Abstract
Interferon-γ secreting T lymphocytes against pox virus-derived synthetic 9-mer peptides were tested by enzyme-linked immunospot in peripheral blood of individuals vaccinated with vaccinia virus more than 30 years ago. The peptides were characterized biochemically as high-affinity human leucocyte antigen (HLA) class I binders (KD ≤ 5 nM). However, five of the individuals tested did not show typical CD8+ T cell-mediated HLA class I-restricted responses. Instead, these donors showed CD4+ T cell-dependent responses against four of a total of eight antigenic 9-mer peptides discovered recently by our group. These latter responses were blocked specifically in the presence of anti-HLA class II antibody. We conclude that long-lived memory responses against pox virus-derived 9-mer peptides, with high binding affinity for HLA class I molecules, are mediated in some cases by CD4+ T cells and apparently restricted by HLA class II molecules.
Keywords: HLA class I, HLA class II, peptide, smallpox virus, vaccine
Introduction
Identification of human leucocyte antigen (HLA) class I binding peptides from infectious agents is of importance for effective vaccine development. We have used a computer-based program to predict HLA class I binding affinities of 9-mer peptides [1], followed by biochemical verification and quantification of the binding using recombinant HLA class I molecules [2,3]. Peptides with high to moderate binding affinities were tested subsequently for antigenicity in healthy individuals vaccinated with vaccinia virus more than 30 years ago [4]. Using these technologies, we have identified previously a number of new antigenic flu-derived peptide epitopes [5] and – more recently – a number (eight) of new pox virus-derived peptide epitopes [4]. During subsequent work on the pox-derived epitopes, we became aware that the measured immune responses of peripheral blood mononuclear cells (PBMC) in vitro by interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) towards high-affinity HLA class I binding peptides were not restricted solely by the HLA class I molecules of the peptide-presenting cells. By the use of blocking CD4 and CD8 antibodies and CD4+ and CD8+ T cell depletion experiments, here we show data which, for the first time, demonstrate clearly that T cells in the peripheral blood of vaccinia virus-vaccinated and responding individuals mediate both the expected typical HLA class I-restricted, CD8+ T cell-dependent responses, as well as unexpected responses which are mediated by CD4+ T cells, and appear to be HLA class II-restricted.
Materials and methods
Collection of blood samples
Buffy coats of 500 ml whole blood from 10 healthy Danish donors (age range: 35–65 years with a sex ratio of 70% female and 30% male; donors gave written informed consent) were obtained from the Blood Bank at the University Hospital (Copenhagen, Denmark) and the blood was used within 24 h to isolate PBMC. The donors were selected according to high-resolution sequence-based typing of HLA-A and -B alleles (Table 1) (Genome Diagnostics, Utrecht, the Netherlands) and sequence-based typing of HLA-DQ and HLA-DR β alleles (Table 3) (Tissue Typing Laboratory, University Hospital, Copenhagen, Denmark). These donors were vaccinated with vaccinia virus more than 30 years ago [4].
Table 1.
Blood donor gender, age and human leucocyte antigen (HLA) class I type for donors used in the present study.
| Donor | Sequence-based typing | |||||
|---|---|---|---|---|---|---|
| # | Sex | Age (years) | HLA-A | HLA-B | ||
| 7 | M | 40 | *0101 | *0101 | *0801 | *5201 |
| 9 | F | 43 | *0301 | *3201a | *0702 | *4402 |
| 13 | M | 60 | *0201 | *0201 | *3901 | *4402 |
| 19 | F | 46 | *0101 | *0201 | *0801 | *4001 |
| 21 | M | 54 | *0101 | *2402 | *0702 | *3901 |
| 23 | F | 58 | *0101 | *2402 | *0702 | *0801 |
| 24 | F | 36 | *0206 | *2601 | *3801 | *5801 |
| 28 | F | 62 | *0301 | *2601 | *0702 | *1401 |
| 30 | F | 49 | *0201 | *1101 | *5201 | *5201 |
| 31 | F | 63 | *0201 | *0301 | *0701 | *5601 |
HLA-A
3201 belongs to the HLA-A1 supertype.
Table 3.
