Abstract
Endomorphin-2 (H-Tyr-Pro-Phe-Phe-NH2) and [Dmt1]EM-2 (Dmt = 2’,6’-dimethyl-l-tyrosine) analogues were synthesized containing alkylated Phe3 derivatives, 2’-monomethyl (2, 2’), 3’,5’- and 2’,6’-dimethyl (3, 3’, and 4’, respectively), 2’,4’,6’-trimethyl (6, 6’), 2’-ethyl-6’-methyl (7, 7’) and 2’-isopropyl-6’-methyl (8, 8’) groups or Dmt (5, 5’). They had the following characteristics: (i) [Xaa3]EM-2 analogues improved μ- and δ-opioid receptor affinities, the latter were inconsequential (Kiδ= 491–3,451 nM); (ii) [Dmt1,Xaa3]EM-2 analogues enhanced μ- and δ-opioid receptor affinities (Kiμ = 0.069–0.32 nM; Kiδ = 1.83–99.8 nM) and lacked interaction with κ-opioid receptors, and (iii) elevated μ-bioactivity (IC50 = 0.12–14.4 nM) and abolished δ-agonism (IC50 > 10 µM; 2’, 3’, 4’, 5’, 6’); however, 4’ and 6’ exhibited mixed μ-agonism/δ-antagonism (4’: IC50μ = 0.12, pA2 = 8.15; 6’: IC50μ = 0.21 nM, pA2 = 9.05), and 7’ was a dual μ-/δ -agonist (IC50μ = 0.17 nM; IC50δ = 0.51 nM). Alteration of EM-2 activity by Dmt1 and alkylated Phe3 residues retained μ-receptor bioactivity and formed dual μ-/δ -agonists and mixed μ-agonists/δ-antagonists.
Introduction
Endomorphin-1 (EM-1: H-Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (EM-2: H-Tyr-Pro-Phe-Phe-NH2) are endogenous opioid peptides with remarkably high selectivity for μ-opioid receptors.1 Since the endomorphins express pharmacological properties similar to morphine and are thought to inhibit pain without its undesirable side effects,2,3 extensive studies have been performed in order to clarify their pharmacological characteristics,4–9 bioactive conformation,10–14 and to improve biological activity in vitro and in vivo by chemical modificaton.15–25
The aromatic amino acid residue in position 3 is the defining structural determinant between EM-1 (Trp3) and EM-2 (Phe3).1,26 Moreover, extensive studies previously indicated that Dmt (2’,6’-dimethyl-l-tyrosine) in lieu of the N-terminal Tyr dramatically enhanced receptor affinity and bioactivity of numerous opioid agonists and antagonists,27,28 and data on endomorphin-2 analogues modified at position 1 with tyrosine analogues alkylated on the tyramine ring improved in vitro biological parameters.29 Similarly, an alkylated Phe analogue, 2’,6’-dimethylphenylalanine (Dmp), was an effective surrogate for phenylalanine in several opioid peptides, such as dynorphin A,30 [Leu5]enkephalin,31 dermorphin, deltorphin II,32 YrFB (H-Tyr-d-Arg-Phe-βAla-NH2),33 and endomorphin-2.34 Furthermore, the replacement of Tyr1 by Dmp in deltorphin II and enkephalin also yielded analogues which were surprisingly nearly as effective as the parental peptides,35 suggesting that alkylation of the aromatic ring enhances hydrophobicity, stability and/or limits rotational freedom in its solution conformation.
Rationale
It is well known that a subtle change in both the hydrophobicity and spatial conformation of an opioid ligand contributes not only to a modification of its overall activity profile (receptor affinity, bioactivity)30,32,34,35 and physicochemical properties (stability, conformation),14,18,21,31 but also to its biological efficacy (in vitro, in vivo) as an antinociceptive agent27,28,36 with an enhanced ability to transit the blood-brain barrier.36,37 In this regard, N,N-alkylation converted a potent δ-opioid antagonist into an inverse agonist,38 and led to the appearance of elevated μ-opioid receptor affinity and increased δ-antagonism among numerous analogues of the Dmt-Tic (1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) pharmacophoric compounds.39 On the other hand, the acquisition of a greater degree of hydrophobicity through alkylation of the tyramine ring of Tyr1 permitted further differentiation of the biological properties between the structurally related endomorphin-1 and -2 molecules.29,36 In light of ability of the dialkylated derivative of phenylalanine (Dmp) to substitute for Tyr1 in deltorphin II35 and for Phe in other opioids,30–35 the importance of the residue in the third position of the endomorphins, which is known to affect their receptor selectivity and bioactivity,1–4,6,7 should be amenable to alterations in the hydrophobic millieu of the aromatic moiety of Phe. A change in overall change in conformation or reduced freedom of rotation imparted by the alkyl substituents should provide a gauge on the potential effect of the substitution on receptor interaction and functional bioactivity parameters (Figure 1). Furthermore, in light of the importance of μ-opioid receptors in modulating pain, appetite and alcohol induced elevation of spontaneous inhibition of postsynaptic currents (IPSC)40 and the involvement of δ-opioid receptors in ameliorating the morphine tolerance and dependence due to the μ-opioid system,41 this study focused exclusively on these two important and interrelated opioid systems; furthermore, as our data reveal (infra vide), these EM-2 analogues are essentially devoid of activity toward κ-opioid receptors. In fact, our data reveal that the combination of Dmt1 and an alkylated Phe3 substitution provides unique evidence for the change in the bioactivities of several analogues, including identification of potent bifunctional μ-opioid receptor agonists containing δ-antagonist or δ-agonist properties.
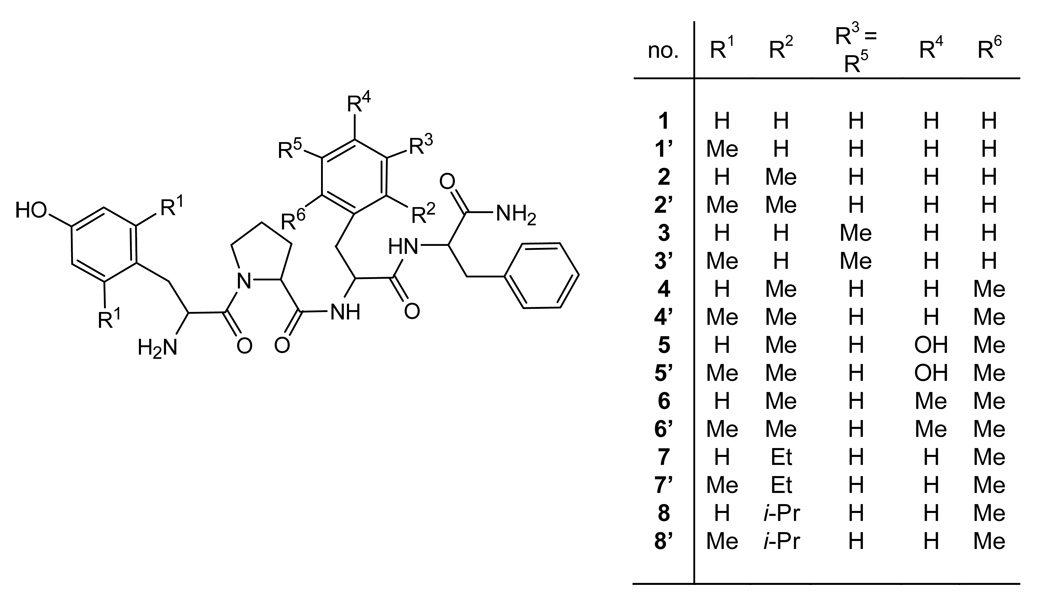
Figure 1.
Schematic Structure of Endomorphin-2 with Alkylation Sites (R1–R6) on Tyr1 and Phe3.
Chemistry
Dmt was synthesized by following the method described by Dygos et al.42 The Phe analogues (Figure 1) containing 2’-methyl (Mmp) (R2), 3’,5’-dimethyl (3,5Dmp) (R3,R5), 2’,6’-dimethyl (Dmp) (R2,R6), 2’,4’,6’-trimethyl (Tmp) (R2,R4,R6), 2’-ethyl-6’-methyl (Emp) (R2,R6) and 2’-isopropyl-6’-methyl (Imp) (R2,R6) were prepared as reported through [Rh(1,5-COD)(R,R-DIPAMP)]BF4, [(R,R)-(−)-1,2-Bis-[(o-methoxyphenyl)(phenyl)phosphino]ethane(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate] mediated asymmetric catalytic hydrogenation of corresponding acetamidoacrylate.43 Boc(tert-butyloxycarbonyl)-Tyr-Pro-OH44 and Boc-Dmt-Pro-OH40 were prepared as reported. Tyr/Dmt-Pro-Xaa-Phe-NH2 (Xaa = Mmp, 3,5Dmp, Dmp, Dmt, Tmp, Emp, Imp) was formed by segment condensation method in solution. Briefly, after deprotection of Boc-Xaa-Phe-NH2 with HCl/dioxane, the resulting H-Xaa-Phe-NH2 was condensed with Boc-Tyr/Dmt-Pro-OH using PyBop (benzotriazol-1-yloxytrispyrrolidinophosphonium hexafluorphosphate) as the coupling reagent. The final Boc protecting group was removed with HCl/dioxane in the presence of anisole, and the resulting peptide was purified by semi-preparative RP(reversed phase)-HPLC.
The identification and purity of the final compounds and their intermediates were verified using MS, NMR, analytical HPLC, and elemental analysis. Some analytical data of the final compounds are summarized in Table 1. The final compounds exhibiting greater than 98% purity were used for all biological assays. Supporting Information provides detailed elemental analyses of all the compounds.
Table 1.
Analytical Data of H-Tyr/Dmt-Pro-Xaa-Phe-NH2 Endomorphin-2 Analogues
| No. | peptide | [α]D | c in H2O | TLCaRf | tof-mass[M+1] | HPLC (min) | ||
|---|---|---|---|---|---|---|---|---|
| (deg) | Calcd | Found | tRb | tRc | ||||
| 2 | H-Tyr-Pro-Mmp-Phe-NH2 | −38.63 | 0.26 | 0.76 | 586.7 | 586.8 | 15.27 | 27.53 |
| 2’ | H-Dmt-Pro-Mmp-Phe-NH2 | −4.18 | 0.23 | 0.76 | 614.8 | 615.0 | 16.08 | 29.50 |
| 3 | H-Tyr-Pro-3,5Dmp-Phe-NH2 | −36.0 | 0.24 | 0.76 | 600.7 | 600.7 | 16.44 | 30.57 |
| 3’ | H-Dmt-Pro-3,5Dmp-Phe-NH2 | −1.51 | 0.22 | 0.78 | 628.8 | 628.9 | 17.33 | 32.59 |
| 4’ | H-Dmt-Pro-Dmp-Phe-NH2 | −2.71 | 0.42 | 0.72 | 628.8 | 628.8 | 16.69 | 30.25 |
| 5 | H-Tyr-Pro-Dmt-Phe-NH2 | −34.22 | 0.34 | 0.75 | 616.7 | 616.8 | 13.67 | 23.26 |
| 5’ | H-Dmt-Pro-Dmt-Phe-NH2 | 0.88 | 0.45 | 0.71 | 644.8 | 644.8 | 14.47 | 25.29 |
| 6 | H-Tyr-Pro-Tmp-Phe-NH2 | −42.67 | 0.23 | 0.77 | 614.8 | 615.0 | 16.63 | 31.10 |
| 6’ | H-Dmt-Pro-Tmp-Phe-NH2 | 0.98 | 0.21 | 0.80 | 642.8 | 642.7 | 17.35 | 32.59 |
| 7 | H-Tyr-Pro-Emp-Phe-NH2 | −36.53 | 0.24 | 0.79 | 614.8 | 614.8 | 16.39 | 30.25 |
| 7’ | H-Dmt-Pro-Emp-Phe-NH2 | −1.97 | 0.24 | 0.80 | 642.8 | 642.9 | 17.19 | 32.70 |
| 8 | H-Tyr-Pro-Imp-Phe-NH2 | −33.25 | 0.21 | 0.76 | 628.8 | 628.9 | 17.26 | 32.81 |
| 8’ | H-Dmt-Pro-Imp-Phe-NH2 | 1.75 | 0.25 | 0.77 | 656.8 | 656.7 | 18.09 | 34.51 |
Solvent: n-BuOH:H2O:AcOH:Pyridine = 4:1:1:2.
The column was eluted at a flow rate of 1 mL/min with a linear gradient of 90% A to 10% A in 30 min.
The column was eluted at a flow rate of 1 mL/min with a linear gradient of 90% A to 50% A in 40 min.
Results and Discussion
Opioid Receptor Affinity
Improved affinities for μ-opioid receptors were observed in the [Xaa3]EM-2 compounds (2, 4, 6–8) compared to the parent peptide (1), except 3 and 5 that exhibited weaker affinities (Table 2). Introduction of a single methyl group at the 2’-position of Phe (2) enhanced μ-opioid receptor affinity 6-fold; a second methyl group at the 6’-position (4) induced a further 5-fold increase. Sterically bulky groups, such as third methyl group at the 4’-position (6), an ethyl (7) or isopropyl (8) at the 6’-position (Figure 1) reduced receptor affinity by a factor of 10 relative to [Dmp3]EM-2 (4), which exhibits the highest μ-selectivity of any known μ-opioid agonist;1,34 nonetheless, the μ-affinities of 6, 7 and 8 were still 3-fold greater than the naturally occurring EM-2 (1) (Table 2). Another dimethylated Phe derivative, [3,5Dmp3]EM-2 (3), displayed the lowest μ-opioid receptor affinity of all the analogues (Table 2), being 141-fold lower than [Dmp3]EM-2 (4) and ca. 5-fold less than 1, suggesting that the position of the alkyl groups on the Phe3 aromatic ring was a critical factor for receptor affinity.
Table 2.
Receptor Affinities of H-Tyr/Dmt-Pro-Xaa-Phe-NH2 Endomorphin-2 Analogues
| no. | Peptide sequence | Kiμ (nM)a | (n) | Kiδ (nM)b | (n) | Kiδ/Kiμ | Kiκ (nM)c | (n) | Kiκ/Kiμ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tyr-Pro-Phe-Phe-NH2 | 1.33 | ± | 0.15 | (3) | 6085 | ± | 1215 | (3) | 4575 | >4000 | >3000 | |||
| 1’ | Dmt-Pro-Phe-Phe-NH2d | 0.26 | ± | 0.01 | (4) | 99.2 | ± | 7.9 | (3) | 382 | 489 | ± | 135 | (3) | 1880 |
| 2 | Tyr-Pro-Mmp-Phe-NH2 | 0.221 | ± | 0.05 | (3) | 1741 | ± | 177 | (5) | 7878 | ND | ||||
| 2’ | Dmt-Pro-Mmp-Phe-NH2 | 0.177 | ± | 0.015 | (3) | 4.61 | ± | 0.3 | (3) | 26 | 176 | ± | 64 | (3) | 994 |
| 3 | Tyr-Pro-3,5Dmp-Phe-NH2 | 6.19 | ± | 0.24 | (5) | 3451 | ± | 624 | (5) | 558 | ND | ||||
| 3’ | Dmt-Pro-3,5Dmp-Phe-NH2 | 0.111 | ± | 0.005 | (3) | 11.6 | ± | 1.6 | (5) | 105 | 830 | ± | 148 | (3) | 7480 |
| 4 | Tyr-Pro-Dmp-Phe-NH2d | 0.044 | ± | 0.003 | (3) | 1440 | ± | 94 | (3) | 32730 | >4000 | >91000 | |||
| 4’ | Dmt-Pro-Dmp-Phe-NH2 | 0.069 | ± | 0.008 | (3) | 2.27 | ± | 0.44 | (4) | 33 | 94.2 | ± | 10.5 | (3) | 1365 |
| 5 | Tyr-Pro-Dmt-Phe-NH2 | 2.63 | ± | 0.25 | (3) | 3020 | ± | 161 | (5) | 1148 | ND | ||||
| 5’ | Dmt-Pro-Dmt-Phe-NH2 | 0.092 | ± | 0.015 | (3) | 80.8 | ± | 6.2 | (4) | 878 | 998 | ± | 363 | (3) | 10850 |
| 6 | Tyr-Pro-Tmp-Phe-NH2 | 0.439 | ± | 0.04 | (4) | 1358 | ± | 128 | (4) | 3093 | ND | ||||
| 6’ | Dmt-Pro-Tmp-Phe-NH2 | 0.182 | ± | 0.004 | (3) | 1.83 | ± | 0.22 | (3) | 10 | 172 | ± | 35 | (3) | 945 |
| 7 | Tyr-Pro-Emp-Phe-NH2 | 0.457 | ± | 0.071 | (3) | 491 | ± | 14 | (4) | 1074 | ND | ||||
| 7’ | Dmt-Pro-Emp-Phe-NH2 | 0.211 | ± | 0.025 | (3) | 3.03 | ± | 0.09 | (3) | 14 | 278 | ± | 66 | (3) | 1320 |
| 8 | Tyr-Pro-Imp-Phe-NH2 | 0.445 | ± | 0.02 | (4) | 543 | ± | 42 | (4) | 1221 | ND | ||||
| 8’ | Dmt-Pro-Imp-Phe-NH2 | 0.32 | ± | 0.027 | (3) | 4.61 | ± | 0.3 | (3) | 14 | 205 | ± | 73 | (3) | 641 |
The italicized compounds represent parental opioid peptides.
Versus [3H]DAMGO.
Versus [3H]Deltorphin-II.
Versus [3H]U-69,593. (n) is the number of independent repetitions conducted for each analogue using 5–8 doses of peptide. ND: not determined.
Among the [Dmt1,Xaa3]EM-2 ligands (1’–8’) (Table 2), alkylated Phe3 analogues essentially enhanced the affinities for both μ- and δ-opioid receptors, although minor discrepancies in μ-opioid receptor affinity were noted for 6’, 7’ and 8’ in comparison to the [Xaa3]EM-2 substances. Although they displayed improved δ-opioid receptor affinity, μ-opioid selectivity remained. As published previously, the presence of Dmt1 in opioids enhanced δ-opioid receptor affinity by orders of magnitude;27–29,45 however, with μ-opioid selective agonists, a N-terminal Dmt residue enhanced affinity to δ-sites to a greater extent than found with the prototype δ-opioid antagonists containing the Dmt-Tic pharmacophore,45 as seen by the 634-fold increase in δ-opioid affinity in 4’ relative to 4 with the concomitant loss of μ selectivity by three orders of magnitude (Table 2). In these Dmt-derivatives, the highest μ-opioid selectivity occurred with [Dmt1,3]EM-2 (5’) (Kiδ/Kiμ = 878), which was less (37-fold) than the most selective [Xaa3]EM-2 compound (4), but nearly 3-fold greater than 1’ (Table 2). One analogue of considerable interest is Tmp3 (6’): with a 44-fold greater interaction than Dmt3 (5’) toward δ-opioid receptors, the data suggest that the hydrogen donor capability of the hydroxyl group of Dmt is apparently less effective in affecting receptor interaction when situated within the peptide than the hydrophobicity of the 4’ methyl group; i.e., the hydroxyl group may contribute a negative influence as an internal residue supporting the original study on the endomorphins.1
As seen in Table 2, κ-opioid receptor affinities for the Dmt-derivatives (1’–8’) and parental compounds (1,4) were quite weak relative to the interaction of these peptides to both μ- and δ-opioid receptors, although the control U-69593 demonstrated good affinity (1.04 ± 0.45 nM). Owing to the lack of high κ-opioid affinity, none of the analogues were investigated for their functional bioactivity.
Functional Bioactivity
The functional bioactivities (Table 3) of the [Xaa3]EM-2 analogues exhibited considerable variation in μ-agonism relative to EM-2 (1): analogues 2, 6, 7 and 8 were 2- to 5-fold more active, while 3 and 5 were inactive. The increase in hydrophobicity and positioning of the methyl groups on Phe3 changed μ-agonism relative to EM-2 (1); i.e., while alkylation at the 3’ and 5’ positions of Phe3 inactivated the molecule (3), substitutions at positions 2’ and 6’ were very well tolerated (4) producing dual μ-/δ-agonism.34 Moreover, trimethylation (6) yielded a highly selective μ-agonist.
