Abstract
The blood-brain barrier (BBB) plays an important role in HIV trafficking into the brain and the development of the central nervous system complications in HIV infection. Tight junctions are the main structural and functional elements that regulate the BBB integrity. Exposure of human brain microvascular endothelial cells (hCMEC/D3 cell line) to HIV-infected monocytes resulted in decreased expression of tight junction proteins, such as junctional adhesion molecule-A (JAM)-A, occludin, and zonula occludens (ZO)-1. Control experiments involved exposure to uninfected monocytes. Alterations of tight junction protein expression were associated with increased endothelial permeability and elevated transendothelial migration of HIV-infected monocytes across an in vitro model of the BBB. Notably, overexpression of the peroxisome proliferator-activated receptor (PPAR)α or PPARγ attenuated HIV-mediated dysregulation of tight junction proteins. With the use of exogenous PPARγ agonists and silencing of PPARα or PPARγ, these protective effects were connected to down-regulation of matrix metalloproteinase (MMP) and proteasome activities. Indeed, the HIV-induced decrease in the expression of JAM-A and occludin was restored by inhibition of MMP activity. Moreover, both MMP and proteasome inhibitors attenuated HIV-mediated altered expression of ZO-1. The present data indicate that down-regulation of MMP and proteasome activities constitutes a novel mechanism of PPAR-induced protections against HIV-induced disruption of brain endothelial cells.—Huang, W., Eum, S. Y., András, I. E., Hennig, B., Toborek, M. PPARα and PPARγ attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities.
Keywords: peroxisome proliferator-activated receptor, human immunodeficiency virus-1, brain endothelial cells, blood-brain barrier
The blood-brain barrier (BBB) is formed by brain capillary endothelial cells in a close association with astrocytes and pericytes. Tight junctions are essential structural components of the BBB. They are located between adjacent endothelial cells and restrict most molecular traffic across the brain endothelium (1). Tight junctions are composed of transmembrane proteins, such as occludin, claudins, and junctional adhesion molecules (JAMs), as well as accessory proteins, such as zonula occludens (ZO)-1. Occludin was the first tight junction protein discovered (2) and appears to be critical for the formation of paracellular barriers (3). JAMs are also important components of tight junctions that regulate transendothelial permeability (4). ZO proteins are the peripheral membrane-associated components of tight junctions (5). The members of the ZO family interact with the transmembrane tight junction proteins and link them with cytoskeleton. For example, ZO-1 binding is required for occludin localization at tight junctions (6). ZO-1 can also interact with JAM through its PDZ (PSD-95/discs large/zona occludens 1) domain (7).
Dysfunction of the BBB in the course of HIV infection has been observed and confirmed in a variety of pathological studies (8, 9) and cerebrospinal fluid (CSF) studies (10) and by dynamic magnetic resonance imaging (11). Disruption of the BBB appears to be critical for HIV trafficking into the brain (12). Alterations of the barrier function of the brain endothelium may also affect brain homeostasis and viability of neurons (1, 13). Thus, disruption of the BBB integrity may be directly involved in the development of central nervous system (CNS) and neurological complications in the later stages of HIV infection (12, 14, 15). At the structural level, the BBB dysfunction is linked to alterations of tight junction structure and functions. Indeed, disruptions of tight junctions are common in HIV-infected patients. For example, fragmentation and decreased immunoreactivity for occludin and ZO-1 were detected in the brains of HIV-infected patients (16, 17). These changes were associated with the accumulation of HIV-infected macrophages, fibrinogen leakage, and apoptosis (16, 18, 19).
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the family of the nuclear receptor gene family. Vascular cells, including endothelial and smooth muscle cells, express two members of the PPAR family, namely, PPARα and PPARγ (20,21,22). One of the major biological effects of PPARs is down-regulation of oxidative stress-sensitive and inflammatory signaling pathways (23, 24). In addition, inflammatory responses induced in brain endothelial cells by treatment with Tat or HIV-infected lymphocytes can be attenuated by stimulation of PPARα and PPARγ activities (25, 26). These effects appear to be mediated at the levels of redox-regulated transcription factors, such as nuclear factor-κB (NF-κB), and/or promoter activities of proinflammatory genes (25, 26). Thus, PPAR agonists emerged as highly promising drugs in HIV infection, especially because they are already being used in treatment of HIV-associated lipodystrophy (27).
Because well-developed tight junctions are responsible for the integrity of the BBB, the aim of the present study was to evaluate the role of PPARα and PPARγ in HIV-mediated disruption of tight junction proteins. Our results indicate that both HIV-induced alterations of tight junction protein expression and transendothelial migration of infected monocytes were attenuated by stimulation of PPARα or PPARγ. Most important, we provide evidence that PPARs can protect against HIV-mediated dysfunction of brain endothelial cells by attenuating matrix metalloproteinase (MMP) and proteasome activities.
MATERIALS AND METHODS
Cell cultures
A human brain microvascular endothelial cell line, called hCMEC/D3, was recently developed and described (28). The cells were cultured in EBM-2 medium (Clonetics, East Rutherford, NJ, USA) supplemented with vascular endothelial growth factor, insulin-like growth factor-1, epidermal growth factor, basic fibroblast growth factor, hydrocortisone, ascorbate, gentamicin, and 2.5% fetal bovine serum (FBS) as originally described (28). Collagen type I (BD Biosciences Pharmingen, San Jose, CA, USA) was used for coating the cell culture dishes.
hCMEC/D3 lines overexpressing PPARα or PPARγ were generated by inserting the full sequence of human PPARα or PPARγ into the pcDNA3 plasmid driven by the cytomegalovirus promoter (26). Cells lines overexpressing PPARα or PPARγ have been named hCMEC/D3/PPARα or hCMEC/D3/PPARγ, respectively. In selected experiments, PPARγ activity was stimulated in normal hCMEC/D3 cells by treatment with 15-deoxy-12,14-prostaglandin J2 (15d-PGJ2; 5 μM; Calbiochem, San Diego, CA, USA) or troglitazone (10 μM; Sigma, St. Louis, MO, USA). To decrease PPAR activity, hCMEC/D3 cells were transfected with 80 nM control small-interfering RNA (siRNA; Dharmacon, Chicago, IL, USA) or with 80 nM PPARα or PPARγ specific siRNA (Invitrogen, Carlsbad, CA, USA) using GeneSilencer (Genlantis, San Diego, CA, USA) as described earlier (29).
