Abstract
Breast cancer development is a complex pathobiological process involving sequential genetic alterations in normal epithelial cells that results in uncontrolled growth in a permissive microenvironment. Accordingly, physiologically relevant models of human breast cancer that recapitulate these events are needed to study cancer biology and evaluate therapeutic agents. Here, we report the generation and utilization of the human breast cancer in mouse (HIM) model, which is composed of genetically engineered primary human breast epithelial organoids and activated human breast stromal cells. By using this approach, we have defined key genetic events required to drive the development of human preneoplastic lesions as well as invasive adenocarcinomas that are histologically similar to those in patients. Tumor development in the HIM model proceeds through defined histological stages of hyperplasia, DCIS to invasive carcinoma. Moreover, HIM tumors display characteristic responses to targeted therapies, such as HER2 inhibitors, further validating the utility of these models in preclinical compound testing. The HIM model is an experimentally tractable human in vivo system that holds great potential for advancing our basic understanding of cancer biology and for the discovery and testing of targeted therapies.
Keywords: cancer model, human in mouse, tissue reconstitution
Tumor development results from the sequential acquisition of multiple genetic and/or epigenetic lesions (http://cgap.nci.nih.gov and www.sanger.ac.uk/) (1–3). This complex, pathobiological process is also influenced profoundly by the tumor's ability to co-opt tissue stromal components, such as endothelium, hematopoietic cells, fibroblasts, and microphages (4). The ability to model this genetic and biological complexity in an in vivo tissue context would greatly enable the understanding of mechanisms underlying cancer initiation, progression, and invasion, as well as the assessment of the impact of specific combinations of genetic alterations on response to conventional and targeted drugs.
Human breast cancer cell line-derived xenografts have long served as the in vivo models of choice for studying the mechanisms that drive the tumorigenesis process and for evaluating preclinical experimental therapeutics (5). Considerable debate has surrounded the utility and relevance of such culture-adapted cell line models. The limited genetic representation of these lines and the acquisition of genetic aberrations during long-term growth in cell culture (6) are thought to contribute to the inadequate nature of these preclinical xenograft models in predicting the effectiveness of anticancer agents in clinical trials (7). Moreover, such cell culture systems are maintained under nonphysiological conditions, which may have an impact on the genetic alterations necessary for transformation. In addition, transformation in vitro failed to audit the complex evolutionary process of tumor development involving the heterotypical interactions between cancer cells and tissue stromal components.
To address the challenges of existing human breast cancer models, we established a humanized in vivo model system in which primary human breast epithelial organoids are engineered with a variety of oncogenes and are then introduced along with immortalized human breast fibroblasts into cleared mammary fat pads to reconstitute genetically altered human breast tissue. By using this model system, we have defined key molecular genetic events required to drive the development of human preneoplastic lesions as well as human breast adenocarcinoma in vivo.
Results
Overexpression of p53sh/HER2 or p53sh/KRAS Creates Ductal hyperplasia and Carcinoma in Situ in Reconstituted Human Breast Tissues.
Previous work has demonstrated that normal human breast tissue can be reconstituted in mice by implanting human breast fibroblasts along with epithelial organoids isolated directly from human reduction mammoplasty tissue (8, 9). The reconstituted human breast tissue typically filled up 5–20% of the mammary fat pad. By employing this tissue recombinant system and a lentiviral gene transduction system ( Fig. S1), we assessed the in vivo biological consequences of specific genetic alterations in the reconstituted human breast tissue. As a starting point, we tested the effects of combining p53 knockdown (targeted in 30–60% of breast cancers) (2) with overexpression of either the NEU/HER2/ERBB2 oncogene (amplified in ≈30% of breast cancers and correlated with poor prognosis) (10) or activated RAS family genes (overexpressed in as many as 67% of breast cancers) (11). Accordingly, human breast epithelial organoids from 1 patient were transduced with a bicistronic modified lentivirus encoding a p53 shRNA (12) in addition to either HER2V659E (p53sh/HER2) or KRASG12V and GFP (p53sh/KRAS/GFP). Infected organoids were implanted, along with immortalized human breast fibroblasts (RMF-HGF; fibroblasts with enforced HGF expression) into cleared and humanized mouse mammary fat pads (n = 40 for each genetic combination).
