Abstract
Sensory transduction in hair cells requires assembly of membrane-bound transduction channels, extracellular tip-links and intracellular adaptation motors with sufficient precision to confer nanometer displacement sensitivity. Here we present evidence based on FM1-43 fluorescence, scanning electron microscopy and RT-PCR that these three essential elements are acquired concurrently between embryonic day 16 and 17, several days after the appearance of hair bundles1, and that their acquisition coincides with the onset of mechanotransduction.
The widely accepted model of hair-cell transduction holds that deflection of the hair bundle, the mechanosensitive organelle, modulates tip-link tension and the open probability of non-selective transduction channels located at either end2. A rise in tip-link tension opens channels and allows calcium to enter and bind to intracellular sites that promote adaptation, a decline in channel open probability. An attractive hypothesis for the development of hair cell transduction suggests that transduction elements are assembled in the cell body and transported by the adaptation motor to the tips of the stereocilia to form a fully functional transduction complex2 (Fig. 1a).
Figure 1.
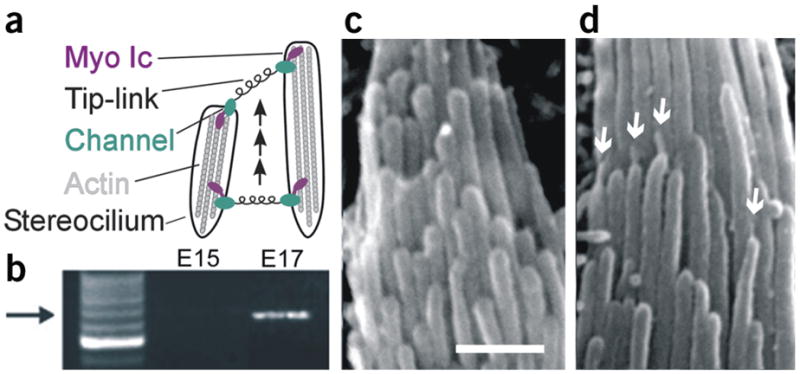
Acquisition of transduction, myosin Ic and tip-links. (a) Diagram illustrating the model for transduction development. (b) Myosin Ic RT-PCR products obtained using mRNA extracted from eight E15 and nine E17 utricles. Arrow indicates position of expected 660-bp product sequenced to confirm identity. (c,d) Scanning electron micrographs of representative hair bundles at E15 and E17, respectively. Scale bar, 1 μm. Arrows indicate several tip-links.
To test for the developmental acquisition of transduction elements, we began with an RT-PCR screen to detect mRNA for myocin Ic (see Supplementary Methods online), a component of the adaptation motor and the only transduction molecule identified thus far3. We examined sensory epithelia dissected from the vestibular organs of embryonic mice on day 15 (E15) and E17. We found that Myosin Ic mRNA was present at E17, but we found no detectable message at earlier stages (Fig. 1b). Myosin VIIa is also present in hair bundles and has been implicated in transduction and adaptation4. However, neither the onset of myosin VIIa expression5 (E10) nor its localization6 coincide with a role in the development of transduction.
Next, we examined the acquisition of tip-links using scanning electron microscopy (Supplementary Methods). Tip-links consist of at least two coiled filaments 150–200 nm in length and 5 nm in diameter7 and connect the tip of one stereocilium along the hair bundle’s morphological axis of sensitivity to the side of its adjacent taller neighbor (Fig. 1a). We scanned sensory epithelia from six E15 mouse utricles, each of which contained several hundred hair bundles, but found no evidence of tip-links (Fig. 1c). By E17, however, tip-links oriented along the bundle’s morphological axis were clearly visible in 86% of the hair bundles examined (Fig. 1d), similar to that reported previously1.
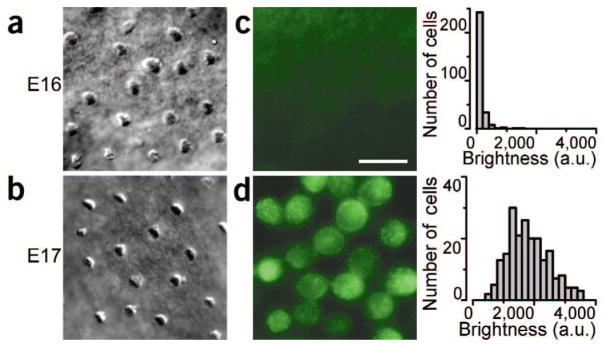
To investigate the acquisition of transduction channels, we examined uptake of the styryl dye FM 1-43, which permeates non-selective cation channels in sensory cells8. We applied 5 μM FM 1-43 for 10 s to vestibular epithelia of embryonic mice and imaged 1,032 hair cells (Supplementary Methods). We observed little fluorescence in E15 (not shown) and E16 hair cells (Fig. 2c), but by E17 there was substantial fluorescence within the cell bodies (Fig. 2d), indicating dye uptake and presumably the presence of non-selective transduction channels. We quantified FM 1-43 fluorescence by calculating the mean brightness of each cell that had an intact hair bundle (Fig. 2, right). At E16, the cells had a mean brightness of 87 ± 185 arbitrary units (n = 289), whereas at E17 the brightness was 1,826 ± 677 (n = 208), similar to that of mature hair cells (not shown). To confirm that the fluorescence observed in E17 cells resulted from FM 1-43 permeation through transduction channels, we blocked dye uptake with 1 mM bath application of the transduction channel blocker gentamicin (Supplementary Fig. 1 online).
