Abstract
An understanding of nuclear reprogramming is fundamental to the use of cells in regenerative medicine. Due to technological obstacles, the time course and extent of reprogramming of cells following fusion has not been assessed to date. Here, we show that hundreds of genes are activated or repressed within hours of fusion of human keratinocytes and mouse muscle cells in heterokaryons, and extensive changes are observed within 4 days. This study was made possible by the development of a broadly applicable approach, species-specific transcriptome amplification (SSTA), which enables global resolution of transcripts derived from the nuclei of two species, even when the proportions of species-specific transcripts are highly skewed. Remarkably, either phenotype can be dominant; an excess of primary keratinocytes leads to activation of the keratinocyte program in muscle cells and the converse is true when muscle cells are in excess. We conclude that nuclear reprogramming in heterokaryons is rapid, extensive, bidirectional, and dictated by the balance of regulators contributed by the cell types.—Adam Palermo, Regis Doyonnas, Nidhi Bhutani, Jason Pomerantz, Ozan Alkan, Helen M. Blau. Nuclear reprogramming in heterokaryons is rapid, extensive, and bidirectional.
Keywords: gene expression, time course, microarrays, somatic fate change, balance of regulators
Strong interest in the mechanisms that regulate nuclear reprogramming has been spurred by recent studies showing that fusion with embryonic stem (ES) cells (1,2,3) or the introduction of a few defined factors (4, 5) can revert somatic cells to a pluripotent state. Somatic cells can also be directly reprogrammed toward a specific differentiated fate following fusion of two distinct cell types to form proliferating hybrids (6,7,8) or postmitotic hybrids called heterokaryons (9,10,11,12,13,14,15,16). The generation of one adult phenotype directly from another as an alternative to reprogramming somatic cells to an intermediate stem cell state will have broad implications in regenerative medicine. A striking limitation of many of the studies published to date is the paucity of information regarding the molecular characterization of reprogramming, due primarily to technological impediments. Although the dominance of ES cells in reprogramming was demonstrated in ES cell-fibroblast tetraploid hybrids by gene expression profiling (2), it was not possible in these same-species fusion products to determine the timing or extent of reprogramming because the hybrids were analyzed after numerous passages and the contribution of the two nuclei could not be distinguished. On the other hand, in studies of nuclear reprogramming toward a differentiated fate in nondividing interspecific heterokaryons, only the changes in expression of a small number of genes were analyzed, using either species-specific reverse transcriptase-polymerase chain reaction (RT-PCR) or immunohistochemistry (9,10,11,12,13,14,15,16), as gene expression microarrays cannot adequately distinguish the expression profiles of human and mouse transcripts due to cross-hybridization (17,18,19,20). As a result, to date it is unclear whether nuclear reprogramming after cell fusion only affects a small subset of genes or changes the global program. A means of elucidating the global gene expression profile would be invaluable for elucidating the early regulatory network that underlies reprogramming. In addition, this knowledge would greatly facilitate the generation from somatic cells of both tissue-specific cells and autologous ES cells for clinical use in regenerative medicine.
Here, we describe species-specific transcriptome amplification (SSTA), a novel approach that permits, for the first time, the specific profiling of gene expression changes from the nucleus of interest in fused cells throughout the time course of reprogramming. By specifically amplifying the human or mouse transcriptional complement from heterokaryon populations and hybridizing it to microarrays, transcript detection is possible within a few hours after fusion, even when the input from one cell type is present at less than 10% of the total. In addition, we capitalize on the use of heterokaryons, nondividing interspecific fusion products in which chromosome loss and rearrangement does not occur and the nuclei of the two cell types remain intact and distinct. Our studies using heterokaryons, which allow changes in reprogramming to be assessed in a population within a few hours of addition of the fusagen, have previously revealed a remarkable degree of plasticity of the differentiated state of numerous somatic cell types; for example, induction of muscle gene expression in hepatocytes (9, 10; also see refs. 21, 22 for review). More recently, using species-specific COBRA assays, we showed that in conjunction with changes in gene expression, human keratinocyte nuclei undergo muscle-specific epigenetic changes in the absence of replication when fused with mouse myotubes, providing unexpected evidence that in somatic cells, as in germ cells, active reversal of DNA methylation states can occur (16). We report here that reprogramming in heterokaryons formed between muscle cells and primary keratinocytes is initiated rapidly and is not limited to a few genes, but is extensive. Notably, this provides the first evidence that reprogramming occurs in either direction, and is ultimately determined by the relative nuclear dosage and not by a particular dominant phenotype or master regulator, such as MyoD. In addition to the reprogramming of keratinocyte gene expression toward a muscle transcriptional state, muscle nuclei can be reprogrammed toward a keratinocyte state, and the 2 phenotypes are mutually exclusive. Thus, reprogramming in heterokaryons is initiated rapidly, involves the activation and repression of hundreds of genes, and can lead to dominance of either phenotypic program.
MATERIALS AND METHODS
Flow cytometry
Heterokaryons were prepared as described previously. To sort GFP+/dsRed+ heterokaryons (16), samples were trypsinized using 1× trypsin EDTA solution (Invitrogen, Carlsbad, CA, USA), and resuspended in PBS with 2.5% v/v goat serum (Hyclone, Logan, UT, USA). Samples were sorted using a modified BD FACSVantage se (BD Biosciences, San Jose, CA, USA) at Stanford’s Shared FACS Facility. To sort NCAM+ heterokaryons, heterokaryons were first incubated on their tissue culture plates for 30 min with the anti-human-NCAM monoclonal antibody 5.1H11 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA) diluted 1:100 in differentiation medium. Cells were then washed 3 times in differentiation medium and incubated with the secondary antibody goat-anti-mouse Alexa488 (Invitrogen). Cells were then washed 3 times, and trypsinized and sorted as above.
