Abstract
Objective
To assess whether men who have sex with men (MSM) prefer a gel or a suppository as a delivery vehicle for a rectal microbicide.
Methods
77 HIV-negative MSM with recent history of inconsistent condom use during receptive anal intercourse (RAI) who acknowledged being at risk of contracting HIV were enrolled in a randomized, crossover acceptability trial. They compared 35 ml of placebo gel with 8 g placebo rectal suppositories used in up to three RAI occasions each.
Results
Participants preferred the gel over the suppository (75% vs. 25%, p <.001), and so did their partners (71% vs. 29%, p <.001). The gel received more favorable ratings overall and on attributes such as color, smell, consistency, feeling in rectum immediately after insertion and/or 30 minutes after insertion, and application process. The gel resulted in less negative ratings in terms of participants being bothered by leakage, soiling, bloating, gassiness, stomach cramps, urge to have bowel movement, diarrhea, pain or trauma. Participants liked the gel more in terms of feelings during anal sex, sexual satisfaction, partners’ sexual satisfaction, and liking the product when condoms were used and when condoms were not used.
Conclusions
In this sample taken from one of the populations most likely to benefit from rectal microbicide availability, gel had higher acceptability than suppository as a potential microbicide vehicle.
Keywords: acceptability, anal intercourse, homosexual, men who have sex with men, microbicide, formulation
Introduction
Rectal microbicides are compounds under development that may decrease the risk of HIV transmission when applied inside the rectum prior to intercourse. Rectal microbicides need to be efficacious against HIV and, equally important, products that people are able and willing to use (“acceptability”). Initial acceptability studies were hypothetical (i.e., potential users reported their likelihood of using a product described to them but which they did not use) 1,2. An exception was Gross et al.’s pioneer rectal trial3 which highlighted the importance of acceptability in microbicide efficacy. While progress in rectal microbicide development lags behind those made for vaginal microbicides4, several clinical trials testing the safety and acceptability of rectal microbicide candidates are underway5.
The rectal compartment is an open conduct with a large surface of mucosa that could be exposed to the virus6. Consequently, large volumes of a rectal topical microbicide have been assumed to be needed to provide coverage and maximize the product’s effectiveness. In a prior study5 we explored what would be the largest acceptable volume of gel for men who practice RAI, and found that 35 ml was the upper limit, although smaller volumes were preferred.
Other than gels, suppositories offer another way to deliver a microbicidal agent intrarectally. Predicting that mucosal coverage would also be a concern with a suppository, we assumed that a suppository would have to be fairly large to accommodate enough active formula. Consequently, we designed a study to compare the relative acceptability of 35 mls of a gel formulation with that of a 8g rectal suppository as delivery vehicles for a rectal microbicide to be used prior to RAI by men who have sex with men.
Method
The study design and procedures were approved by the Institutional Review Boards of both the New York State Psychiatric Institute and Fenway Community Health (FCH) in Boston, Massachusetts.
Recruitment & Eligibility Criteria
Participants were recruited7 in Boston, Massachusetts, between May 2005 and April 2007. Eligible men had to be 18 years of age or older, HIV-negative by self-report, and aware that unprotected RAI with a partner of HIV-positive or unknown serostatus carries HIV-transmission risk; furthermore, they had to report having had unprotected RAI in the prior year and rate it as involving some risk of HIV transmission to themselves, have a male partner with whom they engaged in RAI at least once every two weeks, and be willing to abstain from RAI three days prior to initial clinical exam and three days after finishing using the first product and before using the second one. Participants were excluded if they had had a genital or rectal herpes outbreak in the previous six months, diarrhea or rectal bleeding within the past two months, a history of chronic gastrointestinal disorders, or large hemorrhoids or internal warts; further exclusion criteria were having been involved in a drug research protocol within the past year or having a current male partner also enrolled in the study.