In the majority of experiments, hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells were exposed to HIV-infected monocytes or uninfected controls as described below. In selected experiments, hCMEC/D3 cells were pretreated for 2 h with MMP or proteasome inhibitors at the final concentration of 5 μM. The MMP9-specific inhibitor was purchased from Calbiochem, GM 6001 (a general MMP inhibitor) was from Biomol (Plymouth Meeting, PA, USA), and both proteasome inhibitors [MG-132 and proteasome inhibitor I (PSI)] were from Calbiochem. MG-132 is a reversible inhibitor of the chymotrypsin- and trypsin-like activity (30), and PSI is a reversible inhibitor of the chymotrypsin-like activity (31).
Human monocytic cells (U937 cells) were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). The human astrocytic cell line (SVGA cells) was cultured in Dulbecco modified Eagle’s medium (DMEM; Sigma) supplemented with 10% FBS and penicillin plus streptomycin (100 U/ml and 100 μg/ml, respectively). All cultures were maintained at 37°C and 5% CO2.
HIV-1 stock generation and monocyte infection
HIV-1 stock was generated in human embryonic kidney 293T cells (American Type Culture Collection, Manassas, VA, USA). The cells were grown in DMEM containing 10% FBS and antibiotics mixture (100 U/ml penicillin and 100 μg/ml streptomycin) and maintained at 37°C and 5% CO2. When cultures reached ∼70% confluence, the cells were transfected with the HIV-1 PYK-JRCSF plasmid carrying 0.5 kb of 3′-flanking sequences and 2.2 kb of 5′-flanking DNA (32, 33). Transfection procedure was performed with calcium phosphate according to the procedure provided by the manufacturer (Invitrogen). Briefly, 20 μg PYK-JRCSF plasmid and 36 μl 2M CaCl2 were mixed with sterile H2O in a final volume of 300 μl. The mixture was then slowly added to 300 μl 2× HEPES buffer. The mixture was incubated at room temperature for 30 min and added to the media of 293T cells cultured on 100 mm dishes. After the overnight transfection, cells were washed and incubated for 24 h in RPMI 1640 medium supplemented with 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Cell culture supernatant containing HIV-1 stock was collected, filtered through 0.45 μm filters (Millipore, Bedford, MA, USA), and frozen at −80°C. HIV p24 antigen was assessed by ELISA (ZeptoMetrix, Buffalo, NY, USA) as the marker of HIV infection.
HIV stock was used to infect U937 cells. Briefly, 5 × 106 U937 cells were infected with viral isolate containing 120 ng of p24 by overnight incubation at 37°C and 5% CO2. Cells were then washed, resuspended in fresh medium, and maintained for an additional 3–5 days to facilitate viral replication. The levels of HIV p24 used in all experiments in the present study were between 140–180 ρg/μl, which correspond to those present in serum of AIDS patients (34, 35). Uninfected U937 cells served as controls.
Coculture models to evaluate permeability and monocyte transmigration
The hCMEC/D3 cell line is known to express relatively low transendothelial electrical resistance values (28). Therefore, the integrity of the barrier function was measured by permeability of FITC Dextran-4 across a model system composed of cocultures of hCMEC/D3 cells with astrocytes. The coculture systems result in increased expression of the BBB-specific properties. Indeed, the baseline permeability values across hCMEC/D3 monolayers are higher by ∼40–50% as compared with cocultures of hCMEC/D3 cells with astrocytes (data not shown). Cocultures of hCMEC/D3 cells with astrocytes were generated as described earlier (36) on the opposite sides of inserts of the 12-well Transwell system with collagen-coated polycarbonate membranes (pore size of 0.4 μm for permeability studies or 3 μm for transendothelial migration; Corning Costar, Corning, NY, USA). Astrocytes (1×105 cells) were plated on the lower surface of the membrane and allowed to attach for 5 h. Then, the inserts were placed upright and hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells (1×105 cells) were plated onto the upper site of the membrane. The upper chamber contained complete EBM-2 medium suitable for hCMEC/D3 cells, and the lower chamber contained DMEM containing 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) to support astrocyte growth.
For permeability studies, cocultures of hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells with astrocytes were exposed for 24 h to 0.5 × 106 HIV-infected or uninfected human monocytes (U937 cells) per well added to the top chamber. Then, the cells were washed three times with Kreb-Ringer glucose buffer and incubated for 30 min with FITC Dextran-4 (1 mg/ml; Sigma) added to the upper chamber. Aliquots of 100 μl from the lower chamber were collected for fluorescent measurement using 480 and 530 nm for excitation and emission, respectively.
The cocultures of hCMEC/D3 cells with astrocytes were also used for monocyte transmigration studies. Briefly, HIV-infected or uninfected human monocytes (U937 cells) were labeled with 5 μM calcein-AM (Calbiochem). Then, 1 × 106 labeled monocytes were added to the top chambers of the Transwell system and transmigration was allowed for 24 h in a cell culture incubator. Fluorescence was measured in aliquots of 100 μl collected from the bottom chambers using 488 and 535 nm for excitation and emission, respectively.
Western blotting
Confluent hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells cultured on 6-well plates were exposed for 24 h to 1.5 × 106 HIV-infected or uninfected monocytes per well in the presence or absence of specific MMP or proteasome inhibitors. Protein was extracted using the RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and centrifuged at 15,000 g for 15 min. The supernatants were collected and protein concentrations were determined using BCA protein assay kit (Pierce, Rockford, IL, USA). Samples were separated on 4–15% Tris-HCL Ready SDS-polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA, USA), transferred onto nitrocellulose membrane (Bio-Rad Laboratories), and incubated with the respective antibodies. Anti-ZO-1, JAM-A, and occludin antibodies were purchased from Zymed (San Francisco, CA, USA). Anti-actin antibody was purchased from Sigma, and all secondary antibodies were from Santa Cruz Biotechnology. For visualization of detected proteins, immunoblots were analyzed using an ECL Western blot detection kit (Amersham Biosciences, Piscataway, NJ, USA). Quantification of immunoreactive bands was performed by scanning densitometry using UN-SCAN-IT gel image analysis software (Silk Scientific, Orem, UT, USA).