Human leucocyte antigen (HLA) class II type for donors responding to the peptides depicted in Fig. 1.
| HLA class II | CD4+ T cell epitope in responding donors | |||||
|---|---|---|---|---|---|---|
| HLA-DRB1* | HLA-DQB1* | Donor no. | Pox113 | Pox114 | Pox116 | Pox131 |
| 07, 15 | 03, 06 | Donor 13 | − | − | + | + |
| 15, 51 | 06, 06 | Donor 30 | − | − | + | − |
| 04, 15 | 03, 06 | Donor 31 | − | − | + | − |
| 03, 13 | 02, 06 | Donor 19 | + | + | + | + |
| 03, 04 | 02, 03 | Donor 24 | − | + | − | − |
Shared HLA class II β chains between donors are shown in bold type.
Isolation of PBMC
The PBMC were isolated from buffy coats by density gradient centrifugation using Lymphoprep (NycomedPharma AS, Oslo, Norway). The freshly isolated PBMC were cryopreserved for later use at 20 × 106 cells in 1 ml RPMI-1640 containing 20% fetal calf serum and 10% dimethylsulphoxide at −140°C.
Peptides
The 9-mer peptides were synthesized by standard 9-fluorenylmethyloxycarbonyl chemistry, purified by reverse-phase high-performance liquid chromatography (at least 80%, usually > 95% purity) and validated by mass spectrometry (Shafer-N, Copenhagen, Denmark). Peptides were distributed at 20 µg/vial and stored lyophilized at −20°C until use. Peptides were dissolved just before use.
Biochemical peptide–HLA class I binding assay
The biochemical assay for peptide–major histocompatibility complex (MHC)-I binding was performed as described previously [2,3]. Briefly, denatured and purified recombinant HLA heavy chains were diluted into a renaturation buffer containing HLA heavy chain, β2-microglobulin, graded concentrations of the test peptide and incubated at 18°C for 48 h allowing equilibrium to be reached. We have demonstrated previously that denatured HLA molecules can fold efficiently, however, only in the presence of appropriate peptide [6]. The concentration of peptide–HLA complexes generated was measured in a quantitative enzyme-linked immunosorbent assay and plotted against the concentration of peptide offered [2]. Because the effective concentration of HLA (3–5 nM) used in these assays is below the equilibrium dissociation constant (KD) of most high-affinity peptide–HLA interactions, the peptide concentration leading to half-saturation of the HLA is a reasonable approximation of the affinity of the interaction. An initial screening procedure was employed whereby a single high concentration (20 000 nM) of peptide was tested. If no complex formation was found, the peptide was assigned as a non-binder to the HLA molecule in question; conversely, if complex formation was found in the initial screening, full titration of the peptide was performed to determine the binding affinity.
Depletion of CD4+ or CD8+ T cells from PBMC
CD4+ T cells or CD8+ T cells were positively depleted from PBMC according to the manufacturer's instruction using monoclonal anti-CD4-coated or monoclonal anti-CD8-coated Dynabeads from Dynal Biotech ASA (Oslo, Norway). PBMC depleted of CD4+ T or CD8+ T cells were verified by flow cytometry.
IFN-γ ELISPOT assay
The PBMC were thawed, washed and then used for CD4+ or CD8+ T cell depletion (see Materials and methods) or cultured directly in RPMI-1640 supplemented with 5% heat-inactivated AB serum (Valley Biomedical, Winchester, VA, USA), 2mM l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. PBMC (4–6 × 106) or depleted PBMC were cultured in 1 ml culture medium in 24-well plates (Nunc, Roskilde, Denmark). Individual peptides were added to a final concentration of 20 µg/ml per well, a concentration generally used for this assay [4,5,7–9], and incubated for 10 days at 37°C, 5% CO2 in humidified air. Recombinant human (rh)IL-2 (Proleukin; Chiron, Amsterdam, the Netherlands) 20 U/ml was added on day 1. Cells were harvested on day 10, washed twice in RPMI-1640 and resuspended in complete medium to a final concentration of 1 × 106 cells/ml. The IFN-γ ELISPOT assay was performed as described previously [5] to quantify peptide-specific T cells after in vitro expansion. Briefly, peptides were added at a final concentration of 10 µg/ml in the absence or presence of 10 µg/ml anti-human CD4 monoclonal antibodies (mAb), 10 µg/ml anti-human CD8 mAb or 10 µg/ml isotype control immunoglobulin G1 (all antibodies from BD Pharmingen, San Diego, CA, USA). For PBMC depleted of CD4+ or CD8+ T cells, cells were cultured in the presence or absence of indicated peptides in ELISPOT plates. In order to block MHC-II-restricted responses, 10 µg/ml anti-pan HLA class II monoclonal antibody IVA12 (ATCC, Rockville, MD, USA) was added, and to block MHC-I-restricted responses anti-MHC-I antibody W6/32 ascites (ATCC) was added at a final dilution of 1:40 for 30 min before adding peptides in ELISPOT assays. As positive controls, cells were stimulated with 10 µg/ml phytohaemagglutinin (Sigma-Aldrich, Poole, Dorset, UK). Results are expressed as mean number of spot-forming cells per 105 PBMC.