Table 3.
Functional Bioactivities of H-Tyr/Dmt-Pro-Xaa-Phe-NH2 Endomorphin-2 Analogues
| no. | peptide | GPI assaya | MVD assaya | |||||
|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | IC50 (nM)b | pA2c | ||||||
| 1 | Tyr-Pro-Phe-Phe-NH2 | 6.9 | ± | 0.9 | 344 | ± | 93 | |
| 1’ | Dmt-Pro-Phe-Phe-NH2d | 0.261 | ± | 0.038 | 0.59 | ± | 0.18 | |
| 2 | Tyr-Pro-Mmp-Phe-NH2 | 1.33 | ± | 0.27 | 15.7 | ± | 5.1 | |
| 2’ | Dmt-Pro-Mmp-Phe-NH2 | 0.162 | ± | 0.041 | >10000 (0.00%) | 6.59 | ||
| 3 | Tyr-Pro-3,5Dmp-Phe-NH2 | 389 | ± | 166 | >10000 (8.0%) | |||
| 3’ | Dmt-Pro-3,5Dmp-Phe-NH2 | 14.4 | ± | 5.4 | >10000 (7.1%) | 6.77 | ||
| 4 | Tyr-Pro-Dmp-Phe-NH2e | 0.378 | ± | 0.104 | 1.39 | ± | 0.17 | |
| 4’ | Dmt-Pro-Dmp-Phe-NH2 | 0.123 | ± | 0.020 | >10000 (15.6%) | 8.15 | ||
| 5 | Tyr-Pro-Dmt-Phe-NH2 | 541 | ± | 178 | 978 | ± | 113 | |
| 5’ | Dmt-Pro-Dmt-Phe-NH2 | 1.94 | ± | 0.21 | >10000 (<1%) | 7.06 | ||
| 6 | Tyr-Pro-Tmp-Phe-NH2 | 1.90 | ± | 0.49 | >10000 (36.2%) | |||
| 6’ | Dmt-Pro-Tmp-Phe-NH2 | 0.212 | ± | 0.077 | >10000 (22.2%) | 9.05 | ||
| 7 | Tyr-Pro-Emp-Phe-NH2 | 2.35 | ± | 0.53 | 277 | ± | 29 | |
| 7’ | Dmt-Pro-Emp-Phe-NH2 | 0.170 | ± | 0.072 | 0.51 | ± | 0.24 | |
| 8 | Tyr-Pro-Imp-Phe-NH2 | 2.74 | ± | 1.20 | >10000 (17.7%) | |||
| 8’ | Dmt-Pro-Imp-Phe-NH2 | 0.204 | ± | 0.050 | 5.56 | ± | 2.47 | |
The italicized compounds represent parental opioid peptides
The data are the means of over five independent repetitions used different isolated tissue preparations. IC50: concentration required for 50% inhibition of the electrically induced contraction in muscle derived from a dose-response curve.
Values in parentheses indicate maximal inhibition of the tissue contraction at the concentration of 10,000 nM.
Negative log of the molar concentration required to double the deltorphin II concentration to achieve the original response.
Data cited from ref. 29.
Data cited from ref. 34.
The [Dmt1,Xaa3]EM-2 analogues (Table 3) generally doubled μ-opioid bioactivities (2’, 4’), remained essentially unchanged (5’, 6’, 7’, 8’) or lost bioactivity (3’) relative to 1’.29 Compound 3’ was an anomaly; the biopotency of this analogue correlated with μ-opioid receptor affinity, suggesting that the methyl groups at positions 3’ and 5’ (3, 3’) presented an unfavorable conformation for activation of the μ-opioid receptor, whereas the dimethyl alkylation at positions 2’ and 6’ in Phe3 (4’) (and 4)34 was permitted. This was equally true for the trimethyl- (6’), ethylmethyl- (7’) and isopropylmethyl-Phe3 (8’) derivatives; this observation was repeated in the [Xaa3]EM-2 series (6, 7, 8) reflecting a change that presumably enabled the ligands to adopt a more compatible ligand-receptor conformation to trigger a biological response. Substitution of Phe3 by Dmt3 only yielded a bioactive compound in the presence of Dmt1 (compare 5 and 5’). Moreover, replacement of the 4’ OH of Dmt3 (5’) by a methyl group to yield Tmp3 (6’) displayed slightly enhanced μ-agonism and significantly improved δ-anatgonism. Tmp3 (6’) became a mixed μ-agonist/δ-antagonist with δ-anatgonism being two orders of magnitude greater than Dmt3 (5’).
Recently, Dmt-Pro-Trp-Phe-NH2 ([Dmt1]EM-1) was reported to be a mixed μ-opioid agonist/δ -antagonist (GPI IC50 = 0.272 nM; MVD pA2 = 8.60);36b on the other hand, [Dmt1]EM-2 (1’) is a dual μ-/δ-opioid agonist.29 The only difference between [Dmt1]EM-1 and 1’ is Trp3 or Phe3, respectively. This indicates that the bulky the side chain of Trp in combination with Dmt1 causes either a steric hindrance in the conformation of the peptide, or a shift in hydrophobicity to potentiate the induction of δ-opioid receptor antagonism.
In terms of the δ-functional bioactivities, the majority of the ligands failed to elicit substantial δ-agonism except 7’ and to a lesser extent both 8’ (10-fold loss) and 2 (30-fold decrease), in which 7’ resembled the bioactivity profiles of the reference compounds (1’ cf. ref. 24, and 434). Nonetheless, the [Dmt1,Xaa3]EM-2 compounds established a distinctly different bioactive profile than that of the [Xaa3]EM-2 analogues: (i) the appearance of δ-antagonism (2’, 3’, 4’, 5’, 6’) ranging from pA2 = 6.59 (2’) to a potent pA2 = 9.05 (6’), and (ii) exhibited δ-agonism with 7’ and 8’. The δ-opioid antagonism of [Dmt1,Tmp3]EM-2 (6’) is ca. 8-fold more potent than the prototype δ-antagonist H-Dmt-Tic-OH (pA2 = 8.48).45 Furthermore, [Xaa3]EM-2 analogues remained μ-agonists except for the inactive ligands 3 and 5, and the dual μ-/δ-agonism of 2. An N-terminus Dmt in 4’ eliminated δ-agonism of 4 with the appearance of δ-antagonism (4’); in fact, the loss of δ-agonism in the [Dmt1,Xaa3]EM-2 analogues was associated with the δ-antagonism (6’ > 4’ > 5’, 3’, 2’).
Substitution of Phe3 in EM-2 by other phenylalanine analogues, such as Hfe (homophenylalanine), Phg (phenylglycine), d-Phg,46 N-methyl-Phe,22 d-2-Nal [d-3-(2-naphthyl)-alanine],23 l- and d-α-aminoxy-Phe,21 β-methyl-Phe,18 4’-NH2-Phe and 4’-NCS-Phe,47 failed to improve either the affinity toward the μ-opioid receptor or provide unique functional bioactivities in the resultant ligands. Our results clearly indicated that modification of Phe3 with alkyl groups not only enhnaced receptor affinity, but also transformed the functional bioactivity to elicit pharmacological properties as bifunctional opioid ligands (dual μ-/δ-agonists and mixed μ-agonists/δ-antagonists).
Conclusion
This study demonstrated that alkylated Phe3 residues incorporated into the third position of EM-2 act as effective amino acid surrogates, in particular the [Dmt1,Xaa3]EM-2 analogues, which exhibited significantly higher affinity for both μ- and δ-opioid receptors, yet retained μ-opioid bioactivity. The lack of interaction of the analogues with κ-opioid receptors revealed that these compounds maintained the selectivity of endomorphin for μ-opioid receptors. The most intriguing observation was the unique appearance of δ-antagonism in the endomorphins, which normally lack interaction with δ-opioid receptors and have high μ-opioid receptor selectivity and prominent μ-opioid agonism.1 These bifunctional molecules are targets in the design of a new antinociceptive opioids that could potentially alleviate acute or chronic pain with low physical dependence and tolerance,41 especially due to the acquisition of δ-opioid antagonism. Pharmacological and biochemical evidence further reveal that μ- and δ-opioid receptors readily form heterodimers48 and that the δ-opioid receptors modulate the function of μ-opioid receptors in this complex.49 Thus, our opioid derivatives displaying dual μ-/δ-agonism (7’) and mixed μ-agonism/δ-antagonism (6’, 4’) characteristics which are shown to exhibit a low tendency to develop dependence and tolerance,50 and verified using δ-receptor knockout mice51 and a δ-receptor antagonist52 might be applicable for antinociception with a low degree of dependence and tolerance. Furthermore, structural clarification of the subtle differences among these interrelated opioid ligands14,23 should provide insight on the molecular mechanism of action between agonists and antagonists.53
Experimental Section
Melting points were determined on a Yanagimoto micromelting point apparatus and are uncorrected. TLC was performed on precoated plates of silica gel F254 (Merk, Darmstadt, Germany). Rf1, Rf2, Rf3, and Rf4 values refer to CHCl3:H2O:AcOH (acetic acid) (90:8:2), AcOEt (ethyl acetate), AcOEt:MeOH (methanol) (20:1), and n-BuOH (butanol):H2O:AcOH:Pyridine (4:1:1:2), respectively. Optical rotations were measured with a DIP-1000 automatic polarimeter (Japan Spectroscopic Co.). Analytic RP-HPLC used a Waters Delta 600 with COSMOSIL C18 column (4.6 mm × 250 mm). The solvents for analytical HPLC were: A, 0.05% TFA (trifluoroacetic acid) in water; B, 0.05% TFA in CH3CN. The column was eluted at a flow rate of 1 mL/min with a linear gradient: 90% A to 10% A in 30 min and with a linear gradient: 90% A to 50% A in 40 min; the retention time was reported as tR (min). 1H and 13C NMR spectra were measured on a Bruker DPX-400 spectrometer at 25 °C. Chemical shift values are expressed as ppm downfield from tetramethylsiliane.
General Procedure for Boc Protected Phe Analogues Boc-Xaa-OH (Xaa = Mmp, 3,5Dmp, Dmp, Dmt, Tmp, Emp, Imp)
(Boc)2O (di-tert-butyl dicarbonate: l5.5 mmol) in dioxane (15 mL) was added to the solution of amino acid (5.0 mmol) in H2O (15 mL) containing TEA (triethylamine: 15.0 mmol). The reaction mixture was stirred at 0 °C for 15 min, then room temperature for 2 h and the solvent was removed under vacuum. The residue was adjusted to pH 2–3 by using cooled 10% citric acid; the precipitated product was extracted with AcOEt. The combined extract was washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated. The residue was diluted with hexane, the solid was collected by filtration and dried under vacuum. Elemental analyses of Boc-Xaa-OH are summarized in Supporting Information (Table 1).
Nα-tert-Butyloxycarbonyl-2’-methyl-l-phenylalanine
Yield 1.25 g (89.2%); mp 118–119 °C; Rf1 = 0.56; −15.54° (c = 1.03, MeOH). 1H-NMR (400.1 MHz, CDCl3)δ: 7.20-7.05 (m, 4H), 6.63 (br, 0.38H), 4.93 (br, 0.62H), 4.60-4.37 (m, 1H), 3.33-3.20 (m, 1H), 3.07-2.80 (m, 1H), 2.36 (s, 3H), 1.40, 1.17 (2s, 9H).
Nα-tert-Butyloxycarbonyl-3’,5’-dimethyl-l-phenylalanine
Yield 0.71 g (96.5%); mp 115–116 °C; Rf1 = 0.56; +16.09° (c = 0.67, MeOH). 1H-NMR (400.1 MHz, CDCl3)δ: 6.89 (s, 1H), 6.79 (s, 2H), 4.89 (br, 1H), 4.52 (br, 1H), 3.12 (dd, 1H, J = 5.34, 13.84 Hz), 3.05-2.91 (m, 1H9, 2.28 (s, 3H), 1.42 (s, 9H).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-phenylalanine
Yield 1.3 g (93.0%); mp 135–136 °C; Rf1 = 0.40; −17.85° (c = 0.41, MeOH). 1H-NMR (400.1 MHz, CDCl3)δ: 7.13-6.95 (m, 3.50H), 5.03-4.92 (br, 0.4H), 4.58-4.46 (m, 1H), 3.25-3.02 (m, 2H), 2.40, 2.37 (2s, 6H), 1.36, 1.06 (2s, 9H).
Nα-tert-Butyloxycarbonyl-2’,4’,6’-trimethyl-l-phenylalanine
Yield 1.37 g (88.2%); mp 153–154 °C; Rf1 = 0.56; −13.26° (c = 0.94, MeOH). 1H-NMR (400.1 MHz, CDCl3)δ: 7.03 (br, 0.60H), 6.84 (s, 2H), 4.97 (br, 0.40H), 4.53-4.40 (m, 1H), 3.23-3.13 (m, 1H), 3.05 (dd, 1H, J = 9.95, 14.10 Hz), 2.35 (s, 6H), 2.24 (s, 3H), 1.37, 1.08 (2s, 9H).
Nα-tert-Butyloxycarbonyl-2’-ethyl-6’methyl-l-phenylalanine
Yield 1.32 g (85.7%); mp 117–118 °C; Rf1 = 0.56; −14.33° (c = 1.0, MeOH). 1H-NMR (400.1 MHz, CDCl3)δ: 7.26 (br, 0.77H), 7.14-7.00 (m, 3H), 4.97 (br, 0.23H), 4.60-4.50 (m, 1H), 3.28-3.17 (m, 1H), 3.17-3.06 (m, 1H), 2.86-2.65 (m, 2H), 2.42, 2.39 (2s, 3H), 1.36 (s, 2.2H), 1.22 (t, 3H, J = 7.51 Hz), 1.05 (s, 6.8H).
Nα-tert-Butyloxycarbonyl-2’-isopropyl-6’-methyl-l-phenylalanine
Yield 1.45 g (90.3%); mp 121–122 °C; Rf1 = 0.56; −12.78° (c = 0.93, MeOH). 1H-NMR (400.1 MHz, CDCl3)δ: 7.26 (br, 0.76H), 7.17-7.07 (m, 2H), 7.05-6.95 (m, 1H), 4.95 (br, 0.24H), 4.56-4.45 (m, 1H), 3.47-3.30 (m, 0.76H), 3.30-3.05 (m, 2.24H), 2.43, 2.40 (2s, 3H), 1.35 (s, 2.3H), 1.25 (d, 3H, J = 6.73 Hz), 1.18 (d, 3H, J = 6.42 Hz), 1.05 (s, 6H).
General Procedure for Synthesis of Boc-Xaa-Phe-NH2 (Xaa = Mmp, 3,5Dmp, Dmp, Dmt, Tmp, Emp, Imp)
The H-Phe-NH2 hydrochloride salt (0.25 g, 1.25 mmol) was dissolved in DMF (N,N-dimethylformamide: 10 mL) containing DIPEA (diisopropylethylamine: 0.50 mL, 2.87 mmol), and to this solution Boc-Xaa-OH (1.14 mmol) and PyBop (0.65 g, 1.25 mmol) were added. The reaction mixture was stirred at 0 °C for 10 min, then room temperature for 4 h. After remove of solvent, the residue was diluted with AcOEt (80 mL). The dilution was washed with ice cooled 10% citric acid (3 × 15 mL), 5% Na2CO3 (3 × 15 mL) and saturated NaCl (3 × 20 mL), dried over Na2SO4 and evaporated to dryness. The residue was purified by flash chromatography (SiO2, AcOEt). The compound was precipitated with hexane, collected by filtration and dried under vacuum. Elemental analyses of Boc-Xaa-Phe-NH2 are summarized in Supporting Information (Table 2).
Nα-tert-Butyloxycarbonyl-2’-methyl-l-phenylalanylphenylalanylamide
Yield 470 mg (97.0%); mp 164–166 °C; Rf2 = 0.73; −21.04° (c = 0.34, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 7.89 (d, 0.15H, J = 6.80 Hz, Phe NH), 7.79 (d, 0.84H, J = 8.25 Hz, Phe NH), 7.45, 7.36 (2s, 1.0H, CONH2), 7.30-6.96 (m, 10.9H, CONH2, Mmp NH, Phe Ar-H, Mmp Ar-H), 6.52 (d, 0.1H, J = 6.8 Hz, Mmp NH), 4.60-4.43 (m, 1.0H, Phe αH), 4.11 (dt, 1.0H, J = 4.45, 9.80 Hz, Mmp αH), 3.03 (dd, 1.0H, J = 4.87, 13.72 Hz, Phe βH), 2.90-2.70 (m, 2H, Phe βH, Mmp βH), 2.65 (dd, 1.0H, J = 10.15, 14.12 Hz, Mmp βH), 2.24 (s, 3H, Mmp CH3), 1.29, 1.14 (2s, 9.0H, Boc). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.45, 171.11, 155.01, 137.58, 136.01 (5q), 129.73, 129.25, 127.89, 126.13, 125.39 (5t, Mmp and Phe Ar-C), 78.16 (q, Boc), 4.71 (t, Mmp αC), 53.29 (t, Phe αC), 37.67 (s, Phe βC), 34.78 (s, Mmp βC), 28.00, 27.57 (2p, Boc), 18.90 (p, Mmp CH3).