MMP activity assays
Specific MMP-2 and MMP-9 activities were detected by gelatin zymography (37) performed on premade 10% polyacrylamide gels containing 0.1% gelatin according to the instruction provided by the manufacturer (Invitrogen, Carlsbad, CA). The bands were visualized by staining for 30 min with a solution containing 0.1% Coomassie R-250 in 40% ethanol and 10% acetic acid, followed by destaining for 2 h at room temperature in a solution containing 10% ethanol and 7.5% acetic acid. Band densitometry was determined using UN-SCAN-IT gel image analysis software (Silk Scientific).
Proteasome activity assays
The chymotrypsin-like (ChT-L), trypsin-like (T-L), and peptidylglutamyl-peptide hydrolyzing (PGPH) proteasome activities were measured using specific fluorogenic substrates, Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin, Boc-Leu-Ser-Thr-Arg-AMC, and Z-Leu-Leu-Glu-AMC, respectively, as described previously (38). All substrates were purchased from Sigma. Briefly, cells were cultured on 6-well plates and confluent cultures were exposed to HIV-infected or uninfected monocytes for 24 h. Cells were then washed with cold PBS and lysed in 300 μl proteasome activity buffer (10 mM Tris-HCl, pH 7.8; 1 mM EDTA; 0.5 mM dithiothreitol; 0.5% Triton X-100; and 5 mM MgCl2). Protein concentrations were determined by BCA protein assay kit (Pierce). Then, 200 μg protein extracts were incubated with individual fluorogenic substrates (each at the concentration of 100 μM) for 120 min at 37°C in 200 μl total reaction volume. The reaction was stopped by addition of 1% SDS (final concentration). Fluorescence was monitored on a fluorometer at 360 nm excitation and 450 nm emission.
Statistical analysis
Each experiment was repeated a minimum of three times. Data are expressed as means ± se. Statistical analysis was completed using Sigma-Stat 2.03 (SPSS, Chicago, IL, USA). One-way or two-way ANOVA was used to compare mean responses among the treatments. A statistical probability of P < 0.05 was considered significant.
RESULTS
PPAR overexpression protects against HIV-induced hyperpermeability and increased transendothelial monocyte migration
Viral proteins, chemokines, and cytokines affect endothelial permeability and promote monocyte trafficking in HIV-associated encephalitis. Therefore, we evaluated the effects of increased PPARα and PPARγ activity on HIV-induced alterations of endothelial barrier function and transendothelial migration of monocytes.
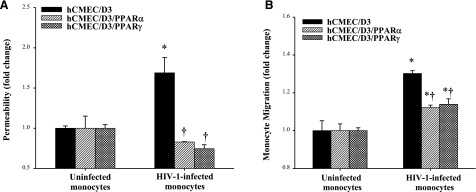
The barrier function was analyzed using an in vitro model of the BBB constructed with cocultures of hCMEC/D3 cell with astrocytes and exposed to HIV-infected or uninfected monocytes. As indicated in Fig. 1A, exposure to HIV-infected monocytes markedly increased permeability across the BBB model. Most important, HIV-induced hyperpermeability was effectively protected by overexpression of PPARα or PPARγ in hCMEC/D3 cells. Indeed, the flux of FITC Dextran-4 was at the same level in hCMEC/D3/PPARα or hCMEC/D3/PPARγ cells exposed to HIV-infected monocytes as in cultures incubated with uninfected monocytes.
Figure 1.
Overexpression of PPARα or PPARγ attenuates HIV-1-induced disruption of transendothelial permeability and monocyte migration. The in vitro BBB model consisted of cocultures of hCMEC/D3 cells and astrocytes and was constructed as described in Materials and Methods. A) hCMEC/D3 cells were exposed to control or HIV-infected monocytes (0.5×106 cells/well) for up to 24 h. The flux of FITC Dextran-4 across the hCMEC/D3 monolayers was assessed as a marker of endothelial permeability. B) hCMEC/D3 cells were exposed to control or HIV-1-infected monocytes (1×106 cells/well) for 24 h. Results are means ± se of 3 separate experiments. *P < 0.05 vs. respective control. †P < 0.05 vs. normal hCMEC/D3 cultures.
The in vitro model of the BBB was also used to assess the effects of PPAR overexpression on transendothelial migration of monocytes. As shown in Fig. 1B, HIV-infected monocytes migrated in a higher number as compared with uninfected controls. However, transendothelial migration of HIV-infected monocytes was significantly reduced in hCMEC/D3 cells overexpressing PPARα or PPARγ (Fig. 1B).
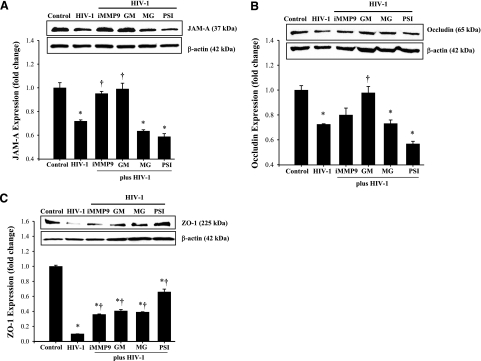
HIV-induced alterations of tight junction protein expression are attenuated in hCMEC/D3 cells overexpressing PPARα or PPARγ
Because tight junctions are the main structural element that regulates the barrier function of the BBB, we studied the effects of increased PPARα and PPARγ activity on HIV-mediated alterations of tight junction protein expression (Fig. 2). Incubation of hCMEC/D3 cells with HIV-infected monocytes for 24 h resulted in a significant decrease in JAM-A (Fig. 2A), occludin (Fig. 2B), and ZO-1 (Fig. 2C). The most affected tight junction proteins were JAM-1 and ZO-1, which decreased to almost nondetectable levels in hCMEC/D3 cells cocultured with HIV-infected monocytes. Notably, overexpression of PPARα or PPARγ markedly attenuated HIV-induced down-regulation of tight junction protein expression (Fig. 2).
Figure 2.