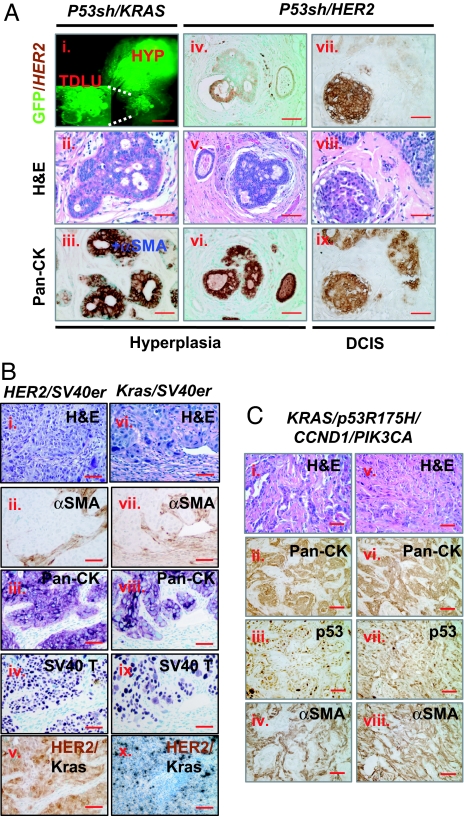
No visible tumors developed over the observation period of up to 12 months after implantation. Tissue recombinants were collected at various time points (spanning 1–10 months after implantation) and subjected to histopathologic analysis (Fig. 1A and Table S1). Both normal and hyperplastic outgrowths were observed in all of the tissue recombinants examined (n = 16 for p53sh/KRAS/GFP tissue recombinants and n = 24 for the p53sh/HER2 tissue recombinants; Fig. 1A i–vi). Histopathological analysis confirmed characteristic normal and hyperplastic human breast ductal architecture in both p53sh/KRAS/GF and p53sh/HER2 tissue recombinants. Lumen formation, basal localized myoepithelial cells, and the presence of multiple layers of luminal cells in the ducts were all evident in the hyperplastic outgrowths, mirroring precisely the histopathologic features of premalignant changes in humans.
Fig. 1.
Human preneoplastic lesions and advanced breast cancers were generated in vivo from genetically engineered human breast tissue recombinants. (A) Premalignant lesions and CIS developed in vivo from human breast tissue recombinants overexpressing HER2 or KRAS with concomitant knocking down of p53. (Ai) GFP whole mount of reconstituted p53sh/KRAS/GFP human breast tissue reveals KRAS-lentivirus expression in both normal Terminal Ductal Lobular Unit (TDLU) structures (Inset) as well as in hyperplasic nodule (HYP). (Aii) H&E of the p53sh/KRAS/GFP hyperplastic outgrowth in Ai. (Aiii). IHC-stained serial section of Aii demonstrating the filling of the luminal space with epithelial cells. (Aiv–Aix) Histological analysis of p53sh/HER2 tissue recombinants revealed both hyperplastic (Aiv–Avi) and carcinoma in situ outgrowth (Avii–Aix) from transduced organoids (Aiv and Avii). (Scale bars: Ai, 0.5 mm; Aii–Aix, 50 μm.) (B) Poorly differentiated, invasive human carcinomas developed in vivo from HER2/SV40er and KRAS/SV40er human breast tissue recombinants. Histological analysis of HER2/SV40er (Bi–Bv) and KRAS/SV40er (Bvi–Bx) tumors. H&E-stained sections (Bi and Bvi) and IHC analysis on serial sections with αSMA (Bii and Bvii), pan-cytokeratin (Pan-CK; Biii and Bviii), SV40 LT (Biv and Bix), and HER2 (Bv) demonstrated that tumors were derived from epithelial cells that overexpressed the transduced oncogenes. RNA in situ analysis (Bx) confirmed the expression of KRAS in KRAS/SV40er tumors. (Scale bar: 100 μm.) (C) Invasive ductal adenocarcinoma developed in vivo from KRAS/p53R175H/CCND1/PIK3CA tissue recombinants. H&E-stained sections of genetically engineered HIM tumor (Ci and Cii) revealed that both samples were invasive ductal adenocarcinoma with prominent glandular architecture and low mitotic index. IHC-stained serial sections of HIM tumors with pan-cytokeratin (Pan-CK; Cii and Cvi), p53 (Ciii and Cvii), and αSMA (Civ and Cviii) revealed mutant p53-positive epithelial cancer cells surrounded by myofibroblastic stroma. (Scale bar: 50 μm.)
In addition to the normal and hyperplastic outgrowths, carcinoma in situ (CIS) was observed in 12% (3 of 24) of the p53sh/HER2 tissue recombinants (Fig. 1A vii–ix). These CIS lesions exhibited histological features that are characteristic of human breast ductal carcinoma in situ (DCIS), presenting as complete lumen filling of monotonous aggregates of large atypical epithelial cells that stained positive for cytokeratin and HER2/neu. The uniform rim of αSMA-positive myoepithelial cells located in the basal layer confirmed the intraductal nature of these CIS lesions. This result is consistent with the prominent biological role of HER2 in human breast cancer pathogenesis, and it gains added significance in that HER2 overexpression is detected in 60–70% of human DCIS specimens (13, 14).
To determine the reproducibility of the results, we generated the p53sh/HER2 and the p53sh/KRAS/GFP tissue recombinants 2 more times by using organoids from 2 additional patients [donor 1 (n = 17 for each genetic combination) and donor 2 (n = 16 for each genetic combination)]. No visible tumors developed from any of the tissue recombinants. Together, these observations demonstrated that this tissue recombinant system, composed of relevant genetic and cellular components, can readily yield early-stage lesions with classical features of the human disease.
HER2/SV40er or KRAS/SV40er Leads to Rapid Onset of Basal-like Invasive Carcinomas in Vivo.