Study Products
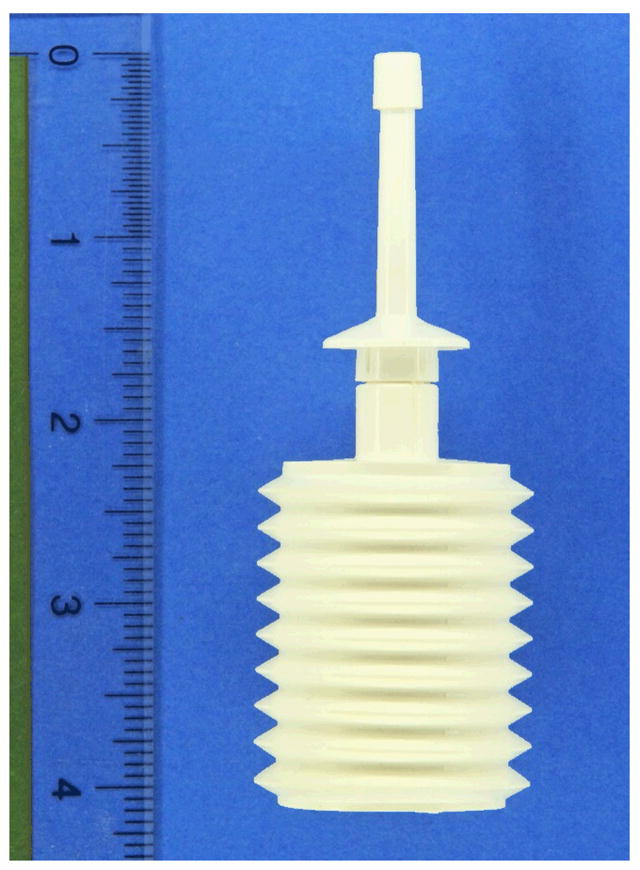
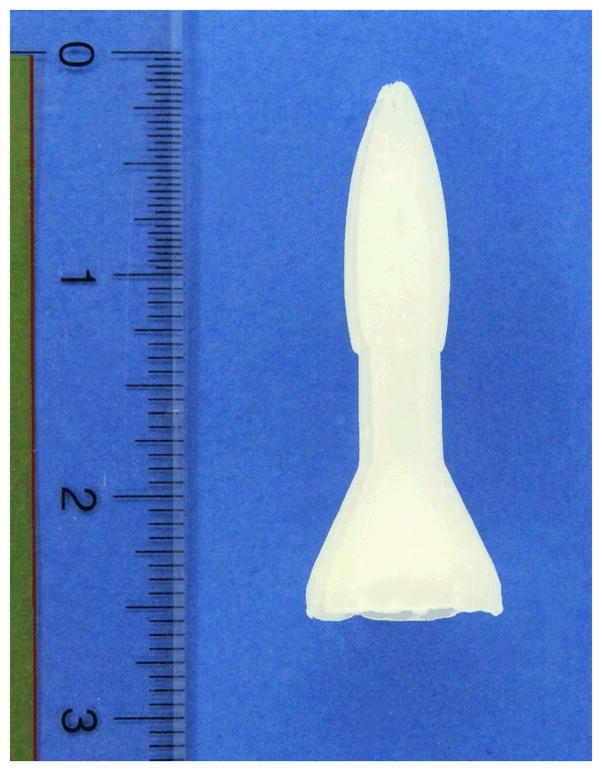
Two placebo products were used: 1) a gel manufactured by Cooper Surgical, Inc. (Trumbull, CT) using deionized (purified) water, Polyoxyethylene, Methylparaben and Sodium-Carbomer that is available on the market as a lubricant and sold as FemGlide or Slippery Stuff; and 2) a suppository manufactured by J.E. Pierce Apothecary, Inc. (Brookline, MA) using two Polyethylene glycol bases (PEG 1450 + PEG 300) with no other ingredients, which can be used as a vehicle for delivery of rectal medications. The suppository, weighing approximately 8 grams and measuring 2 ½ inches (6 cm) in length (see Figure 1) was dubbed “rectal rocket” by its manufacturer. Participants inserted 35 ml of the gel using an enema bottle (see Figure 2) manufactured by Spruyt hillen (Netherlands). The suppository was inserted manually without any applicator.