HIV-1-induced decrease in tight junction protein expression is attenuated in hCMEC/D3 cells overexpressing PPARα or PPARγ. Confluent hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells cultured on 6-well plate Transwell systems were exposed to control or HIV-1-infected monocytes (1.5×106 per well) for 24 h. Expression of JAM-A (A), occludin (B), and ZO-1 (C) was determined by Western blotting. Blots are representative images from 3 independent experiments; bar graphs depict quantified results. Results are means ± se of 3 separate experiments. *P < 0.05 vs. respective control. †P < 0.05 vs. normal hCMEC/D3 cultures exposed to infected monocytes. #P < 0.05 vs. baseline values (hCMEC/D3 cultures exposed to uninfected monocytes).
Overexpression of PPARα or PPARγ diminishes HIV-induced MMP-2 and MMP-9 activities
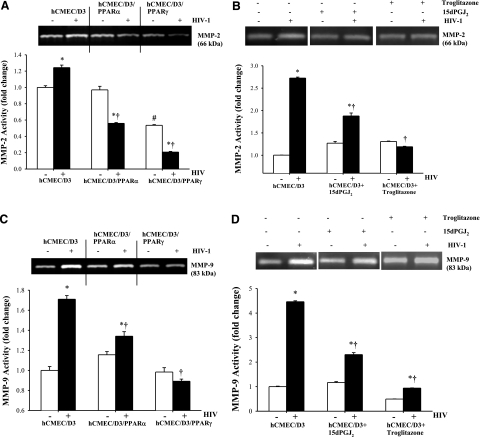
Our studies focused then on the role of MMP-2 and MMP-9 in HIV-induced disruption of tight junction protein expression. Both MMP-2 and MMP-9 are known to be induced in a variety of pathological conditions, including those related to HIV infection of the CNS (39, 40). In addition, recent studies (41,42,43) indicated that MMPs may contribute to the modulation of tight junction protein expression. Gelatinase assays revealed that exposure to HIV-infected monocytes increased both MMP-2 and MMP-9 activities in hCMEC/D3 cells (Fig. 3). Although these effects were attenuated by overexpression of PPARα or PPARγ (Fig. 3A, C), the protective effects of PPARγ were more pronounced. To further confirm these results, PPARγ activity was induced in normal hCMEC/D3 cells by treatment with 15d-PGJ2 or troglitazone. As indicated in Fig. 3B, D, both PPARγ agonists significantly protected against HIV-induced MMP-2 and MMP-9 activities.
Figure 3.
Overexpression of PPARα or PPARγ protects against HIV-1-induced MMP-2 and MMP-9 activities. Confluent hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells were incubated with control or HIV-1-infected monocytic cells for 24 h (A, C). In addition, selected hCMEC/D3 cultures were treated with the PPARγ agonists 15d-PGJ2 (5 μM) or troglitazone (10 μM) for 2 h, followed by a 24 h exposure to HIV-1-infected or control monocytes (B, D). MMP-2 (A, B) and MMP-9 (C, D) activities were detected by zymography in cell culture supernatants as described in Materials and Methods. Specific bands for MMP-2 and MMP-9 were identified by their apparent molecular mass of 66 and 83 kDa, respectively. Results are means ± se of 4 separate experiments. *P < 0.05 vs. respective control. †P < 0.05 vs. normal hCMEC/D3 cultures. #P < 0.05 vs. baseline values.
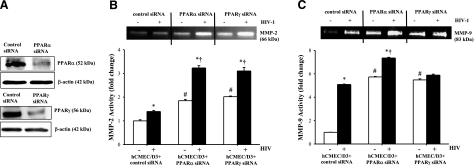
Silencing of PPARα or PPARγ potentiates HIV-induced MMP-2 and MMP-9 activities
To further evaluate the role of PPARs in the regulation of MMP activity, PPARα or PPARγ was silenced in hCMEC/D3 cells, followed by exposure to HIV-infected or uninfected monocytes. Exposure to specific PPARα or PPARγ siRNA resulted in a 70–80% reduction of the individual PPAR proteins (Fig. 4A). Consistent with the results shown in Fig. 3, hCMEC/D3 exposure to HIV-infected monocytes significantly increased both MMP-2 and MMP-9 activities. Notably, these effects were further potentiated in hCMEC/D3 cells with silenced PPARα or PPARγ (Fig. 4B, C).
Figure 4.
Silencing of PPARα or PPARγ potentiates HIV-1-induced MMP activity. PPARα or PPARγ was silenced in normal hCMEC/D3 cells as described in Materials and Methods. Silencing procedure resulted in a 70–80% depletion of individual PPAR proteins (A). hCMEC/D3 cells transfected with control or PPARα- or PPARγ-specific siRNA were exposed to HIV-1-infected or control monocytes for 24 h, followed by determination of MMP-2 (B) and MMP-9 (C) activities by zymography. Results are means ± se of 4 separate experiments. *P < 0.05 vs. respective control. †P < 0.05 vs. control siRNA treatment. #P < 0.05 vs. baseline values.
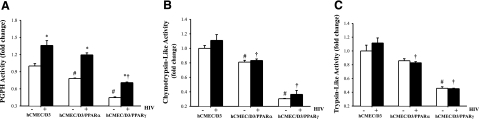
PPAR overexpression decreases baseline proteasome activity and protects against HIV-induced PGPH activation
Proteasome may be another source of proteolytic activity involved in the regulation of tight junction protein levels. Therefore, we evaluated the effects of HIV on proteasome activity in hCMEC/D3 cells. As shown in Fig. 5A, exposure to HIV-infected monocytes increased PGPH activity in hCMEC/D3 cells as compared with uninfected monocytes. In contrast, ChT-L and T-L proteasome activities were not affected by cocultures with HIV-infected monocytes (Fig. 5B, C). Overexpression of PPARγ and, to a lesser extent, PPARα diminished the baseline proteasome activities in hCMEC/D3cells. Indeed, all studied proteasome activities were markedly decreased in hCMEC/D3/PPARγ cells cocultured with HIV-infected monocytes as compared with normal hCMEC/D3 cells exposed to HIV-infected monocytes. A protective influence on ChT-L and T-L activities, but not on PGPH activity, was also observed in hCMEC/D3/PPARα cells cocultured with HIV-infected monocytes.
Figure 5.