Statistics
Wilcoxon's rank-sum test was used to analyse the quantitative differences between the experimental wells and control in ELISPOT assays. A P-value below 0·05 was considered significant.
Results
Responding donors and the effect of anti-CD4 and CD8 antibodies
The PBMC were obtained from donors with reactivity against 9-mer pox virus-derived peptides [4]. Table 2 shows responses in ELISPOT cultures of PBMC from five individual immune donors exposed to seven recently discovered antigenic pox-derived 9-mer peptides with binding affinities for HLA class I at KD ≤ 5 nM [4]. The identification of immune donors and the prediction of immunogenic pox-derived peptides have been reported recently [4]. Table 2 includes data showing the effect on the responses in the presence of blocking anti-CD4 and -CD8 antibodies. As shown in the table, the IFN-γ spot responses were either inhibited significantly in the presence of anti-CD4 antibody (pox peptides 113, 114, 116, 131) or in the presence of anti-CD8 antibody (pox peptides 8, 17, 57) in the ELISPOT culture. Further studies (see below) suggested strongly that the CD4+ and CD8+ T cell responses are mutually independent.
Table 2.
Enzyme-linked immunospot (ELISPOT) responses [spot-forming cell (SFC)] of five donors against seven antigenic pox-peptides in the absence or presence of antibodies blocking either human leucocyte antigen (HLA) class II/CD4 or HLA class I/CD8 ligation.
| Peptide no. | Peptide | Sequence | Predicted HLA supertype | Assayed KD (nM) | Donors | ELISPOT assay: +Peptideb |
||
|---|---|---|---|---|---|---|---|---|
| +Isotype | +Anti-CD4 | +Anti-CD8 | ||||||
| Pox8 | A18R255 | LSDLKKTIY | A1 | 3·5 | Donor 9 | 113 | 89 | 4a |
| Pox113 | E1L213 | FLIDLAFLI | A2 | 3·0 | Donor 19 | 50 | 29a | 52 |
| Pox114 | A47R74 | LMDENTYAM | A2 | 1·0 | Donor 19 | 89 | 35a | 83 |
| Pox116 | P1L71 | RMIAISAKV | A2 | 3·4 | Donor 13 | 16 | 4a | 11 |
| Donor 19 | 80 | 31a | 70 | |||||
| Pox57 | A21R144 | KYQSPVNIF | A24 | 2·2 | Donor 21 | 10 | 5 | 3a |
| Pox17 | A10L637 | DTRGIFSAY | A26 | 1·1 | Donor 28 | 93 | 82 | 51a |
| Pox131 | I1L48 | SEVKFKYVL | B44 | 4·0 | Donor 19 | 95 | 19a | 93 |
Each value represents the mean SFC of four individual ELISPOT cultures. Each individual experiment was repeated for two to three times.
SFC numbers lower than the isotype control, P < 0·05 (Wilcoxon's rank-sum test).
Number of SFC/105 cells.
Depletion of CD4+ and CD8+ T cells from PBMC
To obtain direct evidence on the phenotype of the responding cells depicted in Table 2, PBMC were depleted for either CD4+ or CD8+ T cells prior to incubation with peptides for ELISPOT development. Figure 1 shows the results obtained from PBMC of donor 19, which according to the data in Table 2 reacts with four pox peptides 113, 114, 116 and 131. It is evident that only PBMC depleted of CD8+ T but not CD4+ T cells respond in ELISPOT culture, whereas the CD4+ T cell-depleted PBMC do not respond at all. These data support the data in Table 2, that the responses by this donor (and four other donors, included in Tables 2 and 3) are mediated by CD4+ T cells. In addition, responses were inhibited significantly in the presence of the anti-pan HLA class II antibody IVA12 and anti-CD4 antibody but not in the presence of anti-CD8 antibody. Also, anti-HLA class I antibody W6/32 had no effect (data not included). These data suggest strongly that the responses observed are restricted by HLA class II molecules.
