Abstract
The pathological significance of the mechanisms of tumour immune-evasion and/or immunosuppression, such as loss of T cell signalling and increase in regulatory T cells (Tregs), has not been well established in the nasopharyngeal carcinoma (NPC) microenvironment. To evaluate the Treg immunophenotypes in tumour-infiltrating lymphocytes (TILs), we performed a double-enzymatic immunostaining for detection of forkhead box P3 (FoxP3) and other markers including CD4, CD8, and CD25 on 64 NPC and 36 non-malignant nasopharyngeal (NP) paraffin-embedded tissues. Expression of CD3ζ and CD3ε was also determined. The prevalence of CD4+FoxP3+ cells in CD4+ T cells and the ratio of FoxP3+/CD8+ were increased significantly in NPC compared with those in NP tissues (P < 0·001 and P = 0·025 respectively). Moreover, the ratio of FoxP3+/CD25+FoxP3− in NPC was significantly lower than that in NP tissues (P = 0·005), suggesting an imbalance favouring activated phenotype of T cells in NPC. A significant negative correlation between the abundance of FoxP3+ and CD25+FoxP3− cells (P < 0·001) was also identified. When histological types of NPC were considered, a lower ratio of FoxP3+/CD25+FoxP3− was found in non-keratinizing and undifferentiated carcinomas. Increased CD4+FoxP3+/CD4+ proportion and FoxP3+/CD8+ ratio were associated with keratinizing squamous cell carcinoma. A reduced expression of CD3ζ in TILs was found in 20·6% of the NPC tissues but none of the NP tissues. These data provide evidence for the imbalances of Treg and effector T cell phenotypes and down-regulation of signal-transducing molecules in TILs, supporting their role in suppression of immune response and immune evasion of NPC.
Keywords: CD3ζ, FoxP3, histological type, NPC, Treg
Introduction
Nasopharyngeal carcinoma (NPC) is one of the head and neck carcinomas which occurs rarely in the United States and western Europe but exhibits a high incidence in Southern China and parts of Southeast Asia [1,2]. According to the World Health Organization (WHO) histological classification, NPC is categorized into three groups: keratinizing squamous cell carcinoma (SCC, type I), non-keratinizing carcinoma (NKC, type II) and undifferentiated carcinoma (UC, type III).
Radiotherapy and concomitant chemoradiotherapy can improve overall and disease-free survival for NPC [3]. However, prognosis remains poor in a significant number of patients [4]. Thus, treatment innovations with immunotherapy are currently being assessed to improve outcomes. The Epstein–Barr virus-specific cytotoxic T lymphocyte (CTL) and dendritic cell-based therapies have been demonstrated to be well tolerated [5]. However, clinical benefits have been demonstrated in some patients only. Thus, it is likely that variable clinical responses to immunotherapy may be due to immune evasion by the tumour cells. One of the tumour immune-evasion mechanisms is immunosuppression because of the absence of T cell signalling, for example, via CD3ζ loss or the presence of regulatory T cells (Tregs) in the tumour microenvironment.
The loss of signal-transducing ζ-chains from the T cell receptor (TCR) complex of T cells and FcγRIII (low-affinity Fc receptor for immunoglobulin G) of natural killer cells was found at the tumour site and in the peripheral circulation of patients with malignancies [6]. Although several distinct mechanisms have been postulated to be responsible for ζ-chain loss in T cells of patients with cancer, this phenomenon is still controversial [6,7]. The ζ-chain loss is predictive of poor prognosis and survival in patients with oral and other head and neck carcinomas, ovarian carcinoma and melanoma [7]. This suggests strongly that the loss of ζ-chain expression leads to an impaired tumour-specific immunity.
CD4+ Treg subsets include peripherally induced CD4+ Tregs (termed ‘adaptive’ Tregs), which are derived from peripheral CD4+CD25− precursors and differentiate into functional Tregs following adequate stimulation by antigens and cytokines such as transforming growth factor-β, interleukin (IL)-10 and IL-4. Forkhead box P3 (FoxP3) is a member of the forkhead/winged-helix family of transcription factors, which represents a lineage marker and is crucial for Treg development and function [8]. Upon differentiation, the adaptive Tregs may express CD25 and FoxP3. The CD4+CD25+ Tregs have been shown to suppress the function of CD4+ and CD8+ effector T cells [8,9]. Although multiple suppressive mechanisms including cell–cell contact and soluble factors have been proposed, these mechanisms have not been investigated thoroughly in NPC.
In humans and mice, expression of FoxP3 has been found exclusively in CD4+CD25+ Tregs and correlated strictly with regulatory activity. In contrast to that in mice, FoxP3 expression in humans may also be induced transiently in a high percentage of activated T cells after in vitro stimulation of CD4+CD25− cells and is associated with suppressive function [10,11]. Although recent reports [12–14] showed that these induced cells lack a regulatory phenotype, the immunosuppressive role of FoxP3+ tumour-infiltrating lymphocytes (TILs) within a complex tumour microenvironment is still unknown. Immunohistochemical or flow cytometric detection of tumour-specific effector T cells and Tregs, as well as their activities (e.g. cytokine production and proliferation) in the tumour microenvironment, may be a solution to study the balance between these two cell populations in situ. Because it is difficult to obtain fresh NPC biopsies, the alternative is to use immunohistochemistry on archived paraffin-embedded tissue specimens. The obvious limitation of this approach is the availability of the specific markers for the Tregs and effector T cells and the design of the method of multiple immunostaining on tissue sections. No study had been reported for the presence of Tregs or loss of ζ-chain expression in NPC when we commenced this work.
While this work was being completed, Lau et al.[15] reported an increase of FoxP3+CD4+CD25high Tregs with suppressive activity in the peripheral circulation of 57 NPC patients, as well as at the tumour site in five NPC specimens. However, they did not examine non-malignant nasopharyngeal (NP) tissues for comparison and the relationship between the presence of Tregs and clinicopathological data was also not reported. The objectives of our study are to detect the presence of TILs co-expressing FoxP3 with CD4, CD8 or CD25, as well as the expression of ζ-chain, and to associate their expression with patient and tumour variables. This study will contribute to a better understanding of the mechanisms of immunosuppression in NPC and, thus, provide a better insight towards the development of improved immunotherapeutic strategies.
