest. 1989;96:606–612. doi: 10.1378/chest.96.3.606. [DOI] [PubMed] [Google Scholar]
- Quinlan G J, Lamb N J, Tilley R, Evans T W, Gutteridge J M. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med. 1997;155:479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- Zhu S, Basiouny K F, Crow J P, Matalon S. Carbon dioxide enhances nitration of surfactant protein A by activated alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1025–L1031. doi: 10.1152/ajplung.2000.278.5.L1025. [DOI] [PubMed] [Google Scholar]
- Zhu S, Kachel D L, Martin W J, Matalon S. Nitrated SP-A does not enhance adherence of Pneumocystis carinii to alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 1998;275:L1031–L1039. doi: 10.1152/ajplung.1998.275.6.L1031. [DOI] [PubMed] [Google Scholar]
- Zhu S, Haddad I Y, Matalon S. Nitration of Surfactant Protein A (SP-A) tyrosine residues results in decreased mannose binding ability. Arch Biochem Biophys. 1996;333:282–290. doi: 10.1006/abbi.1996.0392. [DOI] [PubMed] [Google Scholar]
- Matalon S, DeMarco V, Haddad I Y, Myles C, Skimming J W, Schurch S, Cheng S, Cassin S. Inhaled nitric oxide injures the pulmonary surfactant system of lambs in vivo. Am J Physiol Lung Cell Mol Physiol. 1996;270:L273–L280. doi: 10.1152/ajplung.1996.270.2.L273. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ware L B, Geiser T, Matthay M A, Matalon S. Increased levels of nitrate and surfactant protein A nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med. 2001;163:166–172. doi: 10.1164/ajrccm.163.1.2005068. [DOI] [PubMed] [Google Scholar]
- Postle A D, Mander A, Reid K B M, Wang J Y, Wright S M, Moustaki M, Warner J O. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20:90–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- Greene K E, Wright J R, Steinberg K P, Ruzinski J T, Caldwell E, Wong W B, Hull W, Whitsett J A, Akino T, Kuroki Y, Nagae H, Hudson L D, Martin T R. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996;109:1006–1009. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- Herbein J F, Wright J R. Enhanced clearance of surfactant protein D during LPS-induced acute inflammation in rat lung. Am J Physiol Lung Cell Mol Physiol. 2001;281:L268–L277. doi: 10.1152/ajplung.2001.281.1.L268. [DOI] [PubMed] [Google Scholar]
- Hirche T O, Crouch E C, Espinola M, Brokelman T J, Mecham R P, DeSilva N, Cooley J, Remold-O'Donnell E, Belaaouaj A. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J Biol Chem. 2004;279:27688–27698. doi: 10.1074/jbc.M402936200. [DOI] [PubMed] [Google Scholar]
- Griese M, Wiesener A, Lottspeich F, von Bredow C. Limited proteolysis of surfactant protein D causes a loss of its calcium-dependent lectin functions. Biochim Biophys Acta. 2003;1638:157–163. doi: 10.1016/s0925-4439(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Von Bredow C, Wiesener A, Griese M. Proteolysis of Surfactant protein D by cystic fibrosis relevant proteases. Lung. 2003;181:79–88. doi: 10.1007/s00408-003-1008-z. [DOI] [PubMed] [Google Scholar]
- Starosta V, Griese M. Oxidative damage to surfactant protein D in pulmonary diseases. Free Radic Res. 2006;40:419–425. doi: 10.1080/10715760600571248. [DOI] [PubMed] [Google Scholar]
- Greis K D, Zhu S, Matalon S. Identification of nitration sites on surfactant protein A by tandem electrospray mass spectrometry. Arch Biochem Biophys. 1996;335:396–402. doi: 10.1006/abbi.1996.0522. [DOI] [PubMed] [Google Scholar]
- Haddad I Y, Crow J P, Hu P, Ye Y, Beckman J, Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol Lung Cell Mol Physiol. 1994;267:L242–L249. doi: 10.1152/ajplung.1994.267.3.L242. [DOI] [PubMed] [Google Scholar]
- Hartshorn K L, Crouch E C, White M R, Eggleton P, Tauber A I, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E, Tu Y, Briner D, McDonald B, Smith K, Holmskov U, Hartshorn K. Ligand specificity of human surfactant protein D: expression of a mutant trimeric collectin that shows enhanced interactions with influenza A virus. J Biol Chem. 2005;280:17046–17056. doi: 10.1074/jbc.M413932200. [DOI] [PubMed] [Google Scholar]
- Crouch E, McDonald B, Smith K, Cafarella T, Seaton B, Head J. Contributions of phenylalanine 335 to ligand recognition by human surfactant protein D: ring interactions with SP-D ligands. J Biol Chem. 2006;281:18008–18014. doi: 10.1074/jbc.M601749200. [DOI] [PubMed] [Google Scholar]