Fig. 1.
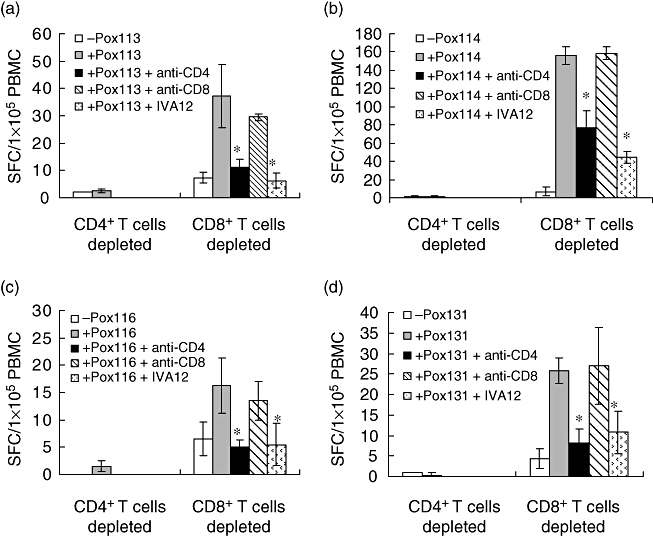
9-mer peptide-induced CD4+ T cell-dependent responses. Peripheral blood mononuclear cells (PBMC) (donor 19) were depleted of CD4+ T cells or CD8+ T cells and incubated with pox113 (a), pox114 (b), pox116 (c) and pox131 (d) respectively. Prior to testing, cells were harvested, washed and exposed to the indicated peptides in enzyme-linked immunospot (ELISPOT) plates for 20 h in the absence or presence of pan anti-human leucocyte antigen class II monoclonal antibody IVA12, anti-CD4 or anti-CD8 antibody. Results are expressed as the mean spot-forming cell values (±standard deviation) of four replicate ELISPOT microcultures, each containing 1 × 105 CD4+ or CD8+ T cell-depleted PBMC. Bars represent standard deviation; *significant inhibition, P < 0·05 (Wilcoxon's rank-sum test).
Figure 2 shows the results obtained from PBMC of three donors (9, 21, 28) measured against pox peptides 8, 57 and 17 respectively. In these cases, depletion of CD4+ T cells did not inhibit responses in the ELISPOT culture, whereas depletion of CD8+ T cells totally removed the responses, supporting the data in Table 2 that these responses are mediated by CD8+ T cells. Also, these latter responses were inhibited fully by both anti-CD8 (Table 2) and anti-MHC-I antibody W6/32 (Fig. 2), but not by anti-CD4 antibody. Also, the anti-pan HLA class II antibody IVA12 had no effect (data not included). These data show that the responses in Fig. 2 are restricted by HLA class I molecules. The data in Figs 1 and 2 also indicate that the anti-CD4 and -CD8 antibodies used are not cytotoxic, but specifically blocking CD4+ T and CD8+ T cell responses respectively. In addition, the data in Figs 1 and 2 show that the same peptide epitope results in either a CD4+ or a CD8+ T cell response. Thus, the two set of responses appear to be mutually independent.
Fig. 2.