Despite the successes in re-creating early premalignant breast lesions in vivo, there was a notable lack of tumor development in all of the p53sh/KRAS/GFP or p53sh/HER2 breast tissue recombinants; this is in contrast to transgenic mouse models in which overexpression of an activated HER2 oncogene produces a highly penetrant breast cancer phenotype (15). These observations raised the possibility that additional genetic events are required to generate advanced disease in the human system. To address this notion, we replaced p53 shRNA with the SV40 early region (SV40er), which encodes for the Large T (LT) and small t (st) antigens to simultaneously disrupt the p53, RB, and PP2A/PI3K pathways (16).
Epithelial organoids from donor 1 were transduced with HER2V659E and SV40er (HER2/SV40er; n = 10; Table 1, experiment set A), KRASG12V and SV40er (KRAS/SV40er), or SV40er alone (SV40er) and were used as donor epithelium to generate human breast tissue recombinants in mice. With the introduction of additional genetic alterations provided by SV40er, tumors developed in all of the HER2/SV40er and KRAS/SV40er tissue recombinants (n = 10 each). Tumors became palpable as early as 5 weeks after implantation. As a negative control, no tumors were observed in the SV40er tissue recombinants (n = 10) over a 6-month observation period. Therefore, the genetic combinations of HER2/SV40er and KRAS/SV40er, but not SV40er alone, were capable of efficiently transforming primary human breast organoids in vivo.
Table 1.
Effect of stromal fibroblasts on tumor development from organoids transduced with Her2/SV40er
| Experimental set | Organoids | Fibroblasts | Total sites | Tumor frequency, no. (%) |
|---|---|---|---|---|
| A | Donor 1 | RMF-HGF | 10 | 10 of 10 (100) |
| B | Donor 1 | RMF-HGF | 4 | 4 of 4 (100) |
| C | Donor 2 | RMF-HGF | 6 | 6 of 6 (100) |
| D | Donor 3 | RMF-HGF | 6 | 5 of 6 (83) |
| E | Donor 4 | RMF-HGF | 6 | 5 of 6 (83) |
| F | Donor 5 | RMF-HGF | 6 | 6 of 6 (100) |
| G | Donor 1 | RMF | 4 | 3 of 4 (75) |
| H | Donor 1 | 1°RMF-1 | 6 | 0 |
| I | Donor 1 | 1°RMF-2 | 6 | 1 of 6 (17) |
| J | Donor 1 | None | 9 | 1 of 9 (11) |
Histological examination of the HER2/SV40er and KRAS/SV40er tumors revealed poorly differentiated invasive carcinomas with anaplastic features (Fig. 1B and Fig. S2). The invasive growth pattern of nests and islands of tumor cells as well as the considerable cellular pleomorphisms are characteristic of highly malignant disease in breast cancer patients (Fig. S3). These anaplastic HER2/SV40er and KRAS/SV40er tumor cells expressed cytokeratins, confirming their epithelial cell origin (Fig. 1B). Furthermore, IHC and RNA in situ hybridization analyses confirmed that these tumors were derived from human breast epithelial cells transduced with HER2 and SV40er (HER2/SV40er) or KRAS and SV40er (KRAS/SV40er). Finally, the tumors contained prominent areas of stromal desmoplasia, a feature present in many human breast carcinomas (Fig. S4).
To further assess the molecular characteristics of the human breast tumors generated, IHC analysis was performed using clinically relevant markers to determine tumor subtype (Fig. S5). Both the KRAS/SV40er and the HER2/SV40er tumors were negative for estrogen receptor (ER) and progensterone receptor (PR). In addition, the KRAS/SV40er tumors were positive for cytokeratin 5/6 and p63 and negative for HER2, exhibiting typical features of basal-like breast cancers in human (ER−/PR−/HER2−/CK5/6+/p63+). Although HER2/SV40er tumors also exhibited a similar IHC expression pattern of basal-type human breast cancer (ER−/PR−/CK5/6+/p63+), they were HER2-positive, resembling basal-HER2 subgroup of human breast cancer (ER−/PR−/HER2+/CK5/6+/p63+) (17).
Because lentiviral gene transfer was used in this model system, it is conceivable that HER2/SV40er and KRAS/SV40er tumors developed as a consequence of a random positional effect of lentiviral integration into the human genome. However, the epithelial organoids used in generating the p53sh/HER2 or p53sh/KRAS/GFP models are from the same donor patient as the ones used in HER2/SV40er and KRAS/SV40er models. Therefore, it is unlikely that positional effect contributed to the tumor development in those models.