Figure 1.
Figure 2.
Procedures
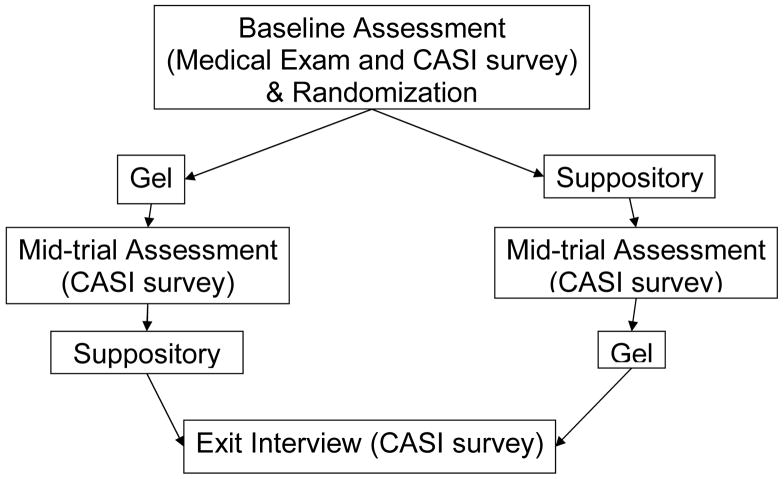
After completing the consent procedures and before being enrolled into the study (see Figure 3), participants underwent a clinical exam. Participants completed a baseline quantitative assessment using a Computer-Administered Self-Interview (CASI) that included questions on demographic information and rectal microbicide intentions. After instruction on the proper use of both products, participants were sequentially randomized to either Group A (gel first, suppository second) or Group B (suppository first, gel second) following the crossover design of the study. Participants were provided either with three filled gel applicators or four suppositories (one extra suppository was supplied in case there were problems during insertion) and asked to insert the product up to two hours prior to intercourse in three different intercourse occasions in the weeks that followed. For the suppositories, participants were asked to wait 30 minutes after insertion before having sex to allow the suppository to dissolve. Participants were reminded that the products were placebos that offered no protection against HIV/STDs, and were given condoms and lubricants. Participants were asked to call the research office within 24 hours of product use to report on product acceptability for the specific sexual episode. Once the three occasions were completed, participants completed a mid-trial CASI acceptability assessment of the first product used. The second product was evaluated with identical procedures. Finally, once participants had the chance to use and evaluate both products, they were asked to use CASI to respond to a product preference measure and discuss any product recommendations.
Figure 3.
Measures
Sexual behavior
Respondents were asked to report their sexual behavior during the previous two months, including number of sexual partners and unprotected RAI occasions.
Rectal microbicide intentions
At baseline, we asked: “If a rectal microbicide were available that provided some protection against HIV, and it looked like the [gel/suppository] we showed you earlier, how likely would you be to use it every time you have receptive anal intercourse?” Answers could range from 1 = extremely unlikely to 10= extremely likely. A slightly edited version of this question was asked after the participant had tried each study product 1 – 3 times.
Product acceptability
A summary measure of acceptability was completed once the participant had finished using each product. This measure included a general question (“Overall, how much did you like the gel/suppository?”), as well as specific questions on the level of like/dislike of the products’ physical properties, application process, and experiences using the product during sex. These questions were answered on a 10-point scale (1=disliked very much to 10= liked very much). We ascertained if any potential problems were experienced (e.g., leakage, soiling of underwear or linens, bloating) and, for those experiencing them, how bothersome they were (1= Not at all to 10= Very much). Finally, participants were asked “How much would you be willing to spend on a gel/suppository per sexual occasion?” (0= less than condoms or nothing to 3= three times as much or more). Questions were worded identically (whenever possible) for both products to make comparison possible.