Nα-tert-Butyloxycarbonyl-3’,5’-dimethyl-l-phenylalanylphenylalanylamide
Yield 493 mg (98.6%); mp 193–195 °C; Rf2 = 0.74; −16.02° (c = 0.39, MeOH). 1H NMR (400.1 MHz, DMSO-d6)δ: 8.0-7.92 (br, 0.14H, Phe NH), 7.86 (d, 0.80H, J = 8.23 Hz, Phe NH), 7.45, 7.35 (2s, 1H, CONH2), 7.30-7.14 (m, 5H, Phe Ar-H), 7.14-6.96 (m, 1H, CONH2), 6.91-6.71 (m, 3.75H, 3,5Dmp Ar-H, 3,5Dmp NH), 6.40-6.32 (br, 0.12H, 3,5Dmp NH), 4.36–4.39 (m, 1H, Phe αH), 4.12-3.98 (m, 1H, 3,5Dmp αH), 3.01 (dd, 1.0H, J = 5.04, 13.75 Hz, Phe βH), 2.84 (dd, 1.0H, J = 8.65, 13.75 Hz, Phe βH), 2.77 (dd, 1.0H, J = 4.26, 13.68 Hz, 3,5Dmp βH), 2.59 (dd, 1.0H, J = 10.09, 13.68 Hz, 3,5Dmp βH), 2.21 (s, 6H, 3,5Dmp CH3), 1.31, 1.16 (2s, 9H, Boc). 13C NMR [100.6 MHz, DMSO-d6] δ: 172.54, 171.14, 155.02, 137.77, 137.62, 136.65 (6q), 129.20, 127.91 (2t, Phe Ar-C), 127.49, 126.77 (2t, 3,5Dmp Ar-C), 126.13 (t, Phe Ar-C), 78.04 (q, Boc), 55.88 (t, 3,5Dmp αC), 53.36 (t, Phe αC), 37.79 (s, Phe βC), 37.16 (s, 3,5Dmp βC), 28.01 (p, Boc), 20.81 (p, 3,5Dmp CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-phenylalanylphenylalanylamide
Yield 491 mg (98.0%); mp 177–179 °C; Rf2 = 0.73; −15.28° (c = 0.58, MeOH). 1H NMR (400.1 MHz, DMSO-d6)δ: 7.95 (d, 0.2H, J = 7.51 Hz, Phe NH), 7.83 (d, 0.75H, J = 8.48 Hz, Phe NH), 7.45 (br, 0.23H, CONH2), 7.33-7.21 (m, 4.68H, CONH2 and Ar-H), 7.21-7.13 (m, 1H, Ar-H), 7.08 (s, 1.0H, CONH2), 7.0-6.86 (m, 3.73H, Ar-H and Dmp NH), 6.54 (d, 0.14H, J = 9.54 Hz, Dmp NH), 4.58-4.45 (m, 1.0H, Phe αH), 4.19-4.03 (m, 1.0H, Dmp αH), 3.02 (dd, 1.0H, J = 5.05, 13.62 Hz, Phe βH), 2.88-2.55 (m, 3.0H, Phe βH and Dmp βH), 2.07 (s, 6.0H, Dmp CH3), 1.26, 1.04 (2s, 9.0H, Boc). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.48, 170.91, 154.64, 137.66, 136.61, 134.68 (6q), 129.28, 127.86, 127.78, 126.12, 125.85 (5t, Ar-C), 78.13 (q, Boc), 54.91 (t, Dmp αC), 53.35 (t, Phe αC), 37.84 (s, Phe βC), 32.30 (s, Dmp βC), 27.97, 27.34 (2p, Boc), 19.80 (p, Dmp CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylphenylalanylamide
Yield 483 mg (93.2%); mp 184–186 °C; Rf2 = 0.93; −19.08° (c = 0.33, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 8.66 (s, 1H, Dmt OH), 7.91 (d, 0.20H, J = 8.18 Hz, Phe NH), 7.79 (d, 0.78H, J = 8.44 Hz, Phe NH), 7.47, 7.32 (2s, 1.0H, CONH2), 7.29-7.02 (m, 6H, Phe Ar-H, CONH2), 6.86 (d, 0.74H, J = 9.30Hz, Dmt NH), 6.43 (d, 0.17H, J = 9.69 Hz, Dmt NH), 6.34 (s, 2H, Dmt Ar-H), 4.58-4.43 (m, 1.0H, Phe αH), 4.10-3.93 (m, 1.0H, Dmt αH), 3.02 (dd, 1.0H, J = 5.09, 13.60 Hz, Phe βH), 2.81 (dd, 1.0H, J = 9.01, 13.60 Hz, Phe βH), 2.65 (dd, 1.0H, J = 5.14, 14.40 Hz, Dmt βH), 2.55 (dd, 1.0H, J = 9.16, 14.40 Hz, Dmt βH), 2.13 (s, 6.0H, Dmt CH3), 1.28, 1.09 (2s, 9.0H, Boc). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.49, 171.13, 154.90, 154.69, 137.65 (5q), 129.26, 127.83, 126.09 (3t, Phe Ar-C), 124.96 (q), 114.61 (t, Dmt Ar-C), 78.05 (q, Boc), 55.38 (t, Dmt αC), 53.30 (t, Dmt αC), 37.82 (s, Phe βC), 31.58 (s, Dmt βC), 27.99, 27.39 (2p, Boc), 19.95 (p, Dmt CH3).
Nα-tert-Butyloxycarbonyl-2’,4’,6’-trimethyl-l-phenylalanylphenylalanylamide
Yield 483 mg (93.5%); mp 224–225 °C; Rf2 = 0.76; −16.38° (c = 0.41, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 7.94 (d, 0.18H, J = 7.62 Hz, Phe NH), 7.83 (d, 0.79H, J = 8.46 Hz, Phe NH), 7.43 (br, 0.20H, CONH2), 7.30-7.13 (m, 5.80H, CONH2 and Tyr Ar-H), 6.92 (d, 0.77H, J = 9.29 Hz, Tmp NH), 6.73 (s, 2H, Tmp Ar-H), 6.48 (d, 0.15H, J = 9.46 Hz, Tmp NH), 4.60-4.40 (m, 1H, Phe αH), 4.15-3.96 (m, 1H, Tmp αH), 3.02 (dd, 1H, J = 5.03, 13.54 Hz, Phe βH), 2.81 (dd, 1H, J = 9.05, 13.54 Hz, Phe βH), 2.72 (dd, 1H, J = 5.31, 14.15 Hz, Tmp βH), 2.63 (dd, 1H, J = 9.22, 14.10 Hz, Tmp βH), 2.18, 2.15 (2s, 9H, Tmp CH3), 1.27, 1.06 (2s, 9H, Boc). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.49, 171.00, 154.67, 153.62, 137.68, 136.40, 134.50, 131.56 (8q), 129.26 (t, Phe Ar-C), 128.48 (t, Tmp Ar-C), 127.84, 126.09 (2t, Phe Ar-C), 78.11 (q, Boc), 55.04 (t, Tmp αC), 53.34 (t, Phe αC), 37.79 (s, Phe βC), 31.91 (s, Tmp βC), 27.96, 27.31 (2p, Boc), 20.33, 19.71 (2p, Tmp CH3).
Nα-tert-Butyloxycarbonyl-2’-ethyl-6’-methyl-l-phenylalanylphenylalanylamide
Yield 481 mg (93.2%); mp 208–210 °C; Rf2 = 0.78; −15.16° (c = 0.35, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 7.93 (d, 0.16H, J = 7.63 Hz, Phe NH), 7.81 (d, 0.77H, J = 8.46 Hz, Phe NH), 7.50-6.88 (m, 10.87H, CONH2, Emp NH, Phe Ar-H, Emp Ar-H), 6.55 (d, 0.16H, J = 9.29 Hz, Emp NH), 4.57-4.40 (m, 1H, Phe αH), 4.16-3.97 (m, 1H, Emp αH), 3.03 (dd, 1H, J = 5.02, 13.62 Hz, Phe βH), 2.85-2.53 (m, 5H, Phe βH, Emp βH, Emp CH2CH3), 2.24 (s, 3H, Emp Ar-CH3), 1.26 (s, 7.38H, Boc), 1.10 (t, 3H, J = 7.42 Hz, Emp CH2CH3), 1.04 (s, 1.62H, Boc). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.46, 170.89, 154.65, 142.64, 137.62, 136.65, 133.90 (7q), 129.29, 127.83 (2t, Phe Ar-C), 127.67 (t, Emp Ar-C), 126.10 (t, Phe Ar-C and Emp Ar-C), 125.94 (t, Emp Ar-C), 78.12 (q, Boc), 55.56 (t, Emp αC), 53.28 (t, Phe αC), 37.88 (s, Phe βC), 31.44 (s, Emp βC), 27.95, 27.35 (2p, Boc), 25.03 (s, Emp, CH2CH3), 19.90 (p, Emp Ar-CH3) 15.03 (p, Emp CH2CH3).
Nα-tert-Butyloxycarbonyl-2’-isopropyl-6’-methyl-l-phenylalanylphenylalanylamide
Yield 495 mg (93.0%); mp 109–110 °C; Rf2 = 0.72; −22.11° (c = 0.33, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 7.93 (d, 0.17H, J = 7.29 Hz, Phe NH), 7.83 (d, 0.76H, J = 8.56 Hz, Phe NH), 7.51, 7.43 (2s, 0.87H, CONH2), 7.30-6.86 (m, 10H, CONH2, Ar-H, Imp NH), 6.60 (d, 0.17H, J = 9.47 Hz, Imp NH), 4.60-4.48 (m, 1H, Phe αH), 4.10-3.97 (m, 1H, Imp αH), 3.22 (hept, 1H, J = 6.68 Hz, CH(CH3)2), 3.04 (dd, 1H, J = 4.98, 13.52 Hz, Phe βH), 2.83 (dd, 1H, J = 9.14, 13.52 Hz, Phe βH), 2.78-2.58 (m, 2H, Imp βH), 2.25 (s, 3H, Imp Ar-CH3), 1.23 (s, 7H, Boc), 1.17 (d, 3H, J = 6.68 Hz, CH(CH3)2), 1.10 (d, 3H, J = 6.68 Hz, CH(CH3)2), 1.02 (s, 2H, Boc). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.53, 170.85, 154.60, 147.38, 137.59, 136.48, 133.01 (7q), 129.34, 127.81, 127.39, 126.22, 126.11, 122.74 (6t, Ar-C), 78.10 (q, Boc), 55.74 (t, Imp αC), 53.19 (t, Phe αC), 38.03 (s, Phe βC), 30.97 (s, Imp βC), 27.92 (t, CH(CH3)2; p, Boc), 27.36 (p, Boc), 24.37, 23.73 (2p, CH(CH3)2), 20.13 (p, Imp Ar-CH3).
General Procedure for Synthesis of Boc-Tyr/Dmt-Pro-Xaa-Phe-NH2 (Xaa = Mmp, 3,5Dmp, Dmp, Dmt, Tmp, Emp, Imp)
Boc-Xaa-Phe-NH2 (0.44 mmol) was treated with 7.7 mol/L HCl/dioxane (1.15 mL, 8.8 mmol) to remove the Boc group at room temperature for 30 minutes. The product was precipitated with ether, filtered and dried under vacuum. The resulted hydrochloride salt was dissolved in DMF (10 mL) containing DIPEA (184 µL, 1.06 mmol), and to this solution Boc-Xaa-Pro-OH (0.44 mmol) and PyBop (252 mg, 0.49 mmol) were added. The reaction mixture was stirred at 0 °C for 10 min, then room temperature for 4 h. After removal of solvent, the residue was diluted with AcOEt. The dilution was washed with ice cooled 10% citric acid (3 × 15 mL), 5% Na2CO3 (3 × 15 mL) and saturated NaCl (3 × 20 mL), dried over Na2SO4 and evaporated to dryness. The residue was purified by flash chromatography (SiO2, AcOEt:MeOH = 10:1). The compound was precipitated with hexane, filtered and dried under vacuum. Elemental analyses of Boc-Tyr/Dmt-Pro-Xaa-Phe-NH2 are summarized in Supporting Information (Table 3).
Nα-tert-Butyloxycarbonyltyrosylprolyl-2’-methyl-l-phenylalanylphenylalanylamide
Yield 289 mg (95.6%); mp 138–140 °C; Rf3 = 0.64; −50.30° (c = 0.64, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.21 (s, 0.19H, Tyr cis-OH), 9,15 (s, 0.76H, Tyr trans-OH), 8.46 (d, 0.16H, J = 8.39 Hz, Phe cis-NH), 8.01 (d, 0.76H, J = 7.57 Hz, Phe trans-NH), 7.86 (d, 0.78H, J = 8.20 Hz, Mmp trans-NH), 7.76 (d, 0.18H, J = 8.12 Hz, Mmp cis-NH), 7.28-7.14 (m, 5.0H, Phe Ar-H), 7.14-6.93 (m, 8.9H, Tyr Ar-H, Mmp Ar-H, CONH2, Tyr NH), 6.68-6.57 (m, 2.1H, Tyr Ar-H, Tyr NH), 4.48-4.34 (m, 2.0H, Phe αH, Tyr αH), 4.34-4.10 (m, 1.77H, Pro trans-αH, Mmp αH), 3.82 (d, 0.17H, J = 7.67 Hz, Pro cis-αH), 3.64-3.53 (m, 0.65H, Pro trans-δH), 3.53-3.37 (m, 0.96H, Pro trans-δH), 3.24-2.54 (m, 6.39H, Pro cis-δH, Phe βH, Tyr βH, Mmp βH), 2.25 (s, 3.0H, Mmp CH3), 1.98-1.87 (m, 0.70H, Pro trans-βH), 1.87-1.66 (m, 2.66H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.48-1.38 (m, 0.27H, Pro cis-γH), 1.38-1.14 (m, 9.2H, Boc, Pro cis-βH), 1.0-0.86 (m, 0.15H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.39, 171.56, 170.86, 170.65, 170.35, 170.25, 155.96, 155.67, 155.36, 155.24, 137.84, 137.65, 136.04, 135.79 (14q), 130.30, 130.09, 129.77, 129.05, 128.94, 127.94, 126.44, 126.20, 126.12, 125.53, 114.89, 114.77 (12t, Ar-C), 78.32, 77.87 (2q, Boc), 59.52 (t, Pro trans-αC), 59.39 (t, Pro cis-αC), 54.10 (t, Tyr trans-αC), 53.88 (t, Tyr cis-αC), 53.71 (t, Phe αC), 53.49 (t, Mmp cis-αC), 53.16 (t, Mmp trans-αC), 46.62 (s, Pro trans-δC), 46.04 (s, Pro cis-δC), 37.60 (s, Phe cis-βC), 37.17 (s, Phe trans-βC), 36.85 (s, Tyr cis-βC), 35.45 (s, Tyr trans-βC), 34.23 (s, Mmp trans-βC), 33.77 (s, Mmp cis-βC), 29.99 (s, Pro cis-βC), 28.54 (s, Pro trans-βC), 28.06 (p, trans-Boc), 27.75 (p, cis-Boc), 24.39 (s, Pro trans-γC), 21.01 (s, Pro cis-γC), 19.01 (p, Mmp trans-CH3), 18.91 (p, Mmp cis-CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-2’-methyl-l-phenylalanylphenylalanylamide
Yield 266 mg (84.5%); mp 222–228 °C; Rf3 = 0.71; −45.81° (c = 0.36, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.05 (s, 0.45H, Dmt cis-OH), 8.86 (s, 0.43H, Dmt trans-OH), 8.33 (d, 0.44H, J = 8.22 Hz, Phe NH), 8.10 (d, 0.15H, J = 7.22 Hz, Phe NH), 8.03 (d, 0.33H, J = 7.52 Hz, Phe NH), 7.83 (d, 0.48H, J = 8.14 Hz, Mmp NH), 7.65 (d, 0.43H, J = 8.06 Hz, Mmp NH), 7.29-7.14 (m, 5.5H, Phe Ar-H, CONH2), 7.14-6.95 (m, 6.0H, Mmp Ar-H, CONH2, Dmt NH), 6.75 (d, 0.33H, J = 8.54 Hz, Dmt NH), 6.43-6.28 (m, 2.1H, Dmt Ar-H, Dmt NH), 4.48-4.23 (m, 3.0H, Pro trans-αH, Mmp αH, Phe αH, Dmt trans-αH), 4.17-4.08 (m, 0.46H, Dmt cis-αH), 3.52-3.40 (m, 0.49H, Pro trans-δH), 3.20-2.65 (m, 8.0H, Pro cis-αH, Pro cis-δH, Pro trans-δH, Phe βH, Mmp βH, Dmt βH), 2.25 (s, 3.0H, Mmp CH3), 2.16, 2.09 (2s, 6.0H, Dmt CH3), 1.96-1.62 (m, 2.53H, Pro cis-βH, Pro trans βH, Pro trans-γH), 1.45-1.07 (m, 9.50H, Boc, Pro cis-γH), 1.05-0.91 (m, 0.51H, Pro cis-βH), 0.86-0.70 (m, 0.47H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.36, 172.19, 171.42, 171.06, 170.63, 170.26, 170.14, 155.51, 155.41, 154.92, 154.65, 138.08, 137.85, 137.81, 137.53, 136.05, 136.01, 135.85 (18q), 129.79, 129.75, 129.07, 129.01, 128.89, 127.94, 126.13, 125.53, 125.50 (9t, Ar-C), 124.24, 122.95 (2q), 114.83, 114.62 (3t, Ar-C), 78.54, 77.88 (2q, Boc), 59.78 (t, Pro trans-αC), 59.14 (t, Pro cis-αC), 53.74 (t, Mmp cis-αC), 53.45 (t, Phe αC), 53.18 (t, Mmp trans-αC), 51.47 (t, Dmt αC), 46.55 (s, Pro trans-δC), 46.18 (s, Pro cis-δC), 37.89 (s, Phe cis-βC), 37.20 (s, Phe trans-βC), 34.12 (s, Mmp cis-βC), 33.48 (s, Mmp trans-βC), 29.93 (s, Pro cis-βC), 28.61 (s, Pro trans-βC), 28.18, 27.98, 27.45 (3p, Boc), 24.34 (s, Pro trans-γC), 20.99 (s, Pro cis-γC), 20.11 (p, Dmt trans-CH3), 19.43 (p, Dmt cis-CH3), 18.99, 18.94 (2p, Mmp CH3).