Exposure to HIV-1 differentially stimulates proteasome activity in hCMEC/D3 cells. Confluent hCMEC/D3, hCMEC/D3/PPARα, or hCMEC/D3/PPARγ cells were exposed to HIV-1-infected or control monocytes for 24 h. Cells were then harvested and lysed, and PGPH (A), ChT-L (B), T-L (C) proteasome activities were determined using fluorogenic substrates, as described in Materials and Methods. Results are means ± se of 3 separate experiments. *P < 0.05 vs. respective controls. †P < 0.05 vs. normal hCMEC/D3 cultures. #P < 0.05 vs. baseline values.
MMP and proteasome activities are differentially involved in HIV-induced alterations of tight junction protein expression
To address the hypothesis that MMPs and proteasome may be involved in HIV-induced disruption of tight junctions, hCMEC/D3 cells were cocultured with HIV-infected monocytes in the presence of specific MMP or proteasome inhibitors. Then, tight junction proteins, such as JAM-A, occludin, and ZO-1, were assessed by Western blotting. As indicated in Fig. 6A, a decrease in JAM-A expression induced by the exposure to HIV-infected monocytes was reversed by both GM6001 (a general inhibitor of MMP activity) and the MMP-9-specific inhibitor. On the other hand, proteasome inhibitors did not affect HIV-induced alterations of JAM-A levels. A decrease in occludin expression in HIV-exposed hCMEC/D3 cells was prevented by inhibition of the total MMP activity by GM6001 (Fig. 6B). Inhibition of MMP-9 activity and blocking of proteasome activity by MG132 and PSI were ineffective. Changes in ZO-1 in hCMEC/D3 cells cocultured with HIV-infected monocytes were attenuated by all MMP and proteasome inhibitors used in the current study (Fig. 6C).
Figure 6.
MMP and proteasome activities differentially regulate HIV-1-induced alterations of tight junction protein expression. Confluent hCMEC/D3 cells were pretreated with MMP or proteasome inhibitors for 2 h and exposed to HIV-1-infected monocytes for 24 h. JAM-A (A), occludin (B), and ZO-1 (C) expression was determined by Western blotting. Blots are representative images from 3 independent experiments; bar graphs depict quantified results. Results are means ± se. *P < 0.05 vs. control. †P < 0.05 vs. exposure to HIV-1 in absence of inhibitors. iMMP9, MMP9 specific inhibitor; GM, GM 6001 (a general MMP inhibitor); MG, MG-132 (proteasome inhibitor); PSI, proteasome inhibitor I.
DISCUSSION
Disruption of the BBB and dysfunction of brain endothelial cells are believed to be involved in HIV trafficking into the brain (14, 15, 44). Indeed, interaction of HIV with brain endothelial cells results in activation of inflammatory responses, such as induction of CCL-2 (45), stimulation of a variety of proinflammatory signaling pathways (16, 46), and disruption of the barrier function of brain endothelial cells (16, 47). These events may directly influence HIV entry into the CNS. In agreement with these earlier reports, our current data support the observations that exposure of brain endothelial cells to HIV-infected monocytes stimulates endothelial hyperpermeability and transendothelial migration (Fig. 1). The novel results of the present study demonstrate that stimulation of PPAR activity can provide protection against HIV-induced alterations of expression of tight junction proteins and disruption of barrier function in an in vitro model of the BBB. Our results also provide evidence that modulation of proteolytic activity of MMPs and proteasome is responsible, at least in part, for these protective effects.
PPARs are ligand-activated transcription factors that are considered to be critical rescue molecules that can down-regulate activation of redox-responsive transcription factors, such as NF-κB, and exert anti-inflammatory effects. PPARγ agonists such as rosiglitazone and metformin have also been used in treatment of lipodystrophy in HIV patients (48). The rationale for this therapy is related to the fact that decreased expression of PPARγ1 and PPARγ2 appears to contribute to the pathogenesis of fibronecrotic changes in insulin-resistant lipodystrophic HIV-infected patients (49). However, there is only limited information indicating that PPARs may be involved in protection against HIV-induced dysfunction of brain endothelial cells (25).
PPARα and PPARγ play a regulatory role in expression of numerous genes, including those involved in inflammatory responses (50). For example, PPAR activation was demonstrated to block CCL2-directed monocyte migration (51). Similarly, retinal leukostasis and leakage were suppressed by treatment with rosiglitazone in experimental diabetic rats (52). Treatment with PPAR agonists has been introduced in a variety of inflammatory disorders, such as colonic inflammation (53) or inflammatory bowel disease (54). PPARγ was also demonstrated to be involved in the regulation of tight junction protein expression in human urothelial cells (55). In agreement with these reports, overexpression of PPARα and PPARγ in the present study markedly protected against HIV-induced endothelial hyperpermeability and transmigration of infected monocytes (Fig. 1).
Novel results of the present study indicate that increased expression of PPARα and PPARγ attenuated the HIV-induced decrease in tight junction expression via protection against enhanced MMP and proteasome activities. Specific proteolytic activities of MMP-2 and MMP-9 were markedly reduced in hCMEC/D3 cells overexpressing PPARα or PPARγ and cocultured with HIV-infected monocytes. The protective effects were also observed in hCMEC/D3 cells exposed to PPARγ agonists 15d-PGJ2 or troglitazone and then exposed to HIV (Fig. 3). However, PPARγ appeared to be more potent in attenuating HIV-1-induced up-regulation of MMP-2 and MMP-9 than PPARα (Fig. 3A, C). Similar effects were observed in comparing the anti-inflammatory effects of PPARα and PPARγ (26). Although PPARα and PPARγ exert extensive homology, these transcription factors are differentially regulated by specific coactivators, which add an additional step in gene regulation and their biological effects (56). The association between PPARs and MMP activation was additionally confirmed in gene silencing experiments (Fig. 4).
MMPs are a family of zinc-dependent endopeptidases that can degrade both basement membranes and extracellular matrix components and thus can facilitate migration of inflammatory cells across the endothelium and contribute to HIV-1 pathology (57, 58). Elevated levels of MMP-9 have been reported in the CSF of HIV-infected children (59) and adult patients (60). In addition, increased CNS levels of MMP-2 and MMP-9 appear to be more frequent in patients with neurological complications of HIV infections, such as HIV-associated dementia (39). Increased production of MMP-2 by astrocytes has also been linked to HIV infection (61). Finally, elevated levels of MMP-2 and MMP-9 were reported in the brains of severe combined immune deficiency mouse model of HIV encephalitis (62). In line with these research reports, our data indicate that exposure of hCMEC/D3 cells to HIV-infected monocytes results in a significant increase in the specific activities of MMP-2 and MMP-9. Most important, our results provide evidence that increased activity of MMPs can directly participate in HIV-induced disruption of tight junction protein expression. Indeed, a broad-spectrum inhibitor GM6001 effectively protected against HIV-induced alterations of JAM-A and occludin levels (Fig. 6A, B). Both JAM-A and occludin are transmembrane tight junction proteins that seal the neighboring cells and are responsible for the barrier function of the brain endothelium.