Product preference
Participants indicated which product they preferred (“Thinking about the gel and suppository, overall which product did you like the most?”) and also reported which product they believed their partner liked the most.
Data analysis
Those who used and rated both products (“completers,” n = 77) were compared to those who did not finish the trial (“attrited,” n = 28) on all demographic variables using t-tests and chi-square tests. We restricted subsequent product comparisons to men who used both products. Binomial tests were used to determine whether one product was preferred by more participants than the other. Wilcoxon tests were used to evaluate whether the ratings for one product were significantly different from the ratings for the other product. We performed a post-hoc correction to decrease the Type-I error.
Results
Sample Description
We present the demographic characteristics of our sample (N = 77) in Table 1. On average, participants were 41 years of age; the majority had completed a high school education or higher; two thirds were employed, having a median income in the $20,001–$40,000 range; two thirds identified as White or European American; and three quarters identified as gay.
Table 1.
Sample Description (N = 77)
| Age in years (range) | M = 40.7, SD = 9.97 (18–60) |
| N (%) | |
| Education | |
| Less than a high school degree | 6 ( 7.8%) |
| High school degree | 24 (31.2%) |
| Some college | 18 (23.4%) |
| College degree | 13 (16.9%) |
| Post-baccalaureate education | 16 (20.8%) |
| Employed | 48 (62.3%) |
| Income | |
| $10,000 or less | 23 (29.8%) |
| $10,001 to $40,000 | 34(44.2%) |
| $40,001 to $80,000 | 12(15.6%) |
| $80,001 or more | 5 ( 6.5%) |
| Did not report | 3 ( 3.9%) |
| Race/Ethnicity | |
| White/European American | 50 (64.9%) |
| Black/African American | 20 (26.0%) |
| Latino | 4 ( 5.2%) |
| Other | 3 ( 3.9%) |
| Identity/Self-Label | |
| Gay | 58 (75.3%) |
| Bisexual | 19 (24.7%) |
Sexual behavior in the two months prior to interview
At baseline, all but one of the participants reported having had at least one male sexual partner (M = 4.40, SD = 5.18) with whom they had engaged in RAI in the prior two months, slightly more than half (56.3%) of RAI occasions were unprotected. Eighty percent (N = 62) of participants reported having insertive anal intercourse (IAI), on average on 5.78 occasions (SD = 7.79), three fifths (59.5%) of them unprotected.
Preference ratings for gel versus suppository
After using each product on up to three separate occasions, significantly more participants reported preferring the gel (N = 58; 75%) than those preferring the suppository (N = 19; 25%; binomial p <.001). In addition, more participants believed their partners also preferred the gel (N = 55; 71%) than those who believed their partners preferred the suppository (N = 22; 29%; binomial p <.001).
At baseline, participants’ intention to use the gel in the future (M = 8.70) was statistically higher (p <.01) than their intention to use the suppository (M = 6.99). After using the product, their intention to use the gel was still higher than the suppository, although the mean ratings for both products went down (i.e., participants were less likely to intend to use the product after having used it).
Participants reported a higher overall acceptability of the gel over the suppository (see Table 2). The gel was preferred in its physical properties and application process prior to RAI. The few participants experiencing problems were more bothered by the suppository. We detected a statistical difference between the gel and suppository in terms of leaking (M = 3.38 vs. 4.14), soiling (M = 2.82 vs. 3.79), feeling bloated (M = 1.49 vs. 2.92), having gas (M = 2.04 vs. 3.62) or cramps (M = 1.30 vs. 2.25), needing to have a bowel movement (M = 2.36 vs. 4.95), diarrhea (M = 1.36 vs. 2.83), and pain or trauma ( M = 1.16 vs. 1.92). The gel was also preferred over the suppository in terms of experiences using the products during sexual intercourse (see Table 2). In general, those participants who had not applied either product prior to sexual intercourse did not report feeling bothered by interrupting the sexual act to apply it. Partner’s sexual satisfaction was also higher for the gel.
