Summary
Perception of the smell of a food precedes its ingestion and perception of its flavor. The neurobiological underpinnings of this association are not well understood. Of central interest is whether the same neural circuits code for anticipatory and consummatory phases. Here, we show that the amygdala and mediodorsal thalamus respond preferentially to food odors that predict immediate arrival of their associated drink (FO+) compared to food odors that predict delivery of a tasteless solution (FO−), and compared to the receipt of the drink. In contrast, the left insula/operculum responds preferentially to the drink, whereas the right insula/operculum and left orbitofrontal cortex respond to FO+ and drink. These findings indicate separable and overlapping representation of anticipatory and consummatory chemosensation. Moreover, since ratings of perceived pleasantness of FO+, FO− and drink were similar, the response in the amygdala and thalamus cannot reflect acquired affective value but rather predictive meaning or biological relevance.
Keywords: conditioning, feeding, reward, motivation, attention, olfaction, flavor, obesity, neuroimaging, functional magnetic resonance imaging
Perception of the smell of a food precedes its ingestion and perception of its flavor. The neurobiological underpinnings of this association are not well understood. However, since separable neural substrates represent the anticipatory and consummatory phase of food reward (Berridge, 1996; Berridge and Robinson, 2003; Kelley and Berridge, 2002; Parkinson et al., 2000), it stands to reason that perception of a food aroma may be represented separately from the perception of its flavor specifically because of the different reward contexts represented by the two sensations; anticipatory vs. consummatory chemosensation (Small et al., 2005).
In a direct comparison between BOLD response to taste anticipation and receipt O’Doherty and colleagues reported that the amygdala, midbrain, and ventral striatum respond preferentially to abstract visual stimuli that predicted the arrival of sugar water compared to neutral cues and compared to the receipt of the sugar water (O’Doherty et al., 2002). They also found distinct responses in the orbitofrontal cortex (OFC) to the cue and the receipt of the taste. However, since the visual stimuli and the sugar water stimulate different sensory systems, represent different objects and differ in perceived pleasantness, it is possible that sensory, perceptual and hedonic factors contribute to the differential effect rather than reward phase per se. It is also not clear if these anticipatory effects will generalize to the sight or aroma of foods, which are well-learned appetitive cues, and flavors, which are more likely to be encountered in everyday life. These issues are important not only for understanding neural encoding of reward and chemosensation but also because subtle differences in the representation of food reward may prove important for understanding how individual differences may contribute to overeating and the current obesity epidemic. For example, it has been shown that obese compared to lean individuals will work harder to obtain food even though they do not find the food more palatable (Saelens and Epstein, 1996). More recently Beaver and colleagues (Beaver et al., 2006) showed that response in the amygdala, striatum, OFC and midbrain to appetitive food pictures correlated with scores of sensitivity to reward. This is important because sensitivity to reward varies as a function of body mass index (Davis et al., 2004). Therefore precisely defining the role of this network in encoding food reward is an important step towards understanding how potential differences in the neurophysiology of reward contribute to overeating.
A related issue is whether anticipatory food sensations differentially engage reward circuits depending upon their affective value or predictive meaning. This is an important question because during conditioning the conditioned cue acquires affective value and predictive meaning (Baeyens et al., 1992; Baeyens et al., 1989; Baeyens et al., 1993; De Houwer et al., 2001); however, in typical conditioning studies, like the one used by O’Doherty and colleagues, it is not possible to determine if preferential response to the cue (e.g. taste anticipation) reflects acquired value or predictive meaning or both. Similarly, while recent work has shown that the predictive representation of the affective value of cues occurs in the ventral midbrain and ventral striatum [O’Doherty, 2006 #722], it is unknown whether there is a separate signal for predictive meaning and whether this signal is independent of value. However, with respect to consummatory chemosensation, a study by Berns and colleagues showed that predictability is a better driver of neural response to juice than affective value (Berns et al., 2001). Additionally, at a more practical level, many studies aiming to uncover differences between neural encoding of food sensations in normal versus abnormal eating use paradigms in which subjects are exposed to food cues that are not predictive of food reward. If predictive meaning and affective value are represented by separable circuits then the results from these studies must be interpreted with this in mind and studies in which both aspects of anticipatory reward are measured will be necessary to provide a more comprehensive understanding of food reward dysfunction in obesity.
Here we performed two fMRI experiments in which we evaluated brain response to food odors that did (FO+) or did not (FO−) predict receipt of its associated drink (Fig 1). We then tested for preferential responses to sensations associated with anticipation (FO+ > FO−) compared to consumption of the drink (drink – tasteless) and vice versa. Further, since we used the same odor quality to represent the FO+, FO− and drink (i.e. retronasal perception of the odor plus sweet taste), our design minimized differential sensory stimulation and equated stimuli for perceived pleasantness and object representation (all stimuli identified the same food because odors represented the flavor of the drink), thus allowing us to focus on effects of predictive meaning and reward context, irrespective of perceived pleasantness and nature of the sensory stimulation. The amygdala has been shown to respond to predictive food cues (Gottfried et al., 2003; O’Doherty et al., 2002) and although the value of the cue is likely represented in the amygdala (Gottfried et al., 2003; LaBar et al., 2001; O’Doherty et al., 2006), response can clearly be driven by factors other than value, such as stimulus intensity (Anderson and Sobel, 2003) and reward context independent of perceived intensity and pleasantness (Small et al., 2005). Thus a specific prediction that we tested and confirmed is that the amygdala (and mediodorsal thalamus) responds preferentially to FO+ compared to FO− and compared to the receipt of the drink. Since these stimuli were similarly pleasant and intense our findings indicate that within the context of conditioning, the amygdala encodes predictive meaning and/or biological relevance and not the perceived pleasantness of a cue.
Figure 1. Paradigm.
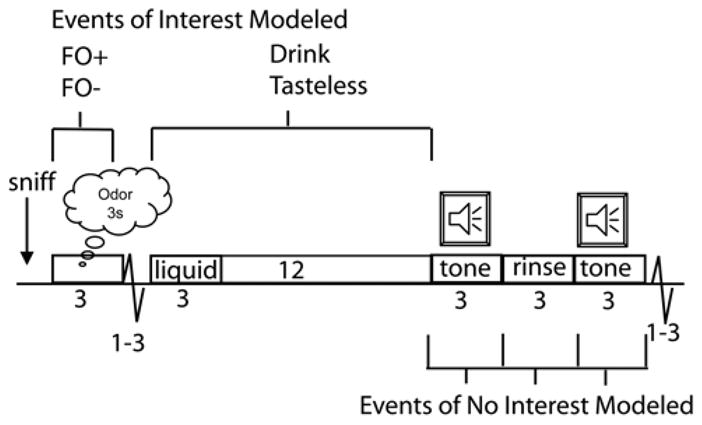
Cartoon of study design. Timeline of stimulus presentation with events of interest indicated above and events of no interest indicated below. One half second into the trial the subject hears the word “sniff” instructing them to sniff the odor about to be delivered. The odor is delivered for 3 seconds while the subject sniffs. Odors are either the fruit odor predicting its drink (FO+) or the fruit odor predicting a tasteless solution (FO−). These are modeled as 3 second mini blocks. One to three seconds following termination of odor delivery a liquid is delivered (0.5cc over 3 seconds). The liquid is either a fruity drink or a tasteless solution. Fruity drinks and tasteless events are modeled as mini blocks extending from the moment of stimulus onset until the onset of the swallow tone 15 seconds later (i.e. 15-sec mini block). The swallow tone plays for 3 seconds, during which time the subject knows they are allowed to swallow. Immediately after the tone a 0.5cc bolus of tasteless solution is delivered as a rinse. This is immediately followed by a second swallow tone instructing subjects to swallow the rinse. These events are modeled as 3 separate events of no interest. A 1 to 3 second jitter of rest follows the onset of the next trial.