Materials and methods
Tissue specimens
Formalin-fixed, paraffin-embedded tissues from 64 NPC biopsy specimens and 38 nasopharyngeal mucosa biopsy specimens with no evidence of malignancy (NP tissues) were used. The NPC biopsy specimens collected between 2000 and 2004 were classified histologically into three types according to WHO classification: SCC (type I; n = 14), NKC (type II; n = 23) and UC (type III; n = 27). The characteristics of NPC patients are shown in Table 1.
Table 1.
Nasopharyngeal carcinoma (NPC) patient profile.
| Characteristic | No. of NPC patients (n = 64) |
|---|---|
| Gender* | |
| Male | 49 |
| Female | 11 |
| Age (years)* | |
| < 50 | 25 |
| ≥ 50 | 35 |
| Race* | |
| Chinese | 29 |
| Non-Chinese | 31 |
| Histological type | |
| SCC (type I) | 14 |
| NKC (type II) | 23 |
| UC (type III) | 27 |
The data were available for only 60 of 64 cases. SCC (type I), squamous cell carcinoma; NKC (type II), non-keratinizing carcinoma; UC (type III), undifferentiated carcinoma.
Immunohistochemistry
The paraffin-embedded tissues were sectioned serially to a thickness of 4 µm. Three serial sections from each paraffin block were subjected to sequential double-immunohistochemical staining to detect the co-expression of FoxP3 with CD4, CD8 and CD25 respectively. The tissue sections were deparaffinized and then boiled with 10 mM Tris, 1 mM ethylenediamine tatraacetic acid (EDTA) buffer (pH 9·0) in a microwave oven for 20 min. After blocking the endogenous peroxidase with 3% hydrogen peroxide, endogenous biotin was blocked by 0·01% avidin and followed by 0·01% D-biotin. Following incubation with 3% bovine serum albumin for 1 h, the first staining sequence was performed by incubating the sections with anti-CD4 (1:30 dilution, clone 4B12; BioGenex Laboratories, San Ramon, CA, USA), anti-CD8 (1:400 dilution, clone C8/144B; Dako, Carpinteria, CA, USA) or anti-CD25 (1:100 dilution, clone 4C9; Novocastra Laboratories, Newcastle, UK) antibody for 2 h at room temperature. The immunoreactivity was detected by the biotin–streptavidin–peroxidase complex method using the LSAB+ Kit (Dako) followed by 3,3′-diaminobenzidine (DAB) solution (Liquid DAB+; Dako) as a chromogen. Both kits were used according to the manufacturer's instructions. For the second staining sequence, the sections were incubated overnight (∼18 h) at 4°C with anti-FoxP3 antibody (1:100 dilution, clone 236A/E7; Abcam, Cambridge, MA, USA). After incubation of the sections with biotinylated secondary antibodies (30 min) from the LSAB+ Kit (Dako) and later with streptavidin–alkaline phosphatase (30 min) (Lab Vision, Fremont, CA, USA), the dark blue staining was developed by using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Amresco, Solon, OH, USA) as a chromogen. Finally, the sections were counterstained with methyl green. Exclusion of the primary antibody from the first or second staining sequence served as the negative control.
For detection of the CD3ζ and CD3ε antigens, immunoperoxidase staining was performed on serial sections as described above for the CD markers. The tissue sections were pretreated by boiling in a microwave oven either with 10 mM citrate buffer (pH 6·0) for anti-CD3ζ antibody (1:400 dilution, clone 6B10.2; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or with 10 mM Tris, 1 mM EDTA buffer (pH 9·0) for anti-CD3ε antibody (1:200 dilution; NeoMarkers, Fremont, CA, USA). After incubation with the primary antibodies for 1 h at room temperature, immunoreactivity was detected by using the LSAB+ Kit (Dako) and DAB (Liquid DAB+; Dako) as a chromogen. Sections were counterstained lightly with haematoxylin. No staining was observed in the negative controls, which were prepared by excluding the primary antibodies.
Quantification of TILs
Each entire tissue section was evaluated for TILs under ×200 magnification. A total of five to 12 photomicrographs of non-overlapping high-power fields (×400 magnification) were captured using a CCD camera (ColorView12; Olympus Soft Imaging Solutions GmbH, Münster, Germany), installed on an Olympus BX51 microscope (Olympus, Tokyo, Japan) and attached to a computer. The photomicrographs represent the densest lymphocytic infiltrates in the tumour tissues or in the epithelium and/or its underlying connective tissue of the NP tissues. For each section, five of the photomicrographs were selected randomly, viewed using image-analytical software (analySIS; Olympus Soft Imaging Solutions GmbH) and evaluated for the numbers of immunostained lymphocytes by counting manually with the assistance of a ‘touch count’ function provided by the software. The CD4+, CD8+ or CD25+ cells were counted first, followed by counts of CD4+FoxP3+, CD8+FoxP3+ or CD25+FoxP3+ cells and FoxP3+CD4−, FoxP3+CD8− or FoxP3+CD25− cells. The average proportions of CD4+FoxP3+/CD4+, CD8+FoxP3+/CD8+ and CD25+FoxP3+/CD25+ were calculated for each tissue. In addition, the ratios of FoxP3+/CD8+ (CD8+FoxP3+ cells were excluded from CD8+ cells) and FoxP3+/CD25+FoxP3− were also calculated. For evaluating the FoxP3+/CD25+FoxP3− ratio, sum of FoxP3+ cell numbers in the five fields was divided by the sum of CD25+FoxP3− cell numbers in the corresponding fields for each tissue. The FoxP3+/CD25+FoxP3− ratio was not calculated for each field because of the lack of CD25+FoxP3− cells in some fields.