RNA samples
RNA from cultured cells was obtained by direct lysis in culture dishes using Qiagen buffer RLT (Qiagen, Valencia, CA, USA) or by FACS sorting into buffer RLT as described in the text. Total RNA was purified using the RNeasy Micro Kit (Qiagen) according to the instructions given. Mouse liver and skin was obtained from an adult C57/BL6 mouse, and immediately pulverized using a mortar and pestle under liquid nitrogen. RNA was extracted from 30 mg of tissue using the RNeasy Mini Kit (Qiagen). Human skeletal muscle RNA was obtained from Clontech Laboratories (Palo Alto, CA, USA).
Human reference RNA was a mixture of Human Control RNA (Invitrogen) and XpressRef Human Universal Total RNA (SuperArray, Frederick, MD, USA). Mouse reference RNA was a mixture of Universal RNA: Mouse Normal Tissues (BioChain, Hayward, CA, USA), Universal Mouse Reference RNA (Stratagene, La Jolla, CA, USA), and RNA from whole mouse E17.5 embryos (a generous gift from Roger Wagner, Stanford University Medical Center, Stanford, CA, USA).
Quantitative PCR
Quantitative PCR was performed on a Rotorgene RG-3000 thermal cycler (Corbett Research, Mortlake, NSW, Australia). Templates were diluted into 15 μl of Platinum Taq buffer containing 2.25 mM MgCl2, 200 μM each dNTP, 0.01% v/v Tween-20 (Sigma-Aldrich, St. Louis, MO, USA), 0.8% v/v glycerol, 7.5 pmol forward and reverse primers, 0.3 U Platinum TaqDNA Polymerase, and 0.002% v/v Sybr Green I Nucleic Acid Gel Stain (Invitrogen). Samples were cycled at 94°C for 2 min, 40× (94°C for 20 s, 60°C for 30 s, 72°C for 45 s).
RESULTS
Development, efficacy, and reproducibility of SSTA
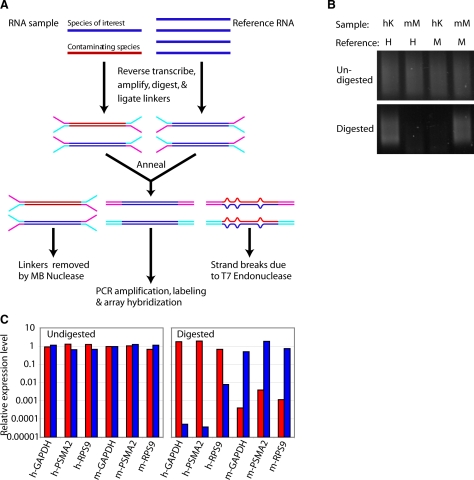
To circumvent the problem of cross-hybridization between human and mouse components in heterokaryons, and to provide an accurate discrimination of the expression profiles of the two species, we developed a method that permits specific, global amplification of the transcriptome of the species of interest. The amplified product can then be labeled and hybridized to an expression microarray. The SSTA method is schematized in Figure 1A. First, the RNA sample of interest is converted into double-stranded cDNA, using a modified version of the SMART technique (23, 24). Double-stranded cDNA is also made from a diverse pool of RNA originating only from the species of interest. This “reference” pool is constructed without bias for any particular tissue or developmental stage (see Materials and Methods). Each cDNA pool is then ligated to ligation-mediated (LM) PCR linkers containing a single-stranded region near the primer binding site. The single-stranded linkers ligated to the sample are complementary to those ligated to the reference, so that when the ligated sample pool is annealed to an excess of reference, the sample-reference hybrids, but not the sample-sample or the reference-reference hybrids, will have double-stranded linkers (Fig. 1A). The annealed pool is digested with a single-strand-specific nuclease (mung bean nuclease) and with the resolvase T7 endonuclease 1, which creates strand breaks at the sites of mismatches or insertions/deletions (25), leading to the selective amplification of only duplexes in which the sample strand is from the species of interest.
Figure 1.
SSTA selectively amplifies sequences from the species of interest. A) SSTA procedure scheme. RNA sample containing the species of interest (blue) and the contaminating species (red) is first converted into double-stranded DNA and ligated to specific PCR linkers. The sample is then annealed to a double-stranded cDNA reference pool of the species of interest, followed by digestion with mung bean nuclease and T7 endonuclease 1. Only the perfectly annealed duplexes in which the sample strand is from the species of interest remain undigested and are then PCR amplified. B) Human keratinocytes (hK) or mouse myoblasts (mM) were annealed to human (H) or mouse reference (M). Samples were digested with MBN/T7 endonuclease I or left undigested, and then PCR amplified for 19 cycles (undigested) or 23 cycles (digested). C) A sample containing 50% human cDNA and 50% mouse cDNA was annealed either to human reference (red bars), mouse reference (blue bars), or human + mouse reference control. Samples were digested or left undigested, and then PCR amplified. Gene abundances were assessed by species-specific quantitative PCR. Each primer pair and treatment was normalized to the human + mouse reference control.