Nα-tert-Butyloxycarbonyltyrosylprolyl-3’,5’-dimethyl-l-phenylalanylphenylalanylamide
Yield 282 mg (91.4%); mp 126–128 °C; Rf3 = 0.67; −44.94° (c = 0.48, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.21 (s, 0.19H, Tyr cis-OH), 9.13 (s, 0.80H, Tyr trans-OH), 8.28 (d, 0.17H, J = 7.94 Hz, Phe cis-NH), 7.94 (d, 0.81H, J = 8.23 Hz, Phe trans-NH), 7.82-7.72 (m, 0.96H, 3,5Dmp NH), 7.30-6.88 (m, 9.90H, Phe Ar-H, Tyr Ar-H, Tyr NH, CONH2), 6.77 (s, 3.0H, 3,5Dmp Ar-H), 6.71-6.54 (m, 2.1H, Tyr Ar-H, Dmt NH), 4.47-4.07 (m, 3.83H, Pro trans-αH, 3,5Dmp αH, Phe αH, Tyr αH), 3.76 (d, 0.17H, J = 7.72 Hz, Pro cis-αH), 3.64-3.53 (m, 0.78H, Pro trans-δH), 3.53-3.47 (m, 1.0H, Pro trans-δH), 3.27-3.16 (m, 0.22H, Pro cis-δH), 3.10-2.52 (m, 6.0H, Phe βH, 3,5Dmp βH, Tyr βH), 2.20, 2.18 (2s, 6.0H, 3,5Dmp CH3), 2.0-1.65 (m, 3.47H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.53-1.42 (m, 0.33H, Pro cis-γH), 1.35, 1.29, 1.23 (3s, 9.0H, Boc), 1.11-0.94 (m, 0.1H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.51, 171.31, 170.89, 170.56, 170.39, 170.27, 170.19, 155.97, 155.68, 155.34, 155.25, 137.81, 137.61, 137.25, 136.76 (15q), 130.25, 130.08, 129.03, 127.95, 127.91, 127.60, 126.80, 126.64, 126.13, 114.92, 114.78 (11t, Ar-C), 78.28, 77.85 (2q, Boc), 59.56 (t, Pro αC), 54.79, 54.10, 54.06, 53.68, 53.42 (5t, 3,5Dmp αC, Phe αC, Tyr αC), 46.60 (s, Pro δC), 37.33 (s, Phe βC), 36.81 (s, 3,5Dmp βC), 35.47 (s, Tyr βC), 28.56 (s, Pro βC), 28.07 (p, trans-Boc), 27.75 (p, cis-Boc), 24.28 (s, Pro γC), 20.79 (p, 3,5Dmp trans-CH3), 20.75 (p, 3,5Dmp cis-CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-3’,5’-dimethyl-l-phenylalanylphenylalanylamide
Yield 281 mg (87.6%); mp 208–210 °C; Rf3 = 0.73; −35.02° (c = 0.31, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.06 (br, 0.47H, Dmt cis-OH), 8.89 (br, 0.39H, Dmt trans-OH), 8.17 (d, 0.47H, J = 7.83 Hz, 3,5Dmp cis-NH), 7.93-7.83 (m, 0.67H, 3,5Dmp trans-NH, Phe trans-NH), 7.74 (d, 0.81H, J = 7.98 Hz, Phe cis-NH), 7.30-6.95 (m, 7.68 H, Phe Ar-H, CONH2, Dmt NH), 6.85-6.72 (m, 3.0H, 3,5Dmp Ar-H), 6.69 (d, 0.34H, J = 8.49 Hz, Dmt NH), 6.39, 6.40 (2s, 2.0H, Dmt Ar-H), 6.24 (d, 0.10H, J = 8.52 Hz, Dmt NH), 4.48-4.19 (m, 3.0H, Pro trans-αH, 3,5Dmp αH, Phe αH, Dmt trans-αH), 4.19-4.10 (m, 0.50H, Dmt cis-αH), 3.55-3.44 (m, 0.50H, Pro trans-δH), 3.20-2.64 (m, 8.0H, Pro cis-αH, Pro trans-δH, Pro cis-δH, Phe βH, 3,5Dmp βH, Dmt βH), 2.21, 2.19 (2s, 3,5Dmp CH3), 2.17, 2.09 (2s, Dmt CH3), 1.96-1.83 (m, 0.50H, Pro trans-βH), 1.83-1.60 (m, 2.0H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.46-1.06 (m, 9.54H, Boc, Pro cis-γH), 1.06-0.92 (m, 0.47H, Pro cis-βH), 0.92-0.77 (m, 0.49H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.46, 172.25, 171.22, 171.08, 170.71, 170.40, 170.22, 170.08, 155.51, 155.43, 154.96, 154.62, 138.06, 137.85, 137.76, 137.51, 137.39, 136.79, 136.78 (19q), 129.04, 127.95, 127.92, 127.57, 127.48, 126.70, 126.61, 126.13 (8t, Ar-C), 124.25, 122.99 (2q), 114.84, 114.66 (2t, Ar-C), 78.46, 77.89 (2q, Boc), 59.78 (t, Pro trans-αC), 59.11 (t, Pro cis-αC), 55.02 (t, 3,5Dmp trans-αC), 54.22 (t, 3,5Dmp cis-αC), 53.72 (t, Phe cis-αC), 53.43 (t, Phe trans-αC), 51.44 (t, Dmt cis-αC), 51.35 (t, Dmt trans-αC), 46.51 (s, Pro trans-δC), 45.94 (s, Pro cis-δC), 37.94 (s, Phe cis-βC), 37.56 (s, Phe trans-βC), 36.61 (s, 3,5Dmp cis-βC), 36.00 (s, 3,5Dmp trans-βC), 31.26 (s, Dmt cis-βC), 30.82 (s, Dmt trans-βC), 30.03 (s, Pro cis-βC), 28.63 (s, Pro trans-βC), 28.19, 27.97, 27.42 (3p, Boc), 24.18 (s, Pro trans-γC), 20.94 (s, Pro cis-γC), 20.78 (p, 3,5Dmp, cis-CH3), 20.76 (p, 3,5Dmp trans-CH3), 20.13 (p, Dmt trans-CH3), 19.39 (p, Dmt cis-CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-2’,6’-dimethyl-l-phenylalanylphenylalanylamide
Yield 284 mg (84.9%); mp 145–146 °C; Rf3 = 0.88; −39.32° (c = 0.52, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.05, 8.86 (2s, 1H, Dmt OH), 8.35 (d, 0.45H, Dmp NH), 8.10-7.94 (m, 0.55H, Dmp NH), 7.88-7.55 (m, 0.40H, Phe NH), 7.70-7.54 (m, 0.60H, Phe NH), 7.40-6.61 (m, 10.9H, Phe Ar-H, Dmp Ar-H, CONH2, Dmt NH), 6.50-6.22 (m, 2.1H, Dmt Ar-H, Dmt NH), 4.65-4.28 (m, 2.80H, Pro trans-αH, Phe αH, Dmp αH, Dmt trans-αH), 4.28-3.98 (m, 0.53H, Dmt cis-αH), 3.57-3.44 (m, 0.47H, Pro trans-δH), 3.28-2.63 (m, 8.20H, Pro cis-αH, Pro trans-δH, Pro cis-δH, Phe βH, Dmp βH, Dmt βH), 2.42-1.98 (m, 12.0H, Dmt CH3, Dmp CH3), 1.98-1.54 (m, 2.54H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.54-1.00 (m, 9.75H, Boc, Pro cis-γH), 1.00-0.82 (m, 0.41H, Pro cis-βH), 0.73-0.53 (m, 0.32H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.21, 172.09, 171.16, 170.97, 170.70, 170.12, 169.98, 155.60, 155.42, 154.92, 138.11, 137.88, 137.82, 137.56, 136.78, 136.54, 134.57, 134.11 (18q), 129.12, 129.00, 128.95, 127.96, 127.91, 127.82, 126.13, 126.07, 125.98, 125.90, 114.83, 114.63 (12t, Ar-C), 78.64, 77.90 (2q, Boc), 59.65 (t, Pro trans-αC), 59.30 (t, Pro cis-αC), 53.82 (t, Phe trans-αC), 53.53 (t, Phe cis-αC), 53.05 (t, Dmp trans-αC), 52.43 (t, Dmp cis-αC), 51.47 (t, Dmt αC), 46.53 (s, Pro trans-δC), 46.34 (s, Pro cis-δC), 37.93 (s, Phe trans-βC), 37.17 (s, Phe cis-βC), 31.89 (s, Dmp cis-βC), 31.40 (s, Dmp trans-βC), 30.93 (s, Dmt trans-βC), 30.65 (s, Dmt cis-βC), 29.66 (s, Pro cis-βC), 28.66 (s, Pro trans-βC), 28.17, 28.00, 27.44 (3p, Boc), 24.35 (s, Pro trans-γC), 20.90 (s, Pro cis-γC), 20.11, 19.84, 19.53 (3p, Dmt CH3, Dmp CH3).
Nα-tert-Butyloxycarbonyltyrosylprolyl-2’,6’-dimethyl-l-tyrosylphenylalanylamide
Yield 307 mg (97.2%); mp 154–156 °C; Rf3 = 0.60; −43.85° (c = 0.33, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.21, 9.15 (2s, 0.96H, Tyr OH), 8.89, 8.84 (2s, 0.95H, Dmt OH), 8.44 (d, 0.21H, J = 8.88 Hz, Dmt cis-NH), 7.91 (d, 0.76H, J = 7.83 Hz, Dmt trans-NH), 7.79 (d, 0.77H, J = 8.10 Hz, Phe trans-NH), 7.69 (d, 0.22H, J = 8.07 Hz, Phe cis-NH), 7.57-7.12 (m, 2.15H, Tyr Ar-H, Tyr NH), 7.10-6.83 (m, 4.84H, Tyr Ar-H, Tyr NH, CONH2), 6.79-6.53 (m, 2.15H, Tyr Ar-H, Tyr NH), 6.35-6.33 (m, 2.0H, Dmt Ar-H), 4.52-4.12 (m, 3.79H, Pro trans-αH, Tyr αH, Phe αH, Dmt αH), 3.88 (d, 0.21H, J = 7.66 Hz, Pro cis-αH), 3.66-3.55 (m, 0.65H, Pro trans-δ), 3.55-3.38 (m, 0.85H, Pro trans-δH), 3.24-3.10 (m, 0.50H, Pro cis-δH), 3.10-2.94 (m, 1.24H, Phe trans-βH, Dmt trans-βH), 2.94-2.54 (m, 4.73H, Phe trans-βH, Phe cis-βH, Tyr βH, Dmt trans-βH), 2.14 (s, 6.0H, Dmt CH3), 2.01-1.88 (m, 0.76H, Pro trans-βH), 1.88-1.77 (m, 1.55H, Pro trans-βH), 1.77-1.64 (m, 0.97H, Pro trans-βH, Pro cis-βH), 1.50-1.40 (m, 0.29H, Pro cis-γH), 1.31, 1.24 (2s, 9.0H, Boc), 1.20-1.06 (m, 0.37H, Pro cis-βH), 0.98-1.83 (m, 0.16H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.34, 172.26, 171.13, 170.93, 170.79, 170.42, 170.18, 155.97, 155.68, 155.46, 155.24, 154.99, 154.90, 137.85, 137.66 (15q), 130.39, 130.11, 129.02, 128.97, 127.91, 126.36, 126.07 (7t, Ar-C), 124.82, 124.72 (2q), 114.86, 114.77, 114.69 (3t, Ar-C), 78.41, 77.87 (2q, Boc), 59.45 (t, Pro αC), 54.12 (t, Tyr αC), 53.81 (t, Phe αC), 53.54 (t, Dmt trans-αC), 52.62 (t, Dmt cis-αC), 46.62 (s, Pro trans-δC), 46.20 (s, Pro cis-δC), 37.15 (s, Phe trans-βC), 36.82 (s, Phe cis-βC), 35.56 (s, Tyr βC), 31.24 (s, Dmt trans-βC), 30.24 (s, Dmt cis-βC), 29.79 (s, Pro cis-βC), 28.58 (s, Pro trans-βC), 28.08 (p, trans-Boc), 27.77 (p, cis-Boc), 24.42 (s, Pro trans-γC), 21.05 (s, Pro cis-γC), 20.00 (p, Dmt, trans-CH3), 19.96 (p, Dmt cis-CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-2’,6’-dimthyl-l-tyrosylphenylalanylamide
Yield 257 mg (80.3%); mp 160–162 °C; Rf3 = 0.63; −34.23° (c = 0.53, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.06, 8.87 (2br, 2H, Dmt OH), 8.24 (d, 0.48H, J = 8.25 Hz, Dmt3 NH), 8.03-7.87 (m, 0.52H, Dmt3 NH), 7.82-7.70 (m, 0.49H, Phe NH), 7.53 (d, 0.51H, J = 7.66 Hz, Phe NH), 7.34-6.81 (m, 7.7H, Phe Ar-H, CONH2, Dmt1 NH), 6.73 (d, 0.30H, J =8.13 Hz, Dmt1 NH), 6.48-6.30 (m, 4.0H, Dmt Ar-H), 4.53-4.03 (m, 3.40H, Pro trans-αH, Dmt3 αH, Phe αH, Dmt1 αH), 3.56-3.43 (m, 0.40H, Pro trans-δH), 3.28-3.17 (m, 0.60H, Pro cis-αH), 3.17-2.62 (m, 7.60H, Pro trans-δH, Pro cis-δH, Phe βH, Dmt3 βH, Dmt1 βH), 2.30-1.98 (m, 12.0H, Dmt CH3), 1.95-1.83 (m, 0.40H, Pro trans-βH), 1.83-1.57 (m, 2.0H, Pro cis-βH, Pro trans-γH), 1.47-1.04 (m, 9.65H, Boc, Pro cis-γH), 1.04-0.88 (m, 0.51H, Pro cis-βH), 0.84-0.65 (m, 0.45H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.26, 172.07, 171.25, 170.96, 170.91, 170.58, 170.29, 170.00, 155.64, 155.44, 154.98, 154.95, 154.65, 138.12, 137.87, 137.81, 137.53 (19q), 129.01, 128.96, 127.95, 127.90, 126.12, 126.05 (6t, Phe Ar-C), 124.80, 122.93 (2q), 114.84, 114.66 (2t, Dmt Ar-C), 78.63, 77.89 (2q, Boc), 59.70 (t, Pro trans-αC), 59.26 (t, Pro cis-αC), 53.84 (t, Dmt3 cis-αC), 53.56 (t, Dmt3 trans-αC), 53.48 (t, Phe cis-αC), 53.14 (t, Phe trans-αC), 51.50 (t, Dmt1 cis-αC), 51.41 (t, Dmt1 trans-αC), 46.48 (s, Pro trans-δC), 46.39 (t, Pro cis-δC), 38.01 (s, Phe cis-βC), 37.17 (s, Phe trans-βC), 31.31 (s, Dmt3 trans-βC), 31.19 (s, Dmt3 cis-βC), 30.95 (s, Dmt1 trans-βC), 29.98 (s, Dmt1 cis-βC), 29.80 (s, Pro cis-βC), 28.65 (s, Pro trans-βC), 28.19, 27.99, 27.47 (3p, Boc), 24.35 (s, Pro trans-γC), 20.95 (s, Pro cis-γC), 20.11, 19.99, 19.51 (3p, Dmt CH3).
Nα-tert-Butyloxycarbonyltyrosylprolyl-2’,4’,6’-trimethyl-l-phenylalanylphenylalanylamide
Yield 237 mg (75.3%); mp 215–217 °C; Rf3 = 0.76; −46.69° (c = 0.35, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.20 (s, 0.23H, Dmt cis-OH), 9.15 (s, 0.72H, Dmt trans-OH), 8.50 (s, 0.20H, J = 8.93 Hz, Phe cis-NH), 7.96 (d, 0.75H, J = 7.87 Hz, Phe trans-NH), 8.22 (d, 0.74H, J = 8.22 Hz, Tmp trans-NH), 7.75 (d, 0.22H, J = 8.16 Hz, Tmp cis-NH), 7.27-7.12 (m, 5.0H, Phe Ar-H), 7.10-6.97 (m, 3.2H, Tyr Ar-H, Tyr-NH), 6.94 (d, 0.70H, J = 8.17 Hz, Tyr NH), 6.80 (br, 0.24H, cis-CONH2), 6.76-6.55 (m, 4.8H, Tmp Ar-H, Tyr Ar-H, CONH2, Tyr NH), 4.58-4.49 (m, 0.23H, Phe cis-αH), 4.43-4.20 (m, 3.38H, Pro tans-αH, Tyr trans-αH, Tmp αH, Phe trans-αH), 4.38-4.10 (m, 0.13H, Tyr cis-αH), 3.92 (d, 0.21H, J = 7.67 Hz, Pro cis-αH), 3.65-3.54 (m, 0.70H, Pro trans-δH), 3.54-3.37 (m, 0.80H, Pro trans-δH), 3.20-3.10 (m, 0.50H, Pro cis-δH), 3.10-2.85 (m, 2.3H, Phe βH, Tmp βH), 2.85-2.53 (m, 3.7H, Phe βH, Tyr βH, Tmp βH), 2.28-2.08 (m, 9.0H, Tmp CH3), 2.0-1.87 (m, 0.71H, Pro trans-βH), 1.87-1.76 (m, 1.56H, Pro trans-γH), 1.76-1.63 (m, 1.0H, Pro cis-βH, Pro trans-βH), 1.48-1.37 (m, 0.23H, Pro cis-γH), 1.30, 1.24 (2s, 9.0H, Boc), 1.20-1.10 (m, 0.29H, Pro cis-βH), 0.97-0.82 (m, 0.17H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.33, 172.24, 171.12, 170.77, 170.23, 170.32, 170.16, 155.95, 155.68, 155.36, 155.23, 137.88, 137.73, 136.59, 136.44, 134.69, 134.54, 131.21 (18q), 130.39, 130.11, 128.99, 128.94, 128.57, 128.48, 127.90, 126.37, 126.05, 114.77 (10t, Ar-C), 78.34, 77.86 (2q, Boc), 59.37 (t, Pro αC), 54.10 (t, Tyr αC), 53.74 (t, Tmp trans-αC), 53.53 (t, Tmp cis-αC), 43.14 (t, Phe trans-αC), 52.16 (t, Phe cis-αC), 46.61 (s, Pro trans-δC), 46.21 (s, Pro cis-δC), 37.42 (s, Phe cis-βC), 37.10 (s, Phe trans-βC), 36.88 (s, Tyr cis-βC), 35.55 (s, Tyr trans-βC), 31.61 (s, Tmp trans-βC), 30.72 (s, Tmp cis-βC), 29.87 (s, Pro cis-βC), 28.58 (s, Pro trans-βC), 28.08 (p, trans-Boc), 27.78 (p, cis-Boc), 24.42 (s, Pro trans-γC), 20.95 (s, Pro cis-γC), 20.36, 20.32, 19.75, 19.71 (4p, Tmp CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-2’,4’,6’-trimethyl-l-phenylalanylphenylalanylamide
Yield 250 mg (76.4%); mp 148–150 °C; Rf3 = 0.80; −35.74° (c = 0.35, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.05 (br, 0.51H, Dmt cis-OH), 8.86 (br, 0.43H, Dmt trans-OH), 8.31 (d, 0.48H, J = 8.76 Hz, Phe NH), 8.06-7.93 (m, 0.51H, Phe NH), 7.86-7.76 (m, 0.49H, Tmp NH), 7.60 (d, 0.49H, J = 8.14 Hz, Tmp NH), 7.28-7.10 (m, 5.59H, Phe Ar-H, Dmt NH, CONH2), 7.06, 6.98 (2s, 1.41H, CONH2), 6.80-6.65 (m, 2.84H, Tmp Ar-H, Dmt NH, CONH2), 6.45-6.28 (m, 2.15H, Dmt Ar-H, Dmt NH), 4.50-4.22 (m, 3.0H, Pro trans-αH, Tmp αH, Phe αH, Dmt trans-αH), 4.16-4.06 (m, 0.49H, Phe cis-αH), 3.55-3.43 (m, 0.56H, Pro trans-δH), 3.27-2.66 (m, 8.55H, Pro cis-αH, Pro cis-δH, Phe βH, Tmp βH, Dmt βH), 2.28-2.05 (m, 15.0H, Dmt CH3, Tmp CH3), 1.95-1.58 (m, 2.74H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.14-1.08 (m, 9.47H, Boc, Pro cis-γH), 1.05-0.9 (m, 0.42H, Pro cis-βH), 0.78-0.63 (m, 0.42H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.25, 172.10, 171.16, 170.95, 170.76, 170.54, 170.20, 169.98, 155.53, 155.41, 154.92, 154.65, 138.11, 137.86, 137.57, 136.57, 136.32, 134.67, 134.60, 131.42, 131.27 (21q), 128.99, 128.95, 128.55, 128.50, 127.95, 127.90, 126.11, 126.06 (8t, Ar-C), 124.22, 122.95 (2q), 114.83, 114.63 (2t, Ar-C), 78.60, 77.90 (2q, Boc), 59.64 (t, Pro trans-αC), 59.23 (t, Pro cis-αC), 53.78, 53.49 (2t, Tmp αC), 53.20, 52.68 (2t, Phe αC), 51.45 (t, Dmt αC), 46.53 (t, Pro trans-δC), 46.40 (t, Pro cis-δC), 37.90 (s, Phe cis-βC), 37.12 (s, Phe trans-βC), 31.59 (s, Tmp cis-βC), 31.46 (s, Tmp trans-βC), 30.97 (s, Dmt trans-βC), 30.41 (s, Dmt cis-βC), 29.81 (s, Pro cis-βC), 28.66 (s, Pro trans-βC), 28.17, 28.00, 27.47 (3p, Boc), 24.36 (s, Pro trans-γC), 20.87 (s, Pro cis-γC), 20.36, 20.31 (2p, Tmp CH3), 20.11 (p, Dmt trans-CH3), 19.75 (p, Tmp CH3), 19.52 (p, Dmt cis-CH3).