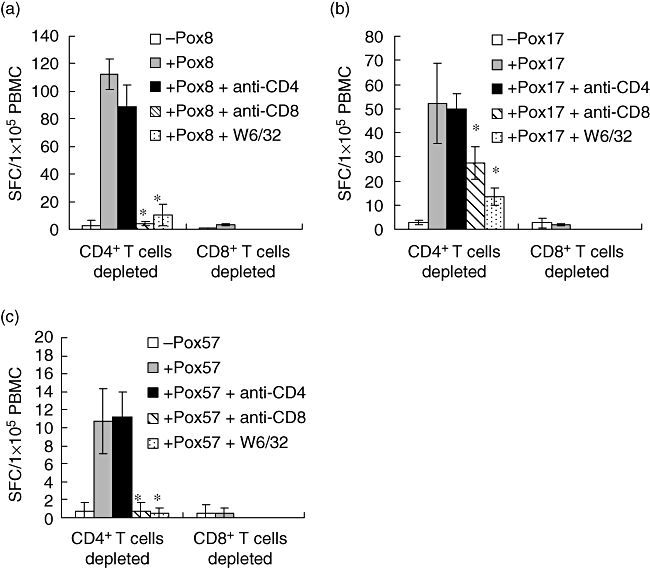
9-mer peptide-induced CD8+ T cell-dependent responses. Peripheral blood mononuclear cells (PBMC) from donors 9, 28 and 21 were depleted of CD4+ T cells or CD8+ T cells and incubated with pox8 (a), pox17 (b) and pox57 (c) respectively. Prior to testing, cells were harvested, washed and exposed to the indicated peptides in enzyme-linked immunospot (ELISPOT) plates for 20 h in the absence or presence of pan anti-human leucocyte antigen class I antibody W6/32, anti-CD4 or anti-CD8 antibody. Results are expressed as the mean spot-forming cell values (±standard deviation) of four replicate ELISPOT microcultures each containing 1 × 105 CD4+ or CD8+ T cell-depleted PBMC. Bars represent standard deviation; *significant inhibition, P < 0·05 (Wilcoxon's rank-sum test).
Discussion
In our previous work [4], a total of 45 pox virus-derived 9-mer peptides were tested for antigenicity in healthy individuals vaccinated with vaccinia virus more than 30 years ago. These peptides were predicted in silico to have high binding-affinities for HLA class I molecules and shown to bind to recombinant HLA class I molecules with an affinity of KD ≤ 5 nM. Eight peptides were found to be antigenic in IFN-γ ELISPOT assay, using peripheral blood T lymphocytes from vaccinated donors as responder cells. Seven of the antigenic peptides are included in the present study. It is assumed generally that about 20% of the possible peptides, which can be generated from a given protein, survive antigen processing and that only half the presented HLA class I binding peptides have a cognate T cell receptor [10,11]. Thus, offering high-affinity 9-mer peptides to the system should result in 0·2 × 0·5 = 10% reactivity. This number is close to the present observation of three HLA class I-restricted, immunogenic peptides of 45 peptides tested.
The major observation of the present study is that four of these seven immunogenic pox virus-derived 9-mer peptides elicit a CD4+ T cell-dependent, apparently class II-restricted response without traces of class I-restricted reactivity. This observation should not be confused with so-called co-receptor mismatched T cells being restricted by HLA class I molecules, but dependent on CD4 binding to HLA class II molecules of the antigen-presenting cells, a phenomenon which has been observed in various experimental settings [12–17].
It is generally accepted that HLA class I binding peptides are composed of eight to 10 amino acids, whereas HLA class II binding peptides consist of 15–20 amino acids being recognized by CD8+ and CD4+ T cells respectively [18–20]. Thus our ELISPOT data, combined with T cell subset depletion experiments, appear to contradict this view by showing that peripheral blood CD4+ T cells from vaccinia virus immune donors react with 9-mer peptides in a seemingly HLA class II-restricted manner, as these responses were blocked by an anti-pan HLA class II and anti-CD4 antibodies. We have no direct proof that the immunogenic 9-mer peptides with high binding affinity for HLA class I molecules also bind to HLA class II molecules. However, we observed that donors with CD4+ T cell responses share certain common HLA class II β chains, as illustrated in Table 3. Thus, the four donors (13, 19, 30 and 31) who showed reactivity against the HLA-A2-binding pox peptide 116 (RMIAISAKV) all expressed the same HLA-DQB1-06 subtype, whereas two donors (19 and 24) who showed reactivity against the HLA-A2-binding pox peptide 114 (LMDENTYAM) both expressed the same HLA-DRB1-03 subtype. The data in Table 3 might suggest that the immunogenic 9-mer pox virus-derived peptides bind to HLA class II molecules expressing the same DR β or DQ β chains, a view supported by the high degree of linkage disequilibrium between DR and DQ α and β chains. However, until our laboratory masters the production of recombinant HLA class II molecules for binding studies we will not have direct proof for HLA class II binding of these peptides.