As a proof of principle, we performed SSTA on a noncomplex mixture of AluI restriction fragments. AluI fragments of human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were ligated to the reference linkers, and subsequently annealed either to AluI fragments of either human GAPDH, mouse GAPDH, or human β-tubulin ligated to the sample linkers. On digestion, amplification was only observed in the case of human GAPDH self-annealing and in the case of a 12 bp fragment of mouse GAPDH, which is identical to the corresponding human fragment (supplemental Fig. 1A), indicating that SSTA selects for the amplification of fragments that are identical between sample and reference.
To determine whether SSTA can produce selectivity for the species of interest in a complex mixture, we annealed samples derived from either human keratinocytes or mouse myoblasts to either the human or mouse reference (Fig. 1B). Without digestion, amplification resulted in a smear from all annealings. However, after digestion, as expected, amplification was only observed when sample and reference were of the same species.
As a quantitative measure of the species selectivity of SSTA, we mixed 50% human keratinocyte cDNA with 50% mouse myoblast cDNA. This mixture was ligated to linkers and annealed to a reference pool containing either both mouse and human reference (normalization control), mouse reference only, or human reference only, and amplified either with or without prior digestion. Using species-specific qPCR, the amounts of the mouse and human ubiquitously expressed genes GAPDH, proteasome subunit alpha type 2 (PSMA2), and ribosomal protein S9 (RPS9) were assessed on each sample (Fig. 1C). After digestion, amplification from the species of interest remained comparable to the control sample, while sequences from the contaminating species were present at 100- to 100,000-fold lower concentrations. The mean difference (geometric mean) was 1960-fold. Thus, for many genes, SSTA introduces a very strong selection in favor of the species of interest.
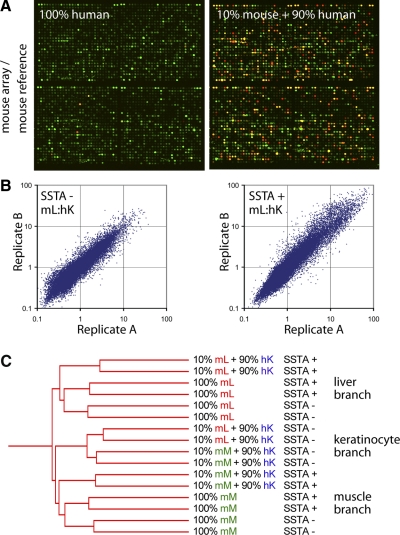
To investigate the reliability and reproducibility of SSTA when used in conjunction with microarrays, we prepared double-stranded cDNA from three samples: mouse liver, mouse myoblasts differentiated for 4 days in culture, and human keratinocytes. We then diluted each mouse sample 1:10 with human keratinocyte cDNA (10% mouse, 90% human). Each sample was brought through the SSTA procedure using a mouse reference, labeled, and applied to the mouse MEEBO microarray printed by Stanford’s Functional Genomics Facility. The same samples were also applied to the array without SSTA. Samples were labeled with Cy5 dye (red), and all samples were cohybridized with the amplified mouse reference, labeled with Cy3 dye (green) (Fig. 2A). To verify that expression measurements were due to the mouse content of each sample, we also performed the SSTA procedure with human keratinocyte cDNA alone (100% human). Cy5 signal was almost undetectable in these control hybridizations.
Figure 2.
Efficacy and reproducibility of SSTA. A) Human keratinocyte cDNA control (left panel) or mouse liver cDNA diluted 1:10 with human keratinocyte cDNA (right panel) were treated with SSTA using a mouse reference, labeled with Cy5 dye (red) and hybridized to microarrays along with a Cy3 common reference (green). B) Scatter plots of replicates of mouse liver (mL) cDNA diluted 1:10 with human keratinocyte (hK) cDNA either SSTA-treated (SSTA+) or untreated (SSTA−). C) Hierarchical clustering of mouse liver or mouse muscle (mM) cDNA diluted 1:10 with human cDNA or left undiluted. Replicates were hybridized to arrays with or without prior SSTA treatment. Hierarchical clustering of samples was performed using average linkage clustering.
To determine the reproducibility of SSTA, we selected the array elements for which the Cy3 signal was substantially above background (fluorescence signal>400 in 14 of 16 hybridizations). Using these elements, similarity between replicates was comparable for SSTA-treated samples and untreated samples (Fig. 2B). Pearson correlation values and average coefficients of variation were also similar with or without SSTA (Supplemental Fig. 1B). We conclude that SSTA does not introduce substantial variability when starting from the same cDNA sample.
To identify a set of informative elements that reliably yielded a significant signal when mouse cDNA was assayed in a species-specific manner, we compared the signal intensities in SSTA-treated samples with 10% mouse cDNA and 90% human cDNA to SSTA-treated samples containing 100% human cDNA. Samples with 10% mouse cDNA had substantial Cy5 signal across many array elements, while signal was reduced in samples with no mouse cDNA (Fig. 2A). Of the 38,785 total mouse array elements, we eliminated 596 array elements with high signal in human-only SSTA hybridizations. Of the remaining elements, we identified 25,495 with Cy5 signal at least 2-fold above the human-only control in at least two of the SSTA hybridizations (Supplemental Fig. 2A). These elements were used for subsequent analysis. We then performed hierarchical clustering (average linkage, Pearson correlation) as a means of assessing sample similarity across all informative elements (Fig. 2C). The samples comprised 3 main branches. The most self-similar branch was the branch in which both the mouse liver and the mouse muscle sample, when diluted with human keratinocyte cDNA, but when untreated by SSTA, clustered together. In contrast, the mouse muscle sample diluted with human keratinocyte cDNA but treated with SSTA clustered with the other muscle samples, and the diluted but SSTA-treated liver sample clustered with the other liver samples. To assess the reproducibility of this clustering pattern, 1000 bootstrapping iterations were performed (Genespring software; Agilent Technologies, Wilmington, DE, USA), and the above branches were preserved 100% of the time. Therefore, after SSTA treatment, the contribution of human cDNA to the global expression program is substantially removed. Similar results were obtained by specifically examining known muscle- and liver-specific genes (Supplemental Fig. 2B).