Nα-tert-Butyloxycarbonyltyrosylprolyl-2’-ethyl-6’-methyl-l-phenylalanylphenylalanylamide
Yield 242 mg (76.7%); mp 222–224 °C; Rf3 = 0.66; −48.52° (c = 0.34, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.20 (s, 0.26H, Tyr cis-OH), 9.15 (s, 0.73H, Tyr trans-OH), 8.50 (d, 0.23H, J = 8.97 Hz, Phe cis-NH), 7.99 (d, 0.75H, J = 7.87 Hz, Phe trans-NH), 7.81 (d, 0.75H, J = 8.13 Hz, Emp trans-NH), 7.73 (d, 0.24H, J = 8.10 Hz, Emp cis-NH), 7.27-7.13 (m, 5.0H, Phe Ar-H), 7.10-6.88 (m, 7.12H, Emp Ar-H, Tyr Ar-H, CONH2), 6.82-6.72 (m, 0.7H, CONH2), 6.70-6.57 (m, 2.10H, Tyr Ar-H, Tyr NH), 4.60-4.52 (m, 0.26H, Phe cis-αH), 4.46-4.10 (m, 3.5H, Pro trans-αH, Phe trans-αH, Tyr αH, Emp αH), 3.90 (d, 0.24H, J = 7.62 Hz, Pro cis-αH), 3.66-3.60 (m, 0.62H, Pro trans-δH), 3.40-3.38 (m, 0.77H, Pro trans-δH), 3.23-3.08 (m, 0.62H, Pro cis-δH), 3.08-2.53 (m, 8.0H, Phe βH, Tyr βH, Emp βH, Emp CH2CH3), 2.26 (s, 3.0H, Emp Ar-CH3), 2.02-1.88 (m, 0.75H, Pro trans-βH), 1.88-1.77 (m, 1.5H, Pro trans-γH), 1.77-1.63 (m, 1.0H, Pro trans-βH, Pro cis-βH), 1.47-1.20 (m, 9.35H, Pro cis-γH, Boc), 1.20-1.02 (m, 3.25H, Pro cis-βH, Emp CH2CH3), 0.9-0.75 (m, 0.15H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.33, 172.18, 171.21, 170.79, 170.61, 170.21, 170.14, 155.96, 155.69, 155.46, 155.24, 142.80, 142.69, 137.84, 137.67, 136.89, 136.75, 133.76, 133.59 (19q), 130.40, 130.10, 129.01, 127.91, 127.79, 126.37, 126.27, 126.07, 126.00, 114.83, 114.78 (11t, Ar-C), 78.39, 77.86 (2q, Boc), 59.43 (t, Pro αC), 54.13 (t, Tyr αC), 53.77 (t, Emp αC), 53.62 (t, Phe trans-αC), 52.67 (t, Phe cis-αC), 46.62 (s, Pro trans-δC), 46.14 (s, Pro cis-δC), 37.54 (s, Phe cis-βC), 37.14 (s, Phe trans-βC), 36.80 (s, Tyr cis-βC), 35.56 (s, Tyr trans-βC), 31.06 (s, Emp trans-βC), 30.13 (s, Emp cis-βC), 29.76 (s, Pro cis-βC), 28.58 (s, Pro trans-βC), 28.06, 27.76 (2p, Boc), 25.18 (s, Emp CH2CH3), 24.43 (s, Pro trans-γC), 21.00 (s, Pro cis-γC), 19.95 (p, Emp trans-Ar-CH3), 19.91 (p, Emp cis-Ar-CH3), 15.56 (p, Emp CH2CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-2’-ethyl-6’-methyl-l-phenylalanylphenylalanylamide
Yield 280 mg (85.6%); mp 135–137 °C; Rf3 = 0.75; −38.20° (c = 0.39, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.06 (br, 0.44H, Dmt cis-OH), 8.87 (br, 0.36H, Dmt trans-OH), 8.30 (d, 0.50H, J = 8.77 Hz, Phe NH), 8.09-7.97 (m, 0.50H, Phe NH), 7.86-7.74 (m, 0.50H, Emp NH), 7.56 (d, 0.50H, J = 8.10 Hz, Emp NH), 7.30-7.10 (m, 5.70H, Phe Ar-H, CONH2, Dmt NH), 7.10-6.87 (m, 4.41H, Emp Ar-H, CONH2), 6.84-6.68 (m, 0.73H, CONH2, Dmt NH), 6.47-6.27 (m, 2.1H, Dmt Ar-H, Dmt NH), 4.50-4.23 (m, 3.0H, Pro trans-αH, Tmp αH, Phe αH, Dmt cis-αH), 4.16-4.04 (m, 0.50H, Dmt cis-αH), 3.23 (d, 0.50H, J = 7.37 Hz, Pro cis-αH), 3.17-2.52 (m, 10.0H, Pro δH, Phe βH, Emp βH, Dmt βH, Emp CH2CH3), 2.25 (s, 3.0H, Emp Ar-CH3), 2.15, 2.11 (2s, 6.0H, Dmt CH3), 1.97-1.84 (m, 0.49H, Pro trans-βH), 1.84-1.58 (m, 2.1H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.47-1.10 (m, 9.67H, Dmt CH3, Emp Ar-CH3, Pro cis-γH), 1.10-1.00 (m, 3.0H, Emp CH2CH3), 1.10-0.84 (m, 0.42H, Pro cis-βH), 0.70-0.53 (m, 0.38H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.17, 172.05, 171.13, 171.02, 170.59, 170.02, 155.63, 155.43, 154.90, 154.65, 142.77, 142.58, 138.11, 137.86, 137.81, 137.51, 136.86, 136.59, 133.74, 133.62 (20q), 128.90, 128.96, 127.96, 127.89, 127.76, 126.24, 126.14, 126.07, 125.96 (9t, Ar-C), 124.21, 122.93 (2q), 114.83, 114.61 (2t, Ar-C), 78.65, 77.89 (2q, Boc), 59.68 (t, Pro trans-αC), 59.26 (t, Pro cis-αC), 53.80 (t, Tmp cis-αC), 53.66 (t, Tmp trans-αC), 53.50 (t, Phe cis-αC), 53.09 (s, Phe trans-αC), 51.48 (t, Dmt αC), 46.54 (s, Pro trans-δC), 46.31 (s, Pro cis-δC), 38.00 (s, Phe cis-βC), 37.16 (s, Phe trans-βC), 31.32 (s, Emp cis-βC), 31.11 (s, Emp trans-βC), 30.09 (s, Dmt trans-βC), 29.77 (s, Dmt cis-βC), 29.71 (s, Pro cis-βC), 28.68 (s, Pro trans-βC), 28.16, 27.99, 27.46 (3p, Boc), 25.20 (s, Emp cis-CH2CH3), 25.14 (s, Emp trans-CH2CH3), 24.37 (s, Pro trans-γC), 20.88 (s, Pro cis-γC), 20.10 (p, Emp trans-Ar-CH3), 19.96 (p, Emp cis-Ar-CH3), 19.95 (p, Dmt trans-CH3), 19.50 (p, Dmt cis-CH3), 15.58 (p, Emp CH2CH3).
Nα-tert-Butyloxycarbonyltyrosylprolyl-2’-isopropyl-6’-methyl-l-phenylalanylphenylalanylamid
Yield 269 mg (83.8%); mp 222–224 °C; Rf3 = 0.62; −45.51° (c = 0.31, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.21 (s, 0.21H, Tyr cis-OH), 9.15 (s, 0.76H, Tyr trans-OH), 8.46 (d, 0.21H, J = 8.89 Hz, Phe cis-NH), 7.84 (d, 0.76H, J = 7.84 Hz, Phe trans-NH), 7.73 (d, 0.77H, J = 8.12 Hz, Imp trans-NH), 7.66 (d, 0.21H, J = 8.07 Hz, Imp cis-NH), 7.30-7.10 (m, 5.0H, Phe Ar-H), 7.10-6.83 (m, 7.9H, CONH2, Imp Ar-H, Tyr Ar-H, Dmt NH), 6.68-6.56 (m, 2.1H, Tyr Ar-H, Dmt NH), 4.58-4.47 (m, 0.21H, Phe cis-αH), 4.45-4.10 (m, 3.60H, Pro trans-αH, Imp αH, Tyr αH, Phe trans-αH), 3.90 (d, 0.19H, J = 7.67 Hz, Pro cis-αH), 3.67-3.50 (m, 0.65H, Pro trans-δH), 3.55-3.40 (m, 0.73H, Pro trans-δH), 3.28-3.11 (m, 1.62H, Pro cis-δH, CH(CH3)2), 3.11-2.92 (m, 2.0H, Phe βH, Imp βH), 2.92-2.67 (m, 3.0H, Phe βH, Tyr βH, Imp βH), 2.67-2.55 (m, 0.82H, Tyr trans-αH), 2.25 (s, 3.0H, Imp Ar-CH3), 2.05-1.90 (m, 0.81H, Pro trans-βH), 1.90-1.77 (m, 1.63H, Pro trans-γH), 1.77-1.63 (m, 0.96H, Pro trans-βH), 1.47-1.36 (m, 0.18H, Pro cis-γH), 1.36-1.02 (m, 15.23H, Pro cis-βH, Boc, Imp CH(CH3)2), 0.90-0.88 (m, 0.18H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.27, 172.20, 171.39, 170.75, 170.51, 170.27, 170.02, 155.96, 155.67, 155.47, 155.25, 147.38, 137.76, 137.57, 136.68, 132.99 (16q), 130.38, 130.06, 129.07, 129.00, 127.90, 127.51, 126.39, 126.09, 122.79, 114.77 (10t, Ar-C), 78.38, 77.86 (2q, Boc), 59.50 (t, Pro αC), 54.32 (t, Imp αC), 54.17 (t, Tyr αC), 53.71 (t, Phe trans-αC), 53.46 (t, Phe cis-αC), 46.62 (s, Pro trans-δC), 46.14 (s, Pro cis-δC), 37.61 (s, Pro cis-βC), 37.24 (s, Pro trans-βC), 36.74 (s, Tyr cis-βC), 35.53 (s, Tyr trans-βC), 30.48 (s, Imp trans-βC), 29.88 (s, Imp cis-βC), 29.74 (s, Pro cis-βC), 28.66 (s, Pro trans-βC), 28.07 (p, trans-Boc), 27.95 (t, CH(CH3)2), 27.73 (p, cis-Boc), 24.43 (s, Pro trans-γC), 24.31, 23.76, 23.65 (3p, CH(CH3)2), 21.00 (s, Pro cis-γC), 20.46 (p, Imp cis-Ar-CH3), 20.23 (p, Imp trans-Ar-CH3).
Nα-tert-Butyloxycarbonyl-2’,6’-dimethyl-l-tyrosylprolyl-2’-isopropyl-6’-methyl-l-phenylalanylphenylalanylamide
Yield 252 mg (75.6%); mp 143–145 °C; Rf3 = 0.67; −35.88° (c = 0.44, MeOH). 1H NMR (400.1 MHz, DMSO-d6) δ: 9.06 (br, 0.35H, Dmt cis-OH), 8.86 (br, 0.37H, Dmt trans-OH), 8.25 (d, 0.37H, J = 8.71 Hz, Phe cis-NH), 8.17-7.98 (m, 0.47H, Phe trans-NH), 7.77-7.53 (m, 0.47H, Imp trans-NH), 7.50 (d, 0.38H, J = 8.09 Hz, Imp cis-NH), 7.30-7.12 (m, 5.5H, Phe Ar-H, Dmt NH), 7.12-6.86 (m, 5.0H, Imp Ar-H, CONH2), 6.75 (d, 0.40H, J = 8.46 Hz, Dmt cis-NH), 6.44-6.30 (m, 2.1H, Dmt Ar-H, Dmt NH), 4.50-4.25 (m, 2.46H, Pro trans-αH, Phe trans-αH, Imp αH, Dmt trans-αH), 4.25-4.17 (m, 0.51H, Phe cis-αH), 4.15-4.07 (m, 0.45H, Dmt cis-αH), 3.56-3.42 (m, 0.56H, Pro cis-δH), 3.26-2.62 (m, 9.0H, Pro cis-αH, Pro trans-δH, Pro cis-δH, Phe βH, Dmt βH, Imp βH, Imp CH(CH3)2), 2.36-2.00 (m, 9.0H, Imp Ar-CH3, Dmt Ar-CH3), 2.00-1.85 (m, 0.59H, Pro trans-βH), 1.85-1.57 (m, 2.0H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.45-1.00 (m, 15.52H, Boc, Imp CH(CH3)2, Pro cis-γH), 1.00-0.85 (m, 0.51H, Pro cis-βH), 0.70-0.55 (m, 0.38H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.32, 172.01, 171.22, 170.50, 170.10, 169.94, 155.63, 155.45, 154.90, 154.68, 147.37, 147.12, 138.11, 137.85, 137.75, 137.44, 136.64, 136.53, 132.95, 132.85 (20q), 129.08, 128.99, 127.96, 127.89, 127.50, 127.40, 126.37, 126.30, 126.15, 126.11 (10t, Ar-C), 124.20 (q), 122.92, 122.79, 114.83, 114.61 (4t, Ar-C), 78.63, 77.91 (2q, Boc), 59.77 (t, Pro trans-αC), 59.24 (t, Pro cis-αC), 54.36 (t, Phe cis-αC), 53.84 (t, Phe trans-αC), 53.76 (t, Imp trans-αC), 53.45 (t, Imp cis-αC), 51.53 (t, Dmt cis-αC), 51.49 (t, Dmt, trans-αC), 46.55 (s, Pro cis-δC), 46.35 (s, Pro trans-δC), 38.04 (s, Phe cis-βC), 37.24 (s, Phe trans-βC), 31.30 (s, Dmt cis-βC), 30.85 (s, Dmt trans-βC), 30.49 (s, Imp trans-βC), 29.82 (s, Pro cis-βC), 29.40 (s, Imp cis-βC), 28.73 (s, Pro trans-βC), 28.17 (p, Boc), 27.98 (p, Boc; t, CH(CH3)2), 27.45 (p, Boc), 24.37 (s, Pro trans-γC), 24.33, 23.78, 23.70 (3p, CH(CH3)2), 20.89 (s, Pro cis-γC), 20.58 (p, Imp cis-Ar-CH3), 20.21 (p, Imp trans-Ar-CH3), 20.07 (p, Dmt cis-Ar-CH3), 19.49 (p, Dmt trans-Ar-CH3).
General Procedure for Synthesis of H-Tyr/Dmt-Pro-Xaa-Phe-NH2·HCl (Xaa = Mmp, 3,5Dmp, Dmp, Dmt, Tmp, Emp, Imp)
Boc-Tyr/Dmt-Pro-Xaa-Phe-NH2 (0.25 mmol) was treated with TFA (0.6 mL, 7.79 mmol) and anisole (40 µL) for 1 h at room temperature. The reaction solution was diluted with hexane, the solid was collected by filtration, dried over KOH pellets and purified by semi-preparative RP-HPLC. The purified peptide was lyophilized from water (3 × 10 mL) containing 1 mol/L HCl (0.25 mL, 0.25 mmol) to give amorphous powder. Elemental analyses of H-Tyr/Dmt-Pro-Xaa-Phe-NH2 are summarized in Supporting Information (Table 4).
Tyrosylprolyl-2’-methyl-l-phenylalanylphenylalanylamide Hydrochloride (2)
Yield 136.5 mg (83.7%); mp 170–172 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.51 (s, 0.36H, Tyr cis-OH), 9.41 (s, 0.64H, Tyr trans-OH), 8.49-8.43 (m, 0.36H, Phe cis-NH), 8.43-8.07 (m, 4.0H, NH3+, Phe trans-NH, Mmp cis-NH), 8.07-7.98 (m, 0.64H, Mmp trans-NH), 7.47-7.40 (m, 0.36H, cis-CONH2), 7.34-7.02 (m, 11.90H, Ar-H, CONH2), 6.95, 6.93 (2s, 0.75H, Ar-H), 6.74-6.67 (m, 2.0H, Ar-H), 4.50-4.33 (m, 2.66H, Pro trans-αH, Mmp αH, Phe αH), 4.15 (t, 0.64H, J = 6.40 Hz, Tyr trans-αH), 3.60-3.48 (m, 1.36H, Pro cis-αH, Tyr cis-αH, Pro trans-δH), 3.35-3.20 (m, 0.72H, Pro cis-δH), 3.10-2.77 (m, 6.64H, Pro trans-δH, Phe βH, Tyr βH, Mmp βH), 2.29, 2.26 (2s, 3.0H, Mmp CH3), 2.0-1.88 (m, 0.64H, Pro trans-βH), 1.82-1.56 (m, 2.28H, Pro cis-βH, Pro trans-βH, Pro γH), 1.56-1.38 (m, 0.72H, Pro cis-βH, Pro cis-γH), 1.34-1.18 (m, 0.36H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ:172.95, 172.38, 170.66, 170.60, 169.99, 167.00, 166.85, 156.67, 156.45, 137.74, 136.06, 135.99, 135.68, 135.60 (14q), 130.75, 130.26, 129.86, 129.75, 129.25, 129.16, 129.12, 127.91, 127.83, 126.37, 126.20, 126.10, 125.58, 125.45 (14t), 124.51, 123.94 (2q), 115.34, 115.18 (2t), 59.44 (t, Pro trans-αC), 59.12 (t, Pro cis-αC), 53.67 (t, Mmp αC), 53.20 (t, Phe αC), 52.36 (t, Tyr trans-αC), 52.31 (t, Tyr cis-αC), 46.70 (s, Pro trans-δC), 46.44 (s, Pro cis-δC), 37.45 (s, Phe cis-βC), 37.24 (s, Phe trans-βC), 36.21 (s, Tyr cis-βC), 35.21 (s, Tyr trans-βC), 34.68 (s, Mmp trans-βC), 34.25 (s, Mmp cis-βC), 31.04 (s, Pro cis-βC), 28.25 (s, Pro trans-βC), 24.32 (s, Pro trans-γC), 21.23 (s, Pro cis-γC), 19.03 (p, Mmp trans-CH3), 18.95 (p, Mmp cis-CH3).
2’,6’-Dimethyl-l-tyrosylprolyl-2’-methyl-l-phenylalanylphenylalanylamide Hodrochloride (2’)
Yield 153.8 mg (93.9%); mp 180–182 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.30 (s, 0.7H, Dmt cis-OH), 9.15 (s, 0.3H, Dmt trans-OH), 8.53 (br, 3.0H, NH3+), 8.30-8.20 (m, Mmp NH, Phe cis-NH), 7.94 (d, 0.3H, J = 8.22 Hz, Phe trans-NH), 7.62 (s, 0.7H, cis-CONH2), 7.34-7.00 (m, 10.3H, CONH2, Ar-H), 6.43 (s, 2.0H, Dmt Ar-H), 4.51 (dt, 0.7H, J = 4.77, 8.98 Hz, Phe cis-αH), 4.47-4.32 (m, 1.6H, Pro trans-αH, Mmp αH, Phe trans-αH), 4.10 (dd, 0.3H, J = 5.23, 10.08 Hz, Dmt trans-αH), 3.61 (dd, 0.7H, J = 4.49, 10.82 Hz, Dmt cis-αH), 3.38-3.24 (m, 1.0H, Pro cis-δH, Pro trans-δH), 3.24-3.13 (m, 0.7H, Pro cis-δH), 3.08-2.75 (m, 6.7H, Pro cis-αH, Phe βH, Mmp βH, Dmt βH), 2.35-2.20 (m, 3.3H, Pro trans-δH, Mmp Ar-CH3), 2.17, 2.05 (2s, 6.0H, Dmt Ar-CH3), 1.87-1.74 (m, 0.3H, Pro trans-βH), 1.67-1.50 (m, 1.7H, Pro cis-βH, Pro trans-βH, Pro trans-γH), 1.50-1.37 (m, 0.7H, Pro cis-γH), 1.25-1.0 (m, 1.3H, Pro cis-βH, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 173.13, 172.36, 170.76, 170.55, 170.33, 169.91, 167.83, 167.28, 155.93, 155.62, 138.43, 138.15, 137.68, 137.64, 135.97, 135.84, 135.77, 135.70 (18q), 129.87, 129.71, 129.32, 129.11, 129.01, 128.72, 127.89, 127.81, 126.29, 126.12, 125.59, 125.48 (12t, Ar-C), 121.38, 120.98 (2q), 115.05, 114.94 (2t, Ar-C), 59.63 (t, Pro trans-αC), 59.04 (t, Pro, cis-αC), 53.71 (t, Mmp cis-αC), 53.54 (t, Phe αC), 53.06 (t, Mmp trans-αC), 49.74 (t, Dmt αC), 46.61 (s, Pro cis-δC), 46.12 (s, Pro trans-δC), 37.59 (s, Phe cis-βC), 37.29 (s, Phe trans-βC), 34.78 (s, Mmp trans-βC), 33.55 (s, Mmp cis-βC), 31.04 (s, Pro cis-βC), 30.70 (s, Dmt cis-βC), 30.01 (s, Dmt trans-βC), 28.85 (s, Pro trans-βC), 24.14 (s, Pro trans-γC), 21.18 (s, Pro cis-γC), 20.02 (p, Dmt trans-CH3), 19.29 (p, Dmt cis-CH3), 19.00 (p, Mmp trans-CH3), 18.91 (p, Dmt cis-CH3).