In light of the present study, it is plausible to suggest that HIV-induced disruption of transmembrane tight junction proteins may result, at least in a major part, from MMP-mediated proteolysis. To support this notion, recent data implicated MMPs in the regulation of tight junction expression (42, 43). As shown in a hypoxia/reperfusion model of stroke in spontaneously hypertensive rats, increased MMP expression may lead to degradation of tight junction proteins and opening of the BBB (42). Moreover, it was demonstrated that oxidative stress, via the tyrosine kinase-dependent mechanisms, can activate MMP-1, -2, and -9 and decrease tissue inhibitors of MMPs (43). These events have been directly linked to enhanced tyrosine phosphorylation of tight junction proteins and the disruption of the BBB (43).
In contrast to the transmembrane tight junction proteins, inhibition of MMP activity was less pronounced in the protection against HIV-mediated alterations of ZO-1 levels (Fig. 6C). ZO-1 belongs to the family of the tight junction accessory proteins that link the transmembrane proteins with the cytoskeleton. Different regulation of these proteins by MMPs indicates a finely tuned involvement of proteolytic enzymes in the regulation of expression of individual tight junction proteins and the integrity of the BBB.
In addition to MMPs, proteasome activity also appears to be involved in the regulation of tight junction protein expression. For example, it was demonstrated that proteasome may modulate the interaction between gap junction and tight junction proteins, such as connexin 43 and ZO-1 (63). Evidence also suggested that proteasome may alter the rate of occludin degradation (64). Therefore, we evaluated the role of proteasome in HIV-mediated alterations of tight junction proteins. The proteolytic core of the proteasome is referred to as the 20S proteasome, and it is composed of multiple a- and b-subunits. Several b-subunits have catalytic sites that exert peptidylglutamyl-hydrolyzing (β1-subunit), trypsin-like (β2-subunit), or chymotrypsin-like (β5-subunit) activity for cleavage after acidic, basic, or hydrophobic residues, respectively (65). Therefore, all three types of enzymatic activity of the proteasome system were included in our experiments (Fig. 5).
The role of proteasome in HIV infection is not fully understood. It has been suggested that inhibition of proteasome in target cells can enhance the infectivity of HIV at the early stages of infection (66). On the other hand, evidence indicates that the proteasome pathway plays a critical role in HIV assembly, maturation, and budding (65). Thus, it is important to note that exposure to HIV-infected monocytes specifically increased proteasome PGPH activity in the present study (Fig. 5). These effects were observed in normal hCMEC/D3 cells as well as in hCMEC/D3 cells overexpressing PPARα or PPARγ. Our studies also indicated that overexpression of PPARα and, even to a greater extent, PPARγ can decrease both the baseline and HIV-induced proteasome activities. The mechanisms of these effects may be associated with the fact that the proteasome is actively involved in proteolysis of damaged (e.g., oxidized) proteins and proteins involved in inflammatory reactions (65). Thus, cellular overexpression of PPARs, due to their antioxidant and anti-inflammatory properties, may protect against baseline oxidative modification of proteins and thus attenuate proteasome activity.
HIV-induced PGPH activity may play a significant pathological role in HIV infection and the disruption of the BBB integrity. For example, increased proteasome activity may contribute to HIV-induced inflammatory responses by proteasome-mediated degradation of IκB, followed by stimulation of NF-κB, a transcription factor involved in stimulation of a variety of proinflammatory genes. These mechanisms are consistent with increased oxidative stress and stimulation of chronic inflammatory responses in HIV infection (65). Taking under consideration the pathological role of proteasome activation, proteasome inhibition was proposed as a therapeutic strategy in HIV infection. Indeed, proteasome inhibitors were demonstrated to interfere with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2 (67). It should be pointed out that several protease inhibitors used for highly active antiretroviral therapy (HAART) also exert inhibitory effects on the proteasome activity (19).
The present results provide a novel observation that proteasome inhibition can attenuate HIV-mediated alterations of ZO-1 expression in hCMEC/D3 cells (Fig. 6C). The mechanisms by which proteasome can affect the expression of tight junction accessory proteins are currently not known. However, ZO protein expression can be regulated by redox-regulated cellular signaling, including the Ras pathway (68). Because inhibition of proteasome can protect against inflammatory reactions, it may indirectly regulate expression of tight junction proteins. In addition, the proteasome can be directly involved in the proteolysis of ZO-1 (69).
In conclusion, exposure of hCMEC/D3 cells to HIV-infected monocytes resulted in endothelial hyperpermeability, increased transendothelial migration, and decreased tight junction protein expression. These effects were associated with increased MMP and proteasome activities. Most important, HIV-mediated alterations of tight junction protein expression and proteolytic activity were markedly attenuated by stimulation of PPARα or PPARγ in hCMEC/D3 cells. The present data indicate that PPARs play important roles in protecting against HIV induced dysfunction of brain endothelial cells by attenuating MMP and proteasome activities. Targeting PPARs may provide a novel therapeutic approach to attenuate the dysfunction of the brain endothelium associated with HIV infection.
Acknowledgments
This work was supported by grants MH-63022, MH-072567, and NS-39254. PYK-JRCSF was obtained through the AIDS Research and Reference Reagent Program, National Institute on Aging, National Institutes of Health.