- Crouch E, McDonald B, Smith K, Roberts M, Mealy T, Seaton B, Head J. Critical role of Arg/Lys343 in the species-dependent recognition of phosphatidylinositol by pulmonary surfactant protein D. Biochemistry. 2007;46:5160–5169. doi: 10.1021/bi700037x. [DOI] [PubMed] [Google Scholar]
- Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch M, Lomonosova E E, Korth H G, Sustmann R, de Groot H. Hydrogen peroxide formation by reaction of peroxynitrite with HEPES and related tertiary amines: implications for a general mechanism. J Biol Chem. 1998;273:12716–12724. doi: 10.1074/jbc.273.21.12716. [DOI] [PubMed] [Google Scholar]
- Kuan S F, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides: surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Head J, Kosma P, Brade H, Muller-Loennies S, Sheikh S, McDonald B, Smith K, Cafarella T, Seaton B, Crouch E. Recognition of heptoses and the inner core of bacterial lipopolysaccharides by surfactant protein D. Biochemistry. 2008;47:710–720. doi: 10.1021/bi7020553. [DOI] [PubMed] [Google Scholar]
- Velsor L W, Postlethwait E M. NO2-induced generation of extracellular reactive oxygen is mediated by epithelial lining layer antioxidants. Am J Physiol Lung Cell Mol Physiol. 1997;273:L1265–L1275. doi: 10.1152/ajplung.1997.273.6.L1265. [DOI] [PubMed] [Google Scholar]
- Crouch E, Wright J R. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- Ofek I, Mesika A, Kalina M, Keisari Y, Podschun R, Sahly H, Chang D, McGregor D, Crouch E. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect Immun. 2001;69:24–33. doi: 10.1128/IAI.69.1.24-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad I Y, Zhu S, Ischiropoulos H, Matalon S. Nitration of surfactant protein A results in decreased ability to aggregate lipids. Am J Physiol Lung Cell Mol Physiol. 1996;270:L281–L288. doi: 10.1152/ajplung.1996.270.2.L281. [DOI] [PubMed] [Google Scholar]
- Davis I C, Zhu S, Sampson J B, Crow J P, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic Biol Med. 2002;33:1703–1713. doi: 10.1016/s0891-5849(02)01170-x. [DOI] [PubMed] [Google Scholar]
- Head J F, Mealy T R, McCormack F X, Seaton B A. Crystal structure of trimeric carbohydrate recognition and neck domains of surfactant protein A. J Biol Chem. 2003;278:43254–43260. doi: 10.1074/jbc.M305628200. [DOI] [PubMed] [Google Scholar]
- Souza J M, Daikhin E, Yudkoff M, Raman C S, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys. 1999;371:169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- Kovacs H, O'Ddonoghue S I, Hoppe H J, Comfort D, Reid K B, Campbell D, Nilges M. Solution structure of the coiled-coil trimerization domain from lung surfactant protein D. J Biomol NMR. 2002;24:89–102. doi: 10.1023/a:1020980006628. [DOI] [PubMed] [Google Scholar]
- Shrive A K, Tharia H A, Strong P, Kishore U, Burns I, Rizkallah P J, Reid K B, Greenhough T J. High-resolution structural insights into ligand binding and immune cell recognition by human lung surfactant protein D. J Mol Biol. 2003;331:509–523. doi: 10.1016/s0022-2836(03)00761-7. [DOI] [PubMed] [Google Scholar]
- Hoppe H J, Barlow P N, Reid K B. A parallel three stranded alpha-helical bundle at the nucleation site of collagen triple-helix formation. FEBS Lett. 1994;344:191–195. doi: 10.1016/0014-5793(94)00383-1. [DOI] [PubMed] [Google Scholar]
- Guo C J, Atochina-Vasserman E N, Abramova E, Foley J P, Zaman A, Crouch E, Beers M F, Savani R C, Gow A J. S-Nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 2008;6:e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut J P, Byun J, Tran H D, Lauber W M, Carroll J A, Hotchkiss R S, Belaaouaj A, Heinecke J W. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rozendaal B A, van de Lest C H, Van Eijk M, Van Golde L M, Voorhout W F, van Helden H P, Haagsman H P. Aerosolized endotoxin is immediately bound by pulmonary surfactant protein D in vivo. Biochim Biophys Acta. 1999;1454:261–269. doi: 10.1016/s0925-4439(99)00042-3. [DOI] [PubMed] [Google Scholar]
- Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- Sahly H, Ofek I, Podschun R, Brade H, He Y, Ullmann U, Crouch E. Surfactant Protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich O-antigens. J Immunol. 2002;169:3267–3274. doi: 10.4049/jimmunol.169.6.3267. [DOI] [PubMed] [Google Scholar]
- Gardai S J, Xiao Y Q, Dickinson M, Nick J A, Voelker D R, Greene K E, Henson P M. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]