Abstract
Because of localized vascular damage and increased tissue oxygen demand, wound healing occurs in a relatively hypoxic microenvironment. These features are particularly relevant to wound healing and fibrosis in chronic inflammatory conditions, such as Crohn’s disease and ulcerative colitis. In these studies, we sought to identify the contribution of hypoxia to mechanisms of wound repair in a model of the intestinal submucosa. Initial studies revealed that hypoxia promotes wound healing, as modeled by an increase in intestinal fibroblast-mediated collagen gel contraction. Guided by results from transcriptional profiling, we identified the selective induction of fibroblast integrin β1 (ITGB1) by hypoxia. Further analysis revealed that hypoxia, as well as pharmacological activators of hypoxia-inducible factor (HIF), induce fibroblast β1 integrin mRNA, protein, and function by as much as 4-fold. Cloning and analysis of the β1 integrin gene promoter revealed a 10 ± 0.8-fold increase in promoter activity in response to hypoxia, and subsequent studies identified a functional DNA binding region for HIF in the ITGB1 gene promoter. Mutational analysis of the HIF binding site within the ITGB1 promoter resulted in a significant loss of ITGB1 hypoxia-inducibility. As proof of principle, studies in a murine model of colitis revealed a correlation between colitic disease severity and tissue ITGB1 expression (R2=0.80). Taken together, these results demonstrate that hypoxia induces fibroblast ITGB1 expression and function by transcriptional mechanisms dependent on HIF.—Keely, S., Glover, L. E., MacManus, C. F., Campbell, E. L., Scully, M. M., Furuta, G. T., Colgan, S. P. Selective induction of integrin β1 by hypoxia-inducible factor: implications for wound healing.
Keywords: inflammation, Crohn’s disease, restitution, transcription, gene promoter
Stiffening and fibrosis of intestinal tissues are common in chronic inflammatory diseases (1, 2). While muscle fibers account for a large percentage of intestinal contractions, wound healing and the associated events of scarring, stricture formation, and tissue remodeling are all associated with nonmuscular tissue contraction (3, 4). Interactions between epithelial cells, fibroblasts, and various leukocyte populations within the mucosa each contribute to tissue contractions and regulated extracellular matrix remodeling (4, 5). At present, the molecular pathways that govern the events leading to wound healing are not fully elucidated.
An important, yet underappreciated, factor in intestinal wound repair is the availability of oxygen (6, 7). A number of factors, including localized vascular damage and increased tissue oxygen demand, significantly shift tissue metabolism during wound healing and can result in hypoxia. As a global regulator of oxygen homeostasis, hypoxia-inducible factor (HIF) has been implicated in multiple physiological and pathophysiological pathways (8,9,10). HIF is stabilized through its interaction with one or more of three HIF-selective iron- and oxygen-dependent prolyl hydoxylation (PHD) enzymes, which act on prolines 564 and 402 within the oxygen-dependent degradation domain (ODD) of the HIF-α subunit (11). In mammalian cells, three PHD isoforms have been identified (PHD -1, -2, and -3), and shown to hydroxylate HIF-α (12, 13). These enzymes have an absolute requirement for oxygen as a substrate. The overall reaction results in the insertion of one oxygen atom into the HIF-α peptide substrate at the proline residue; the other atom generates succinate from 2-OG with the release of CO2 (11).
HIF has been widely shown to promote mucosal barrier protection (14,15,16). Although previous work has shown that HIF is induced at the site of wounds during reepithelialization (17) and that several HIF target genes, such as VEGF and iNOS, have all been shown to play a role in the wound healing process (18, 19), little is known about the role of HIF in tissue reepithelialization (i.e., epithelial restitution). More recent studies by our group and others have shown that induction and stabilization of the alpha subunit of HIF through inhibition of prolyl hydroxylases not only ameliorates disease in murine models of colitis but also promotes intestinal reepithelialization in vivo (20, 21). In vitro models of wound healing suggest that HIF induction also increases fibroblast contraction within a collagen matrix, another important step in the wound-healing process (21). These results not only suggest an important role for HIF in intestinal wound healing, but also implicate HIF stabilization as a potential therapeutic strategy (20, 21).
Here, we hypothesized that HIF activation promotes mucosal wound healing. Employing an established in vitro model for wound healing, we identified ITGB1 as a HIF target gene that contributes to the wound-healing process in both the extracellular matrix (ECM) and epithelium. Our findings suggest that HIF may play an initiating role in intestinal wound healing through induction of the integrin β1, a key protein in fibroblast contraction and epithelial migration, further supporting the strategy of HIF stabilization as a potential therapeutic means of promoting wound healing in mucosal lesions.
MATERIALS AND METHODS
Cell culture
18CO intestinal fibroblast cells and HeLa epithelial cells (18) were grown and maintained in T75 cell culture flasks (Costar Corp., Cambridge, MA, USA) as described previously (22).
Collagen gel contraction assay
18CO fibroblasts were trypsinized, washed with PBS, and resuspended in complete medium at 500,000 cells/ml. Collagen gels were made as described previously (23). All gels contained a final concentration of 150,000 cells/ml and 1.0 mg/ml collagen I with or without indicated concentrations of FG-4497 or dimethyloxalylglycine (DMOG). In some cases, cell cultures were exposed to hypoxia, as described previously (24).