Table 2.
Mean Ratings of Gel and Suppository Characteristics (N = 77)
| Variable | Gel | Suppository | p-value |
|---|---|---|---|
| Overall, how much liked product | 7.61 | 5.32 | <.001 |
| Product properties | |||
| Color | 8.08 | 6.65 | <.001 |
| Smell | 8.00 | 6.18 | .014 |
| Consistency (thick or thin) | 7.58 | 5.60 | <.001 |
| Application Process | |||
| Process of applying | 6.84 | 5.31 | .001 |
| Ease of insertion | 7.60 | 7.52 | ns |
| Feeling in rectum after inserting | 7.78 | 4.71 | <.001 |
| Feeling in rectum 30 min. later | 7.55 | 5.97 | <.001 |
| Ease of carrying around | 6.39 | 6.55 | ns |
| Experiences using product during sex | |||
| Feeling during anal sex | 7.91 | 5.95 | <.001 |
| Sexual satisfaction using product | 8.14 | 6.23 | <.001 |
| Liked with condoms | 7.76 | 6.14 | .001 |
| Liked without condoms | 8.31 | 6.78 | .004 |
| Product improved sex1 | 1.30 | 0.86 | <.001 |
| Penetration was easier2 | 1.04 | 0.74 | .001 |
| Partner’s sexual satisfaction | 8.25 | 6.95 | .001 |
| Overall, how much partner liked | 7.56 | 6.08 | .001 |
| Intentions to use product in the future | |||
| Likely to use similar product | 8.35 | 6.18 | <.001 |
| Likely to use when no condoms | 8.70 | 7.27 | .001 |
| Would spend more than condoms | 1.60 | 1.45 | ns |
Response scale (0 = Worse, 1 = No different, 2 = Better)
Response scale (0=No, 1= Penetration was somewhat easier, 2=Penetration was much easier).
Discussion
Our study was conducted with sexually-active men who reported being HIV-uninfected and engaged in unprotected RAI in circumstances that involved risk of HIV transmission –i.e., one of the populations most likely to benefit from efficacious and acceptable rectal microbicides. Having tried two different placebo formulations (gel and suppository) of a microbicide vehicle during RAI, men preferred the gel. Those who experienced problems reported feeling more bothered by suppository-related issues than gel-related ones. Participants did not appear to be too bothered by problems related to product administration; however, they expressed preference for a product that did not require waiting time before it became active and voiced willingness to spend as much or slighter more for microbicides than what they spend in condoms. Men expressed higher intentionality to use a gel than a suppository after seeing both products but before trying them; this preference persisted after the trial. This seems to indicate that initial attitudes about use of a product that an individual has had the chance to examine may be predictive of future intention to use such product.
While these findings offer promise to an emerging field in HIV prevention, several limitations must be noted. First, our sample consisted exclusively of men who have RAI; generalizations to women who engage in RAI are not warranted. Second, we did not account for men’s previous history with gels or suppositories prior to baseline, which may confound our findings. In addition, at the time this study was designed, it was assumed that large volumes of gel (maybe up to 50 mL) would be necessary to confer mucosal coverage and protection. Current developments, including the likely utilization of anti-retrovirals in microbicide trials, suggest smaller volumes of gel may be sufficient8, thus increasing the potential acceptability of rectal microbicidal gels in the future.