Tyrosylprolyl-3’,5’-dimethyl-l-phenylalanylphenylalanylamide Hydrochoride (3)
Yield 142.5 mg (87.2%); mp 170–172 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.53 (s, 0.34H, Tyr cis-OH), 9.43 (s, 0.66H, Tyr trans-OH), 8.40-8.16 (m, 3.68H, NH3+, 3,5Dmp cis-NH, Phe cis-NH), 8.12 (d, 0.66H, J = 8.22 Hz, 3,5Dmp trans-NH), 7.88 (d, 0.66H, J = 7.91 Hz, Phe trans-NH), 7.47 (s, 0.34H, cis-CONH2), 7.33-7.05 (m, 8.0H, Ar-H, CONH2), 6.94 (d, 0.66H, J = 8.31 Hz, Ar-H), 6.83-6.67 (m, 5.0H, Ar-H), 4.50-4.30 (m, 2.66H, Pro trans-αH, 3,5Dmp αH, Phe αH), 4.15 (t, 0.66H, J = 6.53Hz, Tyr trans-αH), 3.70 (dd, 0.36H, J = 5.68, 8.92 Hz, Tyr cis-αH), 3.60-3.48 (m, 0.66H, Pro trans-δH), 3.48-3.43 (m, 0.34H, Pro cis-αH), 3.35-3.20 (m, 0.68H, Pro cis-δH), 3.07-2.70 (m, 6.66H, Pro trans-βH, Phe βH, 3,5Dmp βH, Tyr βH), 2.20 (s, 6.0H, 3,5Dmp CH3), 2.0-1.87 (m, 0.66H, Pro trans-βH), 1.78-1.59 (m, 1.98H, Pro trans-βH, Pro trans-γH), 1.59-1.44 (m, 0.68H, Pro cis-βH, Pro cis-γH), 1.44-1.32 (m, 0.34H, Pro cis-βH), 1.32-1.16 (m, 0.34H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 173.01, 172.55, 170.67, 170.44, 170.36, 169.97, 167.12, 167.08, 156.70, 156.49, 137.76, 137.50, 137.15, 136.80, 136.76 (15q), 130.69, 130.20, 129.25, 129.12, 127.93, 127.86, 127.61, 126.89, 126.66, 126.12 (10t, Ar-C), 124.63, 123.98 (2q), 115.38, 115.24 (2t, Ar-C), 59.60 (s, Pro trans-αC), 59.35 (t, Pro cis-αC), 55.12 (t, 3,5Dmp cis-αC), 54.05 (t, 3,5Dmp trans-αC), 53.76 (t, Phe αC), 52.43 (t, Tyr trans-αC), 52.36 (t, Tyr cis-αC), 46.74 (s, Pro trans-δC), 46.40 (s, Pro cis-δC), 37.50 (s, Phe cis-βC), 37.38 (s, Phe trans-βC), 37.29 (s, 3,5Dmp trans-βC), 36.63 (s, 3,5Dmp cis-βC), 36.16 (s, Tyr cis-βC), 35.30 (s, Tyr trans-βC), 30.97 (s, Pro cis-βC), 28.85 (s, Pro trans-βC), 24.21 (s, Pro trans-γC), 21.13 (s, Pro cis-γC), 20.81 (p, 3,5Dmp CH3).
2’,6’-Dimethyl-l-tyrosylprolyl-3’,5’-dimethyl-l-phenylalanylphenylalanylamide Hydrochloride (3’)
Yield 141.6 mg (86.3%); mp 180–182 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.30 (s, 0.71H, Dmt cis-OH), 9.18 (s, 0.29H, Dmt trans-OH), 8.50 (br, 3.0H, NH3+), 8.36 (d, 0.71H, J = 8.47 Hz, Phe cis-NH), 8.12 (d, 0.71H, J = 8.35 Hz, 3,5Dmp cis-NH), 8.01 (d, 0.29H, J = 8.22 Hz, Phe trans-NH), 7.84 (d, 0.29H, J = 7.99 Hz, 3,5Dmp trans-NH), 7.63 (s, 0.71H, cis-CONH2), 7.37-7.05 (m, 6.29H, CONH2, Ar-H), 6.84-6.75 (m, 3.0H, Ar-H), 6.45, 6.43 (2s, 2.0H, Dmt Ar-H), 4.51 (dt, 0.71H, J = 4.90, 8.75 Hz, Phe cis-αH), 4.46-4.33 (m, 0.87H, Pro trans-αH, 3,5Dmp trans-αH, Phe trans-αH), 4.33-4.22 (m, 0.71H, 3,5Dmp cis-αH), 4.11 (dd, 0.29H, J = 5.61, 9.50 Hz, Dmt trans-αH), 3.66 (dd, 0.71H, J = 4.01, 11.08 Hz, Dmt cis-αH), 3.44-3.28 (m, 0.8H, Pro cis-δH), 3.25-3.14 (m, 0.8H, Pro cis-δH), 3.10-2.68 (m, 6.7H, Pro cis-αH, Dmt βH, 3,5Dmp βH, Phe βH), 2.88-2.80 (m, 0.4H, Pro trans-δH), 2.20, 2.06 (2s, 12.0H, Dmt and 3,5Dmp CH3), 1.87-1.75 (m, 0.29H, Pro trans-βH), 1.67-1.40 (m, 2.29H, Pro cis-βH, Pro trans-βH, Pro trans-γH, Pro cis-γH), 1.28-1.14 (m, 0.71H, Pro cis-βH), 1.14-1.0 (m, 0.71H, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 173.17, 172.48, 170.83, 170.31, 170.25, 169.98, 167.94, 167.60, 155.95, 138.43, 138.14, 137.74, 137.65, 137.25, 136.78 (15q), 129.28, 129.11, 127.92, 127.82, 127.59, 126.73, 126.61 (8t, Ar-C), 121.43, 121.02 (2q), 115.05 (t, Ar-C), 59.81 (t, Pro trans-αC), 59.08 (t, Pro cis-αC), 55.67 (t, 3,5Dmp cis-αC), 54.02 (t, 3,5Dmp trans-αC), 53.61 (t, Phe αC), 49.81 (t, Dmt αC), 46.65 (s, Pro cis-δC), 46.21 (s, Pro trans-δC), 37.60 (s, Phe cis-βC), 37.41 (s, Phe trans-βC), 37.24 (s, 3,5Dmp trans-βC), 36.01 (s, 3,5Dmp cis-βC), 31.11 (s, Pro cis-βC), 30.58 (s, Dmt cis-βC), 30.03 (s, Dmt trans-βC), 28.83 (s, Pro trans-βC), 23.98 (s, Pro trans-γC), 21.09 (s, Pro cis-γC), 20.81 (p, 3,5Dmp CH3), 20.07 (p, Dmt trans-CH3), 19.23 (p, Dmt cis-CH3).
2’,6’-Dimethyl-l-tyrosylprolyl-2’,6’-dimethyl-l-phenylalanylphenylalanylamide Hydrochloride (4’)
Yield 168 mg (69.5%); mp 194–196 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.33 (s, 0.6H, Dmt cis-OH), 9.15 (s, 0.4H, Dmt trans-OH), 8.58, 8.40 (2br, 3.0H, −NH3+), 8.25 (d, 1.6H, J = 8.62 Hz, Dmp NH, Phe cis-NH), 7.95 (d, 0.4H, J = 8.38 Hz, Phe trans-NH), 7.45 (s, 0.6H, cis-CONH2), 7.37-7.09 (m, 5.6H, Ar-H, CONH2), 7.03 (s, 0.4H, trans-CONH2), 6.98-6.84 (m, 3.0H, Ar-H), 6.68 (s, 0.4H, trans-CONH2), 6.46 (s, 1.2H, Dmt cis-CH3), 6.40 (s, 0.8H, Dmt trans-CH3), 4.50 (dt, 0.6H, J = 4.71, 8.95 Hz, Phe cis-βH), 4.44-4.28 (m, 1.8H, Pro trans-αH, Dmt αH, Phe trans-αH), 4.10 (dd, 0.4H, J = 5.02, 9.59 Hz, Dmt trans-αH), 3.63 (dd, 0.6H, J = 4.30, 10.43 Hz, Dmt cis-αH), 3.38-3.28 (m, 0.6H, Pro cis-δH), 3.28-3.17 (m, 0.6H, Pro cis-δH), 3.08-2.70 (m, 6.6H, Pro cis-αH, Phe βH, Dmt βH, Dmp βH), 2.37-2.0 (m, 12.6H, Pro cis-αH, Dmt and Dmp CH3), 1.87-1.74 (m, 0.4H, Pro trans-βH), 1.67-1.43 (m, 2.4H, Pro cis-βH, Pro trans-βH, Pro trans-γH, Pro cis-γH), 1.30-1.10 (m, 1.2H, Pro cis-βH, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.97, 172.20, 170.69, 170.61, 169.93, 169.81, 167.92, 167.20, 155.99, 155.58, 138.44, 138.15, 137.77, 137.70, 136.76, 136.52, 134.66, 134.30 (18q), 129.30, 129.02, 127.84, 127.79, 126.07, 126.04, 126.02, 125.98 (8t, Ar-C), 121.34, 121.03 (2q), 115.08, 114.90 (2t, Ar-C), 59.62 (t, Pro trans-αC), 59.09 (t, Pro cis-αC), 54.26 (t, Dmp cis-αC), 53.63 (t, Phe αC), 53.01 (t, Dmp trans-αC), 49.72 (t, Dmt αC), 37.47 (s, Pro cis-βC), 37.24 (s, Pro trans-βC), 32.48 (s, Dmp trans-βC), 31.09 (s, Dmp cis-βC), 31.03 (s, Pro cis-βC), 30.69 (s, Dmt cis-βC), 30.10 (s, Dmt trans-βC), 28.90 (s, Pro trans-βC), 24.17 (s, Pro trans-βC), 21.30 (s, Pro cis-βC), 20.04 (p, Dmp CH3), 19.81 (p, Dmt trans-CH3), 19.40 (p, Dmt cis-CH3).
Tyrosylprolyl-2’,6’-dimethyl-l-tyrosylphenylalanylamide Hydrochloride (5)
Yield 143.0 mg (87.3%); mp 188–190 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.52 (s, 0.28H, Tyr cis-OH), 9.41 (s, 0.69H, Tyr trans-OH), 9.00 (s, 0.90H, Dmt OH), 8.40-8.10 (m, 4.0H, -NH3+, Dmt NH, Phe cis-NH), 7.95 (d, 0.73H, J = 8.18 Hz, Phe trans-NH), 7.30-7.04 (m, 8.0H, CONH2, Tyr and Phe Ar-H), 6.99 (d, 0.64H, J = 8.36 Hz, Tyr Ar-H), 6.93 (s, 0.68H, trans-CONH2), 6.73 (d, 0.62H, J = 8.22 Hz, Tyr cis-Ar-H), 6.69 (d, 1.40H, J = 8.31 Hz, Tyr trans-Ar-H), 6.38 (s, 2.0H, Dmt Ar-H), 4.50-4.33 (m, 2.0H, Pro trans-αH, Phe αH, Dmt cis-αH), 4.30 (q, 0.73H, J = 7.30, 14.84 Hz, Dmt trans-αH), 4.15 (t, 0.70H, J = 6.52Hz, Tyr trans-αH), 3.72-3.65 (m, 0.35H, Pro cis-αH), 3.65-3.50 (m, 1.0H, Tyr cis-αH, Pro trans-βH), 3.10-2.55 (m, 6.7H, Pro trans-δH, Phe βH, Tyr βH, Dmt βH), 2.19, 2.16 (2s, 6.0H, Dmt CH3), 2.04-1.90 (m, 0.70H, Pro trans-βH), 1.83-1.69 (m, 1.40H, Pro trans-γH), 1.69-1.36 (m, 1.9H, Pro cis-βH, Pro trans-βH, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.70, 172.78, 170.96, 170.69, 170.32, 169.90, 167.04, 166.84, 156.67, 156.43, 155.02, 154.98, 137.87, 137.82, 137.77, 137.75 (16q), 130.78, 130.36, 129.18, 129.09, 127.87, 127.80, 126.04 (9t, Ar-C), 124.74, 124.49, 124.04 (3q), 115.32, 115.17, 114.76 (3t, Ar-C), 59.48 (t, Pro trans-αC), 59.07 (t, Pro cis-αC), 53.80, 53.70 (2s, Phe αC and Dmt αC), 52.40 (s, Tyr αC), 46.73 (s, trans Pro δC), 46.63 (s, Pro cis-δC), 37.31 (s, Phe cis-βC), 37.25 (s, Phe trans-βC), 36.18 (s, Tyr cis-βC), 35.25 (s, Tyr trans-βC), 31.60 (s, Dmt trans-βC), 31.14 (s, Dmt cis-βC, Pro cis-βC), 28.83 (s, Pro trans-βC), 24.41 (s, Pro trans-γC), 21.41 (s, Pro cis-γC), 20.13 (p, Dmt cis-CH3), 20.04 (p, Dmt trans-CH3).
2’,6’-Dimethyl-l-tyrosylprolyl-2’,6’-dimethyl-l-tyrosylphenylalanylamide Hydrochloride (5’)
Yield 130 mg (67.8%); mp 209–211 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.31 (s, 0.57H, Dmt1 cis-OH), 9.12 (s, 0.39H, Dmt1 trans-OH), 8.99, 8.96 (2s, 0.97H, Dmt3 OH), 8.47 (br, 2.8H, NH3+), 8.20-8.07 (m, 1.58H, Dmt3 NH, Phe cis-NH), 7.86 (d, 0.38H, J = 8.13 Hz, Phe trans-NH), 7.48 (s, 0.54H, cis-CONH2), 6.50-6.30 (m, 4.0H, Dmt1 and Dmt3 Ar-H), 4.49 (td, 0.59H, J = 4.89, 8.81 Hz, Phe cis-αH), 4.43-4.32 (m, 0.82H, Pro trans-αH, Phe trans-αH), 4.32-4.20 (m, 1.0H, Dmt3 βH), 4.10 (dd, 0.39H, J = 5.09, 10.0 Hz, Dmt1 trans-αH), 3.68-3.57 (m, 0.54H, Dmt1 cis-αH), 3.40-3.16 (m, 1.18H, Pro cis-δH), 3.10-2.60 (m, 6.6H, Pro cis-αH, Phe βH, Dmt1 βH, Dmt3 βH), 2.30-2.20 (m, 12.82H, Pro trans-δH, Dmt1 and Dmt3 CH3), 1.87-1.73 (m, Pro trans-βH), 1.67-1.38 (m, 2.64H, Pro cis-βH, Pro trans-βH, Pro trans-γH, Pro cis-γH), 1.32-1.07 (m, 1.24H, Pro cis-βH, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.94, 172.22, 170.92, 170.80, 169.91, 169.82, 167.94, 167.21, 155.99, 155.59, 155.01, 154.98, 138.47, 138.16, 137.80, 137.67, 137.55 (18q), 129.29, 129.04, 127.84, 127.80, 126.08, 126.02 (6t, Ar-C), 124.94, 124.69, 121.37, 121.07 (4q), 115.08, 114.90, 114.75 (3t, Ar-C), 59.67 (t, Pro trans-αC), 59.08 (t, Pro cis-αC), 54.83 (t, Dmt1 αC), 53.56 (t, Phe αC), 49.77 (t, Dmt3 αC), 46.81 (s, Pro cis-δC), 46.08 (s, Pro trans-δC), 37.51 (s, Phe cis-βC), 37.28 (s, Phe trans-βC), 31.08 (s, Dmt3 trans-βC), 31.04 (s, Pro cis-βC), 30.73 (s, Dmt1 cis-βC), 30.48 (s, Dmt3 cis-βC), 30.09 (s, Dmt1 trans-βC), 28.89 (s, Pro trans-βC), 24.20 (s, Pro trans-γC), 21.37 (s, Pro cis-γC), 20.09, 20.02, 19.99, 19.36 (4p, Dmt CH3).
Tyrosylprolyl-2’,4’,6’-trimethyl-l-phenylalanylphenylalanylamide Hydrochloride (6)
Yield 156.1 mg (95.3%); mp 179–181 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.53 (s, 0.3H, Tyr cis-OH), 9.41 (s, 0.7H, Tyr trans-OH), 8.49 (d, 0.3H, J = 8.75 Hz, Tmp cis-NH), 8.40-8.10 (br, 4.0H, NH3+, Tmp trans-NH, Phe cis-NH), 8.00 (d, 0.7H, J = 8.30 Hz, Phe trans-NH), 7.30-6.91 (m, 8.3H, Ar-H, CONH2), 6.80-6.60 (m, 4.7H, Ar-H, CONH2), 4.50-4.32 (m, 2.7H, Pro trans-αH, Tmp αH, Phe αH), 4.15 (t, 0.7H, J = 6.36 Hz, Tyr trans-αH), 3.82-3.75 (m, 0.3H, Pro cis-αH), 3.69 (t, 0.3H, J = 7.08 Hz, Tyr cis-αH), 3.60-3.50 (m, 0.7H, Pro trans-δH), 3.40-3.25 (m, 0.6H, Pro cis-δH), 3.10-2.72 (m, 6.7H, Pro trans-δH, Phe βH, Tyr βH, Tmp βH), 2.30-2.10 (m, 9.0H, Tmp Ar-CH3), 2.02-1.90 (m, 0.7H, Pro trans-βH), 1.85-1.69 (m, 1.4H, Pro trans-γH), 1.69-1.38 (m, 1.9H, Pro cis-βH, Pro trans-βH, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.66, 172.25, 170.76, 170.54, 170.32, 169.97, 166.94, 166.78, 156.69, 156.44, 137.87, 137.81, 136.64, 136.49, 134.71, 131.16, 131.06 (18q), 129.15, 129.04, 128.62, 127.86, 127.79, 126.02 (6t, Ar-C), 124.40, 124.01 (2q), 115.31, 115.15 (2t, Ar-C), 59.41 (t, Pro trans-αC), 59.04 (t, Pro cis-αC), 53.67 (t, Phe αC), 53.43 (t, Pro cis-αC), 53.29 (t, Tmp trans-αC), 52.32 (t, Tyr αC), 46.72 (s, Pro trans-δC), 46.64 (s, Pro cis-δC), 37.15 (s, Phe βC), 36.05 (s, Tyr cis-βC), 35.21 (s, Tyr, trans-βC), 32.01 (s, Tmp trans-βC), 31.62 (s, Tmp cis-βC), 31.13 (s, Pro cis-βC), 28.85 (s, Pro trans-βC), 24.39 (s, Pro trans-γC), 21.39 (s, Pro cis-γC), 20.36, 19.86, 19.78 (3p, Tmp Ar-CH3).
2’,6’-Dimethyl-l-tyrosylprolyl-2’,4’,6’-trimethyl-l-phenylalanylphenylalanylamide Hydrochloride (6’)
Yield 137.6 mg (80.6%); mp 184–186 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.32 (s, 0.6H, Dmt cis-OH), 9.14 (s, 0.4H, Dmt trans-OH), 8.48 (br, 3.0H NH3+), 8.24-8.14 (m, 1.6H, Tmp NH, Phe cis-NH), 7.93 (d, 0.4H, J = 8.38 Hz, Phe trans-NH), 7.40 (s, 0.60H cis-CONH2), 7.33-7.10 (m, 5.6H, Phe Ar-H, cis-CONH2), 7.05 (s, 0.4H, trans-CONH2), 6.75, 6.74 (2s, Tmp Ar-H), 6.60 (s, 0.4H, trans-CONH2), 6.46 (s, 1.2H, Dmt cis-Ar-CH3), 6.40 (s, 0.8H, Dmt trans-Ar-CH3), 4.49 (td, 0.6H, J = 4.76, 8.9 Hz, Phe cis-αH), 4.42-4.24 (m, 1.8H, Pro trans-αH, Tmp αH, Phe trans-αH), 4.00 (dd, 0.4H, J = 5.08, 10.06 Hz, Dmt trans-αH), 3.64 (dd, 0.6H, J = 4.36, 10.88 Hz, Dmt cis-αH), 3.46-3.27 (m, 1.4H, Pro cis-δH, Pro trnas-δH), 3.27-3.18 (m, 0.6H, Pro cis-δH), 3.08-2.68 (m, 6.6H, Pro cis-βH, Phe βH, Tmp βH, Dmt βH), 2.30-2.00 (m, 15.4H, Pro trans-δH, Dmt and Tmp Ar-CH3), 1.87-1.75 (m, 0.4H, Pro trans-βH), 1.70-1.43 (m, 2.4H, Pro cis-βH, Pro trans-βH, Pro trans-γH, Pro cis-γH), 1.30-1.15 (m, 1.2H, Pro cis-βH, Pro cis-γH). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.96, 172.23, 170.74, 170.68, 169.91, 169.83, 167.98, 167.22, 156.00, 155.59, 138.45, 138.16, 137.75, 136.57, 136.33, 134.73, 134.66, 131.55, 131.16 (19q), 129.29, 129.02, 128.59, 127.84, 127.79, 126.07, 126.01 (7t, Ar-C), 121.37, 121.07 (2q), 115.09, 114.90 (2t, Ar-C), 59.62 (t, Pro trans-αC), 59.09 (t, Pro cis-αC), 54.50 (t, Tmp cis-αC), 53.59 (t, Phe αC), 53.10 (t, Tmp trans-αC), 49.78 (t, Dmt cis-αC), 49.73 (t, Dmt trans-αC), 46.80 (s, Pro cis-δC), 46.10 (s, Pro trans-δC), 37.47 (s, Phe cis-βC), 37.18 (s, Phe trans-βC), 32.21 (s, Tmp trans-βC), 31.03 (s, Pro cis-βC), 30.86 (s, Tmp cis-βC), 30.74 (s, Dmt trans-βC), 30.12 (s, Dmt cis-βC), 28.92 (s, Pro trans-βC), 24.18 (s, Pro trans-γC), 21.36 (s, Pro cis-γC), 20.38 (p, Tmp trans-Ar-CH3), 20.35 (p, Tmp, cis-Ar-CH3), 20.01 (p, Tmp trans-Ar-CH3), 19.94 (p, Dmt trans-Ar-CH3), 19.73 (p, Tmp cis-Ar-CH3), 19.36 (p, Dmt cis-Ar-CH3).