In Kuperwasser et al. (8), spontaneous carcinomas developed from 1 of 10 mammoplasty specimens without any genetic modification, a result that authors noted could not be reproduced with materials from the same patient. Preexisting genetic/epigenetic changes in a subpopulation of the donor organoids is likely a factor contributing to tumor development in the first case. To determine the effect of different donor material on tumor development, we repeated the HER2/SV40er model by using epithelial organoids obtained from 4 additional patient samples (donors 2–5; Table 1, experiment sets C, D, E, and F). As a positive control, HER2/SV40er tissue reconstitution was repeated using organoids from donor 1 (n = 4; Table 1, experiment set B). Tumors developed from all 4 of the patient donor epithelia, with similar kinetics and at similar frequencies as those from the original patient sample, donor 1. Together, these findings establish that the HER2/SV40er and KRAS/SV40er genetic combinations can drive efficient transformation of normal human breast epithelial cells into highly aggressive invasive carcinomas in vivo.
To further verify the tumorigenic potential of the HIM tumors, cancer cells were isolated from 6 primary HER2/SV40er tumors and 6 primary KRAS/SV40er tumors and reinjected into humanized fat pads of recipient mice. Tumors developed from both HER2/SV40er and KRAS/SV40er cancer cells, demonstrating their full malignant transformation (Table S2).
Breast tumors represent highly complex tissue systems wherein poorly defined, yet critical, heterotypic interactions take place between tumor cells and their microenvironment (10). To assess the influence of various stromal fibroblasts on tumor development, we reconstituted breast tissue recombinants with HER2/SV40er-transduced organoids either alone or together with various human breast stromal fibroblasts (Table 1). Consistent with previous experiments, tumors were efficiently generated from tissue recombinants when the HER2/SV40er organoids were comixed with immortalized fibroblasts, RMFs with or without enforced expression of HGF (RMF-HGF vs. RMF-EG in Table 1, experiment sets A, B, and G). In contrast, tumor development was rarely observed when the same HER2/SV40er organoids were implanted either alone or comixed with normal primary fibroblasts derived from 2 different patient samples (Table 1, experiment sets H, I, and J). Consistent with previous studies, our results demonstrate that specific stromal properties can dramatically influence the tumorigenic potential of oncogenically primed human breast tissue in vivo (4).
Signature Breast Cancer Genes—p53R175H/CCND1/PI3K/KRAS—Drive the Development of Human Low-Grade Breast Adenocarcinoma.
Although SV40er has been used extensively to transform human cells, it is not considered to play a pathogenetic role in spontaneous human breast cancer in the clinic. We therefore selected a specific set of oncogenes that are implicated in the etiology of human breast cancer and target the pathways that are known to be affected by SV40er (16). In humans, more than 40% of breast cancers exhibit overexpression of cyclin D1 (CCND1), which inhibits RB activity (3); 30–60% harbor mutations of p53 (2); and 20–40% of breast cancers carry mutations of PIK3CA (1). We therefore chose to target both RB and p53 pathways through overexpression of CCND1 and a point mutant allele of p53 (p53R175H). In addition, we targeted the PI3K pathway by overexpressing a constitutively active form of PIK3CA.
Human breast epithelial organoids from patient no. 1 (donor 1) were transduced with KRAS/p53R175H/CCND1/PIK3CA and reconstituted into a total of 20 murine mammary glands. Compared with HER2/SV40er and KRAS/SV40er models, tumor latency in this model was, on average, longer and displayed more variability. Between 2 and 9 months after implantation, tumors developed in 90% (18 of 20) of the tissue recombinants (Fig. 1C). These tumors presented as invasive ductal adenocarcinomas that were highly reminiscent of spontaneous, invasive, low-grade human breast ductal adenocarcinoma in humans. IHC performed on tumor sections verified that the cancer cells were of epithelial cell origin and expressed p53R175H. These tumors also displayed prominent areas of stromal desmoplasia, and the adjacent fibroblasts expressed αSMA. Similar to the KRAS/SV40er tumors, IHC revealed that the KRAS/p53R175H/CCND1/PIK3CA tumors did not express ER/PR or HER2, but were positive for cytokeratin 5/6 and p63 (ER−/PR−/HER2−/CK5/6+/p63+), indicative of basal-like breast cancers (Fig. S5). Stable integration of all of the lentiviral genes (p53R175H, CCND1, PIK3CA, and KRAS) and expression of those integrated genes were confirmed by genomic PCR and RT-PCR analyses (Fig. S6).
To test the reproducibility of the KRAS/p53R175H/CCND1/PIK3CA genetic combination and to evaluate the potential contribution of preexisting genetic changes in donor material, human breast epithelial organoids from a different patient sample (donor 2) were used to generate 20 KRAS/p53R175H/CCND1/PIK3CA tissue recombinants (Fig. S7). Between 2 and 7 months after implantation, the breast tissue recombinants were collected and subjected to histopathologic examinations. In this set of experiments, the KRAS/p53R175H/CCND1/PIK3CA tissue recombinants gave rise to various preneoplastic lesions, such as hyperplasia and cribform subtype of human DCIS. Despite the greater variability of precursor lesions, emerging invasive carcinoma was observed in 95% (19 of 20) of the tissue recombinants.
Robust Telomerase Activity Was Detected in the Spontaneous HIM Tumors.