In subsets of experiments, protein neutralization assays were performed by the addition of mouse anti-human α2β1 integrin (clone PiE6, 1:500 titered concentration) or binding control anti-MHC class I (clone W6/32, 30 μg/ml final concentration) to cell suspensions prior to seeding in collagen gels. Collagen gel suspension medium was similarly supplemented with antibody during contraction assays.
Gels were digitally imaged on setting (t=0) and at various time points thereafter. Gel surface area was quantified in terms of pixel number using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/). Relative changes in surface area are reported as a percentage of the original surface area.
FG-4497 was synthesized at FibroGen, Inc. (San Francisco, CA, USA). DMOG was obtained from Cayman Chemicals (Ann Arbor, MI, USA). Where indicated, cells were exposed to the PHD inhibitors FG-4497 or DMOG at varied concentrations for indicated periods of time. Control conditions included exposure to equivalent concentrations of the appropriate vehicle.
Transcriptional analysis
The transcriptional profile of T84 epithelial cells subjected to control (normoxia, pO2 147 torr) or hypoxia (pO2 20 torr for 6 or 18 h hypoxia) was assessed from total RNA using quantitative genechip expression arrays (Affymetrix, Santa Clara, CA, USA) (25). Results obtained by gene array analysis were subsequently verified in 18CO human fibroblast cells by real-time polymerase chain reaction (PCR) (ITGB1 primer set: forward, 5′-CCTTGGGATGACTTGATTG-3′; reverse, 5′-ACCTTTCGGTCACTTAGGG-3′) using iQ SYBR mix (iCycler; Bio-Rad, Hercules, CA, USA), as described previously (25, 26).
HIF-responsive element plasmid transfection and luciferase reporter assay
18CO cells were passaged into 24-well plates and allowed to attach for 24 h. Transient transfection of 18CO cells and assessment of luciferase activity was carried out as described previously (27).
Cloning of the ITGB1 promoter
Analysis of full-length cDNA [GenBank accession no. NM_002211] identified the transcription start site (TSS) of ITGB1 at position −130 relative to the first codon (ATG). On the basis of these reports, we cloned genomic fragments extending from positions −979 to + 7 relative to the ITGB1 TSS, flanked by restriction sites CTCGAG (XhoI) and AAGCTT (HindIII), respectively. ITGB1 promoter (1016 bp) was generated from T84 cell genomic DNA using standard PCR with Accuprime DNA polymerase (forward, 5′-GGCGCTCGAGGATAGCAGCTTGCCAGTAGC-3′; reverse, 5′-ATAAAAGCTTCGGCGGCTCCTCCTCCTCAGGG-3′); it was then cloned into pGL-3 basic luciferase expression vector (Promega, Madison, WI, USA). Homology to published sequence was determined by sequencing through the University of Colorado Health Science Center genomics core sequencing facility. Reporter assays were performed with HeLa cells transfected with the indicated promoter constructs and exposed to normoxia or hypoxia, as described previously (28). All activity was normalized with respect to cotransfected Renilla plasmid.
Analysis of the ITGBI promoter sequence revealed the existence of a single potential binding site for HIF-1 (core sequence 5′-CACGTGG-3′) at position −548 to −555 relative to the TSS. Where indicated, mutations were performed as described previously (28). We constructed primers for directed mutagenesis of the HIF-1 sequence, from 5′-CACGTGG-3′ to 5′-CAATCAG-3′ flanked 24 and 16 bp on the 5′ and 3′ ends, respectively (forward, 5′-GCCAGTTCCCTTCCAGAAGGACCAATCAGTTTTTGGCCCCATGG-3′; reverse, 5′-CCATGGGGCCAAAAACTGATTGGTCCTTCTGGAAGGGAACTGGC-3′).
Chromatin immunoprecipitation (ChIP) of HIF-1α
ChIP was performed as described previously (29) using anti-HIF-1α antibody (rabbit polyclonal; Novus Biologicals, Littleton, CO, USA). HIF binding to ITGB1 promoter DNA was quantified by both standard and real-time PCR using primers (forward, 5′-CATCTCGGAAGCCCCCTGAGTC-3′; reverse, 5′-TCCCCAGTCTCACCACCCTTCGT-3′) designed to amplify a 166-bp region of the ITGB1 promoter. Chromatin incubated with beads or bead plus rabbit IgG were used to control for nonspecific binding.
Trinitrobenzene sulfonic acid (TNBS) colitis murine model
TNBS colitis was induced in mice with a modification (26) to the model by Morris et al. (30) and Boirivant et al. (31). Control animals received a corresponding volume of vehicle (50% ethanol) alone. Where indicated, animals were dosed with FG4497, as described previously (21). Animal weight was measured daily, and weight loss was employed as a measure of disease progress. Four days post-TNBS, mice were sacrificed; whole colon was excised and homogenized in TriZOL (Invitrogen Life Technologies, Carlsbad, CA, USA) using a 550 sonic dismembrator (Fisher Scientific International, Hampton, NH, USA) followed by phenol-chloroform extraction, as described previously (26). Reverse transcription was performed using Iscript cDNA synthesis kit using the manufacturer’s instructions (Bio-Rad Laboratories). Murine ITGB1 expression was examined by real-time PCR with the following primers: forward, 5′-CTGTGGGTGAATTGTTGC-3′; reverse, 5′-CTAATCTTTTAATGTGTCTGTTTGC-3′.