Tyrosylprolyl-2’-ethyl-6’-methyl-l-phenylalanylphenylalanylamide Hydrochloride (7)
Yield 158.3 mg (96.6%); mp 167–169 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.53 (s, 0.33H, Tyr cis-OH), 9.41 (s, 0.67H, Tyr trans-OH), 8.43 (d, 0.33H, J = 8.96 Hz, Emp cis-NH), 8.37-8.10 (m, 4.0H, NH3+, Emp trans-NH, Phe cis-NH), 7.97 (d, 0.67H, J = 8.30 Hz, Phe trans-NH), 7.30-6.90 (m, 11.33H, Ar-H, CONH2), 6.80-6.64 (m, 2.67H, Ar-H, trans-CONH2), 4.52-4.33 (m, 2.67H, Pro trans-αH, Emp αH, Phe αH), 4.14 (t, 0.67H, J = 6.26 Hz, Tyr trans-αH), 3.68-3.63 (m, 0.33H, Pro cis-αH), 3.60-3.50 (m, 1.0H, Tyr cis-αH, Pro trans-δH), 3.36-3.24 (m, 0.66H, Pro cis-δH), 3.08-2.76 (m, 6.67H, Pro trans-δH, Phe βH, Tyr βH, Emp βH), 2.67-2.55 (m, 2.0H, Emp CH2CH3), 2.31, 2.28 (2s, 3.0H, Emp Ar-CH3), 2.03-1.90 (m, 0.67H, Pro trans-γH), 1.83-1.70 (m, 1.34H, Pro trans-γH), 1.70-1.30 (m, 2.0H, Pro cis-βH, Pro trans-βH, Pro cis-γH), 1.14-1.03 (m, 3.0H, Emp CH2CH3). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.71, 172.18, 170.62, 170.42, 170.38, 169.86, 166.99, 166.85, 156.70, 156.44, 142.84, 142.71, 137.83, 137.75, 136.95, 136.80, 133.53, 133.44 (18q), 130.76, 130.34, 129.21, 130.34, 127.85, 127.78, 126.38, 126.30, 126.04 (9t, Ar-C), 124.47, 123.96 (2q), 115.33, 115.16 (2t, Ar-C), 59.45 (t, Pro trans-αC), 59.08 (t, Pro cis-αC), 53.79 (t, Emp αC), 53.67 (t, Phe αC), 52.38 (t, Tyr, trans-αC), 52.29 (t, Tyr cis-αC), 46.71 (s, Pro trans-δC), 46.58 (s, Pro cis-δC), 37.34 (s, Phe cis-βC), 37.22 (s, Phe, trans-βC), 36.15 (s, Tyr cis-βC), 35.32 (s, Tyr trans-βC), 31.47 (s, Emp trans-βC), 31.13 (s, Emp cis-βC), 30.95 (s, Pro cis-βC), 28.84 (s, Pro trans-βC), 25.30 (s, Emp cis-CH2CH3), 25.12 (s, Emp trans-CH2CH3), 24.38 (s, Pro trans-γC), 21.32 (s, Pro, cis-γC), 20.09 (p, Emp cis-Ar-CH3), 19.99 (p, Emp, trans-Ar-CH3), 15.06 (p, Emp CH2CH3).
2’,6’-Dimethyl-l-tyrosylprolyl-2’-ethyl-6’-methyl-l-phenylalanylphenylalanylamide Hydrochloride (7’)
Yield 143.4 mg (87.0%); mp 176–178 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.32 (s, 0.65H, Dmt cis-OH), 9.14 (s, 0.35H, Dmt trans-OH), 8.52 (br, 3.0H, NH3+), 8.27-8.17 (m, 1.65H, Emp NH, Phe cis-NH), 7.89 (d, 0.35H, Phe trans-NH), 7.51 (s, 0.65H, cis-CONH2), 7.36-7.28 (m, 1.30H, Ar-H), 7.25-7.12 (m, 4.6H, Ar-H), 7.06-6.89 (m, 3.1H, CONH2, Ar-H), 6.65 (s, 0.35H, trans-CONH2), 6.45, 6.40 (2s, 2.0H, Dmt Ar-H), 4.51 (dt, 0.65H, J= 4.75, 9.02 Hz, Phe cis-αH), 4.43-4.26 (m, 1.70H, Pro trans-αH, Emp αH, Phe trans-αH), 4.10 (dd, 0.35H, J = 5.15, 10.02 Hz, Dmt trans-αH), 3.64 (dd, 0.65H, J = 4.01, 10.94 Hz, Dmt cis-αH), 3.36-3.18 (m, 1.65H, Pro cis-δH, Pro trans-δH), 3.10-2.50 (m, 8.65H, Pro cis-αH, Phe βH, Emp βH, Dmt βH, Emp CH2CH3), 2.34-2.18 (m, 3.35H, Emp Ar-CH3, Pro trans-δH), 2.14, 2.09 (2s, 6.0H, Dmt Ar-CH3), 1.87-1.76 (m, 0.35H, Pro trans-βH), 1.68-1.43 (m, 2.35H, Pro cis-βH, Pro trans-βH, Pro trans-γH, Pro cis-γH), 1.27-1.02 (m, 4.30H, Pro cis-βH, Pro cis-γH, Emp CH2CH3). 13C NMR (100.6 MHz, DMSO-d6) δ: 173.03, 172.15, 170.58, 169.95, 169.83, 167.96, 167.20, 156.01, 142.77, 142.64, 138.46, 138.15, 137.74, 137.68, 136.87, 136.61, 133.90, 133.44 (14q), 129.34, 129.02, 127.84, 127.77, 126.31, 126.26, 126.11, 126.02 (8t, Ar-C), 121.36, 121.04 (2q), 115.08, 114.90 (2t, Ar-C), 59.63 (t, Pro trans-αC), 59.09 (t, Pro cis-αC), 54.93 (t, Emp αC), 53.57 (t, Phe αC), 49.76 (t, Dmt αC), 46.76 (s, Pro cis-δC), 46.10 (s, Pro trans-δC), 37.52 (s, Phe cis-βC), 37.24 (s, Phe trans-βC), 31.70 (s, Emp trans-βC), 31.02 (s, Pro cis-βC), 30.68 (s, Dmt cis-βC), 30.27 (s, Emp cis-βC), 30.10 (s, Dmt trans-βC), 28.95 (s, Pro trans-βC), 25.41 (s, Emp cis-CH2CH3), 25.04 (s, Emp trans-CH2CH3),24.18 (s, Pro trans-γC), 21.30 (s, Pro cis-γC), 20.13 (p, Emp trans-CH2CH3), 20.01 (p, Emp cis-CH2CH3), 19.97 (p, Dmt cis-Ar-CH3), 19.36 (p, Dmt trans-Ar-CH3).
Tyrosylprolyl-2’-isopropyl-6’-methyl-l-phenylalanylphenylalanylamide Hydrochloride (8)
Yield 155.7 mg (94.9%); mp 173–175 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.53 (s, 0.3H, Dmt cis-OH), 9.40 (s, 0.7H, Dmt trans-OH), 8.44 (d, 0.3H, J = 8.81 Hz, Imp cis-NH), 8.37-8.05 (m, 4.0H, NH3+, Imp trans-NH, Phe cis-NH), 7.91 (d, 0.7H, J = 8.05 Hz, Phe trans-NH), 7.32-6.87 (m, 12.0H, Ar-H, CONH2), 6.64-6.76 (m, 2.0H, Ar-H), 4.51-4.36 (m, 2.0H, Pro trans-αH, Imp cis-αH, Phe αH), 4.32 (dd, 0.7H, J = 7.23, 15.13 Hz, Imp trans-αH), 4.13 (t, 0.7H, J = 6.33 Hz, Tyr trans-αH), 3.67-3.50 (m, 1.3H, Pro cis-αH, Tyr cis-αH, Pro trans-δH), 3.37-3.16 (m, 1.6H, Pro cis-δH, Imp CH(CH3)2), 3.10-2.78 (m, 6.7H, Pro trans-δH, Phe βH, Tyr βH, Imp βH), 2.31, 2.29 (2s, 3.0H, Imp Ar-CH3), 2.07-1.96 (m, 0.7H, Pro trans-βH), 1.82-1.64 (m, 2.1H, Pro trans-βH, Pro trans-γH), 1.64-1.51 (m, 0.6H, Pro cis-βH, Pro cis-γH), 1.51-1.41 (m, 0.3H, Pro cis-βH), 1.41-1.27 (m, 0.3H, Pro cis-γH), 1.20-1.04 (m, 6.0H, CH(CH3)2). 13C NMR (100.6 MHz, DMSO-d6) δ: 172.75, 172.23, 170.57, 17052, 170.37, 169.94, 167.11, 166.82, 156.70, 156.44, 147.45, 147.24, 137.81, 137.65, 136.76, 136.68, 132.97, 132.71 (18q), 130.73, 130.33, 129.25, 129.17, 127.84, 127.79, 127.56, 127.47, 126.48, 126.41, 126.06 (11t, Ar-C), 124.55, 124.00 (2q), 122.83, 115.33, 115.18 (3t, Ar-C), 59.49 (t, Pro trans-αC), 59.17 (t, Pro cis-αC), 54.46 (t, Imp αC), 53.66 (t, Phe cis-αC), 53.56 (t, Phe trans-βC), 52.44 (t, Tyr trans-βC), 52.32 (t, Tyr cis-βC), 46.72 (s, Pro trans-δC), 46.53 (s, Pro cis-δC), 37.39 (s, Phe βC), 36.20 (s, Tyr cis-βC), 35.34 (s, Tyr trans-βC), 31.05 (s, Pro cis-βC), 30.88 (s, Imp trans-βC), 30.45 (s, Imp cis-βC), 28.93 (s, Pro trans-βC), 27.87 (t, Imp CH(CH3)2), 24.42 (s, Pro trans-γC), 24.36 (p, Imp trans-Ar-CH3), 23.86 (p, Imp cis-Ar-CH3), 21.33 (s, Pro cis-γC), 20.61 (p, Imp cis-CH(CH3)2), 20.27 (p, Imp trans-CH(CH3)2).
2’,6’-Dimethyl-l-tyrosylprolyl-2’-isopropyl-6’-methyl-l-phenylalanylphenylalanylamide Hydrochloride (8’)
Yield 131.3 mg (79.6%); mp 185–187 °C. 1H NMR (400.1 MHz, DMSO-d6) δ: 9.31 (s, 0.54H, Dmt cis-OH), 9.12 (s, 0.46H, Dmt trans-OH), 8.47 (br, 3.0H, NH3+), 8.30 (0.46H, d, J = 8.57 Hz, Imp trans-NH), 8.21 (d, 1.08H, J = 8.72 Hz Imp cis-NH, Phe cis-NH), 7.82 (d, 0.46H, J = 8.31 Hz, Phe trans-NH), 7.55 (s, 0.46H, trans-CONH2), 7.34, 7.32 (2s, 1.08H, Ar-H), 7.26-6.85 (m, 8.46H, Ar-H, CONH2), 6.45, 6.39 (2s, Dmt Ar-H), 4.51 (dt, 0.54H, J = 4.71, 8.98 Hz, Phe cis-αH), 4.45-4.24 (m, 1.92H, Pro trans-αH, Imp αH, Phe trans-αH), 4.09 (dd, 0.46H, J = 5.30, 9.98 Hz, Dmt trans-αH), 3.69 (dd, 0.54H, J = 4.08, 11.20 Hz, Dmt cis-αH), 3.37-3.10 (m, 2.54H, Pro cis-δH, Pro trans-δH, Imp CH(CH3)2), 3.10-2.75 (m, 6.54H, Pro cis-αH, Phe βH, Imp βH, Dmt βH), 2.34-2.17 (m, 3.46H, Pro trans-δH, Imp Ar-CH3), 2.11, 2.09 (2s, 6.0H, Dmt Ar-CH3), 1.90-1.75 (m, 0,46H, Pro trans-βH), 1.68-1.40 (m, 2.46H, Pro cis-βH, Pro trans-βH, Pro trans-γH, Pro cis-γH), 1.27-1.00 (m, 7.08H, Pro cis-βH, Pro cis-γH, Imp CH(CH3)2). 13C NMR (100.6 MHz, DMSO-d6) δ: 173.06, 172.18, 170.54, 170.50, 170.03, 169.85, 168.05, 167.17, 156.03, 155.55, 147.35, 147.14, 138.44, 138.13, 137.74, 137.57, 136.69, 136.50, 133.12, 132.72 (20q), 129.35, 129.11, 127.83, 127.78, 127.53, 127.45, 126.42, 126.36, 126.09, 126.05, 122.87, 122.82 (12t, Ar-C), 121.34, 121.05 (2q), 115.08, 114.90 (2t, Ar-C), 59.59 (t, Pro trans-αC), 59.12 (t, Pro cis-αC), 55.36 (t, Imp cis-αC), 54.12 (t, Imp trans-αC), 53.62 (t, Phe cis-αC), 53.42 (t, Phe trans-αC), 49.80 (t, Dmt, cis-αC), 49.72 (t, Dmt, trans-αC), 46.72 (s, Pro cis-δC), 46.06 (s, Pro, cis-δC), 37.49 (s, Phe, cis-βC), 37.40 (s, Phe trans-βC), 31.11 (s, Imp, trans-βC), 30.95 (s, Pro trans-βC), 30.67 (s, Imp, cis-βC), 30.09 (s, Dmt, trans-βC), 29.87 (s, Dmt cis-βC), 28.94 (s, Pro, cis-βC), 27.93 (t, Imp cis-CH(CH3)2), 27.83 (t, Imp trans-CH(CH3)2), 24.41 (p, Imp trans-CH(CH3)2), 24.17 (p, Imp cis-CH(CH3)2), 24.12 (s, Pro trans-γC), 23.89 (p, Imp trans-CH(CH3)2), 23.82 (p, Imp cis-CH(CH3)2), 21.34 (s, Pro cis-γC), 20.73 (p, Imp cis-Ar-CH3), 20.24 (p, Imp trans-Ar-CH3), 19.96 (p, Dmt trans-Ar-CH3), 19.34 (p, Dmt cis-Ar-CH3).
Opioid Receptor Binding Assays
Opioid receptor affinities were determined under equilibrium conditions [2.5 h at room temperature (22 °C)] in a competition assay using brain P2 synaptosomal membranes prepared from 150–160 g Sprague-Dawley rats.29,40 Synaptosomes were preincubated to remove endogenous opioids for 60 min at 22 °C, washed in excess ice-cold buffer (50 mM Tris-HCl, pH 7.5) containing protease inhibitor (soybean trypsin inhibitor) and stored in a 20% glycerol-containing buffer (50 mM HEPES, pH 7.5) with protease inhibitor at −80 °C as described previously.29,40 The δ- and μ-opioid receptors were radiolabeled with 1.9 nM [3H]deltorphin-II (Tyr-d-Ala-Phe-Glu-Val-Val-Gly-NH2) and 3.5 nM [3H]DAMGO ([d-Ala2,N-Me- Phe4,Gly-ol5]enkephalin), respectively,29,36,40 and excess unlabeled peptide (2 µM) established the level of nonspecific binding. After incubation, the radiolabeled membranes containing both radioligands were rapidly filtered on Whatman GF/C glass fiber filters presoaked in 0.1% polyetheneimine in order to optimize the signal-to-noise ratio, washed with ice-cold Tris-HCl-BSA (bovine serum albumin) buffer, and dried at 75–80 °C for 1 hr. Radioactivity was determined using EcoLume (ICN, Costa Mesa, CA). All compounds are analyzed in duplicate using 5–8 peptide dosages and several synaptosomal preparations in independent repetitions (n values noted in Table 2) to ensure statistical significance. The affinity constants (Ki) were calculated according to Cheng and Prusoff54 using published Kd values for [3H]deltorphin-II (1.4 nM) and [3H]DAMGO (3.5 nM).
κ-Receptor binding assays were carried out using P2 synaptosomal membranes prepared from guinea pig cerebellum (Hartley, male, 260–310 g).30,55 They were labeled with 2.0 nM [3H]U-69,59356 [(5a,7a,8b)-(+)-N-methyl-N-[7-(1-pyrrolidiniyl)-1-oxaspiro[4.5]dec-8yl]-benzeneacetamide, 1.75 TBq/mmol, NEN] and nonspecific binding was estimated in the presence of unlabeled compound (1 µM). Filtration used Whatman GF/B glass fiber filters presoaked in 0.1% polyetheneimine and free radiolabeled compound was washed with ice-cold Tris-HCl buffer. The Kd value for [3H]U-69,593 was 1.3 nM.
Biological Activity in Isolated Tissue Preparation
The myenteric plexus longitudinal muscle preparations (2–3 cm segments) from the small intestine of male Hartley strain guinea pigs (GPI) measured μ-opioid receptor agonism and a single mouse vas deferens (MVD) was used to determine δ-opioid receptor agonism as described previously.33–35 The isolated tissues were suspended in organ baths containing balanced salt solutions in a physiological buffer, pH 7.5. Agonists were tested for the inhibition of electrically evoked contraction and expressed as IC50 (nM) obtained from the dose-response curves. The IC50 values represent the mean ± SE of five to six separate assays. δ–Opioid antagonist potencies in the MVD assay were determined against the δ agonist deltorphin-II and are expressed as pA2 determined using the Schild Plot.57 The Schild slopes of all analogues were 0.88–1.07.
Acknowledgments
These studies were supported in part by a Grant-in-Aid for Japan Society for the Promotion of the Science (JSPS) Fellows (1503306) to T.L., and in part by the Intramural Research Program of the NIH, and NIEHS. The assistance and expertise of the library staff at NIEHS is greatly appreciated.