Results
Two experiments were performed. In both, subjects received odors and drinks while being scanned on a 3T Siemens Trio magnet. Food odors (pineapple and peach odor in experiment 1 and pineapple and chocolate odor in experiment 2) were delivered using air dilution olfactometery as 3 second bursts of air preceded by the instruction “3,2,1, sniff” (see methods section and Fig 1). Drinks associated with the odors (pineapple drink, peach drink, chocolate milkshake) as well as a tasteless baseline solution were delivered using our custom built gustometer as 0.5 cc boluses over 3 seconds. Subjects received odors followed by tastes and were asked to perform a discrimination task in which they pressed button A if they perceived a taste and button B if they perceived tasteless. The first experiment was the main study (N = 12); however, the design did not include unpaired trials and the jitter between the presentation of the food odor (FO+) and the delivery of drink was relatively short (to mimic natural eating conditions) (Figure 1). Therefore we conducted a second experiment (N = 6) in which we included unpaired cue trials and longer jitters between odor and drink events (see methods) to ensure adequate ability to separate neural response to anticipatory and consummatory chemosensation. An additional paradigmatic difference was that midway through experiment 1 the association between food odor and drink was switched (i.e. reversed) so that FO+ became FO− (predicted tasteless rather than its drink). Experiment 2 did not include a reversal. Rather, stimuli were counterbalanced between subjects (FO+ was pineapple for some and chocolate for others). Finally, experiment 1 was pre and post-processed using SPM2 and experiment 2 using SPM5. Despite these differences, the results from both experiments are very similar.
Based upon previous studies we designated the follow structures as regions of interest: the amygdala, mediodorsal thalamus, ventral striatum, midbrain, orbitofrontal cortex (OFC), subcallosal cingulate and hypothalamus (Arana et al., 2003; Gottfried et al., 2003; LaBar et al., 2001; O’Doherty et al., 2006; O’Doherty et al., 2002; Small et al., 2005; Small et al., 2003; Small et al., 2001).
Experiment 1
Behavioral Results
Intensity and Pleasantness Ratings
Intensity and pleasantness ratings of the stimuli were assessed using a numerical 11-point scale for intensity (0 = no taste, 10 = extremely intense) and pleasantness (0 = extremely unpleasant, 5 = neutral, 10 = extremely pleasant). Ratings were taken before the first scan, midway through the study and after the last scan.
We first examined possible effects of reward context (FO+ vs. FO−) on odor ratings with a within-subject repeated measures analysis of variance (ANOVA) with context (FO+ vs. FO−) and rating (intensity/pleasantness) as within-subject variables. Ratings from the middle and end of the experiment were used (i.e. after each odor was experienced in each reward context). There was an effect of rating {F(1,11) = 13.2; p = 0.008}(intensity and pleasantness ratings differed from each other) but not of context {F(1,11) 0.9; p = 0.42}, indicating that the odors were rated as similarly intense and pleasant irrespective of their predictive value. We therefore collapsed across time and reward context and conducted a second repeated measures ANOVA with rating (intensity/pleasantness), fruit (peach/pineapple), and modality (odor/drink) as the 3 within-subject variables. This analyses revealed a main effect of rating (intensity vs. pleasantness), {F(1,11) = 8.0, p = 0.02}, and modality (odor vs. drink), {F(1,11) = 13.2, p = 0.004}. Although the main effect of rating is meaningless (intensity differs from pleasantness), the main effect of modality indicates that there was an overall difference between odor and drink sensations that results from a combination of intensity and pleasantness ratings. However there was no effect of fruit and no interactions were observed. Nevertheless because of the main effect of modality we performed post-hoc anlyses to see what was driving the main effect. Post-hoc inspection showed that the drinks were rated as more intense (mean odor = 5.6 and mean drink = 6.6 with p = 0.01) than the odors. No other significant effects were observed. This suggests that the main effect was largely due to intensity differences but that this difference was not of sufficient magnitude to lead to a significant interaction. Finally, we performed a repeated measures ANOVA independently for intensity and pleasantness ratings for all four stimuli (peach odor, pineapple odor, peach drink and pineapple drink)(Fig 2). In accordance with the omnibus test, the ANOVA of intensity showed an effect of intensity {F (4.9; p = 0.05} with post-hocs showing that the peach drink was perceived as more intensity than the peach (p = 0.01) and pineapple odors (0.008) but not the pineapple drink (p = 0.6). The analysis for pleasantness was not significant.
Figure 2. Intensity and Pleasantness Ratings.
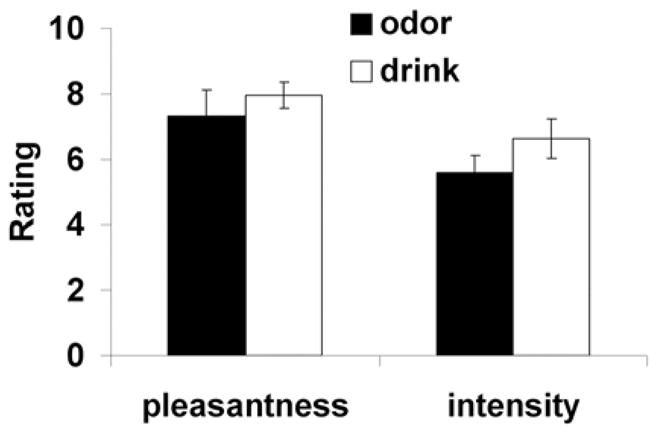
Mean intensity and pleasantness ratings of odors and drinks. Error bars represent standard error of the mean
Response Accuracy and Reaction Times
Due to technical difficulties, response data was only available for 8 of the 12 subjects. The percentage of correct responses was 89% (range 77–100%). Mean reaction times to accurately detected, validly cued drinks (flavored drink following FO+ and tasteless following FO−) were compared to mean reaction times for the invalidly cued drinks (i.e. catch trials = flavored drink following an FO− or tasteless following and FO+) for each of the 8 subjects using a paired two-sample t-test. This analysis revealed a significant effect such that reaction times were consistently faster to validly compared to invalidly cued trials {t(1,7) 2.8; p = 0.03}(Fig. 3).
Figure 3. Reaction Times.