A total of five photomicrographs of independent high-power fields (×400 magnification) with the most abundant infiltrating lymphocytes were captured for evaluating the number of CD3ε+ cells. The CD3ζ+ cells were counted similarly in the identically photographed fields on consecutive sections. The staining intensity was also scored as follows: 1 = mild, 2 = moderate, and 3 = strong. Because the staining intensity of CD3ζ scored 2 or 3 in all NP tissues, TILs with mild staining (score 1) were considered negative for CD3ζ. The CD3ζ expression was considered to be down-regulated when the number of CD3ζ+ cells was < 50% of CD3ε+ cells.
Statistical analysis
As the data were not normally distributed, the association of each proportion or ratio of immunophenotypes with patient and tumour variables and its comparison between NPC and NP tissues were assessed by the Mann–Whitney U-test or the Kruskal–Wallis one-way analysis of variance by ranks. Frequencies of CD3ζ down-regulation were compared between test groups using Fisher's exact test or the Kruskal–Wallis test when necessary. Analysis of the correlation between FoxP3+ and CD8+ or CD25+FoxP3− cell numbers was performed by Spearman's rank correlation test. A two-sided P-value of less than 0·05 was considered statistically significant. Statistical analyses were performed using spss version 11.5 statistical software.
Results
Comparisons of Treg phenotypes between NPC and NP tissues
We analysed the Treg phenotypes from intraepithelial- or stromal-infiltrated lymphocytes in 64 NPC and 38 NP tissues. In the first analysis, we determined the proportions of CD4+FoxP3+/CD4+, CD8+FoxP3+/CD8+, and CD25+FoxP3+/CD25+ in each tissue sample. Double staining identified the presence of CD8+FoxP3+ cells in only some tissues (29 of 64 NPC and 16 of 36 NP tissues). Enumeration of FoxP3+ cells in these 29 NPC and 16 NP tissues showed that only a very small number of FoxP3+ cells (median, 2·07% of FoxP3+ cells) are CD8+FoxP3+ cells. Therefore, the ratio of FoxP3+/CD8+ (CD8+FoxP3+ cells were excluded from CD8+ cells) instead of CD8+FoxP3+/CD8+ proportion was used for analyses. In addition, the double staining indicated that in most tissues a considerably high number of FoxP3+ cells were CD25−. Therefore, in our current study, we also determined the ratio of FoxP3+ cells (independent of CD25 expression) to CD25+FoxP3− cells, which were reported as activated/effector T cells [16]. The distribution of the proportion or ratio values of CD4+FoxP3+/CD4+, FoxP3+/CD8+ and CD25+FoxP3+/CD25+ in NPC and NP tissues are summarized in Fig. 1a, b and c respectively.
Fig. 1.
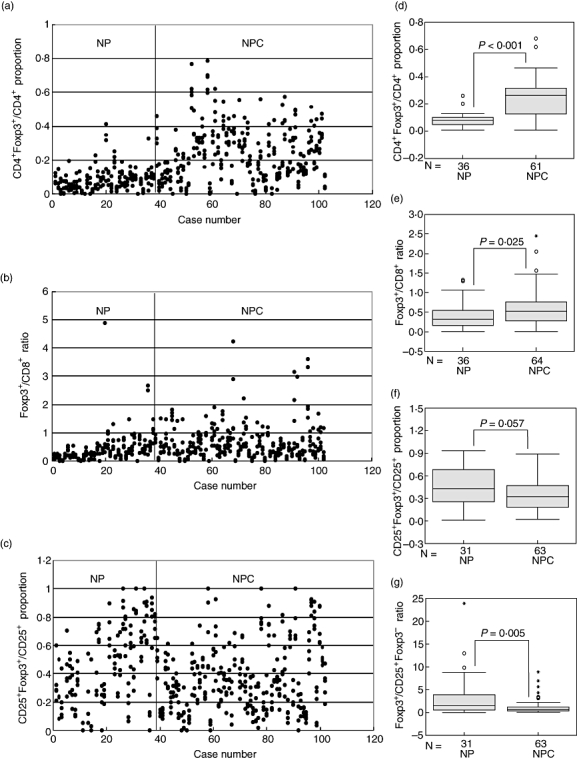
Comparisons of CD4+ forkhead box P3 (FoxP3)+/CD4+, FoxP3+/CD8+, CD25+FoxP3+/CD25+ and FoxP3+/CD25+FoxP3− in nasopharyngeal carcinoma (NPC) versus non-malignant nasopharyngeal (NP) tissues. The scatter-plots show the proportion or ratio values of (a) CD4+FoxP3+/CD4+, (b) FoxP3+/CD8+ and (c) CD25+FoxP3+/CD25+ from the five selected fields (represented by dots) for each tissue specimen. The average of proportion or ratio values for each tissue was used for statistical analysis. The distribution of FoxP3+/CD25+FoxP3− ratios is not presented in a scatter-plot because each tissue specimen acquired only one ratio value, which was calculated by dividing of the total FoxP3+ cell numbers in the five selected fields with the total of CD25+FoxP3− cell numbers in the corresponding fields. The Mann–Whitney U-test was used to compare the proportion or ratio values of (d) CD4+FoxP3+/CD4+, (e) FoxP3+/CD8+, (f) CD25+FoxP3+/CD25+ and (g) FoxP3+/CD25+FoxP3− between NPC and NP tissues. Some cases were excluded because of poor tissue morphology after microwave-boiling treatment. The sample sizes (N) of two groups in the analyses are as indicated.
The CD4+FoxP3+/CD4+ proportion and FoxP3+/CD8+ ratio in NPC (median, 0·26 and 0·52 respectively) were significantly higher than those in NP tissues (median, 0·08; P < 0·001; Fig. 1d and median, 0·32; P = 0·025; Fig. 1e respectively). There was no statistically significant difference in CD25+Foxp3+/CD25+ proportion between NPC and NP tissues (median, 0·32 and 0·43 respectively; P = 0·057; Fig. 1f). However, we observed a significantly higher ratio of Foxp3+/CD25+FoxP3− in the NP tissues (median, 1·47) compared with the NPC (median, 0·61), P = 0·005 (Fig. 1g). Representative immunohistochemical features are shown in Fig. 2.
