Abstract
Objective
The objective was to extract, quantify and characterize the uterine magnetomyographic (MMG) signals that correspond to the electrophysiological activity of the uterus.
Methods
Transabdominal MMG recordings with high spatial-temporal resolution were performed with the use of the 151 non-invasive magnetic sensors system. The extraction, quantification and characterization procedures were developed and applied to representative MMG signals that were recorded from a pregnant woman at regular intervals starting at 37 weeks of gestation until the subject reached active labor.
Results
Multiple MMG recordings were successfully performed on the subject before she went into active labor. The extracted MMG burst activity showed a statistically significant correlation (r=0.2;p<0.001) with the contractile events perceived by mothers. The time frequency analysis of the burst activity showed a power shift towards higher frequency at 48 hours before the subject went into active labor as compared to earlier recordings. Further there was a gradual increase in the synchrony in the higher frequency band as the subject reached close to active labor.
Conclusions
The non-invasive recording of the magnetic signals of pregnant uterus with high spatial-temporal resolution can provide an insight into the preparatory phase of labor and has the potential of predicting term and preterm labor.
Keywords: Uterine contractions, Magnetomyography, Electromyography, Labor
1. Introduction
The uterus is normally able to accomplish the remarkable task of maintaining an environment in which uterine contractile activity is suppressed during the development of the fetus. At term (> 37 weeks of gestation) however, the individual depolarization of myometrial cells is initiated and coordinated to produce organized contractions causing the expulsion of the fetus from the mother’s body [1]. At times, this coordinated activity occurs early in gestation (< 37 weeks) resulting in premature birth of the fetus. Despite aggressive prematurity prevention programs designed to identify patients early and intervene more rapidly, the incidence of preterm birth has increased in America and is now approaching 10% of all births annually [1]. The immature newborn often develops significant medical complications such as long-term neurological and respiratory problems. These infants require long-term neonatal intensive care at a huge emotional and financial cost to families and hospitals.
Currently there are two methods employed to record the electrophysiological activity of the uterine contractions. The first one is electromyography (EMG)[2,3,4] which is recorded by electrodes attached to the abdomen, and the second is the newly established method based on recording magnetic fields corresponding to electrical fields by magnetomyography [5,6]. Both these techniques measure the electrical (magnetic) activity on the surface of the maternal abdomen that is a result of a sequence of bursts or groups of action potentials that are generated and propagated in the uterine muscle.
In this paper we present the techniques and results from the extraction, quantification and characterization of uterine magnetomyographic activity applied to a representative recording. Once the technique is established we can work towards achieving a comprehensive instrumentation and protocol that would aid in prediction of onset of labor. This ability would be of great clinical benefit for the management of the term patient and especially for the management of patients at high risk for premature delivery.
2. Methods
2.1 Uterine Magnetomyography
All electrophysiological phenomena are characterized by the flow of ion currents within the body. These currents are detectable by measuring potentials inside or on the surface of the body. The physics of electromagnetism predicts that the flow of current will also result in a magnetic field. Consequently, common clinical electrophysiological measurements such as the electrocardiogram (ECG), and electroencephalogram (EEG) have magnetic homologues the magnetocardiogram (MCG) and the magnetoencephalogram (MEG), respectively [7]. However, unlike uterine EMG signals, MMG signals are detectable outside the boundary of the skin without making electrical contact with the body. Also the uterine electrical activity reaching the abdominal surface is strongly dependent upon tissue conductivity. The measured electrical activity arises from volume currents flowing in the body to the electrode sites, and not directly due to the primary current generators. It is well known that uterine EMG signals suffer some degree of attenuation by the time they reach the surface of the maternal abdomen. By contrast, magnetic field recordings are more strongly coupled to the primary currents and are much less dependent on tissue conductivity boundaries. Unlike electrical recordings, the magnetic recordings are independent of any kind of reference, thus ensuring that each sensor mainly records localized activity. Further the spatial-temporal resolution obtained from the abdominal surface electrode recordings is limited based on the electrical properties of the abdomen and the practical difficulties of placing several pairs of electrodes on the mother.