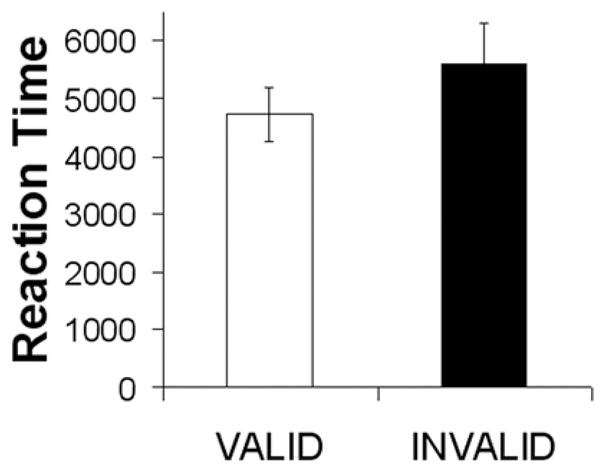
Mean reaction times. Error bars represent standard error of the mean
Imaging
Preferential Response to Odors Predicting the Drink
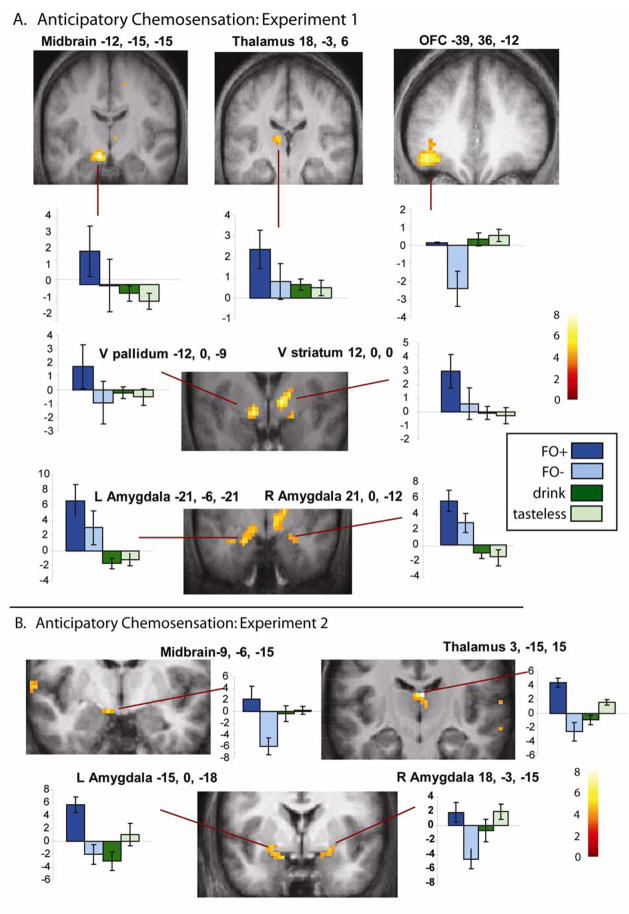
To evaluate the prediction that food aromas immediately predicting receipt of food would be more powerful elicitors of reward circuit activation we contrasted FO+ > FO−. Importantly, since each odor represented FO+ and FO− at some point during the experiment, differential effects cannot be related to sensory, perceptual or hedonic differences. This resulted in three clusters of activation in predicted regions significant at the cluster level after whole brain correction for multiple comparisons (see Fig. 4A and Table 1). Each cluster comprised multiple peaks. Peaks in the first cluster included the left dorsal midbrain, ventral pallidum, and amygdala. The second cluster included the right ventral striatum and thalamus. The third cluster included several peaks within the lateral orbitofrontal cortex extending from a y of 26 to 45. Activation was also present in the right amygdala that was significant after small volume correction. Finally, an unpredicted but significant cluster of activity was observed in the superior parietal cortex. In the reverse contrast (FO− > FO+) there was only one non-significant peak detected and this was in the inferior frontal gyrus at 48, 24, 30; z = 3.9, p < 0.99. Thus the findings clearly support the prediction that food cues are significantly more potent when they are associated with receipt of their associated drink.
Figure 4. Brain Representation of Anticipatory Chemosensation.
A. Results from experiment 1. Coronal brain sections showing significant peaks from the group analysis of FO+ > FO−. Graphs illustrate the response to all four events. Parameter estimates plotted on the x- axis. Error bars represent the standard error of the mean. Color bar represents t-values. All images thresholded a p < 0.001 with a cluster threshold of k > 3. B. Results from experiment 2. Coronal sections showing significant peaks from the group analysis of FO+ > FO−. Graphs illustrate the response to all four events. Parameter estimates plotted on the x- axis. Error bars represent the standard error of the mean. Color bar represents t-values. All images thresholded a p < 0.005with a cluster threshold of k > 3.
Table 1.
Responses to Anticipatory Chemosensation in Experiment 1
| Region/Analysis | MNI Co-ordinate | Cluster | z-value | p-value |
|---|---|---|---|---|
| FO+ > FO− | ||||
| Left Substantia Nigra | −12, −15, −15 | 85 | 4.7 | 0.001 |
| Left Ventral Pallidum | −12, 0, −9 | 4.3 | ||
| Left Amygdala | −21, −6, −21 | 3.6 | ||
| Right Ventral Striatum | 12, 0, 0 | 70 | 4.3 | 0.003 |
| Right Thalamus | 18, −3, 6 | 4.0 | ||
| Right Thalamus | 6, −12, 3 | 3.4 | ||
| Right Amygdala | 21, 0, −12 | 21 | 3.4 | .02* |
| 30, −9, −21 | 3.3 | |||
| Orbitofrontal Cortex | −39, 36, −12 | 104 | 4.3 | 0.0001 |
| −48, 45, −15 | 4.2 | |||
| −33, 42, −3 | 3.7 | |||
| Superior Parietal Lobule | −21, −45, 63 | 69 | 4.1 | 0.003 |
| {(FO+ > FO−) >( drink – tasteless)} | ||||
| Left Ventral Pallidum | −12, 0, −9 | 43 | 4.8 | 0.03 |
| Left Substantia Nigra | −12, −12, 15 | 4.4 | ||
| Left Amygdala | −18, −6, −21 | 3.5 | ||
| Left Thalamus | −9, −21, 9 | 6 | 3.3 | .93 |
| Right Thalamus | 12, −3, 0 | 5 | 3.3 | .98 |
| Orbitofrontal Cortex | −48, 48, −15 | 67 | 4.3 | 0.003 |
| −39, 36, −9 | 4.3 | |||
| −33, 36, −21 | 3.6 | |||
| Superior Parietal Lobule | −24, −45, 66 | 25 | 4.1 | 0.18 |
p-values are whole brain corrected at the cluster level with the exception of the right amygdala, which is uncorrected at the cluster level (indicated by *). Regions in italics indicate separate peaks within the cluster. Thalamic peaks in {(FO+ > FO−) > (drink – tasteless)} and the peak in the superior parietal lobule are not significant after whole brain correction but are reported because they were identified in the contrast of CS+ > CS−.
Anticipatory Sensations of Food
To isolate brain regions responding preferentially to reward anticipation compared to reward receipt, we looked at the random effects group interaction contrast, {(FO+ > FO−) – (drink > tasteless)}. Clusters of activation are reported that surpass a cluster-wise threshold corrected across the whole brain of p<0.05. This analysis yielded a 43-voxel cluster of left-lateralized activity encompassing distinct peaks in the dorsal midbrain, the ventral pallidum, and the amygdala (Table 1). A second 67-voxel cluster was observed in the left lateral OFC (Table 1).
Consummatory Sensations of Food
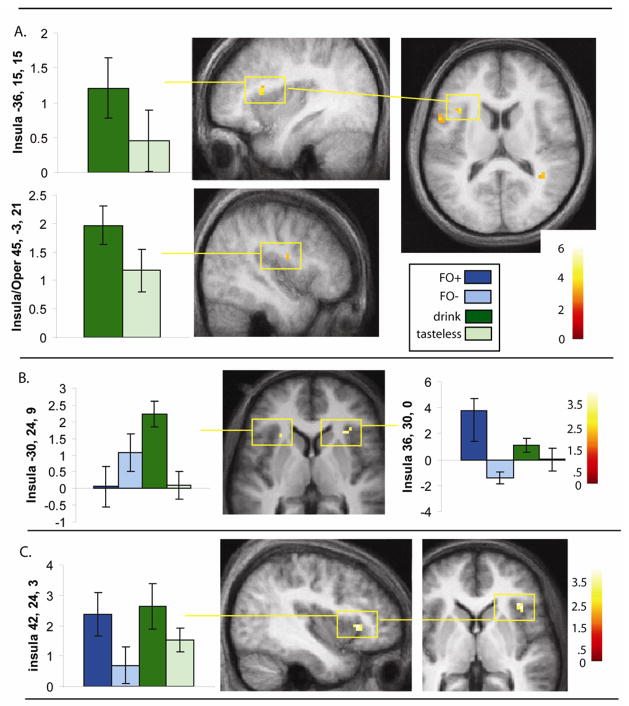
Comparison of drink > tasteless isolated greater response in the left parietal and frontal operculum, bilateral anterior insula, which corresponds to gustatory cortex, and in the right piriform cortex, which corresponds to olfactory cortex (Table 2; Fig. 5). Of these regions, only the left post-central and frontal opercula were also present in the interaction analysis {(drink > tasteless) – (FO+ > FO−)}; however, even when using a small volume correction (defined using a centroid from a previous study of taste(Small et al., 2003), both peaks missed significance (p = 0.06 and 0.08, respectively). There were also no significant activations elsewhere when correcting across the whole brain for multiple comparisons (though we did observe a response in the cerebellum at −9, −75, −27; z = 3.8; p = 0.04 (uncorrected/clusterwise)).