References
- Abbott N J, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B R, Siliciano J D, Mooseker M S, Goodenough D A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Schulz C U, Meyer Zu Brickwedde M K, Pendl G G, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- Rhodes R H, Ward J M. AIDS meningoencephalomyelitis. Pathogenesis and changing neuropathologic findings. Pathol Annu. 1991;26:247–276. [PubMed] [Google Scholar]
- Krebs F C, Ross H, McAllister J, Wigdahl B. HIV-1-associated central nervous system dysfunction. Adv Pharmacol. 2000;49:315–385. doi: 10.1016/s1054-3589(00)49031-9. [DOI] [PubMed] [Google Scholar]
- Singer E J, Syndulko K, Fahy-Chandon B, Schmid P, Conrad A, Tourtellotte W W. Intrathecal IgG synthesis and albumin leakage are increased in subjects with HIV-1 neurologic disease. J Acquir Immune Defic Syndr. 1994;7:265–271. [PubMed] [Google Scholar]
- Avison M J, Nath A, Berger J R. Understanding pathogenesis and treatment of HIV dementia: a role for magnetic resonance? Trends Neurosci. 2002;25:468–473. doi: 10.1016/s0166-2236(02)02234-8. [DOI] [PubMed] [Google Scholar]
- Wu D T, Woodman S E, Weiss J M, McManus C M, D'Aversa T G, Hesselgesser J, Major E O, Nath A, Berman J W. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6:S82–S85. [PubMed] [Google Scholar]
- Hawkins B T, Davis T P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Toborek M, Lee Y W, Flora G, Pu H, Andras I E, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Poluektova L. Immune privilege and HIV-1 persistence in the CNS. Immunol Rev. 2006;213:180–194. doi: 10.1111/j.1600-065X.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber G A, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven L A, Middel J, Verhoef J, De Groot C J, Nottet H S. Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol Appl Neurobiol. 2000;26:356–360. doi: 10.1046/j.1365-2990.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- Dallasta L M, Pisarov L A, Esplen J E, Werley J V, Moses A V, Nelson J A, Achim C L. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini M, Mostert M, Rinaudo M T. Proteasomes as drug targets. Curr Drug Targets. 2003;4:657–671. doi: 10.2174/1389450033490759. [DOI] [PubMed] [Google Scholar]
- Cabrero A, Laguna J C, Vazquez M. Peroxisome proliferator-activated receptors and the control of inflammation. Curr Drug Targets Inflamm Allergy. 2002;1:243–248. doi: 10.2174/1568010023344616. [DOI] [PubMed] [Google Scholar]
- Marx N, Bourcier T, Sukhova G K, Libby P, Plutzky J. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- Israelian-Konaraki Z, Reaven P D. Peroxisome proliferator-activated receptor-alpha and atherosclerosis: from basic mechanisms to clinical implications. Cardiol Rev. 2005;13:240–246. doi: 10.1097/01.crd.0000137255.54390.12. [DOI] [PubMed] [Google Scholar]
- Hayes M M, Lane B R, King S R, Markovitz D M, Coffey M J. Peroxisome proliferator-activated receptor gamma agonists inhibit HIV-1 replication in macrophages by transcriptional and post-transcriptional effects. J Biol Chem. 2002;277:16913–16919. doi: 10.1074/jbc.M200875200. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Madrigal J L, Lizasoain I, Moro M A, Lorenzo P, Leza J C. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry. 2005;57:885–894. doi: 10.1016/j.biopsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Ramirez S H, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol. 2008;180:1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Rha G B, Han M J, Eum S Y, Andras I E, Zhong Y, Hennig B, Toborek M. PPARalpha and PPARgamma effectively protect against HIV-induced inflammatory responses in brain endothelial cells. J Neurochem. 2008;107:497–509. doi: 10.1111/j.1471-4159.2008.05626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin D, Hadigan C, Lehrke M, Mazza S, Lazar M A, Grinspoon S. Resistin levels in human immunodeficiency virus-infected patients with lipoatrophy decrease in response to rosiglitazone. J Clin Endocrinol Metab. 2005;90:3423–3426. doi: 10.1210/jc.2005-0287. [DOI] [PubMed] [Google Scholar]
- Weksler B B, Subileau E A, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male D K, Roux F, Greenwood J, Romero I A, Couraud P O. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Akasaka Y, Ide T, Hara T, Kobayashi N, Utsumi M, Murakami K. Highly sensitive upregulation of apolipoprotein A-IV by peroxisome proliferator-activated receptor alpha (PPARalpha) agonist in human hepatoma cells. Biochem Pharmacol. 2007;74:1738–1746. doi: 10.1016/j.bcp.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Sixt S U, Beiderlinden M, Jennissen H P, Peters J. Extracellular proteasome in the human alveolar space: a new housekeeping enzyme? Am J Physiol Lung Cell Mol Physiol. 2007;292:L1280–L1288. doi: 10.1152/ajplung.00140.2006. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira M E, Berg K A, Wilk S. A new inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces accumulation of ubiquitin-protein conjugates in a neuronal cell. J Neurochem. 1994;63:1578–1581. doi: 10.1046/j.1471-4159.1994.63041578.x. [DOI] [PubMed] [Google Scholar]
- Cann A J, Zack J A, Go A S, Arrigo S J, Koyanagi Y, Green P L, Pang S, Chen I S. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J Virol. 1990;64:4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras I E, Rha G, Huang W, Eum S, Couraud P O, Romero I A, Hennig B, Toborek M. Simvastatin protects against amyloid beta and HIV-1 Tat-induced promoter activities of inflammatory genes in brain endothelial cells. Mol Pharmacol. 2008;73:1424–1433. doi: 10.1124/mol.107.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale J M, Isaacs M D, Contreras P, Gomez B, Lozano L, Austin E, De Martin M C, Gregory R L, McLaughlin G L, Amador A. Immunological markers of disease progression in patients infected with the human immunodeficiency virus. Clin Diagn Lab Immunol. 1997;4:474–477. doi: 10.1128/cdli.4.4.474-477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M M, McKinley G, Englard A, Grieco M H. Effect of azidothymidine (AZT) on P24 antigen levels in patients with AIDS-related complex and AIDS. J Clin Lab Anal. 1989;3:199–201. doi: 10.1002/jcla.1860030402. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Stins M, Way D, Witte M H, Weinand M, Kim K S, Bock P, Gendelman H E, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- Pereira A M, Strasberg-Rieber M, Rieber M. Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin Exp Metastasis. 2005;22:285–295. doi: 10.1007/s10585-005-8672-8. [DOI] [PubMed] [Google Scholar]