Fig. 2.
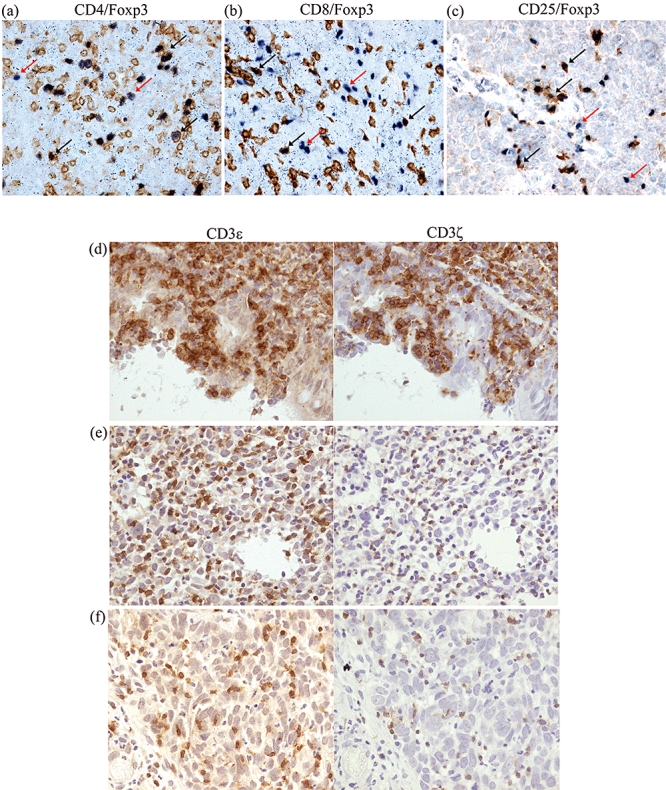
Immunoreactivities of antibodies against forkhead box P3 (FoxP3) and CD markers. (a–c) Representative double positive tumour-infiltrating lymphocytes (TILs) are indicated by black arrows. The brown membranous staining specifies CD antigens and the dark blue-purple nuclear staining identifies FoxP3. The CD8+FoxP3+ cells are rarely present. The single positive FoxP3 cells are as shown by red arrows and in the CD25/FoxP3 picture, these indicate that a proportion of the FoxP3+ cells are CD25−. (d–f) Immunoperoxidase staining of serial paraffin sections of a non-malignant nasopharyngeal (NP) tissue (d) and two nasopharyngeal carcinoma (NPC) tissues (e, f) with anti-CD3ε and anti-CD3ζ antibodies. Note the down-regulation of CD3ζ expression in TILs with significant absence (f) and decrease (e) of CD3ζ staining relative to CD3ε staining and the comparable expression of both CD3ζ and -ε in the NP tissue (d). Original magnification ×400.
Correlation between the abundance of FoxP3+ and CD8+ or CD25+FoxP3− cells in NPC and NP tissues
The numbers of FoxP3+ cells in all fields were correlated with CD8+ or CD25+FoxP3− cell numbers in the corresponding fields for NPC, NP or all cases using the Spearman's rank correlation test. A statistically significant negative correlation between FoxP3+ and CD25+FoxP3− cell numbers (P < 0·001) was identified in NPC as well as in NP and all tissues (Table 2). The FoxP3+ cells were correlated positively with CD8+ cells in NP tissues (P = 0·029) but not in NPC cases.
Table 2.
Correlations between forkhead box P3 (FoxP3)+ and CD8+ or CD25+FoxP3− cell numbers in nasopharyngeal carcinoma (NPC) and non-malignant nasopharyngeal (NP) tissues.
| CD8+ | CD25+FoxP3− | |||
|---|---|---|---|---|
| NPC | FoxP3+ | CC | −0·003 | −0·197 |
| P-value | 0·964 | < 0·001 | ||
| n* | 320a | 315b | ||
| NP tissues | FoxP3+ | CC | 0·163 | −0·395 |
| P-value | 0·029 | < 0·001 | ||
| n* | 180c | 155d | ||
| Total | FoxP3+ | CC | 0·030 | −0·294 |
| P-value | 0·504 | < 0·001 | ||
| n* | 500e | 470f | ||
Total fields (five fields for each case) evaluated from
64 or
63 NPC cases,
36 or
31 NP tissues and
100 or
94 all tissues. CC, correlation coefficient.
Association of CD4+FoxP3+/CD4+ proportion, FoxP3+/CD8+ and FoxP3+/CD25+FoxP3− ratios with patient and tumour variables
Table 3 shows the relationship of the proportion or ratio of CD4+FoxP3+/CD4+, FoxP3+/CD8+ and FoxP3+/CD25+FoxP3− with patient and tumour variables including age at diagnosis, gender, race and histological type. We observed that all three proportion/ratios in NPC tissues were associated significantly with histological type (P = 0·001, P = 0·002 and P = 0·001 respectively) and not with other patient and tumour variables. To analyse this association further, a comparison of the proportion/ratios among type I, type II and type III NPCs and NP tissues was performed. As shown in Fig. 3, the CD4+FoxP3+/CD4+ proportion, FoxP3+/CD8+ and FoxP3+/CD25+FoxP3− ratios were significantly higher in histological type I, SCC, compared with type II, NKC (P = 0·006, P = 0·001 and P = 0·001 respectively) or to type III, UC (P < 0·001, P = 0·001 and P = 0·022 respectively). Figure 3a shows that the CD4+FoxP3+/CD4+ proportions in all histological types were significantly higher than that in NP tissues (P < 0·001). The FoxP3+/CD8+ ratio was higher in histological type I relative to NP tissues (P < 0·001; Fig. 3b). On the other hand, the FoxP3+/CD25+FoxP3− ratio in NP tissues was significantly higher than that in histological type II (P < 0·001) or type III (P = 0·025) (Fig. 3c).