Table 2.
Responses to Consummatory Chemosensation in Experiment 2
| Region/Analysis | MNI Co-ordinate | Cluster | z-value | p-value |
|---|---|---|---|---|
| Analysis: Drink – Tasteless | ||||
| Left Post-Central Operculum | −66, −15, 36 | 49 | 4.5 | 0.04 |
| Right Post-Central Operculum | 69, −15, 24 | 6 | 3.2 | 0.99 (ns) |
| Left Frontal Operculum | −54, 9, 18 | 44 | 4.1 | 0.06 |
| −57, 12, 30 | 3.5 | |||
| Right Frontal Operculum | 63, 6, 30 | 10 | 3.5 | 0.86 (ns) |
| 60, 0, 24 | 3.2 | |||
| Left Anterior Dorsal Insula/operculum | −36, 15, 15 | 11 | 3.8 | 0.008* |
| Right Mid Dorsal Insula/Operculum | 45, −3, 21 | 5 | 3.3 | 0.02* |
| Piriform | 27, 3, −12 | 7 | 3.4 | 0.04* |
| Analysis (Drink > tasteless)–(FO+ > FO−) | ||||
| Left Post-Central Operculum | −66, −18, 36 | 4 | 3.7 | 0.06* (ns) |
| Left Frontal Operculum | −60, 12, 27 | 3.6 | 3 | 0.08* (ns) |
p-values are whole brain corrected at the cluster level with the exception of the insula/operculum peaks, which are corrected across a small volume (15mm diameter sphere) at the cluster level (indicated by a *). Peaks from a previously published study of taste response were used to define the center of the small volumes15. Regions in italics indicate separate peaks within the cluster. Note that the right frontal and parietal operculum are not significant activations but are reported because we do not want to give the impression that the activations are unilateral in these regions. ns = not significant
Figure 5. Brain Representation of Conusmmatory Chemosensation and Signal Integration.
A. Results from experiment 1. Sagittal and axial sections showing response in the anterior insula isolated in the group analysis of drink – tasteless. Graphs illustrate the response at significant peaks. Parameter estimates plotted on the x- axis. Error bars represent the standard error of the mean. Color bar represents t-values. All images thresholded a p < 0.001 with a cluster threshold of k > 3. B. Results from experiment 2. Axial section shows bilateral response in the anterior insula isolated in the group analysis of drink – tasteless. Since the insular peaks were also significant in the interaction analysis {(drink – tasteless) > (FO+ - FO−)} graphs illustrate response of all four events of interest. Error bars represent the standard error of the mean. Color bar represents t-values. All images thresholded a p < 0.005 with a cluster threshold of k > 3. C. Results from experiment 2. Sagittal and axial sections showing response in the right anterior insula/frontal operculum that was isolated in the conjunction analysis of (FO+ - FO−) AND (drink – tasteless) when the conjunction null was stipulated. The graph illustrates the response at the peak for all four events. Parameter estimates plotted on the x- axis. Error bars represent the standard error of the mean. Color bar represents t-values. Images thresholded a p < 0.005 with a cluster threshold of k > 3.
Regions Responding to Anticipatory and Consummatory Chemosensation
To identify regions that responded to both FO+ and drink we performed a conjunction analysis between FO+ and drink. This did not result in any significant responses.
Experiment 2
Results from the primary experiment clearly support our prediction of separable circuits representing anticipatory and consummatory chemosensation of food. However, because FO+ and FO− were always followed by their drink and because we used a relatively short jitter between events we were concerned that we did not adequately separate the hemodynamic response functions to each phase. We therefore conducted a follow-up control experiment in which we used a similar design but included paired and unpaired odor trials and used a longer jitter between events (see Methods). Importantly only unpaired trials were used in the analyses so that we could be absolutely certain that response to the receipt of the drinks does not influence response to the odors. Additionally, in the original experiment we had counterbalanced stimuli within subjects by reversing the associations between odor and drink midway through the experiment. Here we decided to counterbalance stimuli across subjects (i.e. half the subjects had chocolate odor as FO+ and strawberry as FO− and half had the reverse association). This allowed us to rule out any effects of reversal learning that might have contributed to effects observed in experiment 1.
Stimulus delivery, data acquisition and analyses were similar to the original experiment. Paired and unpaired trials were modeled separately, and only unpaired odor trials (i.e. odors not followed by drinks) and drinks were included as events of interest in the analyses. To probe for replications we used the peaks from activations reported in Tables 1 and 2 as centroids for 10 mm small volume searches of maps generated from respective contrasts of the new dataset. Peaks were considered significant at a p < 0.05 corrected across the small volume. Because the follow-up included only 6 subjects we used a t-map threshold of p < 0.005, but restricted our analysis to searching for replications.
Behavioral Results
Intensity and Pleasantness Ratings
All subjects participated in a training session in which they rated all stimuli for intensity and pleasantness, and then performed one mock run during which one of the odors (FO+) predicted delivery of the associated milkshake drink with 100% accuracy and the other odor (FO−) predicted delivery of the tasteless solution with 100% accuracy. Following the training session subjects reported being explicitly aware of the predictive nature of the odors (i.e. that one odor was sometimes followed by its drink and the other by a tasteless solution). Pleasantness and intensity ratings were also assessed on the scanning day before and after the experiment. To determine if conditioning changed the perceived pleasantness of the odors we performed a paired t-test of the pleasantness rating of the FO+ before training compared to before scanning (i.e., after conditioning) was conducted. Although this did not reveal a significant effect, there was a trend for conditioning to increase perceived pleasantness (t 1.8; p 0.07 one tailed) with perceived pleasantness tending to be higher following conditioning (mean 4.1, SEM 1.5 before training and mean 5.8, SEM 0.74 before scanning). A repeated measures ANOVA was then run on the intensity and pleasantness ratings collected before and after scanning to determine if 1) ratings changed over the course of scanning, 2) intensity or pleasantness differed across stimuli. Time (before and after), stimulus (FO+, FO− and drink), and rating (intensity and pleasantness) were entered as within-subject variables. No significant effects of time (p = 0.59); time by rating (p = 0.86), time by rating by stimulus (p = 0.37), stimulus (p =0.86), or stimulus by rating (p = 0.31) were observed. There was a main effect of rating (p = 0.008), again due to the fact that intensity and pleasantness ratings differed from each other. In sum, there were no significant differences in perceived intensity or pleasantness ratings of the FO+, FO− or drink (mean pleasantness/SEM collapsed across time of FO+ = 5.2/0.74, FO− = 5.4/0.86, drink = 5.9/1.5; mean intensity/SEM of FO+ = 23.4/4.4, FO− = 27.6/3.2, drink =21.5/4.4).