RESULTS
Correlation of HIF stabilization and collagen gel contraction
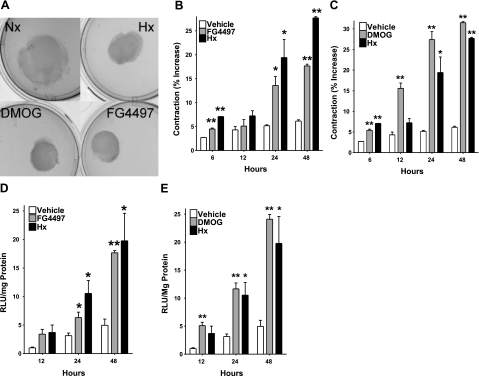
In previous studies, we demonstrated that pharmacological activation of HIF (PHD inhibition) increases contraction of collagen gels seeded with mouse embryonic fibroblast cells (NIH-3T3) (21). Here, we sought to examine the molecular mechanisms of this model in the context of intestinal wound healing and employed the use of CCD-18CO cells as a model of human colonic fibroblast collagen gel contraction. When seeded in collagen gels, 18CO cells showed a significant increase and time-dependent level of contraction when treated with PHD inhibitors DMOG and FG4497 or exposed to hypoxia (Fig. 1A–C) This contraction correlated directly with increases in HIF stabilization, as measured by luciferase reporter assay (Fig. 1D, E).
Figure 1.
Influence of hypoxia and HIF activation on fibroblast-mediated collagen gel contraction. Collagen gels seeded with 18CO fibroblasts and exposed to contraction of 18CO-seeded collagen gels (A), 10 μM FG4497 (B), or 1 mM DMOG (C) contracted in a time-dependent manner over 48 h. Hypoxia served as a control throughout and showed comparable levels of contraction. Vehicle (DMSO) and normoxia-treated cells showed only baseline contraction. Parallel levels of HIF activation in 18CO fibroblasts were measured by transient transfection with an HRE-luciferase reporter plasmid, with exposure to 10 μM FG4497 (D) and 1 mM DMOG (E). *P < 0.025, **P < 0.01; Student’s t test; n = 6.
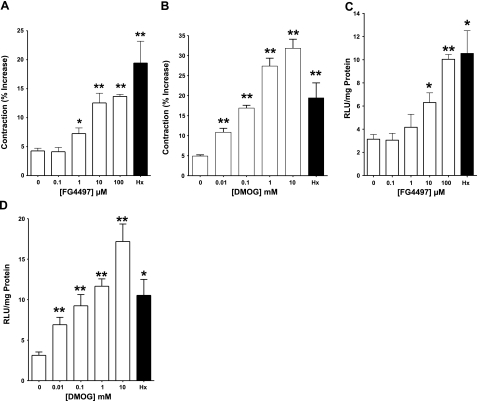
In additional experiments, concentration response assays for the PHD inhibitors showed a dose dependence between HIF activation and collagen gel contraction for both DMOG (P<0.01 by ANOVA) and FG4497 (P<0.01 by ANOVA), thereby implicating HIF activation in the process of collagen remodeling and fibroblast-mediated collagen gel contraction (Fig. 2).
Figure 2.
Influence of prolyl hydoxylation (PHD) inhibitors on fibroblast-mediated collagen gel contraction. Collagen gels seeded with 18CO fibroblasts and exposed to FG4497 (A) or DMOG (B) contracted in a concentration-dependent manner over 12 h. Hypoxia was a positive control and showed comparable levels of contraction to both inhibitors. Vehicle (DMSO) and normoxia-treated cells showed only baseline contraction. Contraction correlated with the concentration-dependent induction of HIF-1 in 18CO fibroblasts transfected with an HRE-luciferase reporter plasmid, seeded into collagen gels, and treated with FG4497 (C) or DMOG (D). Again, hypoxia was employed as a positive control and normoxia/vehicle-treated cells showed only baseline HIF induction. *P < 0.025, **P < 0.01; Student’s t test; n = 6.
Hypoxia-induced collagen gel contraction is transcriptionally dependent
To gain insight into the mechanisms of HIF-mediated collagen gel contraction, we systematically assessed the contribution of soluble and nonsoluble mediators to this process. First, the role of secreted soluble factors to fibroblast-mediated collagen gel contraction was examined. Freshly seeded fibroblast collagen gels were incubated in 50 and 100% culture medium from “conditioned” fibroblast collagen gels incubated in hypoxia or normoxia for 24 h. Conditioned medium derived from hypoxic cultures, relative to normoxia, transferred to naive gels did not significantly contribute to overall contraction of the gels (data not shown).
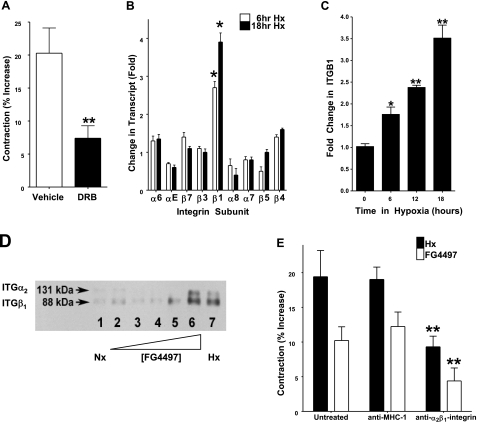
Next, we examined whether HIF-induced contraction was a consequence of transcriptional processes. To do this, fibroblast collagen gels were exposed to the transcription inhibitor dichloro-beta-d-ribofuranosylbenzimidazole (DRB), and the level of gel contraction was measured in hypoxia. As shown in Fig. 3A, DRB inhibited HIF-dependent contraction of collagen gels by 66 ± 8% (P<0.01 compared to vehicle controls), thereby strongly implicating transcriptional dependence in HIF-mediated collagen gel contraction.