Table 3.
Association of CD4+ forkhead box P3 (FoxP3)+/CD4+ proportion, FoxP3+/CD8+ and FoxP3+/CD25+FoxP3− ratios with patient and tumour variables.
| CD4+FoxP3+/CD4+ | FoxP3+/CD8+ | FoxP3+/CD25+FoxP3− | ||||
|---|---|---|---|---|---|---|
| Characteristic | MR | P-value | MR | P-value | MR | P-value |
| Gender* | ||||||
| Male | 27·89 | 0·275 | 29·88 | 0·560 | 30·04 | 0·968 |
| Female | 34·20 | 33·27 | 29·80 | |||
| Age (years)* | ||||||
| < 50 | 28·42 | 0·821 | 27·16 | 0·211 | 30·88 | 0·746 |
| ≥ 50 | 29·42 | 32·89 | 29·40 | |||
| Race* | ||||||
| Chinese | 25·07 | 0·079 | 26·55 | 0·090 | 29·48 | 0·820 |
| Non-Chinese | 32·79 | 34·19 | 30·50 | |||
| Histological type† | ||||||
| SCC (type I) | 45·79 | 0·001 | 48·14 | 0·002 | 45·57 | 0·001 |
| NKC (type II) | 29·96 | 28·17 | 23·04 | |||
| UC (type III) | 23·38 | 28·07 | 32·62 | |||
Mann–Whitney U-test;
Kruskal–Wallis test.
MR, mean rank. SCC (type I), squamous cell carcinoma; NKC (type II), non-keratinizing carcinoma; UC (type III), undifferentiated carcinoma.
Fig. 3.
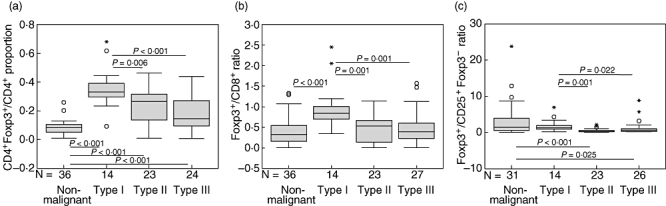
Relationship between CD4+ forkhead box P3 (FoxP3)+/CD4+, FoxP3+/CD8+ or FoxP3+/CD25+FoxP3− and histological type. Comparisons of the means of (a) CD4+FoxP3+/CD4+ proportion, (b) FoxP3+/CD8+ and (c) FoxP3+/CD25+FoxP3− ratios among the histological types and non-malignant nasopharyngeal (NP) tissues were performed by Mann–Whitney U-test. The differences without statistical significance are not indicated.
Reduced CD3ζ expression in TILs and its association with patient and tumour variables
Reduced immunoreactivity of CD3ζ in TILs was found in 20·6% (13 of 63) of the NPC tissues (Table 4). In contrast, none of the NP tissues (n = 36) showed reduced CD3ζ staining. This difference was statistically significant (P = 0·004). All the 13 NPCs with reduced ζ-chain expression showed mildly CD3ζ-stained cells (score 1) with an almost equal number of CD3ε+ cells (Fig. 2e), except in one case, with a number less than 50% of CD3ε+ cells (Fig. 2f). The strong or moderate staining of CD3ε in the same areas of consecutive tissue sections indicated good antigen preservation and uniform staining of our formalin-fixed tissue sections (Fig. 2d–f). All NP tissues had a score of 3 for the staining intensity of CD3ζ and CD3ε, except in three cases, where a score of 2 for CD3ζ was found. Among the patient and tumour variables, we identified a statistically significant relationship between down-regulation of CD3ζ expression and histological type (P = 0·034; Kruskal–Wallis test). The CD3ζ expression was decreased significantly in type III NPC compared with type I NPC (P = 0·016; Fisher's exact test) (Table 4).
Table 4.
Expression of CD3ζ in nasopharyngeal carcinoma (NPC) and non-malignant nasopharyngeal (NP) tissues and its association/relationship with patient and tumour variables.
| Characteristic | Normal CD3ζ | Reduced CD3ζ | P-value |
|---|---|---|---|
| Malignancy status* | |||
| Non-malignant (NP) | 36 | 0 | 0·004† |
| Malignant (NPC) | 50 | 13 | |
| Gender‡ | |||
| Male | 39 | 10 | 1·000† |
| Female | 8 | 2 | |
| Age (years)‡ | |||
| < 50 | 18 | 6 | 0·521† |
| ≥ 50 | 29 | 6 | |
| Race‡ | |||
| Chinese | 22 | 6 | 1·000† |
| Non-Chinese | 25 | 6 | |
| Histological type | |||
| SCC (type I) | 14 | 0 | |
| NKC (type II) | 19 | 4 | 0·034§ |
| UC (type III) | 17 | 9 |
Only 63 of 64 NPC and 36 of 38 NP tissues were available for analysis. The others were excluded because of poor preservation of tissue morphology after microwave-boiling treatment.
Fisher's exact test;
four of 63 NPC cases were excluded from analysis because of the lack of data in the medical records.
Kruskal–Wallis test; using Fisher's exact test, type I versus type III, P = 0·016, type I versus type II, P = 0·276, and type II versus type III, P = 0·209. SCC (type I), squamous cell carcinoma; NKC (type II): non-keratinizing carcinoma; UC (type III): undifferentiated carcinoma.
Discussion
The presence of TILs within the NPC microenvironment may indicate an active immune response directed at antigens expressed on tumour cells [17,18]. Conversely, the reciprocal interactions between tumour cells and TILs might create an immunosuppressive network that results in tumour evasion and attenuation of immunotherapeutic efficacy. In the present study, we performed an immunohistochemical analysis on NPC biopsies to determine the alterations in (a) the prevalence of Treg phenotypes, (b) the ratios of Treg and effector T cell phenotypes and (c) the expression of signal-transducing ζ-chain in TILs. These imbalances in the tumour microenvironment might lead to immune tolerization in NPC patients.