Both the CD4+ and CD8+ T cell responses generated in the ELISPOT culture system most probably represent true immunological memory achieved in vivo following vaccinia virus vaccination more than 30 years ago, and not primary immune responses generated from naive T cells in the in vitro culture. Thus, assuming that T cells represent approximately 50% of the mononuclear cells in peripheral blood, the present T cell subset depletion experiments indicate that the level of responding cells per ELISPOT culture is approximately 100–300/105 T cells. With four to six cell divisions during the period of in vitro expansion, it follows that five to 20 virus-specific T cells/105 T cells are circulating in the peripheral blood of vaccinia virus immune individuals, a cell number which is far above the assumed frequency of naive T cells (1:105–1:106) of any particular specificity [21].
In previous work we demonstrated immunological memory for only those pox peptides with very high binding affinity for class I molecules (KD ≤ 5 nM), but not peptides of lower affinities [4]. The present observation, that CD4+ T cells have memory for pox virus-derived 9-mer peptides, might reflect the inherent cross-reactivity in the CD4+ T cell population; thus, estimates suggest that one single T cell clone can react productively (i.e. secrete IFN-γ) with approximately 1 million different MHC-associated peptide ligands [22,23]. Accordingly, the CD4+ T cell reactivity observed in the present study might reflect this high degree of cross-reactivity, and the peptide specificity observed might not reflect reactivity against pox virus-derived peptides per se. If, however, our data reflect specific vaccine-induced memory for pox virus-derived 9-mer peptides, CD4-dependent, class II-restricted immune responses are probably induced by viral antigens in dying vaccinia virus-infected cells which, during the process of vaccination, are taken up by dendritic cells, and even 9-mer peptides might be presented by class II molecules. Alternatively, vaccinia virus might infect viable dendritic cells and some viral peptides might reach the class II compartment by an endogenous route. Whatever the mechanism, the same peptide might be presented by both HLA class I and class II molecules. In donors for whom only peptide-specific CD4+ T cell responses are observed, the peptide might not be immunogenic when presented by the MHC class I and thereby spare the dendritic cell for CTL-mediated killing. The vaccine responses, as observed here, lead to initiation of a sustained CD4+ T cell-dependent, apparently HLA class II-restricted memory response favouring vaccine-induced long-lived memory responses. The advantages of CD4+ T cell triggering are obvious: the virus-infected dendritic cells are not killed by anti-viral CD8+ T cells, and the responding CD4+ T cells benefit from the supportive environment provided by class II/peptide-expressing dendritic cells.
In the majority of studies which employ IFN-γ ELISPOT technology to monitor HLA class I responses against class I binding peptides, the investigators should also take into account potential CD4-dependent class II responses. Our present data show that high-affinity class I binding peptides (as we found no immunity against intermediate affinity peptides [4]) might end up as immunogenic peptides in the HLA class II complex. Thus, ELISPOT-based analyses of reactivity against 9-mer class I binding peptides should include either anti-CD4/CD8 blocking or CD4/CD8 T cell subset depletion experiments or, alternatively, perforin- or granzyme B-ELISPOT analyses to obtain the true phenotype of the antigen-specific T cells.
In conclusion, long-lived memory responses against vaccinia virus-derived peptides with high affinity-binding for HLA class I are, in some cases, mediated by CD4+ T cells and apparently restricted by HLA class II molecules.
Acknowledgments
This work was supported by NIAID contracts HHSN266200400083C, HHSN266200400025C and EU 6FP 503231. We are grateful to Ms Trine Devantier for her excellent technical assistance.