Figure 3.
Identification of HIF-dependent induction of ITGB1 in fibroblast-mediated collagen gel contraction. Collagen gels were seeded with 18CO fibroblasts and treated with the transcriptional inhibitor DRB (30 μg/ml in culture medium) or vehicle (DMSO). Cells were incubated in hypoxic conditions for 24 h. A) DRB-treated gels showed a significantly lower level of contraction over vehicle controls in response to hypoxia; n = 6. B) Microarray screen of hypoxia-inducible integrins identifies β1 integrin as selectively induced at 6 and 18 h. C) Time-dependent induction of ITGB1 by hypoxia. 18CO fibroblasts were incubated in hypoxia and mRNA isolated at 6, 12, and 18 h for cDNA synthesis. D) Analysis of α2β1 integrin protein expression in response to HIF activation. Collagen gels seeded with 18CO fibroblasts were incubated with FG-4497 or exposed to hypoxia over a 24-h period. Gels were lysed, and α2β1 integrin was examined by immunoprecipitation and Western blot analysis. E) Functional role for α2β1 in fibroblast-collagen gel contraction. Fibroblast-embedded collagen gels were pretreated with a functionally inhibitory anti-human α2β1 integrin antibody (10 μg/ml) or binding control antibody directed against MHC-I and examined for contraction. *P < 0.025, **P < 0.01; Student’s t test.
In an attempt to identify HIF targets that may be involved in the regulation of collagen gel contraction, we turned to microarray results obtained from a comparison of intestinal epithelial cells subjected to normoxia or hypoxia for 6 or 18 h. Since integrins are the primary surface molecules in collagen binding, we focused our attention toward integrin transcripts found within this microarray. As shown in Fig. 3B, of the 10 integrin subunits expressed, only integrin β1 (ITGB1) was significantly changed by hypoxia. Indeed, ITGB1 was induced in a time-dependent fashion by hypoxia (2.6±0.3 and 3.7±0.4-fold increase at 6 and 18 h of hypoxia, respectively; P<0.025 for both, relative to normoxia). As guided by these results, we examined the induction of ITGB1 in the 18CO colonic fibroblast cell line. Fibroblast-imbedded collagen gels were subjected to hypoxia (0, 6, 12, and 18 h) and examined for ITGB1 mRNA levels by real time-PCR. As depicted in Fig. 3C, ITGB1 was significantly induced by hypoxia, with a 3.5 ± 0.4-fold induction at 18 h (P<0.01). Such findings identify ITGB1 as a potential target gene for hypoxia-induced collagen gel contraction.
Analysis of α2β1 integrin protein expression in fibroblast-collagen gels
We next investigated induction of the heterodimeric α2β1 integrin protein by hypoxia. For these purposes, 18CO cells were exposed to FG4497, DMOG, hypoxia, or normoxia, and a2β1 integrin protein was immunoprecipitated and detected using Western blot analysis. As shown in Fig. 3D, the α2β1 integrin heterodimer increased in an FG4497 concentration-dependent manner. Higher concentrations of FG4497 (10 μM) showed comparable increases to hypoxia, and DMOG-induced α2β1 integrin protein expression was similar to that of FG4497 (data not shown). These data suggest that hypoxia and HIF activation induce α2β1 integrin at the protein level and that such induction manifests at the level of the integrin heterodimer.
HIF activation elicits functional induction α2β1 integrin
While α2β1 integrin induction by HIF correlates with fibroblast gel contraction, such findings do not demonstrate that α2β1 integrin mediates functional collagen gel contraction. To ascertain whether α2β1 integrin played a functional role in contraction, 18CO fibroblast cells were preincubated with functionally inhibitory antibody directed against the α2β1 integrin heterodimer, or surface-binding controls directed against MHC class I (W/632) prior to seeding within collagen gels and exposure to FG4497 or hypoxia. The medium was similarly supplemented with antibody. As shown in Fig. 3E, cells pretreated with anti-α2β1 integrin antibody showed a significant reduction in contraction when compared to untreated and binding control antibody in response to both hypoxia (P<0.025) and to HIF activation by FG4497 (P<0.025), thereby indicating that induction of α2β1 integrin is functionally relevant in increased collagen gel contraction observed in response hypoxia and HIF activation.