2.2 Instrumentation
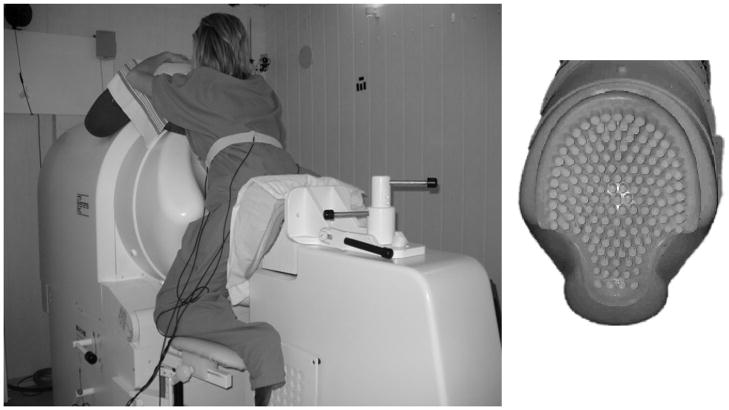
At the University of Arkansas for Medical Sciences (UAMS) we have installed the world’s first biomagnetic sensing system that was specifically designed to study the various aspects of maternal-fetal health. The SARA (SQUID Array for Reproductive Assessment) system [5] (Figure 1) has a high spatial-temporal resolution with 151 sensors that passively record the weak biomagnetic signals and is based on a technique called magnetoencephalography [7]. SQUID is an acronym for Superconducting Quantum Interference Device. SARA is designed to cover the entire maternal abdomen thus providing us with electrophysiological signals from the maternal heart, fetal heart, fetal brain and uterine smooth muscle activity.
Figure 1.
Left: Mother positioned on the SARA system Right: A cut-out view of the sensor lay-out.
2.3 General Recording Procedure and Data Analysis
Recording Methods
After obtaining a written consent, the subject was asked to sit comfortably and lean forward on to the sensor array. The first recording on this subject was performed at 37 weeks of gestation and the subject returned for repeated measurements till she reached active labor. The last recording performed on subject was at 39 weeks. Each recording session lasted approximately 20 minutes with a sampling rate of 250 Hz. The measured spatial-temporal signals are a complex superposition of different sources, which must be separated efficiently into their constituent waveforms. As a first step the interfering maternal and fetal cardiac signals are attenuated by band pass filtering the data between 0.1–1 Hz. A notch filter was applied to suppress the maternal breathing artifact (0.25–0.35 Hz) and the data was down sampled to 32 Hz for further analysis. A contour map of the field distribution was plotted to help determine the areas of activity over the abdomen. In the next step we proceed to extract, quantify and characterize the MMG signals.
Extraction
In order to extract the uterine electrophysiological burst patterns, we apply Hilbert-Wavelet transform (HWT) and affinity propagation based cluster analysis to the MMG data [8]. To accomplish this we decompose the MMG signals using discrete wavelet transform and quantify the power in each frequency band by computing the Hilbert amplitude of the signal and integrating the power over all sensors.
Quantification
Using spectral analysis Garfield et al [4,9,10] have shown that the power density of uterine EMG bursts in patients during active labor peaked at 0.71 ± 0.05 Hz as compared to non laboring term (0.48 ± 0.03 Hz) patients. Also, the power density peak values were comparatively low for patients not in labor with respect to patients in active labor. Based on these findings we performed the time frequency analysis on the spatial-temporal data obtained from uterine MMG recordings to understand the variation of frequency of the process as a function of time. To accomplish this we considered the signals reconstructed at the different levels of wavelet decomposition (as done in the MMG burst identification) that correspond to different frequency bands and computed the Hilbert transform to quantify the signal power in that band. In a wavelet analysis, the signal is decomposed into different frequency bands, in different levels of decomposition. However, in general, the decomposition levels vary in most of the wavelets in a dyadic manner. Mathematically, the frequency bins of the subsequent bands (level, j) vary as follows: fs/2(j−1) to fs/2j, where fs is the sampling frequency of the signal. In case the frequency bands defaulted in the wavelet analysis is not within the frequency range of interest, the original data is resampled to a suitable frequency so as to aid the wavelet analysis to decompose the signal in the desired frequency range. In our study the original signal was sampled at 250 Hz. Based on the earlier study by Garfield et al, [4,9,10] we included the following two frequency bands;(i) low frequency 0.1–0.4 Hz and high frequency 0.4–0.8 Hz. However, maternal breathing is a prominent signal around the frequency of 0.33 Hz and hence to suppress this activity we used a notch filter between 0.25–0.4 Hz. In order to obtain (or at least close) these two frequency bands from the wavelet analysis, we downsampled the signals to 32 Hz. Based on the above considerations the decompositions closest to our low and high frequency bands of interests were 0.25–0.5 Hz and 0.5–1 Hz. To quantify the power we computed the average of the Hilbert amplitude in both the bands. In order to understand the shift in the power from lower-frequency to higher-frequency we computed the ratio of the power (high to low) in the two bands. This ratio was calculated for MMG signals from each SARA sensor.