Taken together, these results suggest that SSTA eliminates the cross-hybridization from the contaminating species and identifies the expression profile of the species of interest. We therefore concluded that this method would be useful for investigating reprogramming in the heterokaryon system.
The keratinocyte genome is extensively reprogrammed toward muscle gene expression in heterokaryons
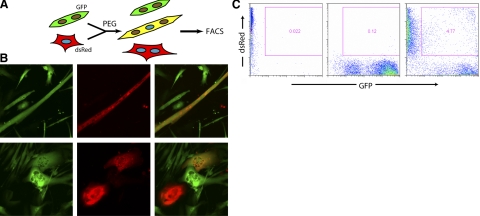
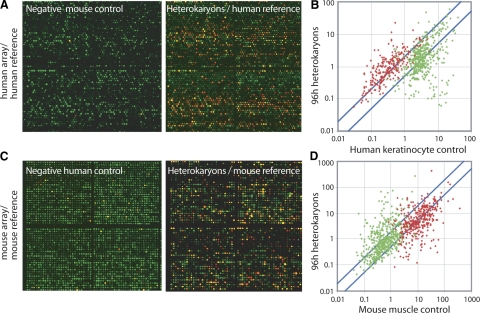
To determine whether alterations in gene expression during reprogramming are limited to a small number of genes or more broadly affect hundreds of genes, we generated heterokaryons according to the method of Zhang et al. (16) between mouse myotubes expressing GFP and human primary keratinocytes expressing dsRed (Fig. 3A). Heterokaryons were identifiable in the culture dish as cells expressing both fluorescent proteins (Fig. 3B). To enrich for heterokaryons, duplicate cultures were trypsinized 4 days after heterokaryon formation and double-positive populations were isolated by FACS (Fig. 3C). As negative controls, human keratinocytes alone for 4 days and cocultures of mouse muscle and human keratinocytes just prior to fusion were also collected in duplicate. Three separate human myoblast cultures and a sample of human muscle RNA were used as positive controls. cDNA was generated from these samples, and the proportion of human cDNA in each was determined by quantitative PCR. The proportion of human cDNA in all samples was equalized by addition of mouse muscle cDNA, and human expression profiles were generated by human-specific SSTA. We identified 22,823 informative elements in a manner analogous to the mouse array defined above (Fig. 2A), by comparison of mouse muscle only control hybridizations to samples containing 15% human cDNA (Fig. 4A and Supplemental Fig. 3A).
Figure 3.
Generation and isolation of heterokaryons. A) Scheme of heterokaryon formation. Human keratinocytes (dsRed) were fused with mouse myotubes (GFP) in differentiation medium, using polyethylene glycol (PEG). B) Images of GFP (green) and dsRed (red) heterokaryon cultures 4 days after fusion. Top panels: characteristic heterokaryon with myotube morphology. Bottom panels: heterokaryons with keratinocyte morphology. C) FACS profiles of human keratinocytes alone (left panel), mouse muscle cells alone (center panel), or heterokaryons (right panel). Sorting gate used for heterokaryon isolation is indicated.
Figure 4.
Global analysis of reprogramming in heterokaryons. A) Mouse muscle cDNA control (left panel) or cDNA from 96 h heterokaryons (right panel) were treated with SSTA using a human reference, labeled with Cy5 dye (red) and hybridized to human microarrays along with a Cy3 common reference (green). B) Cy5/Cy3 expression values of muscle genes (red) or keratinocyte genes (green) are plotted in 4 day heterokaryons vs. human keratinocyte control. C) Human keratinocyte cDNA control (left panel) or cDNA from 96 h heterokaryons (described in A, right panel) were treated with SSTA using a mouse reference, labeled with Cy5 dye (red) and hybridized to mouse microarrays along with a Cy3 common reference (green). D) Cy5/Cy3 expression values of muscle genes (red) or keratinocyte genes (green) are plotted in 4 day heterokaryons vs. mouse muscle control.
From these informative elements, we identified sets of putatively muscle-specific and keratinocyte-specific elements by selecting elements strongly differentially expressed between the unreprogrammed human keratinocyte negative controls and the human muscle tissue or myoblast positive controls. We called elements with higher expression in muscle controls “muscle genes” and those with higher expression in keratinocyte controls “keratinocyte genes.” We then analyzed the behavior of these elements in reprogrammed heterokaryons (Fig. 4A, B). Of 273 muscle genes, 171 (63%) were induced at least 2-fold in heterokaryons. This is a significant enrichment over the proportion (22%) of genes on the whole array displaying a 2-fold induction between these conditions (P≪0.001 by Fisher’s exact test). Conversely, of 494 keratinocyte genes, 237 (48%) decreased in expression at least 2-fold in heterokaryons, a significant enrichment over the array average of 13% (P≪0.001). Keratinocyte genes (n=28) increased by 2-fold (Supplemental Fig. 3C). We therefore concluded that the reprogramming of keratinocytes toward muscle is extensive and is not limited to changes in a small number of genes, but instead involves both the activation of muscle genes and the repression of hundreds of keratinocyte genes.