ITGB1 promoter studies
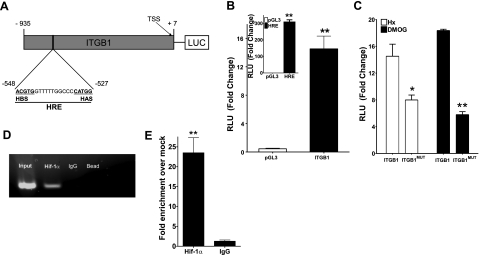
In view of the likelihood of a transcription-mediated induction of ITGB1 during hypoxia, attention was directed at the 5′-region of the ITGB1 gene for potential hypoxia-regulated transcription factor sequences. Available public databases (32) and analysis of full-length cDNA (GenBank Accession AL365203) identified the TSS of ITGB1 at position −149 relative to the start codon (ATG, see Fig. 4A). On the basis of these reports, we cloned genomic fragments extending from positions −935 to +7 into a pGL3 luciferase reporter vector, and we examined transcriptional activity following transient transfection into HeLa cells. As shown in Fig. 4B, ITGB1-transfected cells demonstrated a 14 ± 2-fold increase in luciferase activity in response to hypoxia. No response was observed in response to hypoxia in empty vector-transfected controls. As a positive control for hypoxia, HeLa cells were transfected with a pGL3- HIF-responsive element (HRE) plasmid containing 4 tandem HIF-1 enhancer sequences from the 3′ region of the erythropoiten gene (15) (inset, Fig. 4B).
Figure 4.
Isolation, cloning, and functional analysis of the human ITGB1 promoter. A) Construct map of the ITGB1 promoter sequence showing the HRE site relative to the TSS. B) Influence of hypoxia (solid bars) on ITGB1 promoter activity. Inset: HRE and empty PGL3 vector served as positive and negative controls, respectively. C) Influence of site-directed mutagenesis of the HIF binding site on hypoxia (open bars) and DMOG (1 mM)-induced ITGb1 activity. For all experiments, a Renilla plasmid was employed as a cotransfection control. D) ChIP analysis to examine HIF-1α binding to the ITGB1 promoter in hypoxic Caco2 cells. Reaction controls included immunoprecipitations using a nonspecific IgG and beads only, as well as PCR performed using total Caco2 DNA (input). One of three experiments is shown. E) Real-time PCR analysis of ChIP DNA samples. Results were calculated as a fold enrichment over IgG control ± se. Results are derived from three experiments. *P < 0.025, **P < 0.01; Student’s t test.
To further identify the contribution of HIF to induction of ITGB1 in hypoxia, we performed site-directed mutagenesis of the putative HIF binding site in the ITGB1 promoter. The HIF-binding region of the ITGB1 promoter was mutated to a non-HIF binding sequence and examined for transcriptional activity compared to the wild-type ITGB1 promoter following transient transfection into HeLa cells. As shown in Fig. 4C, the ITGB1-HIF mutant promoter (ITGB1MUT)-transfected cells showed a 49 ± 3% and 70 ± 2% decrease in activity elicited by hypoxia and DMOG, respectively (both P<0.025 compared to wild-type plasmid). These data further support our findings of HIF-dependent regulation of ITGB1.
HIF-1α binds directly to the ITGB1 promoter
We next determined whether this region of the ITGB1 promoter binds HIF-1α. For these purposes, we utilized ChIP to analyze HIF-1α binding in live cells. As shown in Fig. 4D, ChIP analysis of nuclei derived from Caco2 cells exposed to hypoxia revealed a prominent band of 166 bp. No bands were evident in control IgG immunoprecipitates bead-only controls, and input samples (preimmunoprecipitation) revealed the predictable 166-bp band under these conditions. Real-time PCR quantification of this ChIP assay from genomic DNA showed a 0.22 ± 0.036% amplification of total genomic input (Fig. 1B), which corresponds to a 25.5 ± 3.8-fold enrichment over mock controls (P<0.001, Fig. 4E). Taken together, these results provide strong evidence for a functional hypoxia-inducible activity, mediated by HIF, in the distal 5′-region the ITGB1 promoter.
ITGB1 is induced in a murine model of colitis
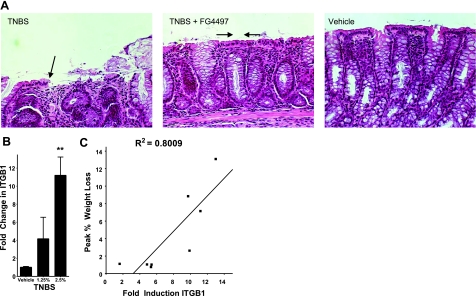
As proof of principle for the relevance of β1-integrin induction in vivo, we investigated ITGB1 expression in the TNBS murine model of colitis, a model we have previously shown to result in severe ulceration and concomitant tissue hypoxia (25). Moreover, as an extension of our previous work (21) implicating PHD inhibition and enhanced reepithelialization of colitic wounds (Fig. 5A), we determined whether ITGB1 was induced in response to colitis. As shown in Fig. 5B, murine ITGB1 was induced in a TNBS concentration-dependent manner, with a 4.2 ± 2.3-fold induction in response to 1.25% TNBS and an 11.1 ± 1.8-fold induction in response to 2.5% TNBS (P<0.025 compared to vehicle controls). When plotted against disease severity, a significant correlation between induction of ITGB1 and peak weight loss in these animals (R2=0.80, P<0.01) was observed (Fig. 5C). These results demonstrate an in vivo relevance to hypoxic induction of ITGB1.
Figure 5.
Correlation of ITGB1 expression in murine colitis. A) PHD inhibition in murine TNBS colitis promotes intestinal wound healing and epithelial restitution. Shown here are histological sections from animals administered TNBS (left), TNBS with PHD inhibitor (60 g/kg FG4497; middle panel), or vehicle alone (no TNBS, no FG4497). B) Induction of ITGB1 in TNBS colitis. mRNA was isolated from whole colon from mice 4 days after induction of TNBS colitis. ITGB1 expression was examined by real-time PCR and compared to healthy mice. C) ITGB1 induction correlation with disease severity (weight loss). **P < 0.01; Student’s t test.