References
- 1.Larsen MV, Lundegaard C, Lamberth K, et al. An integrative approach to CTL epitope prediction: a combined algorithm integrating MHC class I binding, TAP transport efficiency, and proteasomal cleavage predictions. Eur J Immunol. 2005;35:2295–303. doi: 10.1002/eji.200425811. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester-Hvid C, Kristensen N, Blicher T, et al. Establishment of a quantitative ELISA capable of determining peptide–MHC class I interaction. Tissue Antigens. 2002;59:251–8. doi: 10.1034/j.1399-0039.2002.590402.x. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester-Hvid C, Nielsen M, Lamberth K, et al. SARS CTL vaccine candidates; HLA supertype-, genome-wide scanning and biochemical validation. Tissue Antigens. 2004;63:395–400. doi: 10.1111/j.0001-2815.2004.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang ST, Wang M, Lamberth K, et al. MHC I restricted epitopes conserved among variola and other related orthopoxviruses are recognised by T cells 30 years after vaccination. Arch Virol. 2008;153:1833–44. doi: 10.1007/s00705-008-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Lamberth K, Harndahl M, et al. CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine. 2007;25:2823–31. doi: 10.1016/j.vaccine.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Ostergaard PL, Nissen MH, Hansen NJ, et al. Efficient assembly of recombinant major histocompatibility complex class I molecules with preformed disulfide bonds. Eur J Immunol. 2001;31:2986–96. doi: 10.1002/1521-4141(2001010)31:10<2986::aid-immu2986>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Hammond AS, Klein MR, Corrah T, et al. Mycobacterium tuberculosis genome-wide screen exposes multiple CD8 T cell epitopes. Clin Exp Immunol. 2005;140:109–16. doi: 10.1111/j.1365-2249.2005.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alter G, Merchant A, Tsoukas CM, et al. Human immunodeficiency virus (HIV)-specific effector CD8 T cell activity in patients with primary HIV infection. J Infect Dis. 2002;185:755–65. doi: 10.1086/339338. [DOI] [PubMed] [Google Scholar]
- 9.Brice GT, Graber NL, Carucci DJ, Doolan DL. Optimal induction of antigen-specific CD8+ T cell responses requires bystander cell participation. J Leukoc Biol. 2002;72:1164–71. [PubMed] [Google Scholar]
- 10.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Lauemoller SL, Kesmir C, Corbet SL, et al. Identifying cytotoxic T cell epitopes from genomic and proteomic information: ‘the human MHC project’. Rev Immunogenet. 2000;2:477–91. [PubMed] [Google Scholar]
- 12.Boyle LH, Goodall JC, Opat SS, Gaston JS. The recognition of HLA-B27 by human CD4(+) T lymphocytes. J Immunol. 2001;167:2619–24. doi: 10.4049/jimmunol.167.5.2619. [DOI] [PubMed] [Google Scholar]
- 13.Boyle LH, Hill Gaston JS. Breaking the rules: the unconventional recognition of HLA-B27 by CD4+ T lymphocytes as an insight into the pathogenesis of the spondyloarthropathies. Rheumatology (Oxf) 2003;42:404–12. doi: 10.1093/rheumatology/keg097. [DOI] [PubMed] [Google Scholar]
- 14.Boyle LH, Goodall JC, Gaston JS. The recognition of abnormal forms of HLA-b27 by CD4+ T cells. Curr Mol Med. 2004;4:51–8. doi: 10.2174/1566524043479257. [DOI] [PubMed] [Google Scholar]
- 15.Darrow TL, bdel-Wahab Z, Quinn-Allen MA, Seigler HF. Recognition and lysis of human melanoma by a CD3+, CD4+, CD8- T-cell clone restricted by HLA-A2. Cell Immunol. 1996;172:52–9. doi: 10.1006/cimm.1996.0214. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Kimura S, Aoki N, Sato K, Celis E, Katagiri M. Existence of MHC class I-restricted alloreactive CD4+ T cells reacting with peptide transporter-deficient cells. Immunogenetics. 2001;53:626–33. doi: 10.1007/s00251-001-0379-7. [DOI] [PubMed] [Google Scholar]
- 17.Moore RL, Fox BS. CD4+ class I-restricted T cells specific for HIV gp160 315-329. Cell Immunol. 1994;154:43–53. doi: 10.1006/cimm.1994.1055. [DOI] [PubMed] [Google Scholar]
- 18.Chicz RM, Urban RG, Lane WS, et al. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992;358:764–8. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 19.Rudensky AY, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–7. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 20.York IA, Goldberg AL, Mo XY, Rock KL. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol Rev. 1999;172:49–66. doi: 10.1111/j.1600-065x.1999.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 21.Lammermann T, Sixt M. The microanatomy of T-cell responses. Immunol Rev. 2008;221:26–43. doi: 10.1111/j.1600-065X.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 22.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DB, Wilson DH, Schroder K, et al. Specificity and degeneracy of T cells. Mol Immunol. 2004;40:1047–55. doi: 10.1016/j.molimm.2003.11.022. [DOI] [PubMed] [Google Scholar]