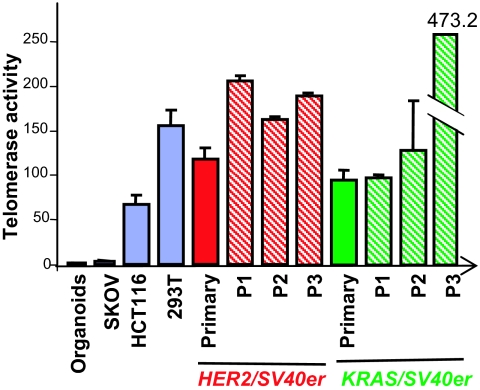
Unlike reported cell culture-based transformation systems (18), enforced transduction of hTERT was not required for transformation of primary breast epithelial cells in vivo for both the HER2/SV40er and KRAS/SV40er models and the KRAS/p53R175H/CCND1/PIK3CA model. Given the frequent activation of telomerase in advanced human cancers, we analyzed telomerase activity in normal human breast organoids, in primary HER2/SV40er and KRAS/SV40er tumors (1 tumor each), and in the same tumors propagated up to 3 times in secondary animals (Fig. 2). Although the donor breast epithelial organoid lysates possessed barely detectable levels of telomerase, both HER2/SV40er and KRAS/SV40er primary tumor lysates exhibited telomerase activity levels comparable to those of HCT116 and HEK293T human cancer cell lines (19). In addition, high telomerase activity was maintained throughout propagation of the primary tumors. Therefore, HIM tumors exhibited high telomerase activity without enforced transduction of hTERT. This observation is intriguing in light of the well-established requirement for enforced hTERT in the transformation of cultured human cells, a difference that may reflect the presence of antioncogenic stresses in cell culture and/or in vivo factors capable of driving robust activation of hTERT gene expression.
Fig. 2.
Telomerase activities in both HER2/SV40er and KRAS/SV40er tumors are comparable to that in 2 human cancer cell lines: HCT116 and HEK293T. Proteins were extracted from HER2/SV40er and KRAS/SV40er primary tumors as well as propagated tumors at passage 1 (P1), passage 2 (P2), and passage (P2). Telomerase activity in those protein lysates was analyzed by a PCR-based quantitative telomerase detection method (Allied Biotech Inc.). All telomerase activity was normalized to human breast organoids, the primary human breast epithelial cells from which the tumors were derived. Human cell lines with low (SKOV), medium (HCT116), and high (293T) telomerase activities were used in this study as controls.
Tumor Development Progresses Through Distinct Stages.
Analysis across many human breast cancer specimens points to a clear disease evolution process defined by distinct histopathological stages; thus, the creation of a model system that captures the natural developmental history of this disease would facilitate the study of breast cancer on many levels. To assess the extent in which the HIM model recapitulates human disease progression in vivo, we monitored outgrowth development in the HER2/SV40er tissue recombinants over time. Six reconstituted breast tissues were collected at each time point on days 7, 10, 17, 25, 35, and 45 after implantation and subjected to histopathological analysis.
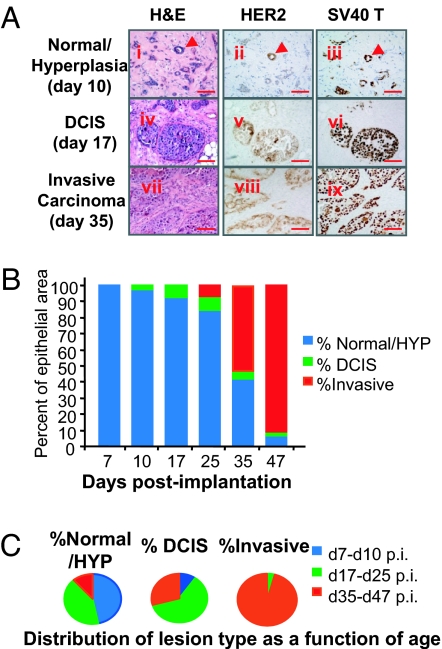
On day 7 after implantation, the organoids were still undergoing remodeling to form human breast acini, which by day 10 after implantation had matured to the stage of lumen formation and primitive ductal outgrowth (Fig. 3A i–iii). Immunohistochemistry on cross-sections for HER2 and SV40 LT expression revealed that HER2+ organoids and SV40er+ organoids participated in the formation of histologically normal human breast acini. There were noticeably more SV40er+ epithelial cells than HER2+ or double-stained HER2+/SV40er+ cells. This may be the result of the higher titer of the SV40er lentiviruses used to infect human breast organoids prior to injection. Nonetheless, HER2+/SV40er+ acini/hyperplasia were readily detectable in those glands. By day 17 after infection, DCIS-like outgrowths from breast tissue recombinants became readily detectable (Fig. 3A iv–vi). These structures consisted of more than 5 layers of relatively uniform epithelial cells filling the ductal lumen and exhibiting features of nuclear atypia, and sometimes central necrosis, while maintaining a prominent basement membrane. By day 35 after infection, invasive carcinomas became the prominent outgrowth in the tissue recombinants (Fig. 3A vii–ix). All of the DCIS and invasive lesions stained positive for both HER2 and SV40er expression, indicating they were indeed derived from the infected organoids.