Time taken to indicate delivery of milkshake or tasteless were recorded but we did not include invalid trials due to time constraints (subjects were already in the scanner for over an hour to accommodate inclusion of paired and unpaired trials). Thus, as in experiment 1, subjects performed a task but there were no invalid trials, only trials on which no drink or tasteless occurred (i.e. unpaired trials).
Imaging Results
Preferential Response to Odors Predicting the Drink
Comparison of unpaired FO+ with unpaired FO− again isolated responses in the left midbrain (−9, −6, 15; z = 3.2), right mediodorsal thalamus (3, −15, 15; z = 4.5) and bilateral amygdala/piriform (Left at −15, 0 – 18; z = 3.2 and −24, 0, −9; z = 3.2 // Right at 18, 3, −21; z = 3.1; 24, 0, −12; z = 3.0; and 12, −3, −15; z = 2.8) (Fig 4B).
Anticipatory Sensations of Food
As in experiment 1, to isolate brain regions responding preferentially to reward anticipation compared to reward receipt, we looked at the random effects group interaction contrast, {(FO+ > FO−) – (drink > tasteless)}.
This revealed significant responses in the mediodorsal thalamus at 3, −15, 15; z = 4.7; and bilateral amygdala/piriform (Left at −9, −6, 15; z = 3.6 and −21, 3, −18; z = 3.0 // Right at 24, 0, −15; z = 3.2).
Consummatory Sensations of Food
Comparison of drink > tasteless resulted in activation of bilateral insular cortex (Left at −30, 24, 9; z = 2.7 // Right at 36, 33, −9; z = 3.3 and 36, 30 0; z = 3.1) and in the piriform cortex −24, 18, −12; z = 2.9 (Fig 5B). The interaction analysis {(drink > tasteless) – (FO+ > FO−)} again yielded bilateral insular responses (Left at −27, 30, 12; z = 4.1; −27, 21, 6; z = 3.9; and −39, 27, 6; z = 3.3 // Right = 36, 33, −9; z = 4.3; 45, 30, −9; z = 3.1; and 42, 27, 0 z = 3.0).
Regions that respond to both anticipatory and consummatory chemosensation
To identify regions that respond to both anticipatory and consummatory chemosensation we performed a conjunction analysis of the contrasts FO+ > FO− and drink - tasteless using the conjunction null option in SPM5. This resulted in a significant response in the right anterior insula/frontal operculum at 42, 24, 3; z = 3.2 (Fig 5C). Since we used the conjunction null option the analysis confirms that this region responds significantly to both FO+ and drink (Nichols et al., 2005). We then re-ran the analysis using the global null option, which is less stringent in that it isolates regions common to both events but not necessarily significant in response to both events. This produced bilateral responses in the anterior insula/frontal operculum as well as a response in the left anterior OFC at −24, 48, −9; z = 3.5. This response was adjacent to the OFC peak identified in response to anticipatory chemosensation observed in experiment 1, but did not overlap.
Discussion
The goal of this study was to test the prediction that pleasant food aromas immediately predicting receipt of their drink (FO+) would be more powerful elicitors of reward circuit activation than equally pleasant food aromas that did not predict receipt of their drinks (FO−), and that at least a subset of these regions would be preferentially responsive to FO+ compared to the receipt of the caloric drink. A specific prediction was that the amygdala would respond preferentially to anticipatory chemosensation. In support of our predictions, in two independent fMRI experiments, we found that the mediodorsal thalamus, amygdala and midbrain (possibly substantia nigra) displayed greater response to the food odors when they predicted immediate receipt of their associated caloric drink compared to when they predicted the arrival of a tasteless solution (Fig 4). Responses in the mediodorsal thalamus and amygdala were also greater to FO+ compared to the receipt of its associated caloric drink. In contrast, the left anterior insula responded to the drink but not the predictive odor (Table 1; Fig 5B), while the right anterior insula and left OFC responded to both predictive food odor and drink (Fig 5C). These results support the existence of separable neural networks encoding anticipatory vs. consummatory food reward and identify candidate sites where integration of the separable reward signals may take place. The findings also extend previous work by highlighting a role for the mediodorsal thalamus in encoding predictive food odors, and by showing separable responses not only to abstract cues predicting sweet taste but also to predictive food odors and drinks that are likely encountered in everyday life. Finally, our results contribute to the larger field of reward learning by showing that response to predictive food odors in the mediodorsal thalamus and amygdala can be driven by predictive meaning independent of the affective value of the cue. This is important because it suggests that during conditioning it is not acquisition of affective value that is being encoded by the amygdala but rather predictive meaning or biological relevance.
Anticipatory Chemosensation
In experiment one we observed preferential response in the ventral striatum, ventral pallidum, amygdala, OFC and mediodorsal thalamus to food odors that predicted immediate arrival of their drinks compared to equally pleasant and intense food odors that predicted a tasteless solution and compared to receipt of the similarly pleasant and intense drink (Fig 4A). In experiment 2 this effect was replicated in the amygdala and mediodorsal thalamus. Further, although the midbrain did not respond selectively to anticipatory chemosensaton in experiment 2, the response in this region partially replicated in that it responded preferentially to FO+ compared to FO−. Although all of these regions have been previously implicated in anticipatory food reward (Everitt et al., 1989; Gottfried et al., 2003; Holland and Gallagher, 2004; McClure et al., 2003; Schoenbaum, 2004; Schoenbaum et al., 1998, 1999, 2000; Schoenbaum et al., 2003; Tindell et al., 2004; Tindell et al., 2006), we have chosen to focus the discussion on the regions in which replications were observed because it is possible that inadequate separation of odors and drinks contribute to effects observed in experiment one.
There is a wealth of data from nonhuman animals showing that the amygdala plays a critical role in both appetitive and aversive Pavlovian conditioning (Baxter and Murray, 2002; Holland and Gallagher, 2004). With respect to feeding, human neuroimaging work has shown that the amygdala responds preferentially to abstract cues that predict arrival of a taste stimulus (O’Doherty et al., 2002). Accordingly, we predicted and confirmed that the amygdala responds preferentially to anticipatory compared to consummatory chemosensation. This finding extends the work of O’Doherty and colleagues in two ways. First it shows that the effect generalizes to well-learned predictive cues that are encountered in everyday life. Second, it provides definitive evidence that the preferential response in the amygdala to taste anticipation does not reflect differences in perceived pleasantness of between the CS+ and CS− or between the CS+ and the USC. This is important because during conditioning the conditioned cue acquires both affective value and predictive meaning (Baeyens et al., 1992; Baeyens et al., 1989; Baeyens et al., 1993; De Houwer et al., 2001). In O’Doherty’s 2002 study subjects were not asked to provide ratings of the perceived pleasantness of the cues; however, in a subsequent study, using a similar paradigm, they showed that abstract visual cues do acquire affective value during conditioning [O’Doherty, 2006 #722]. Therefore it is likely that the CS+ was perceived to be more pleasant than the CS− in the 2002 taste anticipation study. As a result it is not possible to know whether the network that they isolated as responding preferentially to taste anticipation reflected acquisition of value or predictive meaning. Furthermore, assuming that the CS+ did become more pleasant it is unlikely to be as pleasant as the USC. Therefore, the direct comparison between anticipatory and consummatory food reward is confounded by differences in perceived pleasantness. A unique feature of our study was that, in addition to being ecologically relevant, the stimuli that represented FO+, FO− and drink were perceptually identical (pineapple and peach aroma as FO+ and FO− in experiment 1 and chocolate or strawberry milkshake odor in experiment 2), or similar (e.g. pineapple and peach odor as FO+ and pineapple and peach drink as the unconditioned stimulus). Consequently, the design allowed us to examine the effect of conditioning while minimizing differences in sensory, semantic and hedonic features between stimuli. We found that the aromas were rated as equally pleasant when they represented FO+ and FO−; however, since response times were slower during catch trials (experiment 1 and Fig. 3), and since subjects explicitly reported being aware of the association (experiment 2), it was clear that conditioning had occurred. Within this context we can rule out the possibility that differences in sensory, semantic or hedonic features lead to the strong preferential response and can conclude that response was driven by predictive meaning.