We demonstrate that the prevalence of CD4+FoxP3+ cells (CD4+FoxP3+/CD4+) was increased significantly in the microenvironment of NPC compared with the surrounding areas of NP epithelium. This is consistent with a previous study showing an increase of CD4+CD25highFoxP3+ Treg population in the peripheral blood of NPC patients [15]. The Treg phenotype was also detected in TILs by flow cytometry, but only five NPC biopsies were examined and no NP tissues were used for comparison [15].
The CD25+ T cells have been found to be increased in NPC compared with NP tissues [17,19]. However, it is unknown whether these CD25+ T cells co-expressed FoxP3. Because CD25+FoxP3+ cells have been considered to represent Tregs in previous reports [20,21], we analysed the prevalence of CD25+FoxP3+ cells in the CD25+ population. Although the CD25+FoxP3+/CD25+ proportion in NP tissues tended to be higher than that in NPC tissues, this difference was not statistically significant (Fig. 1f). However, we observed a significant number of CD25−FoxP3+ cells in NPC and NP tissues (Fig. 2c). The CD25−FoxP3+ cells were also found previously in normal human lymphoid tissues and peripheral blood [22,23] but, to our knowledge, their functions are still unclear. There is a possibility that these CD25−FoxP3+ cells could exert suppressive activity, as demonstrated in murine CD4+CD25low/−FoxP3+ cells [16,24]. Thus, we used FoxP3 independently of CD25 as the marker for the Tregphenotype.
To elucidate further the balance between Treg and effector cells, we evaluated FoxP3+ and CD25+FoxP3− cells as representing Treg and activated effector T cell phenotypes respectively [10,11,16], and analysed the FoxP3+/CD8+ and FoxP3+/CD25+FoxP3− ratios in the tissue samples. We found a significantly higher ratio of FoxP3+/CD8+ in NPC compared with NP tissues (Fig. 1e). This suggests that the balance is tilted in favour of Tregs (high Tregs and low CD8+ CTLs) in NPC compared with NP tissues. This result is also in line with recent reports on hepatocellular carcinoma (HCC), whereby an abundant accumulation of FoxP3+ Tregs was found with a concomitantly low infiltration of CD8+ T cells in tumour regions relative to non-tumour regions [25,26]. Our data also demonstrated a positive correlation between FoxP3+ cell and CD8+ T cell infiltration in NP tissues but not in NPC tissues (Table 2). In the study by Gao et al.[25], a similar correlation was also found in both the non-cancerous peri-tumour region and tumour centre of the HCC tissues. In contrast to the FoxP3+/CD8+ ratio, the ratio of FoxP3+/CD25+FoxP3− was found to be significantly lower in NPC compared with the NP tissues, indicating that the balance is tilted towards activated effector T cells (low Tregs and high activated effector T cells) in NPC. This imbalance is due apparently to the significantly increased number of CD25+FoxP3− cells in NPC versus NP tissues, as there is no significant difference in FoxP3+ cell number (data not shown). In a recent study by Siddiqui et al.[27], the CD4+CD25+FoxP3− T cells were correlated positively with worse pathological features of renal cell carcinoma and poorer patient survival. It is unclear whether this is true for NPC, as we were not able to obtain survival data. We also observed that a high number of FoxP3+ cells was correlated significantly with a low number of CD25+FoxP3− cells in the NPC as well as NP tissues. Further investigations are required to determine whether FoxP3+ cells have the ability to inhibit the activation of T cells by inhibiting both CD25 expression and IL-2 production, as demonstrated previously in CD4+CD25+ Tregs[9].
Next, we analysed the association of the proportion/ratios with histological types. Our data showed that the FoxP3+/CD25+FoxP3− ratio is lower in non-keratinizing (NKC, type II) and undifferentiated (UC, type III) NPCs compared with the NP tissues. There was no difference when a comparison was made between keratinizing SCC (type I) and NP tissues. In undifferentiated NPC, TILs expressed a relatively high CD25 and interferon-γ, which is indicative of an activated status [17]. Furthermore, the presence of a high percentage of tumour-infiltrating activated granzyme B+ CTLs is a very strong and independent predictor of a rapid fatal clinical outcome in NKC (type II) and UC (type III) [28]. Taken together, our data suggest that the imbalance favouring higher CD25+FoxP3− cells (activated T cells) is associated with the pathogenesis of NPC with prominent lymphoid stroma (types II and III NPCs). Tumours with more activated T cells may be more aggressive because of T cell-derived cytokines or other proinflammatory factors, such as IL-1 and CD40 ligand, that favour tumour progression [29–31]. Furthermore, expression of IL-1 and CD40 ligand in TILs and CD40 expression in tumour cells have been demonstrated in NKC (type II) and/or UC (type III) [32–34].
We also found that the proportion of CD4+FoxP3+/CD4+ and FoxP3+/CD8+ ratio were highest in SCC. This may suggest that the high FoxP3+/CD8+ ratio and high prevalence of CD4+FoxP3+ Treg phenotype were associated with the pathogenesis of SCC of the nasopharynx. NPC patients with this histological type had a poorer survival rate because of a higher incidence of locoregional failure compared with those with NKC and UC [1,2,35]. A poorer prognosis has been observed in patients with a high prevalence of FoxP3+ Treg phenotype in pancreatic ductal, ovarian and hepatocellular carcinomas [36–38], although this was not found consistently in other malignancies [39–41]. Thus, the association of Treg phenotypes with the clinical outcome of NPC patients is worthy of further investigation.