Fig. 3.
HER2/SV40er tumors develop through a multistep tumorigenesis process in the reconstituted human breast tissue. HER2/SV40er tissue recombinants were subjected to histopathological analysis at various days after implantation. (A) Histopathological analysis of representative breast tissue outgrowth of normal/hyperplasia (Ai–Aiii), DCIS (Aiv–Avi), and invasive carcinoma (Avii–Aix) from tissue recombinants collected at the indicated time points. (Scale bar: 50 μm.) (B) Percent of epithelial area coverage for each lesion type at individual time points. At day 7 after implantation, only normal and hyperplastic outgrowth was observed. DCIS began to emerge at day 10 after implantation, and it became more abundant at days 17 and 25 after implantation. Invasive carcinoma emerged at day 25 after implantation, at which point the lesion grew rapidly, such that by day 47 after implantation, the invasive area took over the majority of the gland area. (C) Distribution of lesion type as a function of age. DCIS outgrowth was most frequently detected between days 17 and 25 after implantation (p.i.), whereas invasive carcinoma was most frequently observed after day 35 after implantation.
To quantify the frequency of different subtypes of breast tissue outgrowth over time, 3 to 6 cross-sections for each of the total 36 tissue recombinants in this study were stained with H&E and were subjected to blinded histopathological examination. Tumor progression was quantified by counting the percent epithelial area for each lesion type in the tissue recombinants over time (Fig. 3 B and C). Normal/hyperplastic outgrowths predominated at the early observation times (days 7–10 after infection and days 17–25 after infection), and these were gradually replaced by more advanced outgrowths at later times. DCIS-like structures were most frequently detected between days 17 and 25 after infection. By day 35 after infection, invasive tumor outgrowths were the predominant outgrowth in the reconstituted human breast tissue. Collectively, these data indicate that the HIM model system recapitulates the multistep process of human breast cancer progression.
HER2/SV40er Primary Tumors Respond to both Small Molecule and Biological HER2 Antagonists.
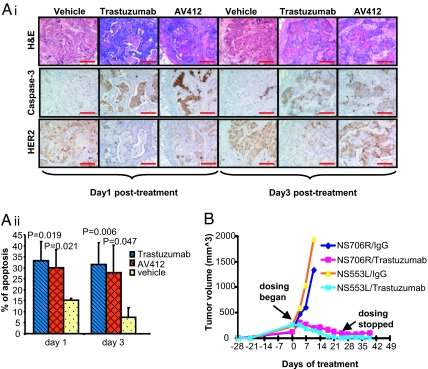
Given the remarkable similarities between the HIM tumors and human breast cancers, we next sought to determine the utility of HIM tumors in targeted drug treatment studies. We first treated mice bearing HER2/SV40er primary human breast tissue recombinants with 2 HER2 antagonists, trastuzumab (20) or AV412 (21), for 4 days within the period during which DCIS-like/invasive cancer lesions were prominent but gross palpable tumors not yet evident (days 26–29 after infection). The treated tissue recombinants were collected 1 and 3 days following the last day of drug treatment (n = 6). The anticancer effect of drug treatment was assessed directly on serial tumor tissue sections (Fig. 4Ai). At both 1 and 3 days following treatment, both trastuzumab- and AV412-treated tumors exhibited significantly more apoptotic regions compared with tumors from mice treated with vehicle alone. Furthermore, the number of carcinoma cells expressing HER2 was reduced following treatment with either trastuzumab or AV412. The difference between treatment and control groups was quantified by measuring percent of apoptotic areas in all tissue recombinants (Fig. 4Aii). Both trastuzumab- and AV412-treated groups exhibited a significant response to drug compared with the vehicle-treated cohort 1 and 3 days following treatment. These data show that short-course treatment schedules with 2 different anti-HER2 therapies provokes apoptosis in the HER2-driven HIM tumors, mirroring clinical observations with trastuzumab (22). In addition, the comparable effectiveness of AV412 encourages its evaluation as a potential therapeutic agent in the treatment of breast cancers driven by HER2.
Fig. 4.
HER2/SV40er HIM tumors responded to the treatments of targeted therapies. (A) Primary HER2/SV40er HIM tumors responded to short treatment with HER2 antagonists. (Ai) Mice carrying HER2/SV40er human breast tissue recombinants were treated with trastuzumab, AV412, or vehicle for 4 days at the period during which DCIS-like/invasive cancer lesions are prominent but no palpable tumors are evident. Compared with vehicle control group, both trastuzumab and AV412 treatment groups exhibited extensive apoptotic areas (labeled by activated caspase-3 positivity) and significantly fewer tumor cells (labeled by HER2 positivity). (Scale bar: 100 μm.) (Aii) Quantization of apoptotic areas in primary human breast tissue recombinants among all dosing groups. There were 6 tissue recombinants in each group. (B) Two independent HER2/SV40er HIM tumor lines (NS706R and NS553L) regressed upon treatment with trastuzumab. Tumor-bearing mice were treated with either trastuzumab or human IgG control at 20 mpk. Tumor growth curves of treated mice were plotted as a function of time.