Characterization
In order to characterize the uterine burst activity we investigated the phase synchronization of the spatio-temporal MMG signals. This analysis would reveal the level of synchrony in signals across the sensors in the longitudinal recordings. The assumption [3] is that infrequent and unsynchronized low uterine electrical activity occurs throughout most of the pregnancy. However, at term, changes in the uterine physiology results in better propagation and synchronization of electrical burst activity throughout the uterus causing rhythmic contractions leading to the delivery of the fetus. Thus the efficiency of contractions leading to labor depends on the synchronous burst activity over a large area of the uterus.
To study the synchronization [11], the MMG data was divided into 15 sec disjoint time windows and for each sensor (center) phase synchrony (synchronization index, SI) was computed with six (sensors) of its spatial neighbors. The average of these six indexes was assigned as the SI of the chosen center. The analysis was repeated for all time windows. To correlate these results with the number of days from the last recording to the time of active labor, in each window a 99 percentile of the SIs was computed. The median value of this quantity from all the windows was taken as a quantification of the SI of the data. A contour plot of SI on the sensor space will generate an iso-synchronization index (ISI) map which could be compared across different days of the longitudinal study. Again based on studies by Garfield et al [4,9,10], our analysis was done in two different frequency bands – (0.1–0.4 Hz) and (0.4–0.8 Hz) with a notch filter set at 0.25–0.4 Hz to eliminated maternal breathing signals. To facilitate comparison, the color scale of all the maps is set to the same value.
3. Results
3.1 Extraction of Uterine MMG activity
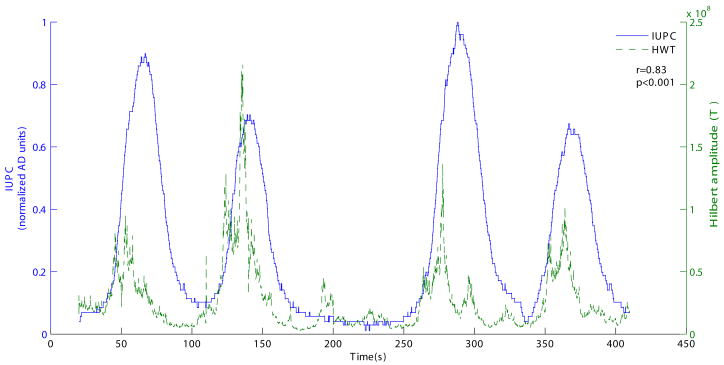
The performance of the extraction algorithm was first tested by applying it to a gold standard data set from our database that was obtained by simultaneous recording of IUPC (intrauterine pressure catheter) and MMG. To facilitate the comparison of the result with IUPC data (Figure 2), we sum the results of HWT from all the sensors. In order to quantify the correlations between the Hilbert energy and IUPC data, we performed cross-correlation analysis. There was a maximum correlation (coefficient) of 0.83 (p<0.001) with a delay of − 9.72 seconds between the two signals, indicating that mechanical activity (IUPC) follows the electrical activity (magnetic signal). Further, to assess the degree of agreement in the contractions identified by IUPC and HWT approach, we constructed binary decision signal (BDS) which assumed a value “1” in the instances the methods identified a contraction and “0” in the non-contractile instances. With the BDS constructed from both approaches we computed the following four statistics: - True Positive (TP) 0.71, True Negative (TN) 0.92, False Negative (FN) 0.29, False Positive (FP) 0.08. These statistics indicate that there is a high degree of sensitivity and specificity between the two approaches.
Figure 2.
Comparison of the results of extraction algorithm with (Top) Intra-Uterine Pressure Catheter Data (IUPC) and (bottom) linear combination of Hilbert Energy from all the SARA sensors.
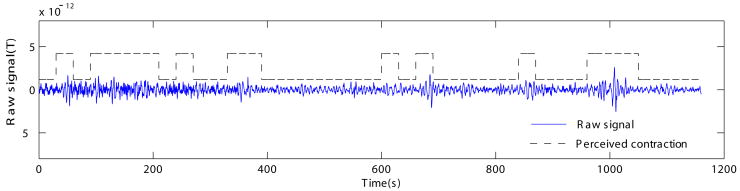
After this test, the algorithm was applied to the recorded data set described in this study. Figure 3 shows a representative channel showing uterine magnetomyographic activity along with the instances of maternal perception. Quantitatively, there was a significant degree of correlation (r=0.2 and p<0.001) between the contractile events perceived by mothers and those identified by HWT. However, in parts of the data, HWT also identified the low-amplitude contractions, which mothers could not perceive.
Figure 3.