DISCUSSION
This study aimed to identify mechanisms of wound repair in an in vitro model of the intestinal submucosa. Previous work demonstrated that HIF induction by hypoxia offers protection to the mucosal barrier and that the pharmacological stabilization of HIF accelerates the healing process in models of murine colitis (20, 21). Here, we extended these studies and identified ITGB1 as a HIF transcriptional target. As such, HIF functions to regulate β1-integrin expression and control fibroblast contraction during wound healing.
Ongoing inflammatory responses are characterized by dramatic shifts in tissue metabolism, resulting in localized hypoxia. Recent studies have focused on the contribution of hypoxia to the inflammatory response (16, 33, 34) and have shown a role for HIF in endogenous protective pathways within a variety of inflammatory diseases (35). Multiple lines of evidence have implicated HIF as a physiologically protective factor (15, 21, 26, 27, 36) that may also have a mediatory role in wound healing (20, 21, 37, 38), Our initial insights of accelerated wound healing were gained from observations of mucosal healing as a result of pharmacological HIF induction in murine colitis (20, 21). To study molecular mechanisms of this response, we adapted a well-characterized collagen contraction assay employing imbedded human intestinal fibroblasts. This model predicted a correlation between HIF stabilization and the extent of wound healing, reflected as an increase in collagen gel contraction.
Previous studies have shown that soluble factors, such as TGF-β, are released in response to hypoxia and can modulate extracellular matrix interactions (39,40,41). TGF-β has been shown to induce α2-integrin in osteogenic cells, presumably also facilitating greater β1 integrin expression, to promote wound healing (42). In our studies, incubation of naive fibroblast-collagen gels in normoxia with conditioned supernatants from hypoxia-incubated gels were unrevealing, suggesting that soluble factors do not play a significant role in fibroblast-mediated collagen gel contraction. Studies using DRB demonstrated a convincing role for transcriptional activation in hypoxia and HIF-mediated increases in collagen gel contraction. Guided by microarray analysis, we identified a selective induction of ITGB1 by hypoxia and HIF stabilization. β1 integrins belong to a family of heterodimeric cell surface receptors comprising 18α and 8β chains that function as direct lines of cell-ECM communication (43, 44). β1 integrins link ECM ligands mechanically to the cell by triggering formation of intracellular cytoskeletal scaffolds that facilitate interactions between various intracellular signaling proteins (45, 46). In the context of the intestinal mucosa, β1 integrins play a role in epithelial migration (47, 48) and fibroblast-collagen interactions (23), both key process in wound restitution and tissue reepithelialization (49, 50). On the basis of initial findings of β1 integrin induction by hypoxia and HIF activators, we examined expression of α2β1-integrin, the primary collagen-binding integrin (51, 52). This analysis identified a prominent induction of α2β1-integrin by hypoxia and HIF activation and revealed a physiological role in collagen gel contraction through utilization of a functionally inhibitor antibody directed against α2β1-integrin.
Several lines of evidence directly implicate HIF in ITGB1 induction by hypoxia. First, hypoxia and two separate and structurally distinct PHD inhibitors (DMOG and FG4497) prominently induce ITGB1 mRNA, protein, and promoter activity. Second, findings of inhibition by DRB strongly implicate one or more hypoxia-mediated transcriptional elements in ITGB1, for which HIF is a major target. Third, sequence analysis and site-directed mutagenesis of the cloned ITGB1 promoter revealed the existence of a functional HRE. Fourth, ChIP analysis revealed prominent HIF-1α binding to the proximal portion of the integrin promoter containing the putative HIF binding site. Important in this regard, HIF may not act as a stand-alone factor in such regulation. For example, since functional integrins exist only as heterodimeric complexes, induction of functional ITGB1 in this model also implicates the parallel induction of an alpha-subunit (i.e., α2 integrin). At present, we do not know the molecular mechanism responsible for α2 integrin induction. Our sequence analysis of the regulatory region of the α2 integrin gene revealed no apparent binding sites for HIF. The lack of an obvious HRE suggests that their induction by hypoxia might be mediated by mechanisms different from those controlling expression of β1 integrin.
As part of our analysis, we extended our previous work with TNBS colitis (21, 26). Specifically, in this model of colitis, it was determined that significant tissue hypoxia, as measured using nitroimidazole dyes, is associated with inflammatory lesions (53). This finding established the concept of “inflammatory hypoxia” (54) and as such, serves as an appropriate model to examine ITGB1 regulation in vivo. In the present studies, analysis of ITGB1 induction in colonic tissue revealed a >10-fold increase in ITGB1 mRNA during severe disease. Moreover, such expression correlated with disease severity. Although we have not determined whether induction of ITGB1 is associated with specific cell populations, it is likely that several mucosal compartments contribute to this increase in expression. β1-integrin, for example, is widely expressed on epithelia and fibroblasts (43, 44, 47, 48) and can also be expressed on infiltrating T-cell populations (55, 56) and other leukocytes in experimental colitis (57). That both genetically deficient and conditional mutants of murine Itgb1 are embryonic or postnatally lethal (58, 59) makes direct assessment of these issues more limited. Additional work will be necessary to define the exact contribution HIF-mediated induction of ITGB1 to mucosal diseases such as colitis.