Absence or reduction of signal-transducing ζ-chain from the TCR complex may alter or impair cytoplasmic signalling, resulting in decreased proliferation and cytokine production and, therefore, leads to an impaired tumour-specific immunity [42–44]. The level of ζ-chain expression on PBLs obtained from patients with undifferentiated NPC was also found in healthy controls [45]. However, the ζ-chain expression in the TIL compartment of NPC has not yet been reported. In the present study we demonstrate, for the first time, that TILs from 20·6% of the NPC tissues had down-regulated ζ-chain expression. In addition, the loss of ζ-chain expression in TILs has a significant association with the UC of nasopharynx. The mechanisms that are responsible for ζ-chain loss in TILs remain largely unclear and controversial, although many different mechanisms have been proposed to account for the aberrations of ζ-chain expression [7]. Several studies analysing peripheral blood and TILs from cancer patients indicate that decreased ζ-chain expression is associated with T cell apoptosis [46,47] which may be induced by Fas ligand (FasL)-expressing tumour cells [48–50]. The mechanisms implicated in down-regulation of ζ-chain expression include proteolytic degradation of ζ-chain by caspases [50–53] and modulation by activated macrophages through release of hydrogen peroxide or cell-contact manner in the tumour microenvironment [54,55]. Previous reports have shown that in non-keratinizing or undifferentiated NPC, tumour cells expressed membranous FasL and the intense leucocyte infiltration comprising predominantly T cells and macrophages [18,56]. The mechanisms involved in down-regulation of ζ-chain in the NPC remain to be established.
In conclusion, we have demonstrated an increase in the prevalence of CD4+FoxP3+ cells and in FoxP3+/CD8+ ratio, indicating that the balance favours the FoxP3+ Treg phenotype rather than CD8+ cells. Interestingly, we also observed an increase in activated effector phenotype in NPC as indicated by the decreased FoxP3+/CD25+FoxP3− ratio. Further characterization of the CD25+FoxP3− cells may help to elucidate their role in immune response or in tumour immune evasion within the NPC microenvironment. The increase of CD4+FoxP3+/CD4+ proportion and FoxP3+/CD8+ ratio also showed an association with SCC (type I), whereas the decrease of FoxP3+/CD25+FoxP3− ratio was associated with NKC (type II) and UC (type III), suggesting a difference in the immune response in different histological types of NPC. Thus, this implies that different immunotherapeutic strategies need to be considered for different histological types of NPC. However, caution should be taken not to over-interpret these results because of small sample size. The partial loss of ζ-chain expression on TILs may be another factor which results in an impaired anti-tumour response of effector cells at the tumour site of NPC patients. Further studies are required to dissect the mechanisms and cellular effects of ζ-chain loss and the role of CD4+FoxP3+, CD25−FoxP3+ and CD25+FoxP3− cells in immune regulation in NPC. Our findings are certainly worthy of further investigation to validate the use of Treg immunophenotypes and/or ζ-chain as a predictor of response or an immunological biomarker in the selection of patients for immunotherapy.
Acknowledgments
We thank Associate Professor Dr Saidi Moin, a biostatistician at the Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, for his constructive comments on the statistical analysis. This study was supported by a research grant (06-02-04-0636-PR0054/05-03) from the Ministry of Science, Technology and Innovation, Malaysia.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. The scatter-plots show the average of proportion or ratio values (represented by dots) of (a) CD4+forkhead box P3 (FoxP3)+/CD4+, (b) FoxP3+/CD8+ and (c) CD25+FoxP3+/CD25+ from the five selected fields for each tissue specimen.
Fig. S2. Comparison of the mean of (a) CD4+forkhead box P3 (FoxP3)+/CD4+, (b) FoxP3+/CD8+, (c) CD25+FoxP3+/CD25+ and (d) FoxP3+/CD25+FoxP3- proportions/ratios between nasopharyngeal carcinoma (NPC) and nonmalignant nasopharyngeal (NP) tissues.
Fig. S3. The scatter-plots show the number of cells with immunoreactivities of various markers in each field (represented by dot) for individual tissues doubly stained with (a) forkhead box P3 (FoxP3)/CD4, (b) FoxP3/CD8 and (c) FoxP3/CD25 antibodies.
Table S1. Information summarizing the discussion section.
Table S2. Association of CD4+ forkhead box P3 (FoxP3)+/CD4+, FoxP3+/CD8+, CD25+FoxP3+/CD25+ and FoxP3+/CD25+FoxP3- proportions/ratios with CD3ζ expression in nasopharyngeal carcinoma (NPC).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29–35. doi: 10.1093/annonc/mdl320. [DOI] [PubMed] [Google Scholar]
- 2.Shanmugaratnam K, Chan SH, de-The G, et al. Histopathology of nasopharyngeal carcinoma correlations with epidemiology, survival rates and other biological characteristics. Cancer. 1979;44:1029–44. doi: 10.1002/1097-0142(197909)44:3<1029::aid-cncr2820440335>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Agulnik M, Siu LL. State-of-the-art management of nasopharyngeal carcinoma: current and future directions. Br J Cancer. 2005;92:799–806. doi: 10.1038/sj.bjc.6602449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Li J, Hong X, et al. Retrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinoma. Oral Oncol. 2008;44:464–70. doi: 10.1016/j.oraloncology.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Masmoudi A, Toumi N, Khanfir A, et al. Epstein–Barr virus-targeted immunotherapy for nasopharyngeal carcinoma. Cancer Treat Rev. 2007;33:499–505. doi: 10.1016/j.ctrv.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4:675–87. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 7.Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–78. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 10.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 13.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 15.Lau KM, Cheng SH, Lo KW, et al. Increase in circulating Foxp3+CD4+CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer. 2007;96:617–22. doi: 10.1038/sj.bjc.6603580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Tang KF, Chan SH, Loh KS, et al. Increased production of interferon-gamma by tumour infiltrating T lymphocytes in nasopharyngeal carcinoma: indicative of an activated status. Cancer Lett. 1999;140:93–8. doi: 10.1016/s0304-3835(99)00056-7. [DOI] [PubMed] [Google Scholar]