The overall preference of a gel as a rectal microbicide vehicle in this sample should not completely discount a suppository as an alternate mode of delivery. The suppository used for our study was relatively large given our wish to make it somewhat comparable to the large volume of gel participants were using. Smaller suppositories or suppositories with different characteristics (e.g., solubility, mode of application) may result in different acceptability ratings. Future research exploring the acceptability of different suppositories and other delivery vehicles should be conducted. For example, rectal douching prior to intercourse is a habitual behavior among MSM9, and even some participants in this trial suggested that douching instructions be given as part of the microbicide use recommendations. A rectal douche that could deliver a microbicidal agent should also be considered. As biochemical progress is made by selecting an effective anti-retroviral to serve as a rectal microbicide in clinical trials10, behavioral research on the acceptability and adherence of a potential microbicide delivery vehicle should continue if we are to understand what other practices or barriers to product use and adherence exist and may hamper the effectiveness of a microbicide.
Key messages
A rectal microbicide formulated as a gel is likely to have higher acceptability among MSM than a suppository formulation.
A high volume of gel (35 mL) appears acceptable for intrarectal use prior to anal intercourse.
Partner’s acceptability of a microbicide gel also appears to be high.
Footnotes
Authors’ Contributions
Carballo-Diéguez, A. was the Principal Investigator of the study and the researcher who took primary responsibility for reporting its results; Dolezal, C. was responsible for data analysis and interpretation; Bauermeister, J.A. contributed significantly to the writing of the paper and to interpretation of results; O’Brien, B. was the Project Director of the study and contributed to the writing of the methods section of the manuscript; Ventuneac, A. contributed to the implementation of assessment procedures and in the writing of the article; Mayer, K. was the co-Principal Investigator of the study and also contributed to the editing of its final report.
Publisher's Disclaimer: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in STI and any other BMJPGL products and sub-licences such use and exploit all subsidiary rights, as set out in our licence http://sti.bmjjournals.com/ifora/licence.pdf.
References
- 1.Carballo-Diéguez A, Stein Z, Saez H, Dolezal C, Nieves-Rosa L, Diaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000 Jul;90(7):1117–1121. doi: 10.2105/ajph.90.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks G, Mansergh G, Crepaz N, Murphy S, Miller LC, Appleby PR. Future HIV prevention options for men who have sex with men: Intention to use a potential microbicide during anal intercourse. AIDS & Behavior. 2000 Sept;4(3):279–287. [Google Scholar]
- 3.Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR. Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET Vaccine Preparedness Study Protocol Team. Sex Transm Dis. 1998 Jul;25(6):296–302. doi: 10.1097/00007435-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Alliance for Microbicide Development. [Accessed January 18, 2008]; Available at: http://www.microbicide.org [permanent: stable content]
- 5.Carballo-Diéguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability: results of a volume escalation trial. Sex Transm Dis. 2007 Apr;34(4):224–229. doi: 10.1097/01.olq.0000233715.59239.83. [DOI] [PubMed] [Google Scholar]
- 6.Anton PA. The other compartment: Challenges and progress in rectal microbicide development. Retrovirology. 2005;2(Suppl 1):S89. [Google Scholar]
- 7.O’Brien W, Box M, Dolezal C, Bauermeister JA, Mayer K, Carballo-Dieguez A. Microbicides 2008. New Delhi, India: 2008. Recruitment and retention strategies utilized in a rectal microbicide study: Enrolling men who have sex with men (MSM) [Google Scholar]
- 8.Klasse PJ, Shatoock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 9.Carballo-Diéguez A, Bauermeister JA, Ventuneac A, Dolezal C, Balan I, Remien RH. The Use of Rectal Douches among HIV-uninfected and Infected Men who Have Unprotected Receptive Anal Intercourse: Implications for Rectal Microbicides. AIDS & Behavior. doi: 10.1007/s10461-007-9301-0. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrow KM, Ruiz MS. Assessing microbicide acceptability: A comprehensive and integrated approach. AIDS & Behavior. doi: 10.1007/s10461-007-9266-z. In press; Available online. [DOI] [PMC free article] [PubMed] [Google Scholar]