In total, these studies demonstrate that HIF is a key regulator of ITGB1, itself critical to wound healing. ITGB1 is induced in murine models of colitis, and its regulation, through HIF targeting, may yield further therapeutic strategies for the management of inflammatory diseases such as irritable bowel disease.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants HL60569, DK50189, and DE DE13499 and by a grant and research fellowship from the Crohn’s and Colitis Foundation of America. The authors acknowledge the generous gift of FG4497 from Fibrogen Corp.
References
- Lawrance I C, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:226–236. doi: 10.1097/00054725-200108000-00008. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55–60. doi: 10.1097/00054725-200401000-00009. [DOI] [PubMed] [Google Scholar]
- Ngo P, Ramalingam P, Phillips J A, Furuta G T. Collagen gel contraction assay. Methods Mol Biol. 2006;341:103–109. doi: 10.1385/1-59745-113-4:103. [DOI] [PubMed] [Google Scholar]
- Moore R, Carlson S, Madara J L. Villus contraction aids repair of intestinal epithelium after injury. Am J Physiol Gastrointest Physiol. 1989;257:G274–G283. doi: 10.1152/ajpgi.1989.257.2.G274. [DOI] [PubMed] [Google Scholar]
- Olson A D. Contraction of collagen gels by intestinal epithelial cells depends on microfilament function. Dig Dis Sci. 1993;38:388–395. doi: 10.1007/BF01316489. [DOI] [PubMed] [Google Scholar]
- Steinbrech D S, Longaker M T, Mehrara B J, Saadeh P B, Chin G S, Gerrets R P, Chau D C, Rowe N M, Gittes G K. Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J Surg Res. 1999;84:127–133. doi: 10.1006/jsre.1999.5627. [DOI] [PubMed] [Google Scholar]
- Tandara A A, Mustoe T A. Oxygen in wound healing—more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- Zinkernagel A S, Johnson R S, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- Meissner U, Allabauer I, Repp R, Rascher W, Dotsch J. Inducible expression of hypoxia-inducible factor 1alpha (HIF-1alpha) as a tool for studying HIF-1alpha-dependent gene regulation during normoxia in vitro. Pharmacology. 2003;69:74–78. doi: 10.1159/000072359. [DOI] [PubMed] [Google Scholar]
- Semenza G L. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Semenza G L. Hypoxia-inducible factor 1 (HIF-1) pathway. [Online] Sci STKE 2007. 2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Appelhoff R J, Tian Y M, Raval R R, Turley H, Harris A L, Pugh C W, Ratcliffe P J, Gleadle J M. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Maxwell P H, Ratcliffe P J. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- Furuta G T, Turner J R, Taylor C T, Hershberg R M, Comerford K, Narravula S, Podolsky D K, Colgan S P. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N A, Hamilton K E, Canny G, Shekels L L, Ho S B, Colgan S P. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–1627. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- Taylor C T, Colgan S P. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- Elson D A, Ryan H E, Snow J W, Johnson R, Arbeit J M. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–6195. [PubMed] [Google Scholar]
- Albina J E, Mastrofrancesco B, Vessella J A, Louis C A, Henry W L, Jr, Reichner J S. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-α. Am J Physiol Cell Physiol. 2001;281:C1971–C1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Cummins E P, Seeballuck F, Keely S J, Mangan N E, Callanan J J, Fallon P G, Taylor C T. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Robinson A, Keely S, Karhausen J, Gerich M E, Furuta G T, Colgan S P. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclachlan T, Narayanan B, Gerlach V L, Smithson G, Gerwien R W, Folkerts O, Fey E G, Watkins B, Seed T, Alvarez E. Human fibroblast growth factor 20 (FGF-20; CG53135-05): a novel cytoprotectant with radioprotective potential. Int J Radiat Biol. 2005;81:567–579. doi: 10.1080/09553000500211091. [DOI] [PubMed] [Google Scholar]
- Phillips J A, Bonassar L J. Matrix metalloproteinase activity synergizes with α2β1 integrins to enhance collagen remodeling. Exp Cell Res. 2005;310:79–87. doi: 10.1016/j.yexcr.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Colgan S P, Dzus A L, Parkos C A. Epithelial exposure to hypoxia modulates neutrophil transepithelial migration. J Exp Med. 1996;184:1003–1015. doi: 10.1084/jem.184.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N A, Robinson A M, MacManus C F, Karhausen J, Scully M, Colgan S P. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- Karhausen J O, Furuta G T, Tomaszewski J E, Johnson R S, Colgan S P, Haase V H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford K M, Wallace T J, Karhausen J, Louis N A, Montalto M C, Colgan S P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- Louis N A, Hamilton K E, Kong T, Colgan S P. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 2005;19:950–959. doi: 10.1096/fj.04-3251com. [DOI] [PubMed] [Google Scholar]