Representative channel showing uterine magnetomyographic activity and its correlation with maternal perception
3.2 Quantification of Uterine MMG activity - Time Frequency Analysis
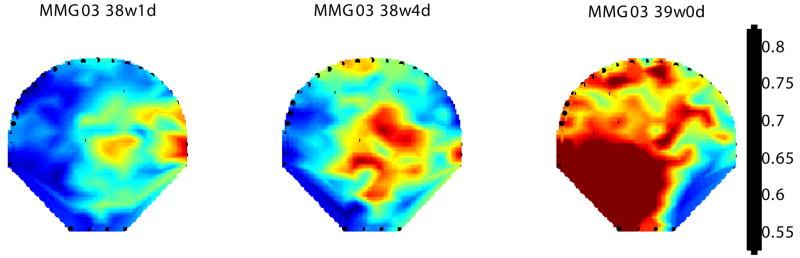
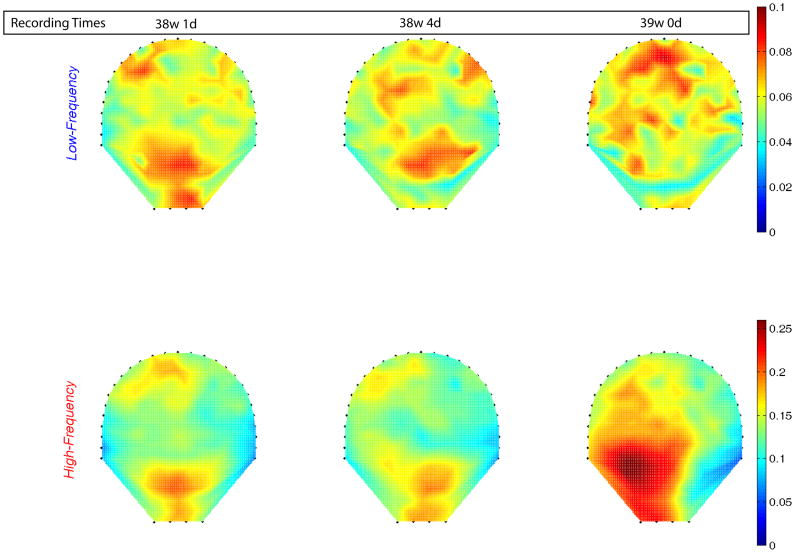
Figure 4 shows a contour diagram of this ratio overlaid on the 151 sensor map covering the maternal abdomen for the subject undergoing longitudinal measurements before she approached active labor. We quantified the power and computed its ratio (high to low) in the two bands. The map reflects the progression of change in the power shift towards higher frequency starting from the earlier recording (38 weeks 1 day) to the last recording at 48 hours (39 weeks 0 days) before the subject went into active labor. In addition, based on the enhanced SARA spatial resolution, we also observe a wider distribution of the increased-MMG power ratio over larger sensor area as the uterus prepares to approach towards active labor.
Figure 4.
Contour plot of the ratio of power in higher-frequency (0.5–1 Hz) to lower-frequency (0.25–0.5 Hz) for MMG data from 38 weeks 1 day until 48 hours before the subject went into active labor. The frequency content of the MMG bursts are color coded and the contour plot is overlaid on a 151 channel sensor map to show the spatial distribution.
3.3 Characterization - Synchronization of Uterine MMG Activity
In Figure 5 we present the ISI maps computed in both the bands for MMG data from 38 weeks 1 day to 39 weeks 0 days (48 hours prior to active labor). Synchrony in the 0.4–0.8 Hz band was higher than the 0.1–0.25 Hz band. Figure 5 shows a gradual increase in the synchrony in the higher frequency band as the subject reached close to active labor. There was no consistent change observed in the low frequency band. Thus, based on the high frequency band, the synchronization analysis shows that close to the onset of labor there is synchronization across different areas of the uterus which indicates that the uterus goes through a preparatory phase.
Figure 5.
ISI maps computed in lower and higher frequency bands from 38 weeks 1 day to 48 hours before active labor (39 weeks 0 days). There is a gradual increase in the synchrony and its spatial distribution as well, as the subject progressed toward active labor.
4. Discussion
Our past studies have demonstrated the feasibility of performing non-invasive MMG recordings corresponding to the electrical activity of the uterine smooth muscle [5]. We have shown that the relative strength of MMG activity increased in patients in active labor (>= 3 cm cervical dilation) as compared to patients with contractions but not in active labor (both term and pre-term) [6]. These patients were recruited from UAMS Labor and Delivery triage area where they had all presented for evaluation of onset of labor. The observed increase in peak amplitude values of magnetic activity of uterus in these subjects correlated with the development of labor within 48 hours of the recording. This would indicate an increased electrical activation of the myometrium close to the onset of labor.