Figure 2.
DIC and FM 1-43 fluorescence images and analysis of E16 and E17 hair cells. (a,b) DIC image of E16 and E17 hair bundles viewed from above. (c,d) Fluorescence image of E16 and E17 hair cells focused at the cell body level. Scale bar (10 μm) applies to all images. Right, histograms of the mean fluorescence of 289 E16 (top) and 208 E17 (bottom) cells. Bin width, 200 arbitrary units (a.u.).
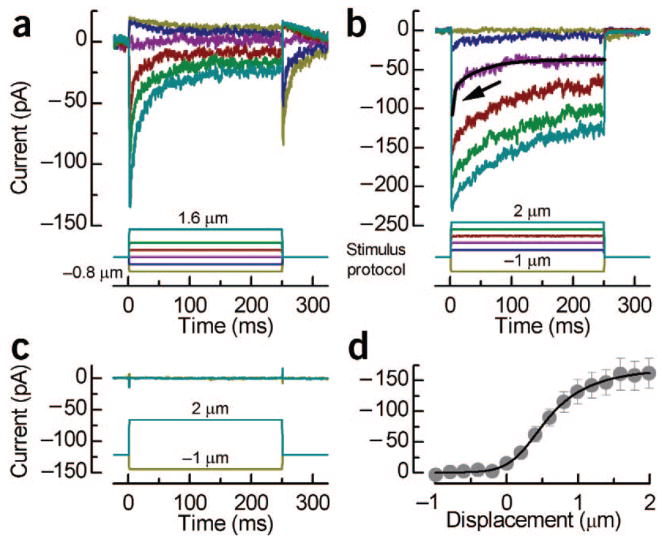
Since dye uptake at rest requires an open channel probability greater than zero, the possibility remained that at earlier stages transduction channels were present but that they remained in a closed conformation. To test for the acquisition of channels and functional mechanotransduction, we deflected hair bundles using either a fluid-jet or a stiff probe mounted on a piezoelectric bimorph and recorded current using tight-seal, whole-cell electrodes in voltage-clamp mode (Supplementary Methods). We recorded currents from 11 E15 (Fig. 3c) and 4 E16 hair cells, but were able to evoke transduction in only 2 cells. Interestingly, both cells with transduction were among the 5 (of 289 total) E16 cells that displayed FM1-43 fluorescence. In sharp contrast, recordings from 12 of 12 E17 hair cells revealed robust transduction currents (Fig. 3a,b) with maximal amplitudes that ranged between 83 and 278 pA with a mean of −164 ± 76 pA (n = 8 cells stimulated with stiff probe). Based on a single channel conductance of 112 pS (ref. 9), this corresponded to acquisition of ~24 channels. Remarkably, the currents had properties entirely consistent with transduction in mature cells. They activated and deactivated with submillisecond kinetics. The cells were sensitive to displacements as small as 10 nm and only along the bundle’s morphological axis. They had a mean resting open probability of 9.1 ± 6.5% (n = 8) and a mean 10–90% operating range of 1.3 ± 0.4 μm (n = 8; Fig. 3d), similar to that of mature cells: 1.1 ± 0.2 μm (ref. 10). Lastly, we observed two temporal components of adaptation11,12, evident as the current decay during step displacements. The more prominent slow adaptation, which involves myosin Ic3, was fit with exponential functions that had time constants that ranged between 18 and 136 ms with a mean of 49 ± 37 ms (n = 8, step size ~0.5 μm), which is well within the range of slow adaptation in mature cells3,10. The fast component of adaptation was evident in only a few cells and only for intermediate bundle deflections (Fig. 3b, arrow), consistent with its appearance in a subset of mature mouse utricle cells3. The fast component had a time constant of 3.1 ± 0.8 ms (n = 4, step size ~0.6 μm) and may result from calcium binding directly on or near the channel to confer a closed conformation11,13.