- Kong T, Eltzschig H K, Karhausen J, Colgan S P, Shelley C S. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of β2 integrin gene expression. Proc Natl Acad Sci U S A. 2004;101:10440–10445. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G P, Beck P L, Herridge M S, Depew W T, Szewczuk M R, Wallace J L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Boirivant M, Fuss I J, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D A, Boguski M S, Lipman D J, Ostell J, Ouellette B F, Rapp B A, Wheeler D L. GenBank Nucleic Acids Res. 1999;27:12–17. doi: 10.1093/nar/27.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J J. Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for hypoxia-inducible factor-1alpha. Crit Care. 2003;7:47–54. doi: 10.1186/cc1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi S, Wrenshall L E, Platt J L. Regional manifestations and control of the immune system. FASEB J. 2003;16:849–856. doi: 10.1096/fj.01-0690hyp. [DOI] [PubMed] [Google Scholar]
- Semenza G L, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta G T, Comerford K M, Louis N, Karhausen J, Eltzschig H K, Hansen K R, Thompson L F, Colgan S P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 (HIF-1) mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chang Q, Cox R A, Gong X, Gould L J. Hyperbaric Oxygen Attenuates Apoptosis and Decreases Inflammation in an Ischemic Wound Model. J Invest Dermatol. 2008;128:2102–2112. doi: 10.1038/jid.2008.53. [DOI] [PubMed] [Google Scholar]
- Mace K A, Yu D H, Paydar K Z, Boudreau N, Young D M. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15:636–645. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- Chen C P, Yang Y C, Su T H, Chen C Y, Aplin J D. Hypoxia and transforming growth factor-beta 1 act independently to increase extracellular matrix production by placental fibroblasts. J Clin Endocrinol Metab. 2005;90:1083–1090. doi: 10.1210/jc.2004-0803. [DOI] [PubMed] [Google Scholar]
- Papakonstantinou E, Aletras A J, Roth M, Tamm M, Karakiulakis G. Hypoxia modulates the effects of transforming growth factor-beta isoforms on matrix-formation by primary human lung fibroblasts. Cytokine. 2003;24:25–35. doi: 10.1016/s1043-4666(03)00253-9. [DOI] [PubMed] [Google Scholar]
- Kan C, Abe M, Yamanaka M, Ishikawa O. Hypoxia-induced increase of matrix metalloproteinase-1 synthesis is not restored by reoxygenation in a three-dimensional culture of human dermal fibroblasts. J Dermatol Sci. 2003;32:75–82. doi: 10.1016/s0923-1811(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Riikonen T, Koivisto L, Vihinen P, Heino J. Transforming growth factor-beta regulates collagen gel contraction by increasing alpha 2 beta 1 integrin expression in osteogenic cells. J Biol Chem. 1995;270:376–382. doi: 10.1074/jbc.270.1.376. [DOI] [PubMed] [Google Scholar]
- Zutter M M, Santoro S A. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990;137:113–120. [PMC free article] [PubMed] [Google Scholar]
- Eckes B, Zweers M C, Zhang Z G, Hallinger R, Mauch C, Aumailley M, Krieg T. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006;11:66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- Davis G E, Senger D R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Turner C E. Paxillin interactions. J Cell Sci. 2000;113:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- Tong Q, Vassilieva E V, Ivanov A I, Wang Z, Brown G T, Parkos C A, Nusrat A. Interferon-gamma inhibits T84 epithelial cell migration by redirecting transcytosis of beta1 integrin from the migrating leading edge. J Immunol. 2005;175:4030–4038. doi: 10.4049/jimmunol.175.6.4030. [DOI] [PubMed] [Google Scholar]
- Lotz M M, Nusrat A, Madara J L, Ezzell R, Wewer U M, Mercurio A M. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am J Pathol. 1997;150:747–760. [PMC free article] [PubMed] [Google Scholar]
- Imaizumi F, Asahina I, Moriyama T, Ishii M, Omura K. Cultured mucosal cell sheet with a double layer of keratinocytes and fibroblasts on a collagen membrane. Tissue Eng. 2004;10:657–664. doi: 10.1089/1076327041348329. [DOI] [PubMed] [Google Scholar]
- Montandon D, D'Andiran G, Gabbiani G. The mechanism of wound contraction and epithelialization: clinical and experimental studies. Clin Plast Surg. 1977;4:325–346. [PubMed] [Google Scholar]
- Grinnell F, Ho C H, Lin Y C, Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274:918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Ho C H. Transforming growth factor beta stimulates fibroblast-collagen matrix contraction by different mechanisms in mechanically loaded and unloaded matrices. Exp Cell Res. 2002;273:248–255. doi: 10.1006/excr.2001.5445. [DOI] [PubMed] [Google Scholar]
- Laughlin K M, Evans S M, Jenkins W T, Tracy M, Chan C Y, Lord E M, Koch C J. Biodistribution of the nitroimidazole EF5 (2-[2-nitro-1H-imidazol-1-yl]-N-(2,2,3,3,3-pentafluoropropyl) acetamide) in mice bearing subcutaneous EMT6 tumors. J Pharmacol Exp Ther. 1996;277:1049–1057. [PubMed] [Google Scholar]
- Karhausen J, Haase V H, Colgan S P. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- Hemler M E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Stemme S, Holm J, Hansson G K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Krieglstein C F, Cerwinka W H, Sprague A G, Laroux F S, Grisham M B, Koteliansky V E, Senninger N, Granger D N, de Fougerolles A R. Collagen-binding integrin α1β1 regulates intestinal inflammation in experimental colitis. J Clin Invest. 2002;110:1773–1782. doi: 10.1172/JCI200215256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R G, Li X, Gray P D, Kuang J, Clayton F, Samowitz W S, Madison B B, Gumucio D L, Kuwada S K. Conditional deletion of β1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T R, Hu H, Braren R, Kim Y H, Wang R A. Cell-autonomous requirement for β1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development. 2008;135:2193–2202. doi: 10.1242/dev.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]