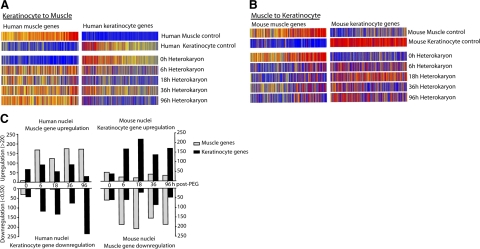
Unlike cell hybrids that must be propagated to select for the fused cells, heterokaryons can be sorted by flow cytometry at any time point following fusion, allowing an analysis of gene expression changes at early time points. Heterokaryon populations were sorted at 6, 18, 36, and 96 h after fusion, and SSTA was used to examine the mouse and human gene expression patterns at these time points, focusing on the previously defined classes of human and mouse muscle and keratinocyte genes. (Fig. 5A, C; left panel). This analysis indicates that substantial reprogramming is already evident at 6 h following fusion, and persists and increases throughout the time course. We therefore concluded that reprogramming is initiated very rapidly, presumably due to the direct action of preexisting cytoplasmic transcriptional regulators on the target genome.
Figure 5.
Time course and extent of bidirectional reprogramming. A) Expression of muscle- or keratinocyte-specific human genes from heterokaryon profiles at various time points during reprogramming. Each vertical bar represents a gene. Red indicates high expression, blue indicates low expression. B) Expression of mouse genes in mouse muscle and keratinocyte control samples, and at various time points in heterokaryons after PEG fusion. C) Left panel: number of human muscle genes that are upregulated (≥2×) or human keratinocytes that are downregulated (≤0.5×) in comparison to human keratinocyte. Right panel: numbers of upregulated mouse keratinocytes genes and mouse muscle genes downregulated in comparison to control mouse muscle controls.
The muscle genome is extensively reprogrammed toward keratinocyte gene expression in heterokaryons
We hypothesized that contrary to dogma reprogramming could be bidirectional and not restricted to the muscle fate. The potent family of bHLH promyogenic transcription factors has widely been assumed to be dominant over other tissue-specific factors, precluding reprogramming of muscle to another fate (9, 12, 26). Additionally, the concept of “phenotypic exclusion” in cell hybrids suggests that a cell is only likely to express features of one differentiated phenotype at any given time (27), presumably the dominant phenotype.
To test the hypothesis that a keratinocyte gene expression profile could be induced in muscle cells, we sorted heterokaryon populations as described above and assessed the expression of genes by the mouse muscle nuclei. As negative controls, mouse muscle cells alone (4 days after onset of fusion), and cocultures of mouse muscle and human keratinocytes just prior to fusion, were collected in duplicate. As positive controls, we used duplicate cultures of mouse keratinocytes as well as a sample of mouse skin. As in Fig. 4A, the proportion of mouse cDNA in each sample was equalized by the addition of human keratinocyte cDNA. These samples were then profiled using mouse-specific SSTA (Fig. 4C). For the mouse array, 25,557 usable elements were identified (Supplemental Fig. 3B). From these elements, we identified 374 muscle genes and 518 keratinocyte genes by comparison of the positive and negative controls (Fig. 4D). Of the muscle elements, 188 (50%) were at least 2-fold lower in expression in heterokaryons, a significant enrichment over the 20% of all array elements decreased by 2-fold in this comparison (P≪0.001). Of the keratinocyte elements, 170 (33%) were increased at least 2-fold in heterokaryons, a significant enrichment over the array average of 15% (P≪0.001) (supplemental Fig. 3C). Again, extensive changes in gene expression were seen as early as 6 h after fusion that persisted and increased over time (Fig. 5B, C; right panel).
From this analysis, we concluded that contrary to expectation, in heterokaryons formed by fusing myotubes and keratinocytes, significant and extensive reprogramming of the muscle nuclei toward a keratinocyte fate can be detected. Whether reprogramming toward a keratinocyte phenotype occurs within the same or different populations of heterokaryons from those that are reprogrammed toward a muscle phenotype is not addressed by these experiments, as a bulk population of mixtures of heterokaryons was analyzed.
The dominant phenotype on reprogramming is dependent on cellular ratio
The observed reprogramming of both genomes in the mixed population of heterokaryons raised the question of whether in individual heterokaryons both gene expression patterns (phenotypes) were coexpressed, or whether expression was an “either/or” decision, in accordance with the concept of phenotypic exclusion. To address this question, we first determined whether the direction of reprogramming was separable in heterokaryon populations. Critical to these experiments was a means of distinguishing among heterokaryons based on nuclear ratio. Our rationale for this distinction was based on previous studies that revealed that the likelihood of a heterokaryon expressing human muscle-specific NCAM was greater when the ratio of myonuclei to ectopic nuclei was higher (10, 28). This finding suggested that reprogramming toward the keratinocyte fate might occur more frequently if this ratio was lower (human keratinocytes>mouse myoblasts).