Similar to the amygdala, the midbrain has been shown to respond to food cues (Beaver et al., 2006; Gottfried et al., 2003; O’Doherty et al., 2006; O’Doherty et al., 2002). Consistent with this work, in the current study we observed preferential response in the midbrain to FO+ compared to FO−. However, it appears that our peak was slightly more dorsal than peaks reported in previous studies (possibly originated from the substantia nigra rather than the ventral tegmental area). Response in the midbrain was not selective to anticipatory chemosensation, but was also not identified in the conjunction analysis between anticipatory and consummatory chemosensation even when the t-map threshold was lowered to p <0.01.
A novel finding that we observed was the consistent recruitment of the mediodorsal thalamus during anticipatory food reward. The mediodorsal thalamus receives inputs from the amygdala and striatum and projects to the OFC (Ongur and Price, 2000; Ray and Price, 1992). Like the amygdala, striatum and OFC, the mediodorsal thalamus receives olfactory inputs(Gottfried et al., 2006) and has been implicated in conditioning (Corbit et al., 2003). More recently, Plailly and colleagues reported that selective attention to odor enhances the strength of the connection between piriform cortex and mediodorsal thalamus and suggested that the thalamic relay plays an important role in olfactory attention and conscious sensation of smell (Plailly et al., 2007). Given the purported role for medial dorsal thalamus in olfactory attention and the possibility that the primary role of the amygdala in conditioning is not acquisition of an emotional meaning but rather in signaling biological relevance (Sander et al., 2003), it is tempting to speculate that the role of the mediodorsal thalamus and amygdala in anticipatory chemosensation is to increase attentional allocation to predictive odors because they are biologically more relevant. Unfortunately it is not possible to disentangle the relative contribution of predictive meaning or biological saliency to the amygdala and mediodorsal thalamic response within the current paradigm. We also cannot rule out the possibility that the amygdala, mediodorsal thalamus and midbrain encode some nonconscious or implicit representation of reward value. This possibility is consistent with the proposal by Berridge and Robinson that motivation consists of implicit and explicit components that are hypothesized to be represented separately in the brain (Berridge and Robinson, 2003). Nevertheless, we can definitively rule out contributions of conscious perceived pleasantness.
Consummatory Chemosensation
In both experiments the anterior insular cortex responded to drink – tasteless (Fig 5). This region corresponds to primary gustatory cortex in humans (Petrides and Pandya, 1994; Small et al., 1999) and is also sensitive to the texture of foods (de Araujo and Rolls, 2004). Its recruitment here likely reflects the encoding of the taste and texture of the caloric drinks compared to the tasteless solution. However, the insular cortex has also been implicated in affective representation of foods in that response to chocolate consumption decreases as a function of satiety as indexed by a negative correlation between response and pleasantness ratings (Small et al., 2001). It is therefore possible that the response isolated here reflects differences in the reward value between the drinks and tasteless solution. However, we feel that this is unlikely because the effect was preferential for consummatory compared to anticipatory chemosensation, despite the fact that FO+ and drink were matched for pleasantness.
A slightly more anterior region of insular cortex in the right hemisphere was the only region of the brain to survive a conjunction analysis in which the conjunction null hypothesis was stipulated. In addition to taste (Small et al., 1999), food (Small et al., 2001), and oral texture (de Araujo and Rolls, 2004) this region of insula (i.e. primary gustatory cortex) also responds to appetizing pictures of foods (Killgore and Yurgelun-Todd, 2006; Simmons et al., 2005), to resting changes in internal state (Tataranni et al., 1999) and to food odors (Small et al., 2005). It is therefore possible that this region represents an area where integration of anticipatory and consummatory food reward signals take place. When we re-ran the conjunction using the global null hypothesis, which is less stringent because it does not depend upon significant responses being present in both contrasts, we also observed response in the left anterior OFC in addition to the anterior insula. This same region was shown to be preferentially responsive to receipt compared to anticipation of sweet taste in the O’Doherty study (O’Doherty et al., 2002). This suggests that the more general response observed here (i.e. response to anticipatory and consummatory food reward) may be specific to chemosensory stimuli. In other words, the region may be specialized for integrating anticipatory and consummatory chemosensory signals. Alternatively, it may be that the critical feature of recruitment is that the stimulus be a food object rather than an abstract symbol (as was used in the O’Doherty study). This is consistent with the view that the orbital cortex integrates multiple sensory inputs to create food concepts (Carmichael and Price, 1996; Simmons et al., 2005; Small et al., 2007).
Finally, we note that our findings have important implications for the study of food reward as a function of weight. First, they suggest that differences in ratings of perceived pleasantness of food and food cues may not accompany underlying neurophysiological differences in the representation of food reward in lean compared to obese subjects. Therefore it will be important to assay neurophysiological responses in an effort to understand the potential contribution of individual differences in reward to the obesity epidemic. Second, they suggest that response in the amygdala, midbrain, and thalamus to food cues is highly sensitive to reward context. Consequently, studies aiming to test for the presence of differences in neurophysiological response to food cues as a function of eating style or body weight should incorporate a predictive component in order to maximize activation of this network. Accomplishing this would be relatively straightforward to do this by having a meal available after a scanning session and thus does not necessarily need to involve delivery of odors and flavors in the scanner.
Conclusions
In this study we show that the amygdala, midbran and mediodorsal thalamus represent predictive encoding of food reward independently from encoding the subjective pleasantness of food cues. We also show that the amygdala and mediodorsal thalamus respond preferentially to anticipatory compared to consummatory chemosensation of food. These findings support the notion that there are separable neural substrates representing anticipation versus consumption of food in humans and that there may be distinct networks representing implicit and explicit components of these two phases of food reward. They further indicate that during conditioning the amygdala encodes acquired predictive meaning rather than acquired value.
Materials and Methods
Experiment 1
Subjects
All procedures were approved by the Northwestern University Institutional Review Board. Fifteen subjects gave informed written consent before participating. Three subjects were excluded due to excessive movement in the scanner, resulting in 12 subjects available for analysis. Subjects were between the ages of 21 and 33 (mean= 25 years); five were men and seven were women. All were right-handed and none had a prior history of neurological disorders or taste/smell impairment.
Stimuli
The odors were two fruity odors, peach (Unilever Foods) and pineapple (Grandma’s Choice imitation pineapple, Shank’s Extracts Inc., Lancaster, PA). Drinks consisted of one of the fruit odors mixed with a sweet beverage powder developed by Unilever Foods (e.g. sweet peach drink and sweet pineapple drink). The tasteless solution consisted of 12.5 mM KCl and 1.25 mM NaHCO3 in distilled water. Intensity and pleasantness ratings of the stimuli were assessed using a numerical 11-point scale for intensity (0 = no taste, 10 = extremely intense) and pleasantness (0 = extremely unpleasant, 5 = neutral, 10 = extremely pleasant). Ratings were taken before the first scan, midway through the study and after the last scan.