To investigate the long-term response of HIM tumors to trastuzumab, cancer cells from 2 different primary HER2/SV40er tumors (NS706R and NS553L) were propagated into new recipient mice and were treated with either trastuzumab (20 mg/kg) or control human IgG for 25 days (Fig. 4B). Dosing of trastuzumab was then stopped at day 25, and the mice were continually monitored for an additional 2 weeks. Remarkably, all mice treated with trastuzumab showed signs of tumor regression after 1 week and no palpable tumors detectable by the end of treatment at day 25. All of the mice remained tumor-free for at least an additional 2 weeks. In contrast, due to excessive tumor burden, the experiment in which mice were treated with human IgG had to be terminated 11 days after the first dosing. Thus, the HER2-driven HIM model is responsive to trastuzumab.
Discussion
We have established a robust human breast cancer in mouse model system in which human preneoplastic lesions and advanced breast cancers can be generated in vivo from genetically engineered human breast epithelial organoids in association with an appropriate human stromal microenvironment (Table S3). The unique combination of genetic engineering and tissue reconstitution has enabled us to gain better understanding of mechanisms involving human breast cancer tumorigenesis.
By using the HIM model approach, we analyzed the genetic requirements for transforming human breast epithelial cells in vivo. Disruption of the p53 pathway and HER2 or KRAS oncogenic functions is not sufficient to generate human breast lesions beyond DCIS (p53sh/HER2 and p53sh/KRAS models). Development of invasive carcinoma requires additional alterations targeting the pRB and PI3K pathways, conferred by the enforced expression of either CCND1/PIK3CA or SV40er (KRAS/p53R175H/CCND1/PI3K, KRAS/SV40er and HER2/SV40er models). Therefore, deregulation of the p53, pRB, and PI3K pathways, as well as overexpression of HER2 or KRAS oncogenes is sufficient for the development of human breast tumors in this in vivo model system.
Widespread introduction of high-quality mammography screening has caused a dramatic increase in the diagnosis of DCIS. Even though most DCISs do not progress to invasive cancer, most patients are treated with lumpectomy plus radiotherapy. Overtreatment of DCIS patients continues to be an important clinical issue (23). One major contributing factor to this limitation is the lack of model systems that generate both low-risk and high-risk DCIS outgrowths resembling those in humans.
Despite the powerful natural selection that occurs in tumorigenesis, the DCIS in p53sh/HER2 tissue recombinants did not progress into invasive tumors, thus representing a model to study the low-risk DCIS observed in patients. On the other hand, the HER2/SV40er model yielded high-risk DCISs that readily evolved into invasive carcinoma. Molecular profiling of these different forms of DCIS outgrowth may help to define biomarkers that can identify DCIS that will develop into invasive tumors. Moreover, the ability to manipulate the genetic profile of organoids affords an opportunity to discover and validate the genetic requirements needed for progression from DCIS to invasive tumors in the p53sh/HER2 model.
Human breast cancer comprises tumors with complex histopathology and genetic changes. The HIM system mirrors this complexity in that tumors generated with different genetic combinations gave rise to invasive tumors of different histological features, ranging from well/moderately differentiated carcinoma (KRAS/p53R175H/CCND1/PIK3CA) to invasive carcinoma (HER2/SV40er and KRAS/SV40er). In addition, IHC analysis classified the KRAS/p53R175H/CCND1/PIK3CA and the KRAS/SV40er tumors as basal-type breast cancer and the HER2/SV40er tumors as the basal-HER2 subtype of human breast cancer. How those histological variations reflect somatic changes, as well as drug treatment outcome, remains to be determined.
One goal for the creation of genetically engineered human tumors is to provide a preclinical model to evaluate the efficacy of candidate therapies in breast cancer. With the increasing knowledge of specific genetic alterations in breast cancer, there is now an opportunity to correlate activity of anticancer agents with specific genetic alterations. As a preliminary proof of principle, we showed that the approved anti-HER2 antibody trastuzumab is effective at increasing apoptosis and inhibiting tumor growth of engineered HER2-expressing tumor, demonstrating that these experimental tumors exhibit a drug response modeling the clinical response of HER2+ breast cancers to trastuzumab. Furthermore, the experimental small molecule HER2 antagonist AV-412 is also effective at increasing apoptosis of HER2-driven tumors, suggesting that this experimental agent may also show promise against HER2-driven breast tumors.
The ability of the HIM system to generate human tumors with defined genetic modifications, that follow the multistep tumor progression process, and whose development is strongly influenced by the specific stromal microenvironment, holds potential as an improved preclinical model system for more rigorous and accurate drug testing.
Materials and Methods
Tissues.
All human breast tissue procurement for these experiments was obtained in compliance with laws and institutional guidelines as approved by the institutional review board from Brigham & Women's Hospital.
Infection and Injection of Human Breast Organoids.
Freshly isolated or freshly thawed organoids were subjected to infection with lentiviruses as described previously (SI Methods). Within 4–18 h after the last infection, the infected organoids were injected into humanized fat pads without selection.