Figure 3.
Transduction currents from embryonic hair cells. (a) Family of transduction currents from an E17 hair cell showing the fastest myosin Ic–type adaptation we observed, an open probability of ~10%, adaptation to negative deflections and rebound currents at the end of the step. (b) Transduction currents from an E17 cell showing slow and fast adaptation. The purple trace was fit by a double exponential (black line) with time constants of 2.8 ms (arrow) and 43.6 ms. (c) Representative currents recorded from an E15 hair cell. (d) Mean current–displacement relationship from eight E17 hair cells fit with a second-order Boltzmann equation (10–90% operating range, 1.36 μm; resting open probability, 8.4%). All experiments were approved by the University of Virginia Animal Care and Use Committee.
Since our data suggested that acquisition of mechanotransduction occurs within one embryonic day, we measured both FM 1-43 uptake and transduction currents at 6-h intervals between E16 and E17. We found that the number of fluorescent cells increased from a few percent to nearly 100% within about 18 h (Supplementary Fig. 2). We recorded currents from ten cells at intermediate stages and found that they either had transduction and adaptation with all the properties of mature cells (n = 6, Supplementary Fig. 2), except smaller, or they lacked transduction entirely. We never observed transduction or adaptation with intermediate properties (i.e., broad operating range, lack of directional sensitivity, lack of adaptation, and the like).
The concurrent acquisition of transduction elements and the rapid, all-or-nothing onset of fully functional mechanosensitivity between E16 and E17 contrast with the gradual and later acquisition (postnatal day 10–20) of sensory transduction in photoreceptors14, but are consistent with the transduction assembly model (Fig. 1a). Furthermore, we propose that myosin Ic may mediate the developmental assembly of the transduction apparatus. Myosin Ic may climb up the actin core, transduction channel and tip-link in tow, until the complex at the lower end of the tip-link reaches the tip of the shorter pair of stereocilia. In this scenario, the upper motor would continue to climb until sufficient tension developed to yield an open probability of ~10%, thus positioning the transduction apparatus within the region of greatest sensitivity. Based on the unloaded climbing rate of the adaptation motor (0.32 μm/s)3 we predict that transduction elements assembled in the cell body will ascend to the tips of the stereocilia within ~22 s. One test of this notion would be to block transduction acquisition by selective inhibition of myosin Ic activity during development, perhaps using a chemical-genetic strategy3. Further tests of this model will be facilitated by molecular identification of the tip-link and transduction channel, which in turn may benefit from knowledge of the precisely defined onset of sensory transduction presented here. Finally, these results draw attention to a critical period (E16–E17) in the normal development of sensory transduction in the inner ear, which may lead to a better understanding of congenital hearing and balance deficits and guide efforts focused on hair-cell regeneration and restoration of hearing and balance function.
Supplementary Material
Acknowledgments
We thank D. Abraham, J. Assad, J. Risner and E. Stauffer for comments on an earlier version of the manuscript. This work was supported by NIH grants to J.R.H. (DC05439-03) and to G.S.G.G.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Denman-Johnson K, Forge A. J Neurocytol. 1999;28:821–835. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- 2.Denk W, Holt JR, Shepherd GM, Corey DP. Neuron. 1995;15:1311–1321. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 3.Holt JR, et al. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 4.Kros CJ, et al. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- 5.Sahly I, El-Amraoui A, Abitbol M, Petit C, Dufier JL. Anat Embryol (Berl) 1997;196:159–170. doi: 10.1007/s004290050088. [DOI] [PubMed] [Google Scholar]
- 6.Hasson T, et al. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG. Proc Natl Acad Sci USA. 2000;97:13336–13341. doi: 10.1073/pnas.97.24.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers JR, et al. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Géléoc GSG, Lennan GWT, Richardson GP, Kros CJ. Proc R Soc Lond. 1997;264:611–621. doi: 10.1098/rspb.1997.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt JR, Corey DP, Eatock RA. J Neurosci. 1997;17:8739–8748. doi: 10.1523/JNEUROSCI.17-22-08739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YC, Ricci AJ, Fettiplace R. J Neurophysiol. 1999;82:2171–2181. doi: 10.1152/jn.1999.82.5.2171. [DOI] [PubMed] [Google Scholar]
- 12.Holt JR, Corey DP. Proc Natl Acad Sci USA. 2000;97:11730–11735. doi: 10.1073/pnas.97.22.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford AC, Evans MG, Fettiplace R. J Physiol. 1989;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratto GM, Robinson DW, Yan B, McNaughton PA. Nature. 1991;351:654–657. doi: 10.1038/351654a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.