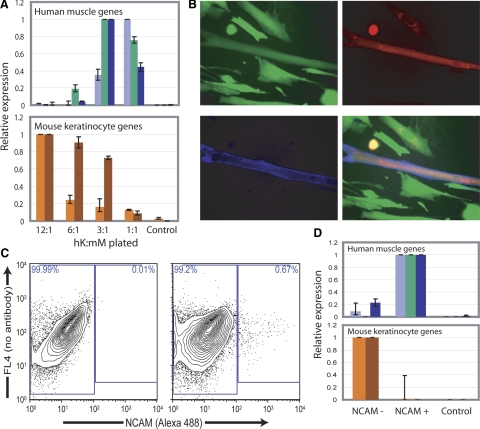
To test this hypothesis, we analyzed heterokaryons produced by inducing fusion in cocultures of different ratios of myoblasts and keratinocytes (Fig. 6A). To analyze gene expression in these limited populations, we designed species-specific quantitative PCR primers to the mouse keratinocyte markers keratin 5 and periplakin as well as to the human muscle markers MyoD, muscle creatine kinase (MCK), and myosin heavy chain 2 (MHC2). In accordance with our hypothesis, higher proportions of muscle cells led to increased reprogramming of keratinocytes toward the muscle fate and decreased reprogramming to the keratinocyte fate (Fig. 6A, top). Conversely, higher proportions of keratinocytes resulted in increased reprogramming of mouse muscle cells to a keratinocyte fate (Fig. 6A, bottom).
Figure 6.
Reprogramming toward muscle or keratinocyte gene expression occurs in separable populations. A) Gene expression level of human muscle genes and mouse keratinocyte genes in different populations of heterokaryons produced by varying ratios of human keratinocytes to mouse myoblasts (hK:mM). Top panel: human muscle creatine kinase (light blue), myosin heavy chain 2 (green), and MyoD (dark blue), measured by quantitative RT-PCR. Bottom panel: mouse keratin 5 (orange) and periplakin (red) measured by quantitative RT-PCR. B) Expression of human muscle-specific NCAM assayed by immunohistochemistry using a human specific antibody in heterokaryons. NCAM (blue), GFP (green), dsRed (red). C) Right panel: FACS isolation of heterokaryons using a human muscle specific NCAM antibody. Left panel: mouse muscle cells are shown as a negative control. D) Reprogramming toward a muscle or keratinocyte fate assessed in NCAM+ and NCAM− populations by quantitative RT-PCR. Genes assayed and controls are as in A.
As a second approach to determining whether the relative proportions of nuclei of the 2 cell types determined the direction of reprogramming, we separated heterokaryons based on muscle NCAM expression using a human-specific antibody (10, 16). In dsRed/GFP-labeled heterokaryon cultures, two morphological heterokaryon phenotypes were observed: an elongated, myotube-like morphology, and a flat morphology with nuclei clustered near the center (Fig. 3B). Heterokaryons of the first morphology frequently expressed human-specific muscle NCAM, whereas heterokaryons of the second morphology did not express human NCAM (Fig. 6B). This finding suggests that the two populations of heterokaryons have distinct reprogramming patterns, perhaps due to a predominance of nuclei of muscle or keratinocyte nuclei, respectively. To further explore this possibility, heterokaryons with or without human NCAM expression were separated by FACS using a human-specific NCAM antibody (Fig. 6C). Reprogramming toward a muscle or a keratinocyte fate was analyzed in the small number of heterokaryons obtained using species-specific quantitative PCR (Fig. 6D). The NCAM-positive population expressed other human muscle-specific transcripts, but only low amounts of mouse keratinocyte-specific transcripts. Conversely, the NCAM-negative population expressed only low amounts of human muscle transcripts, but was reprogrammed to express mouse keratinocyte transcripts.
Taken together, these results suggest that reprogramming is an all-or-none phenomenon dictated by nuclear ratio, in which morphology, surface marker expression, and transcript levels are correlated.
DISCUSSION
We analyzed the kinetics and extent of nuclear reprogramming of cells fused in stable nondividing heterokaryons. Key to the findings was the use of interspecific heterokaryons, the postmitotic fusion products of cells of different species. The rationale behind the use of species differences is that it allows the gene products of the two nuclei to be distinguished. To achieve this goal, however, we had to develop a novel method, SSTA, for assaying the RNA of the species of interest, even when it comprised a small fraction of the total. This was necessary, because gene dosage or nuclear ratio determined the direction of reprogramming within heterokaryons and was purposefully skewed to achieve reprogramming either toward a keratinocyte or toward a muscle phenotype.
SSTA overcomes limitations of existing methods. Studies examining cross-hybridization on either cDNA (17, 19), or oligonucleotide (18, 20) microarrays have shown that hybridization of mouse and human transcripts with sequence similarity near the level of 85–95% produces substantial and detectable signal, precluding their use for this type of analysis. As an alternative method, array elements can be selected that have regions of high dissimilarity between the two genomes, but this approach has several drawbacks (29). First, it requires extensive a priori knowledge of the 3′UTR sequences of both species in question, which may not always be available. Second, because this approach relies solely on increasing the specificity of the hybridization, the measured array signal decreases in proportion to the fraction of the sample RNA that is comprised of the species of interest. Detection issues are therefore a concern when studying a mixture of transcripts from two species as in heterokaryons, where one fraction is low. By contrast, using SSTA, we were able to convert cDNA, comprised of as little as 10% of the species of interest and 90% of the contaminating species, into an essentially pure population where the contaminating fraction was reduced to near 0. By quantitative PCR, we measured enrichment ratios averaging around 2000-fold, exceeding the dynamic range of most microarrays. We showed that, when applied to a microarray, SSTA-treated samples generate robust signals and have a reproducibility similar to non-SSTA-treated samples. Furthermore, expected expression differences were reproduced after SSTA treatment, both when looking at small numbers of preannotated genes and when looking at expression patterns of hundreds of genes by hierarchical clustering. Thus, SSTA is an effective method for species-specific expression profiling.