- Vieira O, Escargueil-Blanc I, Jurgens G, Borner C, Almeida L, Salvayre R, Negre-Salvayre A. Oxidized LDLs alter the activity of the ubiquitin-proteasome pathway: potential role in oxidized LDL-induced apoptosis. FASEB J. 2000;14:532–542. doi: 10.1096/fasebj.14.3.532. [DOI] [PubMed] [Google Scholar]
- Conant K, McArthur J C, Griffin D E, Sjulson L, Wahl L M, Irani D N. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Liuzzi G M, Mastroianni C M, Santacroce M P, Fanelli M, D'Agostino C, Vullo V, Riccio P. Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. J Neurovirol. 2000;6:156–163. doi: 10.3109/13550280009013159. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol S M, Khazen S, Dijkstra C D, de Vries H E. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J. 2006;20:2550–2552. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada E Y, Thompson J F, Liu W, Rosenberg G A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez S H, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Banks W A, Ercal N, Price T O. The blood-brain barrier in neuroAIDS. Curr HIV Res. 2006;4:259–266. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- Eugenin E A, Osiecki K, Lopez L, Goldstein H, Calderon T M, Berman J W. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne G D, Schall K, Leibhart J, Knipe B, Gendelman H E, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras I E, Pu H, Tian J, Deli M A, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- Van Wijk J P, de Koning E J, Cabezas M C, op't Roodt J, Joven J, Rabelink T J, Hoepelman A I. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med. 2005;143:337–346. doi: 10.7326/0003-4819-143-5-200509060-00009. [DOI] [PubMed] [Google Scholar]
- Lemoine M, Barbu V, Girard P M, Kim M, Bastard J P, Wendum D, Paye F, Housset C, Capeau J, Serfaty L. Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS. 2006;20:387–395. doi: 10.1097/01.aids.0000206503.01536.11. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol. 2006;6:421–427. doi: 10.1016/j.coph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Goetze S, Wakino S, Kim S, Nagpal S, Chandraratna R A, Graf K, Fleck E, Hsueh W A, Law R E. Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. Eur J Pharmacol. 2000;401:259–270. doi: 10.1016/s0014-2999(00)00461-1. [DOI] [PubMed] [Google Scholar]
- Muranaka K, Yanagi Y, Tamaki Y, Usui T, Kubota N, Iriyama A, Terauchi Y, Kadowaki T, Araie M. Effects of peroxisome proliferator-activated receptor gamma and its ligand on blood-retinal barrier in a streptozotocin-induced diabetic model. Invest Ophthalmol Vis Sci. 2006;47:4547–4552. doi: 10.1167/iovs.05-1432. [DOI] [PubMed] [Google Scholar]
- Takaki K, Mitsuyama K, Tsuruta O, Toyonaga A, Sata M. Attenuation of experimental colonic injury by thiazolidinedione agents. Inflamm Res. 2006;55:10–15. doi: 10.1007/s00011-005-0002-8. [DOI] [PubMed] [Google Scholar]
- Lytle C, Tod T J, Vo K T, Lee J W, Atkinson R D, Straus D S. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis. 2005;11:231–243. doi: 10.1097/01.mib.0000160805.46235.eb. [DOI] [PubMed] [Google Scholar]
- Varley C L, Garthwaite M A, Cross W, Hinley J, Trejdosiewicz L K, Southgate J. PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol. 2006;208:407–417. doi: 10.1002/jcp.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A C, Palinski W. Peroxisome proliferator-activated receptors: how their effects on macrophages can lead to the development of a new drug therapy against atherosclerosis. Annu Rev Pharmacol Toxicol. 2006;46:1–39. doi: 10.1146/annurev.pharmtox.46.120604.141247. [DOI] [PubMed] [Google Scholar]
- Rumbaugh J, Turchan-Cholewo J, Galey D, St Hillaire C, Anderson C, Conant K, Nath A. Interaction of HIV Tat and matrix metalloproteinase in HIV neuropathogenesis: a new host defense mechanism. FASEB J. 2006;20:1736–1738. doi: 10.1096/fj.05-5619fje. [DOI] [PubMed] [Google Scholar]
- Zozulya A L, Reinke E, Baiu D C, Karman J, Sandor M, Fabry Z. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J Immunol. 2007;178:520–529. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoig C, Castrejon M M, Saavedra-Lozano J, Castano E, Baez C, Lanier E R, Saez-Llorens X, Ramilo O. Cerebrospinal fluid and plasma concentrations of proinflammatory mediators in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23:114–118. doi: 10.1097/01.inf.0000109247.67480.7a. [DOI] [PubMed] [Google Scholar]
- Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel F D, Pfister H W. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–857. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- Lopez-Herrera A, Liu Y, Rugeles M T, He J J. HIV-1 interaction with human mannose receptor (hMR) induces production of matrix metalloproteinase 2 (MMP-2) through hMR-mediated intracellular signaling in astrocytes. Biochim Biophys Acta. 2005;1741:55–64. doi: 10.1016/j.bbadis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, Rasmussen J, Zheng J, Gearing A, Gendelman H E. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol. 2001;114:57–68. doi: 10.1016/s0165-5728(00)00454-9. [DOI] [PubMed] [Google Scholar]
- Girao H, Pereira P. The proteasome regulates the interaction between Cx43 and ZO-1. J Cell Biochem. 2007;102:719–728. doi: 10.1002/jcb.21351. [DOI] [PubMed] [Google Scholar]
- Lui W Y, Lee W M. cAMP perturbs inter-Sertoli tight junction permeability barrier in vitro via its effect on proteasome-sensitive ubiquitination of occludin. J Cell Physiol. 2005;203:564–572. doi: 10.1002/jcp.20254. [DOI] [PubMed] [Google Scholar]
- Louie J L, Kapphahn R J, Ferrington D A. Proteasome function and protein oxidation in the aged retina. Exp Eye Res. 2002;75:271–284. [PubMed] [Google Scholar]
- Dueck M, Guatelli J. Evidence against a direct antiviral activity of the proteasome during the early steps of HIV-1 replication. Virology. 2007;361:1–8. doi: 10.1016/j.virol.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Ott D E, Chertova E N, Welker R, Tessmer U, Princiotta M F, Bennink J R, Krausslich H G, Yewdell J W. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci U S A. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Smart E J, Weksler B, Couraud P O, Hennig B, Toborek M. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci. 2008;28:7788–7796. doi: 10.1523/JNEUROSCI.0061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]