To take this a step further we are continuing to perform longitudinal studies starting as early as 37 weeks of gestation to track the changes in the uterine electrophysiological activity until the subject reaches active labor. As shown in this report we have now developed and demonstrated the techniques that would enhance the extraction, quantification and characterization of the spatial and temporal parameters obtained from the longitudinal recording of uterine MMG signals. We now plan to apply these techniques to larger number of recordings and correlate the results with the clinical outcomes.
Since the MMG activity is recorded in the shielded room and is very sensitive to other mechanical and electrical interferences it is not practical to perform combined IUPC/TOCO measurements with MMG. Again due to the need for a gold standard comparison we performed one gold standard simultaneous IUPC/MMG recordings with special arrangements to minimize interference. This procedure is too cumbersome to perform on a routine basis on every subject hence we have to currently rely on maternal perception.
In summary, we have shown that it is possible to non-invasively record the magnetic signals corresponding to the electrophysiological activity of pregnant uterus with high spatial-temporal resolution. In future, this technique may have the potential of predicting term and preterm labor. However the underlying physiological mechanism of the uterine contraction is only partially understood and we believe that substantial increase in the knowledge of this field could be gained by improving the computational and analysis techniques recorded by SARA. This advancement could lead to the establishment of this technology as a clinical tool by providing the linkage between basic electrophysiology of the uterine contractions and data acquired using SARA.
Acknowledgments
This study is funded by National Institutes of Health/NIBIB grant R01 EB007264-01A2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Devedeux D, Marque C, Mansour S, et al. Uterine electromyography - A critical review. Am J Obstet Gynecol. 1993;169:1636–53. doi: 10.1016/0002-9378(93)90456-s. [DOI] [PubMed] [Google Scholar]
- 2.Steer CM. The electrical activity of the human uterus in normal and abnormal labor. Am J Obstet Gynecol. 1954;68:867–90. doi: 10.1016/s0002-9378(16)38329-6. [DOI] [PubMed] [Google Scholar]
- 3.Garfield RE, Chwalisz K, Shi L, Olson G, Saade GR. Instrumentation for the diagnosis of term and preterm labour -Review. J of Perinat Med. 1998;26(6):413–36. doi: 10.1515/jpme.1998.26.6.413. [DOI] [PubMed] [Google Scholar]
- 4.Garfield RE, Saade G, Buhimschi C, Buhimschi I, et al. Control and assessment of uterus and cervix during pregnancy and labour. Human Reproduction Update. 1998;4(5):673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- 5.Eswaran H, Preissl H, Wilson JD, Murphy P, Lowery CL. First Magnetomyographic Recordings of the Uterine Activity with Spatial-Temporal Resolution Using a 151 Channel Sensor Array. Am Journal of Obstet Gynecol. 2002;187(1):145–151. doi: 10.1067/mob.2002.123031. [DOI] [PubMed] [Google Scholar]
- 6.Eswaran H, Preissl H, Wilson JD, Murphy P, Lowery CL. Prediction of labor it term and preterm pregnancies using non-invasive magnetomyographic recordings of uterine contractions Am. Journal of Obstet Gynecol. 2004;190(6):1598–1602. doi: 10.1016/j.ajog.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 7.Williamson SJ, Romani GL, Kaufman L. Biomagnetism: an interdisciplinary approach. New York-London: Plenum Press; 1983. [Google Scholar]
- 8.Daubechies I. Orthonormal bases of compactly supported wavelets. Comm Pure Appl Math. 1988;41:909–996. [Google Scholar]
- 9.Garfield RE, Maner WL, MacKay LB, Schlembach D, Saade GR. Comparing uterine electromyography activity of antepartum patients versus term labor patients. Am J Obstet Gynecol. 2005;193(1):23–9. doi: 10.1016/j.ajog.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Maner WL, MacKay LB, Saade GR, Garfield RE. Characterization of abdominally acquired uterine electrical signals in humans, using a non-linear analytic method. Med Biol Eng Comput. 200;44(1–2):117–23. doi: 10.1007/s11517-005-0011-3. [DOI] [PubMed] [Google Scholar]
- 11.Ramon C, Preissl H, Wilson JD, Murphy P, Lowery CL, Eswaran H. Synchronization Analysis of Uterine Magnetic Activity During Contractions. BioMedical Engineering OnLine. 2005;4:55–59. doi: 10.1186/1475-925X-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]