Stimulus Delivery
Olfactometer
Odors were presented by a custom-built MRI-compatible olfactometer programmed in Labview (National Instruments, TX). The design is based upon that described by Johnson and colleagues (Johnson and Sobel, 2007), which was adapted from (Kobal, 1981). The machine sits in the MR control room and is connected to the subject via a 25 foot tubing bundle. The olfactometer is programmed with Labview (National Instruments, TX) running on a PC. The tubing and fittings within the machine were constructed from 316 stainless steel parts. All parts that lie in the MR scanner room were constructed from Teflon and other non-ferromagnetic materials.
Air-dilution olfactometery
The flow of compressed air is controlled with 6 mass flow controllers (MKS Instruments, Andover, MA) and humidified with a sparging humidifier. The temperature of the air is adjusted by a space heater that heats the enclosed interior of the machine. Air flow is split into three channels: one to deliver clean air to the subject, one to odorize air, and one to dilute the odorized air with clean air. Each channel goes through 4 street-tees (Ham-Let, San Jose, CA). Each street-tee in the odorized air channel holds 2 mls of odor-containing liquid, resulting in vapor from the liquid odorizing the air passing over it. The street-tees in the dilution and clean air channels are empty. The odorized channel is then mixed with the dilution channel to control the concentration of the odor. The two resulting channels, clean air and diluted odor, are passed out of the machine and through the 25 foot tubing trunk into the MR scanning room. The temperature in the air tubes is maintained throughout the length of the trunk by running heated water tubes (connected to a water recirculator) alongside the air tubes and insulating the trunk. Airflow rate is maintained at 10 L/min.
The trunk terminates in a custom-built Teflon manifold (Teqcom, Santa Ana, CA). In addition to the air line, the trunk contains a vacuum line that also terminates at the manifold. A 3-way solenoid valve (Asco Red-Hat, Florham Park, NJ), situated below and to the right of the odor wells, controls this vacuum that evacuates air from either the clean air or diluted odor channels. This manifold rests on the subject’s chest. Because switching between odorized and clean air occurs very close to the subject’s nose, the rise time for odor delivery is on the order of milliseconds. The subject receives the air via a nasal mask (Sleepnet, Manchester, NH).
Gustometer
The gustometer system is a fully portable device that consists of a laptop computer and 11 independently programmable BS-8000 syringe pumps (Braintree Scientific, Braintree, MA). Each pump holds a 60 mL syringe connected to a 25-foot length of Tygon beverage tubing (Saint-Gobain Performance Plastics, Akron, OH). The current experiment required three liquid lines (pineapple drink, peach drink and tasteless). The lines (i.e. the tubes) were bundled with the odor lines and passed through the waveguide to the magnet room. The tubes were anchored to the headcoil using medical tape so that the ends rested comfortably in the subjects’ mouths. This gustometer and a similar paradigm has been used successfully in a previous imaging studies (Small et al., 2003).
Procedure
Subjects smelled fruit odors that either predicted (FO+) the arrival of its associated fruit drink or the arrival of a tasteless solution (FO−). Figure 1 depicts the paradigm, which was modified from our previous design (Small et al., 2003; Small et al., 2004) to include delivery of odors in the vapor phase prior to liquid onsets. The paradigm is designed to enable dissociation of the liquid events of interest (odors and liquids) from movements related to swallowing by asking subjects to wait to swallow until they heard the cue to swallow (a tone). The odors were delivered for 3 sec to the nasal mask fitted over each Ss nose. At 0.5 seconds before the onset of the odor, subjects heard the audio instruction “Sniff.” The fruity drinks and tasteless solutions were delivered as a 0.5 mL bolus over 3 sec with their onsets jittered such that they followed the presentation of the odor by between 1 and 3 seconds. The jitter between odor and liquid was employed to aid in separation of the hemodynamic response functions. To minimize movement and artifact, subjects did not swallow the liquid until cued by an audio tone 15 seconds after the onset of the liquid. The swallow cue lasted 3 seconds and was immediately followed by a 0.5 mL tasteless rinse delivered over 3 sec and another swallow cue.
For the first half the study, one fruit odor (FO+) was paired with its corresponding sweet fruity drink while the other odor (FO−) was paired with a neutral tasteless solution. Subjects performed a mock run to learn the contingency. In the second half of the experiment, odor contingencies were reversed and a second mock run was performed so subjects could learn the new contingency. The odor that first predicted the fruity drink was counterbalanced across subjects. When a subject moved beyond criteria (1cm in any direction) in any run their entire dataset was excluded from the analyses. Three subjects were excluded due to movement. Thus the remaining 12 subjects had 50% of the trials FO+ and 50% of the trials FO−. During scanning subjects performed a detection task in which they pressed one button as soon as they received the fruity drink and another button when they received the tasteless solution. Each run was composed of 12 trials. The MRI session consisted of 8 runs, each lasting 5 minutes 25 seconds. During each run there was one catch trial in which an unexpected pairing occurred (e.g. the fruit odor that should have predicted the fruity drink was instead followed by tasteless, or vice versa). These were modeled as events of no interest. Four events of interest were modeled 1) FO+ collapsed across odorant, 2) FO− collapsed across odorant, 3) drink (collapsed across flavors), and 4) tasteless. There were 44 repeats of each event type.
Imaging
Data were acquired on a 3T Trio Siemens magnet using procedures identical to previous studies, in which activity in OFC and amygdala have been isolated (Small et al., 2003). Echo planar imaging was used to image the regional distribution of the BOLD signal with TR = 2100 ms, TE = 20ms, flip angle = 80°, FOV = 220, matrix = 64X64, slice thickness = 3 mm, and acquisition of 36 contiguous slices. Slices were acquired in an interleaved mode to reduce the cross talk of the slice selection pulse. At the beginning of each functional run, the MR signal was allowed to equilibrate over 6 scans for a total of 12.6 sec, which was then excluded from analysis. For each subject, a high resolution, T1 weighted 3D volume was acquired in less than 8 minutes (MP-RAGE with a TR/TE of 2.1sec, flip angle of 15°, TI of 1100ms, matrix size of 256×256, FOV of 22cm, slice thickness of 1mm). Data were pre and post-processed with SPM2 (Wellcome Department of Imaging Neuroscience, London, UK) using standard methods described elsewhere (Small et al., 2005; Small et al., 2003). The functional images were time-acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the scan immediately before the anatomical T1 image. The images (anatomical and functional) were then normalized to the Montreal Neurological Institute template (MNI-305), which approximates the anatomical space delineated by Talairach and Tournoux (1988) (Talairach and Tournoux, 1988). Functional images were smoothed with a 10mm FWHM isotropic Gaussian kernel. For the time series analysis on all subjects, a high-pass filter was included in the filtering matrix (according to convention in SPM2) to remove low-frequency noise and slow drifts in the signal that could bias the estimates of the error. Condition-specific effects at each voxel were estimated using the general linear model. The response to events was then modeled by a canonical hemodynamic response function, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 s and the subsequent undershoot. The temporal derivative of the hemodynamic function was also included as part of the basis set to provide a better model of the data (Henson et al., 2002).
Post-processing of the neuroimaging data was performed using group random effects analyses. We applied Gaussian random field theory as implemented in SPM2. The main contrasts of interest were FO+ > FO−, drink > tasteless, and the interactions between them, {(FO+ > FO−) - (drink > tasteless)} and {(drink > tasteless) - (FO+ > FO−)}, which were performed in each individual subject and then entered into second-level analyses using one-sample Student’s t tests. To create plots of parameter estimates, contrast parameter estimate images from each subject were entered into analyses of variance (ANOVAs). A t-map threshold of p = 0.001 (uncorrected) was used. Peaks were considered significant at p < 0.05(with respect to clusters) corrected for multiple comparisons across the entire brain (Worsley et al., 1996). When necessary, small volume corrections (SVC) were defined using coordinates from previously published papers to determine the significance of predicted peaks. Based upon previous studies we predicted that the amygdala, mediodorsal thalamus, ventral striatum, midbrain and lateral orbitofrontal cortex (OFC) would be preferentially engaged during anticipation whereas the medial OFC, subcallosal cingulate and hypothalamus would be preferentially engaged during consumption (Arana et al., 2003; Gottfried et al., 2003; O’Doherty et al., 2002; Small et al., 2005; Small et al., 2001). We predicted that there would be responses to both anticipatory and consummatory sensations in the insula and OFC, reflecting the importance of these regions in chemosensory processing and in predictive encoding of food reward (Gottfried et al., 2003; Gottfried et al., 2006; O’Doherty et al., 2002; Small et al., 2005; Small et al., 2001).