- 18.Tang KF, Tan SY, Chan SH, et al. A distinct expression of CC chemokines by macrophages in nasopharyngeal carcinoma: implication for the intense tumor infiltration by T lymphocytes and macrophages. Hum Pathol. 2001;32:42–9. doi: 10.1053/hupa.2001.20886. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Han HX. Expression of CD25+ lymphocytes in nasopharyngeal carcinoma and its association with EBV infection. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:94–7. [PubMed] [Google Scholar]
- 20.Sato e, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourmouras V, Fimiani M, Rubegni P, et al. Evaluation of tumour-infiltrating CD4+CD25+FOXP3+ regulatory T cells in human cutaneous benign and atypical naevi, melanomas and melanoma metastases. Br J Dermatol. 2007;157:531–9. doi: 10.1111/j.1365-2133.2007.08057.x. [DOI] [PubMed] [Google Scholar]
- 22.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci USA. 2005;102:4091–6. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 26.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-infiltrating Foxp3–CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–81. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 28.Oudejans JJ, Harijadi H, Kummer JA, et al. High numbers of granzyme B/CD8-positive tumour-infiltrating lymphocytes in nasopharyngeal carcinoma biopsies predict rapid fatal outcome in patients treated with curative intent. J Pathol. 2002;198:468–75. doi: 10.1002/path.1236. [DOI] [PubMed] [Google Scholar]
- 29.Ito R, Kitadai Y, Kyo E, et al. Interleukin 1 alpha acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 1993;53:4102–6. [PubMed] [Google Scholar]
- 30.Oberyszyn TM, Sabourin CL, Bijur GN, Oberyszyn AS, Boros LG, Robertson FM. Interleukin-1 alpha gene expression and localization of interleukin-1 alpha protein during tumor promotion. Mol Carcinog. 1993;7:238–48. doi: 10.1002/mc.2940070406. [DOI] [PubMed] [Google Scholar]
- 31.Bereznaya NM, Chekhun VF. Expression of CD40 and CD40L on tumor cells: the role of their interaction and new approach to immunotherapy. Exp Oncol. 2007;29:2–12. [PubMed] [Google Scholar]
- 32.Huang YT, Sheen TS, Chen CL, et al. Profile of cytokine expression in nasopharyngeal carcinomas: a distinct expression of interleukin 1 in tumor and CD4+ T cells. Cancer Res. 1999;59:1599–605. [PubMed] [Google Scholar]
- 33.Caggiari L, Guidoboni M, Vaccher E, et al. High serum levels of soluble CD40-L in patients with undifferentiated nasopharyngeal carcinoma: pathogenic and clinical relevance. Infect Agent Cancer. 2007;2:5. doi: 10.1186/1750-9378-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young LS. Expression of immune regulatory molecules in Epstein–Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am J Pathol. 1995;147:1152–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol. 1995;16:103–8. doi: 10.1016/0196-0709(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 36.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 37.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–31. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki A, Tanaka F, Mimori K, et al. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:173–9. doi: 10.1016/j.ejso.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 40.Gjerdrum LM, Woetmann A, Odum N, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–18. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 41.Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12:3355–60. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 42.Tartour E, Latour S, Mathiot C, et al. Variable expression of CD3-zeta chain in tumor-infiltrating lymphocytes (TIL) derived from renal-cell carcinoma: relationship with TIL phenotype and function. Int J Cancer. 1995;63:205–12. doi: 10.1002/ijc.2910630210. [DOI] [PubMed] [Google Scholar]
- 43.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–73. [PubMed] [Google Scholar]
- 44.Zea AH, Curti BD, Longo DL, et al. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res. 1995;1:1327–35. [PubMed] [Google Scholar]
- 45.Zanussi S, Vaccher E, Caffau C, et al. Interferon-gamma secretion and perforin expression are impaired in CD8+ T lymphocytes from patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol Immunother. 2003;52:28–32. doi: 10.1007/s00262-002-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–45. [PubMed] [Google Scholar]
- 47.Dworacki G, Meidenbauer N, Kuss I, et al. Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7:947s–57s. [PubMed] [Google Scholar]
- 48.Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL. Lymphocyte apoptosis induced by Fas ligand-expressing ovarian carcinoma cells. Implications for altered expression of T cell receptor in tumor-associated lymphocytes. J Clin Invest. 1998;101:2579–88. doi: 10.1172/JCI1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atarashi Y, Kanaya H, Whieside TL. A modified JAM assay detects apoptosis induced in activated lymphocytes by FasL+ human adherent tumor cells. J Immunol Methods. 2000;233:179–83. doi: 10.1016/s0022-1759(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 50.Reichert TE, Rabinowich H, Johnson JT, Whiteside TL. Mechanisms responsible for signaling and functional defects. J Immunother (1997) 1998;21:295–306. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Caspase-mediated degradation of T-cell receptor zeta-chain. Cancer Res. 1999;59:1422–7. [PubMed] [Google Scholar]
- 52.Takahashi A, Kono K, Amemiya H, Iizuka H, Fujii H, Matsumoto Y. Elevated caspase-3 activity in peripheral blood T cells coexists with increased degree of T-cell apoptosis and down-regulation of TCR zeta molecules in patients with gastric cancer. Clin Cancer Res. 2001;7:74–80. [PubMed] [Google Scholar]
- 53.Taylor DD, Bender DP, Gercel-Taylor C, Stanson J, Whiteside TL. Modulation of TcR/CD3-zeta chain expression by a circulating factor derived from ovarian cancer patients. Br J Cancer. 2001;84:1624–9. doi: 10.1054/bjoc.2001.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kono K, Salazar-Onfray F, Petersson M, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell- and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–13. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 55.Kiessling R, Kono K, Petersson M, Wasserman K. Immunosuppression in human tumor–host interaction: role of cytokines and alterations in signal-transducing molecules. Springer Semin Immunopathol. 1996;18:227–42. doi: 10.1007/BF00820668. [DOI] [PubMed] [Google Scholar]
- 56.Tsai ST, Fang SY, Jin YT, Su IJ, Yang BC. Analysis of the expression of Fas-L in nasopharyngeal carcinoma tissues. Oral Oncol. 1999;35:421–4. doi: 10.1016/s1368-8375(99)00016-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