Footnotes
Supporting Information Available: The elemental analyses of all the new compounds are summarized in Table 1–4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A Potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AM, Soignier RD, Zadina JE, Kastin AJ, Nores WL, Olson RD, Olson GA. Dissociation of analgesic and rewarding effects of endomorphin-1 in rats. Peptides. 2000;21:1871–1874. doi: 10.1016/s0196-9781(00)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Carrigan KA, Nelson CJ, Lysle DT. Endomorphin-1 induces antinociception without immunomodulatory effects in the rat. Psychopharmacology. 2000;151:299–305. doi: 10.1007/s002130000487. [DOI] [PubMed] [Google Scholar]
- 4.Harrison LM, Kastin AJ, Zadina JE. Differential effects of endomorphin-1,endomorphin-2, and Tyr-W-MIF-1 on activation of G-proteins in SH-SY5Y human neuroblastoma membranes. Peptides. 1998;19:749–753. doi: 10.1016/s0196-9781(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 5.Kakizawa K, Shimohira I, Sakurada S, Fujimura T, Murayama K, Ueda H. Parallel stimulations of in vitro and in situ [35S]GTPγS binding by endomorphin 1 and DAMGO in mouse brains. Peptides. 1998;19:755–758. doi: 10.1016/s0196-9781(97)00482-8. [DOI] [PubMed] [Google Scholar]
- 6.Soignier RD, Vaccarino AL, Brennan AM, Kastin AJ, Zadina JE. Analgesic effects of endomorphin-1 and endomorphin-2 in the formalin test in mice. Life Sci. 2000;67:907–912. doi: 10.1016/s0024-3205(00)00689-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen J-C, Tao P-L, Li J-Y, Wong C-H, Huang EY-K. Endomorphin-1 and -2 induced naloxone-precipitated withdrawal syndromes in rats. Peptides. 2003;24:477–481. doi: 10.1016/s0196-9781(03)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Kosson P, Bonney I, Carr DB, Lipkowski AW. Endomorphins interact with tachykinin receptors. Peptides. 2005;26:1667–1669. doi: 10.1016/j.peptides.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Botros M, Hallberg M, Johansson T, Zhou Q, Lindeberg G, Frändberg P-A, Tömböly C, Tóth C, Grevès P-L, Nyberg Fred. Endomorphin-1 and endomorphin-2 differentially interact with specific binding sites for substance P (SP) amino terminal SP1–7 in the rat spinal cord. Peptides. 2006;27:753–759. doi: 10.1016/j.peptides.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Leitgeb B, Szekeres A, Tóth G. Conformational analysis of endomorphin-1 by molecular dynamics methods. J. Peptide Res. 2003;62:145–157. doi: 10.1034/j.1399-3011.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Podlogar BL, Paterlini G, Ferguson DM, Leo GC, Demeter DA, Brown FK, Reitz AB. Conformational analysis of the endogenous μ-opioid agonist endomorphin-1 using NMR spectroscopy and molecular modeling. FEBS Lett. 1998;439:13–20. doi: 10.1016/s0014-5793(98)01202-2. [DOI] [PubMed] [Google Scholar]
- 12.Fiori S, Renner C, Cramer J, Pegoraro S, Moroder L. Preferred conformation of endomorphin-1 in aqueous and membrane-mimetic environments. J. Mol. Biol. 1999;291:163–175. doi: 10.1006/jmbi.1999.2951. [DOI] [PubMed] [Google Scholar]
- 13.Keller M, Boissard C, Patiny L, Chung NN, Lemieux C, Mutter M, Schiller PW. Pseudoproline-containing analogues of morphiceptin and endomorphin-2: evidence for a cis-Tyr-Pro amide bond in the bioactive conformation. J. Med. Chem. 2001;44:3896–3903. doi: 10.1021/jm000332e. [DOI] [PubMed] [Google Scholar]
- 14.In Y, Minoura K, Ohishi H, Minakata H, Kamigauchi M, Sugiura M, Ishida T. Conformational comparison of μ-selective endomorphin-2 with its C-terminal free acid in DMSO solution, by 1H NMR spectroscopy and molecular modeling calculation. J. Peptide Res. 2001;58:399–412. doi: 10.1034/j.1399-3011.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 15.Janecka A, Fichna J, Kruszynski R, Sasaki Y, Ambo A, Costentin J, do-Rego J-C. Synthesis and antinociceptive activity of cyclic endomorphin-2 and morphiceptin analogs. Biochem. Pharmacol. 2005;71:188–195. doi: 10.1016/j.bcp.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q-Y, Chen Q, Yang D-J, Feng Y, Long Y, Wang P, Wang R. Endomorphin 1[ψ] and Endomorphin 2[ψ], endomorphins analogues containing a reduced (CH2NH) amide bond between Tyr1 and Pro2, display partial agonist potency but significant antinociception. Life Sci. 2005;77:1155–1165. doi: 10.1016/j.lfs.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Cadillo G, Gentilucci L, Tolomelli A, Spinosa R, Calienni M, Qasen A, Spampinato S. Synthesis and evaluation of the affinity toward μ -opioid receptors of atypical, lipophilic ligands based on the sequence c[-Tyr-Pro-Trp-Phe-Gly-] J. Med. Chem. 2004;47:5198–5203. doi: 10.1021/jm0498811. [DOI] [PubMed] [Google Scholar]
- 18.Tömböly C, Kövér KE, Péter A, Tourwé D, Biyashev D, Benyhe S, Borsodi A, Al-Khrasani M, Rónai AZ, Tóth G. Structure-activity study on the Phe side chain arrangement of endomorphins using conformationally constrained analogues. J. Med. Chem. 2004;47:735–743. doi: 10.1021/jm0310028. [DOI] [PubMed] [Google Scholar]
- 19.Lengyel I, Orosz G, Biyashev D, Kocsis L, Al-Khrasani M, Rónai A, Tömböly C, Fürst Z, Tóth G, Borsodi A. Side chain modifications change the binding and agonist properties of endomorphin 2. Biochem. Biophys. Res. Commun. 2002;290:153–161. doi: 10.1006/bbrc.2001.6136. [DOI] [PubMed] [Google Scholar]
- 20.Cardillo G, Gentilucci L, Qasem AR, Sgarzi F, Spampinato S. Endomorphin-1 analogues containing β-proline are μ-opioid receptor agonists and display enhanced enzymatic hydrolysis resistance. J. Med. Chem. 2002;45:2571–2578. doi: 10.1021/jm011059z. [DOI] [PubMed] [Google Scholar]
- 21.Wei J, Shao X, Gong M-Z, Zhu B-B, Cui Y-X, Gao Y-F, Wang R. Structure-activity relationships of novel endomorphin-2 analogues with N-O turns induced by α-aminoxy acids. Bioorg. Med. Chem. Lett. 2005;15:2986–2989. doi: 10.1016/j.bmcl.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Kruszynski R, Fichna J, do-Rego J-C, Janecki T, Kosson P, Pakulska W, Costentin J, Janecka A. Synthesis and biological activity of N-methylated analogs of endomorphin-2. Bioorg. Med. Chem. 2005;13:6713–6717. doi: 10.1016/j.bmc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 23.(a) Fujita Y, Motoyama T, Takahashi M, Shimizu Y, Tsuda Y, Yokoi T, Li T, Sasaki Y, Ambo A, Kita A, Jinsmaa Y, Bryant SD, Lazarus LH, Okada Y. Development of potent bifunctional endomorphin-2 analogs with mixed μ-/δ-opioid agonist/δ-opioid antagonist properties. J. Med. Chem. 2004;47:3591–3599. doi: 10.1021/jm030649p. [DOI] [PubMed] [Google Scholar]; (b) Fichna J, Piestrzeniewicz M, Gach K, Poels J, Burgeon E, Broeck JV, Janecka A. [d-1-Nal4]endomorphin-2 is a potent μ-opioid receptor antagonist in the aequorin luminescence-based calcium assay. Life Sci. 2006;79:1094–1099. doi: 10.1016/j.lfs.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Okada Y, Fujita Y, Motoyama T, Tsuda Y, Yokoi T, Li T, Sasaki Y, Ambo A, Jinsmaa Y, Bryant SD, Lazarus LH. Structural studies of [2’,6’-dimethyl-l-tyrosine1]endomorphin-2 analogues: enhanced activity and cis orientation of the Dmt-Pro amide bond. Bioorg. Med. Chem. 2003;11:1983–1994. doi: 10.1016/s0968-0896(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y-F, Liu X, Wei J, Zhu B-B, Chen Q, Wang R. Structure-activity relationship of the novel bivalent and C-terminal modified analogues of endomorphin-2. Bioorg. Med. Chem. Lett. 2005;15:1847–1850. doi: 10.1016/j.bmcl.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Hruby VJ, Gehrig CA. Recent development in the design of receptor specific opioid peptides. Med. Res. Rev. 1989;9:343–401. doi: 10.1002/med.2610090306. [DOI] [PubMed] [Google Scholar]
- 27.(a) Bryant SD, Salvadori S, Cooper PS, Lazarus LH. New δ opioid antagonists as pharmacological probes. Trends Pharmacol. Sci. 1999;19:42–46. doi: 10.1016/s0165-6147(97)01156-5. [DOI] [PubMed] [Google Scholar]; (b) Bryant SD, Jinsmaa Y, Salvadori S, Okada Y, Lazarus LH. Dmt and opioid peptides: A potent alliance. Biopolymers (Pept. Sci.) 2003;71:86–102. doi: 10.1002/bip.10399. [DOI] [PubMed] [Google Scholar]
- 28.(a) Lazarus LH, Bryant SD, Salvadori S, Attila M, Jones LS. Opioid infidelity. Novel opioid agonists with dual high affinities for δ and μ receptors. Trends Neurosci. 1998;19:31–35. doi: 10.1016/0166-2236(96)81864-9. [DOI] [PubMed] [Google Scholar]; (b) Lazarus LH, Bryant SD, Cooper PS, Guerrini R, Balboni G, Salvadori S. Design of δ-opioid peptide antagonists for emerging drug applications. Drug Discov. Today. 1998;3:284–294. [Google Scholar]; (c) Balboni G, Guerrini R, Salvadori S, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Conversion of the potent δ-opioid agonist H-Dmt-Tic-NH-CH2-Bid into δ-opioid antagonists by N1-benzimidazole alkylation. J. Med. Chem. 2005;48:8112–8114. doi: 10.1021/jm058259l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Balboni G, Onnis V, Salvadori S, Zotti M, Sasaki Y, Ambo A, Bryant SD, Jinsmaa Y, Lazarus LH. Effect of lysine at C-terminus of the Dmt-Tic opioid pharmacophore. J. Med. Chem. 2006;49:5610–5617. doi: 10.1021/jm060741w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Fujita Y, Tsuda Y, Miyazaki A, Ambo A, Sasaki Y, Jinsmaa Y, Bryant SD, Lazarus LH, Okada Y. Development of potent μ-opioid receptor ligands using unique tyrosine analogues of endomorphin-2. J. Med. Chem. 2005;48:586–592. doi: 10.1021/jm049384k. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki Y, Kawano S, Kohara H, Watanabe H, Ambo A. ORL1 and opioid receptor preferences of nociceptin and dynorphin A analogues with Dmp substituted for N-terminal aromatic residues. Bioorg. Med. Chem. 2006;14:2433–2437. doi: 10.1016/j.bmc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki Y, Hirabuki M, Ambo A, Ouchi H, Yamamoto Y. Enkephalin analogues with 2’,6’-dimethylphenylalanine replacing phenylalanine in position 4. Bioorg. Med. Chem. Lett. 2001;11:327–329. doi: 10.1016/s0960-894x(00)00665-x. [DOI] [PubMed] [Google Scholar]
- 32.Ambo A, Murase H, Niizuma H, Ouchi H, Yamamoto Y, Sasaki Y. Dermorphin and deltorphin heptapeptide analogues: replacement of Phe residue by Dmp greatly improves opioid receptor affinity and selectivity. Bioorg. Med. Chem.Lett. 2002;12:879–881. doi: 10.1016/s0960-894x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 33.Ambo A, Niizuma H, Sasaki A, Kohara H, Sasaki Y. Dermorphin tetrapeptide analogues with 2’,6’-dimethylphenylalanine (Dmp) substituted for aromatic amino acids have high μ opioid receptor binding and biological activities. Bioorg. Med.Chem. Lett. 2003;13:1269–1272. doi: 10.1016/s0960-894x(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki Y, Sasaki A, Niizuma H, Goto H, Ambo A. Endomorphin 2 analogues containing Dmp residues as an aromatic amino acid surrogate with high μ-opioid receptor affinity and selectivity. Bioorg. Med. Chem. 2003;11:675–678. doi: 10.1016/s0968-0896(02)00601-6. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki Y, Sasaki A, Ariizuma T, Igari Y, Sato K, Kohara H, Niizuma H, Ambo A. 2’,6’-Dimethylphenylalanine (Dmp) can mimic the N-terminal Tyr in opioid peptides. Biol. Pharm. Bull. 2004;27:244–247. doi: 10.1248/bpb.27.244. [DOI] [PubMed] [Google Scholar]
- 36.(a) Jinsmaa Y, Shiotani K, Fujita Y, Miyazaki A, Li T, Tsuda Y, Okada Y, Ambo A, Sasaki Y, Bryant SD, Lazarus LH. Differentiation of opioid receptor preference by [Dmt1]endomorphin-2-mediated analgesia in the mouse. Eur. J. Pharmacol. 2005;509:37–42. doi: 10.1016/j.ejphar.2004.12.015. [DOI] [PubMed] [Google Scholar]; (b) Jinsmaa Y, Marczak E, Fujita Y, Shiotani K, Miyazaki A, Li T, Tsuda Y, Ambo A, Sasaki Y, Bryant SD, Okada Y, Lazarus LH. Potent in vivo antinociception and opioid receptor preference of the novel analogue [Dmt1]endomorphin-1. Pharmacol. Biochem. Behav. 2006;84:252–258. doi: 10.1016/j.pbb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 37.(a) Banks WA, Audus KL, Davis TP. Permeability of the blood-brain barrier to peptides: an approach to the development of therapeutically useful drugs. Peptides. 1992;13:1289–1292. doi: 10.1016/0196-9781(92)90037-4. [DOI] [PubMed] [Google Scholar]; (b) Abbruscato T, Thomas S, Hruby V, Davis T. Blood-brain barrier permeability and bioavailability of a highly potent and μ-selective opioid receptor antagonist, CTAP: comparison with morphine. J.Pharmacol. Exp. Ther. 1997;280:402–409. [PubMed] [Google Scholar]; (c) Egleton RD, Abbruscato TJ, Thomas SA, Davis TP. Transport of opioid peptides into the central nervous system. J. Pharm. Sci. 1998;87:1433–1439. doi: 10.1021/js980062b. [DOI] [PubMed] [Google Scholar]
- 38.(a) Labarre M, Butterworth J, St-Onge S, Payza K, Schmidhammer H, Salvadori S, Balboni G, Guerrini R, Bryant SD, Lazarus LH. Inverse agonism by Dmt-Tic analogues and HS 378, a naltrindole analogue. Eur. J. Pharmacol. 2000;406:R1–R3. doi: 10.1016/s0014-2999(00)00636-1. [DOI] [PubMed] [Google Scholar]; (b) Troyen-Tóth P, Décaillor FM, Fillol D, Lazarus LH, Schiller PW, Schidhammer H, Keiffer BL. Inverse agonism and neutral antagonism at wild-type and constitutively mutant delta opioid receptors. J. Pharmacol. Exp. Ther. 2005;313:410–421. doi: 10.1124/jpet.104.077321. [DOI] [PubMed] [Google Scholar]
- 39.(a) Salvadori S, Balboni G, Guerrini R, Tomatis R, Bianchi C, Bryant SD, Cooper PS, Lazarus LH. Evolution of the Dmt-Tic pharmacophore: N-terminal methylated derivatives with extraordinary δ opioid antagonist activity. J. Med. Chem. 1997;40:3100–3108. doi: 10.1021/jm9607663. [DOI] [PubMed] [Google Scholar]; (b) Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Synthesis and opioid activity of N,N-dimethyl-Dmt-Tic-NH-CH(R)-R’ analogues: acquisition of potent δ antagonism. Bioorg. Med. Chem. 2003;11:5435–5441. doi: 10.1016/j.bmc.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Jinsmaa Y, Nedachi M, Tsuda Y, Ambo A, Sasaki Y, Bryant SD, Marczak E, Li Q, Swartzwelder HS, Lazarus LH, Okada Y. Transformation of μ-opioid receptor agonists into biologically potent μ-opioid receptor antagonists. Bioorg. Med. Chem. 2007 doi: 10.1016/j.bmc.2006.11.019. in press. [DOI] [PubMed] [Google Scholar]
- 41.Fundytus M, Schiller P, Shapiro M, Wetrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective δ-opioid receptor antagonist TTPP[Ψ] Eur. J. Pharmacol. 1995;286:105–108. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- 42.Dygos JH, Yonan EE, Scaros MG, Goodmonson OJ, Getman DP, Periana RA, Beck GR. A convenient asymmetric synthesis of the unnatural amino acid 2,6-dimethyl-l-tyrosine. Synthesis. 1992:741–743. [Google Scholar]
- 43.Li T, Tsuda Y, Minoura K, In Y, Ishida T, Lazarus LH, Okada Y. Enantioselective synthesis of a phenylalanine library containing alkyl groups on the aromatic moiety: confirmation of stereostructure by X-ray analysis. Chem. Pharm. Bull. 2006;54:873–877. doi: 10.1248/cpb.54.873. [DOI] [PubMed] [Google Scholar]
- 44.Salvadori S, Marastoni M, Balboni G, Borea P, Tomatis R. Opioid peptides: synthesis and biological properties of dermorphin related hexapeptides. Eur. J. Med.Chem. 1990;25:171–177. [Google Scholar]
- 45.Salvadori S, Attila M, Balboni G, Bianchi C, Bryant SD, Crescenzi O, Guerrini R, Picone D, Tancredi T, Temussi PA, Lazarus LH. δ opioid antangonists: prototypes for designing a new generation of ultraselective opioid peptides. Mol. Med. 1995;1:678–689. [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y-F, Liu X, Liu W-X, Qi Y-M, Liu X-F, Zhou Y-F, Wang R. Opioid receptor binding and antinociceptive activity of the analogues of endomorphin-2 and morphiceptin with phenylalanine mimics in the position 3 or 4. Bioorg. Med.Chem. Lett. 2006;16:3688–3692. doi: 10.1016/j.bmcl.2006.04.063. [DOI] [PubMed] [Google Scholar]
- 47.Chao H, Murray TF, Aldrich JV. Synthesis and evaluation of potential affinity labels derived from endomorphin-2. J. Peptide Res. 2003;61:58–62. doi: 10.1034/j.1399-3011.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 48.(a) Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portogehese PS. A bivalent ligand (KDN-21) reveals spinal δ and κ opioid receptors are organized as heterodimers that give rise to δ1 and κ2 phenotypes. Selective targeting of δ-κ heterodimers. J. Med. Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]; (b) Law P-Y, Ericson-Herbandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerizaiton of μ- and δ-opioid receptors occurs at the cell surface only and requires receptor-G protein interaction. J. Biol. Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]; (c) Snook LA, Milligan G, Kieffer BL, Massotte D. μ-δ opioid receptor functional interaction: Insight using receptor-G protein fusions. J. Pharmacol. Exp. Ther. 2006;318:683–690. doi: 10.1124/jpet.106.101220. [DOI] [PubMed] [Google Scholar]
- 49.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role of heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. USA. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniels DJ, Lenard NR, Etienne CL, Law P-Y, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. USA. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Edle RP, Unterwald E, Pasternak GW, Pinter JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 52.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 53.Bryant SD, Salvadori S, Okada Y, Takahshi M, Sasaki Y, Lazarus LH. Computational chemistry and opioidmimetics: Receptor ligand interaction of three classes of biologically potent peptides. In: Martinez J, Fehrentz J-A, editors. Peptides 2000. Editions EDK. Montpellier: 2000. pp. 407–408. [Google Scholar]
- 54.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibition which cause 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3180. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 55.Ambo A, Adachi T, Sasaki Y. Synthesis and opioid activities of [d-Leu8]Dynorphin(1–8) analogs containing a reduced peptide bond, ψ(CH2-NH) Chem. Pharm. Bull. 1995;43:1547–1550. doi: 10.1248/cpb.43.1547. [DOI] [PubMed] [Google Scholar]
- 56.Lahti RA, Mickelson MM, McCall JM, Von Voigtlander PF. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur. J.Pharmacol. 1985;109:281–284. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- 57.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br. J.Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]