Experiment 2
Subjects
All procedures were approved by the Yale University School of Medicine Institutional Review Board. Six subjects gave informed written consent before participating. Subjects were between the ages of 23 and 41 (mean = 28.8 years); 3 were men and 3 were women. All were right-handed and none had a prior history of neurological disorders or taste/smell impairment. All were nonsmokers.
Stimuli
Food odorants included chocolate cookie, strawberry and cream, rose (6002335, 6106524, 6104579 from Bell Labs Flavors and Fragrances, Inc, IL) and lilac aromas (lilac oil 34371433 International flavors and fragrances - IFF). The chocolate cookie and strawberry and cream aromas served as either the FO+ or the FO− (counterbalanced across Ss). Pilot testing has shown that these aromas are rated as consistently similar in pleasantness and familiarity. The drinks included 100ml of Hershey’s Cookies – n Cream milkshake diluted with 10mL distilled water and 100mL Garelick Ultimate Strawberry milkshake, plus 5 g sucrose and 1ml strawberry flavor (Galaxy flavors). Pilot testing showed that all stimuli are rated as similarly pleasant and intense and that the drinks match their aromas. The exact concentration of aromas were yoked so that all are rated 25 (±5) on the general labeled magnitude scale(Green et al., 1996). Odors were yoked by adding dilution air to the odorized air (see stimulus delivery above). The tasteless solution was the same as in Experiment 1.
Stimulus delivery
The same methods and equipment were used to delivery vapors and liquids as in experiment 1, except that instead of having tubes end in the subject’s mouths we used a mouthpiece that we recently designed to help reduce cross-contamination and increase comfort during liquid delivery in the supine position. The mouthpiece has been described in detail elsewhere (Veldhuizen et al., 2007). In brief, it is a manifold made entirely of Teflon with 9 ports into which beverage tubing is secured. The ports narrow into 1-mm channels that converge at a central point at the bottom of the manifold just above a 7mm plastic sphere. The subject’s tongue rests against the bottom surface of the sphere to receive the stimulus, which drips onto the tongue. The manifold is anchored to the head coil using a Teflon device with two knobs that allow adjustment in vertical and horizontal planes. The primary difference, compared to experiment 1, was that we included unpaired trials and included a longer jitter between events. There were 6 events of interest: FO+ paired, FO+ unpaired, FO− paired and FO− unpaired, milkshake (drink) and tasteless. In the FO+ paired trials the subjects heard “3,2,1 Sniff”, received a 3-second odorant delivery (FO+) followed by a 1–5 second jitter, a 3-second drink delivery and a 5–9 second jitter before the beginning of the next trial. FO− paired trials were identical but included tasteless rather than drink. Unpaired trials included the cue followed by the 3-second odor delivery and a 5–9 second jitter before the start of the next trial. The training run lasted just over 11 minute and consisted of 12 trials of both the FO+ paired and FO− paired events. After the training run all subjects were questioned about the contingency and were accurately able to report what drink followed each odor. During scanning the runs consisted of 35 repeats of the paired trials and 28 repeats of the unpaired trials. Four events of interest were modeled 1) FO+ unpaired, 2) FO− unpaired, 3) drink, and 4) tasteless. Paired trials were modeled as events of no interest.
Imaging
Data acquisition and analysis were similar to experiment 1 except that the 3T Trio Scanner was at Yale University and we used SPM5 instead of SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). We also chose to smooth functional images with a 6mm FWHM isotropic Gaussian kernel rather than a 10 mm kernel. We also reduced the threshold of the t-map to p < 0.005 to increase sensitivity but restricted analysis to small volume searches using peaks identified from the previous study as centroids for 10mm spheres of interest. Peaks were considered significant at p < 0.05 corrected across the small volume.
References
- Anderson AK, Sobel N. Dissociating intensity from valence as sensory inputs to emotion. Neuron. 2003;39:581–583. doi: 10.1016/s0896-6273(03)00504-x. [DOI] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens F, Eelen P, Crombez G, Van den Bergh O. Human evaluative conditioning: acquisition trials, presentation schedule, evaluative style and contingency awareness. Behaviour Research & Therapy. 1992;30:133–142. doi: 10.1016/0005-7967(92)90136-5. [DOI] [PubMed] [Google Scholar]
- Baeyens F, Eelen P, Van Den Bergh O, Crombez G. Acquired affective-evaluative value: conservative but not unchangeable. Behaviour Research & Therapy. 1989;27:279–287. doi: 10.1016/0005-7967(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Baeyens F, Hermans D, Eelen P. The role of CS-US contingency in human evaluative conditioning. Behaviour Research & Therapy. 1993;31:731–737. doi: 10.1016/0005-7967(93)90003-d. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. Journal of Neuroscience. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience & Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of Macaque monkeys. Journal of Comparative & Physiological Psychology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. European Journal of Neuroscience. 2003;18:1286–1294. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- de Araujo E, Rolls ET. Representation in the human brain of food texture and oral fat. Journal of Neuroscience. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J, Thomas S, Baeyens F. Associative learning of likes and dislikes: a review of 25 years of research on human evaluative conditioning. Psychological Bulletin. 2001;127:853–869. doi: 10.1037/0033-2909.127.6.853. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Small DM, Zald DH. The Chemical Senses. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; 2006. pp. 125–171. [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chemical Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Sobel N. Methods for building an olfactometer with known concentration outcomes. Journal of Neuroscience Methods. 2007;160:231–245. doi: 10.1016/j.jneumeth.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. [see comment] Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. International Journal of Eating Disorders. 2006;39:357–363. doi: 10.1002/eat.20240. [DOI] [PubMed] [Google Scholar]
- Kobal G. Electrophysiologische Untersuchungen des menschlichen Geruchssinns. Stuttgart: Theime Verlag; 1981. [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. [see comment] Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the miminum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Progress in Brain Research. 2000;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Comparitive architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- Plailly J, Howard JD, Gottfried JA. Piriform to orbitofrontal transthalamic pathway involved in olfactory attentional processing. Chemical Senses. 2007;32:A1–A125. [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. Journal of Comparative Neurology. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning and the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning. 2. Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–69. [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G. Affect, action, and ambiguity and the amygdala-orbitofrontal circuit. Focus on “combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys”. [comment] Journal of Neurophysiology. 2004;91:1938–1939. doi: 10.1152/jn.01263.2003. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. Journal of Neuroscience. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning & Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Small DM, Bender G, Veldhuizen MG, Rudenga K, Nachtigal D, Felsted J. The role of the human orbitofrontal cortex in taste and flavor processing. Annals of the New York Academy of Sciences. 2007 doi: 10.1196/annals.1401.002. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber J, Mak YE, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish TB, Gitelman DR. Experience-dependent neural integration of taste and smell in the human brain. Journal of Neurophysiology. 2004;92:1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. Journal of Neuroscience. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. Journal of Neurophysiology. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: Modulation of early gustatory cortex by attention to taste. Chemical Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]