Currently, distinguishing expression profiles from mixed cell populations is often difficult, if not impossible, to achieve, when the proportion of the cell type of interest is in relatively low abundance. SSTA overcomes this problem. It should prove useful not only for nuclear reprogramming studies as shown here, but also more broadly for generating expression profiles of cells that comprise a small proportion of a species mixture, for example cells grown on feeder layers, tumor cells in xenograft models surrounded by host stroma or vasculature, tissue-inflammatory cell interactions after bone marrow xenotransplantation, or distinction of specific species in complex microbial communities.
Extensive nuclear reprogramming in heterokaryons
This study describes extensive nuclear reprogramming that encompasses hundreds of genes and is bidirectional, with the direction determined by the relative ratio of the two types of nuclei in the fused cells. Previous microarray profiles of gene expression in the fusion products of ES cells and fibroblasts (2, 30) lacked the ability to distinguish gene expression of the two contributing genomes, as both genomes were of the same species origin. Thus, the detection of an ES expression profile revealed that under the conditions used (cell ratio, culture media), the ES cell type was dominant, but could not evaluate the extent of the contribution of the fused fibroblasts to that profile. In addition, the time course of reprogramming could not be assessed, as the hybrid cells underwent multiple rounds of cell division. By contrast, in the heterokaryons used here, gene expression could be assessed within hours of the onset of reprogramming, since after fusion the nuclei remain distinct and intact. In previous studies, only products of selected genes were analyzed by RT-PCR or by species-specific antibodies or differential enzyme electrophoresis (9,10,11,12,13,14,15,16). The current study allows an assessment in fused cells of a pattern of activation and extinction of hundreds of genes during nuclear reprogramming in a manner previously not possible.
We have shown that muscle gene activation in keratinocytes reprogrammed toward muscle is extensive. We have additionally shown substantial silencing of keratinocyte genes, thus suggesting that the activation of muscle genes is not a matter of genome-wide derepression, but instead is part of a directed and concerted change from the expression program of a keratinocyte to that of a muscle cell. Remarkably, we have also shown that in the same population of heterokaryons, reprogramming of muscle nuclei toward the keratinocyte fate can also occur. To our knowledge, this is the first demonstration that keratinocytes have reprogramming activity and that the ‘dominant’ myogenic regulators can be overridden.
Finally, we have shown that the heterokaryons reprogrammed toward the keratinocyte fate are largely separable from those reprogrammed toward the muscle fate, suggesting that the 2 fates do not coexist and that the cell’s transcriptional control network includes feedback mechanisms that fortify the dominant transcriptional program at the expense of competing programs. We know of one demonstration of a related phenomenon, termed “phenotypic exclusion,” in which melanin and albumin were each sometimes produced in a proliferating melanoma-hepatoma hybrid, but never at the same time (27); however, this study involved the repeated subcloning of the fused cell line. In contrast, in muscle-lymphocyte heterokaryons the two phenotypic programs were coexpressed, a finding that might have been due to the continuous presence of HDAC inhibitors and consequent blocking of gene repression (15). Our studies show that under normal circumstances, clearly one phenotypic program—either the muscle or keratinocyte—predominates over the other. Thus, distinct populations of heterokaryons that have activated either the keratinocyte or the muscle phenotype are separable.
Taken together, our results suggest that the gene expression of differentiated somatic cells is highly plastic when in contact with foreign factors. Reprogramming is initiated rapidly, is extensive, and can cross germ layers. Previous studies reported that muscle dominates over other phenotypes (9, 12, 26). Our results provide novel evidence that reprogramming activity is not limited to a particular cell type such as muscle and that dominance arises from an excess of cytoplasmic factors or nuclear dosage. Heterokaryons offer a unique opportunity to gain insights into the regulatory networks underlying nuclear reprogramming and the mechanisms that maintain cellular memory while allowing for plasticity. An understanding of the molecular events governing reprogramming is crucial in order to maximize the potential to induce pluripotency in somatic cells or direct reprogramming to specific somatic fates for the derivation of cells for use in regenerative medicine.
Supplementary Material
Acknowledgments
We thank Sally Pennypacker and Walt Holleran (Department of Dermatology, University of California, San Francisco, CA, USA) for providing human neonatal foreskin keratinocytes. We are grateful to the members of the H.M.B laboratory for helpful discussions and critical reading of the manuscript. This work was supported by a National Science Foundation fellowship (to A.T.P.); National Institutes of Health National Research Service Award AF051678 (to J.H.P.); NIH grants AG009521, AG020961, AG024987, and HD018179; MDA grant MDA4320; and the Baxter Foundation (to H.M.B).