Constructs and Virus Production.
See SI Methods for details.
Whole Mounts and IHC.
Whole-mount analysis and IHC were performed as described previously (19). Antibodies used were pan-cytokeratin (catalogue no. A0575; DAKO), αSMA (catalogue no. NCL-SMA; Novo Castra), HER2 (catalogue no. 2242; Cell Signaling Technology), SV40T Ag (catalogue no. sc-147; Santa Cruz Biotechnology), p53 (catalogue no. sc-6243; Santa Cruz Biotechnology), and Cleaved Caspase-3 (catalogue no. CP-229B; Biocare Medical).
Telomerase Assay.
Telomerase activity was analyzed by using the PCR-based Quantitative Telomerase Detection Kit (Allied Biotech Inc.). Each tumor sample was assayed in quadruplicates.
Tumor Treatment.
AV412 was resuspended in 0.5% tragacanth and dosed daily at 100 mg/kg. Trastuzumab was dosed twice a week at 20 mg/kg.
Supplementary Material
Acknowledgments.
We gratefully acknowledge Geoffrey Boynton and Edyta Tyminski for expert technical assistance, and Steve Clark, William M. Rideout 3rd, Joerg Heyer, and Rónán C. O'Hagan for critical reading of the manuscript.
Footnotes
Conflict of interest statement: M.W., L.B., A.B.C., C.F., L.J., L.C., S.V., S.B., Q.S., K.C., Z.C., and M.O.R. are employees of AVEO Pharmaceuticals Inc.; C.K. and M.B. are consultants for AVEO Pharmaceuticals Inc.; R.A.D. is a cofounder of AVEO Pharmaceuticals Inc. M.W., C.K., and M.O.R. have filed a patent application based on the work reported in this manuscript.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811785106/DCSupplemental.
References
- 1.Campbell IG, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 2.Coles C, et al. p53 mutations in breast cancer. Cancer Res. 1992;52:5291–5298. [PubMed] [Google Scholar]
- 3.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Overexpression of cyclinD1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients. Int J Cancer. 2002;98:415–418. doi: 10.1002/ijc.10151. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Slamon DJ. Monoclonal antibody therapy for breast cancer: Herceptin. Cancer Chemother Biol Response Modif. 2003;21:223–233. doi: 10.1016/s0921-4410(03)21010-3. [DOI] [PubMed] [Google Scholar]
- 6.Truong K, et al. Evidence for in vitro selection during cell culturing of breast cancer: Detection by flow and image cytometry. Cancer Genet Cytogenet. 1999;114:154–155. doi: 10.1016/s0165-4608(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 7.Sharpless NE, Depinho RA. The mighty mouse: Genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 8.Kuperwasser C, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmar H, et al. A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology. 2002;143:4886–4896. doi: 10.1210/en.2002-220570. [DOI] [PubMed] [Google Scholar]
- 10.Bacus SS, et al. HER-2/neu oncogene expression, DNA ploidy and proliferation index in breast cancer. Anal Quant Cytol Histol. 1992;14:433–445. [PubMed] [Google Scholar]
- 11.Miyakis S, Sourvinos G, Spandidos DA. Differential expression and mutation of the ras family genes in human breast cancer. Biochem Biophys Res Commun. 1998;251:609–612. doi: 10.1006/bbrc.1998.9527. [DOI] [PubMed] [Google Scholar]
- 12.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 13.Ménard S, et al. HER2 overexpression in various tumor types, focussing on its relationship to the development of invasive breast cancer. Ann Oncol. 2001;12:S15–S19. doi: 10.1093/annonc/12.suppl_1.s15. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda H, Hirohashi S. Multiple developmental pathways of highly aggressive breast cancers disclosed by comparison of histological grades and c-erbB-2 expression patterns in both the non-invasive and invasive portions. Pathol Int. 1998;48:518–525. doi: 10.1111/j.1440-1827.1998.tb03943.x. [DOI] [PubMed] [Google Scholar]
- 15.Guy CT, Cardiff RD, Muller WJ. Activated neu induces rapid tumor progression. J Biol Chem. 1996;271:7673–7678. doi: 10.1074/jbc.271.13.7673. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JJ, et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, et al. Basal-HER2 phenotype shows poorer survival than basal-like phenotype in hormone receptor-negative invasive breast cancers. Hum Pathol. 2008;39:167–174. doi: 10.1016/j.humpath.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Elenbaas B, et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Mar V, Zhou W, Harrington L, Robinson MO. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, et al. American Association for Cancer Research Annual Meeting: Proceedings. Philadelphia: American Association for Cancer Research; 2007. AV-412, a potent EGFR/HER2 TK inhibitor causes tumor regression in novel genetically engineered EGFRL858R and EGFRL858R&T790M lung tumor models. abstr 4008. [Google Scholar]
- 22.Mohsin SK, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–2468. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 23.Napoli M. Overdiagnosis and overtreatment. Am J Nurs. 2001;101:11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.