References
- Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Cowan C A, Atienza J, Melton D A, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- Silva J, Chambers I, Pollard S, Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Ephrussi B, Davidson R L, Weiss M C, Harris H, Klein G. Malignancy of somatic cell hybrids. Nature. 1969;224:1314–1316. doi: 10.1038/2241314a0. [DOI] [PubMed] [Google Scholar]
- Peterson J A, Weiss M C. Expression of differentiated functions in hepatoma cell hybrids: induction of mouse albumin production in rat hepatoma-mouse fibroblast hybrids. Proc Natl Acad Sci U S A. 1972;69:571–575. doi: 10.1073/pnas.69.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G J, Bernard H P, Ruddle F H. Human serum albumin phenotype activation in mouse hepatoma–human leukocyte cell hybrids. Science. 1974;185:859–862. doi: 10.1126/science.185.4154.859. [DOI] [PubMed] [Google Scholar]
- Blau H M, Chiu C P, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blau H M, Pavlath G K, Hardeman E C, Chiu C P, Silberstein L, Webster S G, Miller S C, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Baron M H, Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986;46:591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Wright W E. Expression of differentiated functions in heterokaryons between skeletal myocytes, adrenal cells, fibroblasts and glial cells. Exp Cell Res. 1984;151:55–69. doi: 10.1016/0014-4827(84)90355-0. [DOI] [PubMed] [Google Scholar]
- Wright W E. Induction of muscle genes in neural cells. J Cell Biol. 1984;98:427–435. doi: 10.1083/jcb.98.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B T, Tilghman S M. Role of alpha-fetoprotein regulatory elements in transcriptional activation in transient heterokaryons. Mol Cell Biol. 1990;10:5047–5054. doi: 10.1128/mcb.10.10.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova R, Pereira C F, Du Roure C, Merkenschlager M, Fisher A G. Acquisition and extinction of gene expression programs are separable events in heterokaryon reprogramming. J Cell Sci. 2006;119:2065–2072. doi: 10.1242/jcs.02945. [DOI] [PubMed] [Google Scholar]
- Zhang F, Pomerantz J H, Sen G, Palermo A T, Blau H M. Active tissue-specific DNA demethylation conferred by somatic cell nuclei in stable heterokaryons. Proc Natl Acad Sci U S A. 2007;104:4395–4400. doi: 10.1073/pnas.0700181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Bak S, Decker A, Paquette S M, Feyereisen R, Galbraith D W. Microarray-based analysis of gene expression in very large gene families: the cytochrome P450 gene superfamily of Arabidopsis thaliana. Gene. 2001;272:61–74. doi: 10.1016/s0378-1119(01)00516-9. [DOI] [PubMed] [Google Scholar]
- Ji W, Zhou W, Gregg K, Yu N, Davis S, Davis S. A method for cross-species gene expression analysis with high-density oligonucleotide arrays. Nucleic Acids Res. 2004;32:e93. doi: 10.1093/nar/gnh084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjaye J, Herwig R, Herrmann D, Wruck W, Benkahla A, Brink T C, Nowak M, Carnwath J W, Hultschig C, Niemann H, Lehrach H. Cross-species hybridisation of human and bovine orthologous genes on high density cDNA microarrays. BMC Genomics. 2004;5:83. doi: 10.1186/1471-2164-5-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev D N, Ma S F, Simon B A, Irizarry R A, Ye S Q, Garcia J G. In vitro identification and in silico utilization of interspecies sequence similarities using GeneChip technology. BMC Genomics. 2005;6:62. doi: 10.1186/1471-2164-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H M, Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991;112:781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H M. Differentiation requires continuous active control. Annu Rev Biochem. 1992;61:1213–1230. doi: 10.1146/annurev.bi.61.070192.010025. [DOI] [PubMed] [Google Scholar]
- Chenchik A, Zhu YY, Diatchenko L, Li R, Jill J, Siebert P D. Natick, MA, USA: Eaton; RT–PCR Methods for Gene Cloning and Analysis. 1998 [Google Scholar]
- Zhu Y Y, Machleder E M, Chenchik A, Li R, Siebert P D. Reverse transcriptase template switching: a SMART approach for full-length cDNA library construction. BioTechniques. 2001;30:892–897. doi: 10.2144/01304pf02. [DOI] [PubMed] [Google Scholar]
- Mashal R D, Koontz J, Sklar J. Detection of mutations by cleavage of DNA heteroduplexes with bacteriophage resolvases. Nat Genet. 1995;9:177–183. doi: 10.1038/ng0295-177. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Tapscott S J, Davis R L, Thayer M J, Adam M A, Lassar A B, Miller A D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougere C, Weiss M C. Phenotypic exclusion in mouse melanoma-rat hepatoma hybrid cells: pigment and albumin production are not reexpressed simultaneously. Cell. 1978;15:843–854. doi: 10.1016/0092-8674(78)90269-6. [DOI] [PubMed] [Google Scholar]
- Pavlath G K, Blau H M. Expression of muscle genes in heterokaryons depends on gene dosage. J Cell Biol. 1986;102:124–130. doi: 10.1083/jcb.102.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef F, Huelsken J. Cell-type-specific transcriptomics in chimeric models using transcriptome-based masks. Nucleic Acids Res. 2005;33:e111. doi: 10.1093/nar/gni104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi D J, Tanasijevic B, Kaur A, Obergfell C, O'Neill R J, Krueger W, Rasmussen T P. Genome-wide reprogramming in hybrids of somatic cells and embryonic stem cells. Stem Cells. 2007;25:1104–1113. doi: 10.1634/stemcells.2006-0532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.