Abstract
The objective of this study was to assess protein levels for candidate cytokines, chemokines, growth factors, matrix metalloproteinases and their inhibitors in bronchoalveolar lavage fluid (BALF) in patients with polar forms of pulmonary sarcoidosis, i.e. Löfgren's syndrome (LS) and more advanced chest X-ray (CXR) stage III disease. Twenty-four inflammatory molecules were analysed in unconcentrated BALF samples from 10 sarcoidosis patients with CXR stage III and 10 patients with LS by semiquantitative protein array. Four novel molecules [CC chemokine ligand (CCL)15, CCL16, macrophage migration inhibitory factor (MIF) and macrophage stimulating protein (MSP)], detected for the first time in association with sarcoidosis, were then quantified by enzyme-linked immunosorbent assay in a second cohort of 68 sarcoidosis patients and 17 control subjects. The protein levels of CCL15, CCL16, CCL24, CXCL8, CXCL9, CXCL10, interleukin-16, MIF, MSP and matrix metallopeptidase 1 were increased in CXR stage III patients when compared with patients with LS. CCL15 and MSP up-regulation in CXR stage III patients in comparison with LS patients and controls was confirmed by enzyme-linked immunosorbent assay. Moreover, MSP was associated with treatment requirement (P = 0·001) and CCL15 was elevated in patients with disease progression at 2-year follow-up (P = 0·016). CCL16 levels were increased in sarcoidosis versus controls (P < 0·05), but no difference was observed between patient subgroups. MIF up-regulation was not confirmed in a larger patient group. In conclusion, chemokines CCL15, CCL16 and MSP were found elevated for the first time in BALF from sarcoidosis patients; our results showed that CCL15 and MSP may affect disease course.
Keywords: bronchoalveolar lavage fluid, chemokines, cytokines, Löfgren's syndrome, matrix metalloproteinases
Introduction
Sarcoidosis is an inflammatory disorder characterized by the accumulation of CD4+ helper T cell type 1 lymphocytes and macrophages with subsequent granuloma formation at the site of disease, notably in the lung [1]. This multi-system disorder of unknown aetiology has a prevalence of 63·1 cases per 100 000 in the Czech Republic [2]. Spontaneous remission of the disease occurs in approximately two-thirds of cases, but up to 30% of patients develop a chronic functional deficit with progression to fibrosis [3].
Recently, there has been ongoing discussion about the definition of clinical severity of sarcoidosis. Radiological assessment of lung involvement is often used for estimation of clinical severity of sarcoidosis because of its precise definition and clinical importance in outcome prediction [3]. It has been graded from chest X-ray (CXR) stage I to stage IV, with stages III and IV being the most advanced disease manifestation forms, characterized by parenchymal involvement and fibrosis with a low rate of spontaneous resolution (less than 20% for stage III, zero for stage IV) [1,3]. Löfgren's syndrome (LS), characterized by an acute onset, fever, erythema nodosum, arthralgias and bilateral hilar lymphadenopathy, is a distinct sarcoidosis clinical phenotype, which has a better long-term prognosis than other disease forms [1]. There is strong evidence that a wide spectrum of inflammatory molecules is involved in sarcoidosis development and progression [4]. A number of immune mediators, namely chemotactic cytokines, have been implicated in T cell and macrophage infiltrations at sites of inflammation in sarcoidosis, thus leading to granuloma formation (e.g. [5–7]). We hereby hypothesize that the intensity of inflammation may reflect the pathogenic events in different clinical phenotypes of sarcoidosis. This speculation is supported by our previous observation of differential bronchoalveolar lavage fluid (BALF) protein profiles in distinct sarcoid CXR stages and in LS [8].
In order to reveal plausible differences in inflammatory processes ongoing in the two polar clinical forms of pulmonary sarcoidosis we screened for protein levels of 24 molecules including cytokines, chemokines, growth factors, matrix metalloproteinases and their inhibitors in BALF samples from 10 patients with CXR stage III and 10 subjects with LS. The selection of investigated markers was based on their proinflammatory and regulatory function with plausible role in pathogenesis of immune-mediated diseases, in particular of sarcoidosis. For example, CC and CXC chemokines and interleukin (IL)-16 regulate trafficking of inflammatory cells into sites of injury [9], and many of them have already been shown to be involved in sarcoidosis development [10]. Matrix metalloproteinases and their inhibitors are responsible for the deposition and degradation of connective tissue under normal and pathological conditions [11], thus may be important in lung tissue remodelling in sarcoidosis. Macrophage stimulating protein (MSP) and macrophage migration inhibitory factor (MIF) are cytokines known to be initiators and regulators of the immune response [12,13] and may, therefore, be involved in the pathogenesis of sarcoidosis.
In the second phase of the study, the candidate molecules from the first screening phase were quantified subsequently by enzyme-linked immunosorbent assay (ELISA) in a bigger cohort of 68 sarcoidosis patients and 17 control subjects. Additionally, we explored if the newly identified candidate proteins are associated with sarcoidosis disease course assessed according to the chest radiographic stages, requirement for treatment and 2-year outcomes of disease.
Subjects and methods
Study subjects
In total, 20 patients (10 patients with CXR stage III (SIII) and 10 patients with LS) were included in the screening phase studies. The second cohort consisted of sarcoidosis patients [n = 68, LS = 17, CXR stage I (SI) = 13, CXR stage II (SII) = 22, CXR stage III (SIII) = 16] and controls (n = 17); for the basic characteristics of patients and controls, including demographics and bronchoalveolar lavage (BAL) cytology, see Table 1. The diagnosis of pulmonary sarcoidosis was in compliance with the criteria presented in a consensus joint statement on sarcoidosis [3]: typical clinical features together with granulomas on lung biopsies and supported by the BAL cellular profile.
Table 1.
Clinical and laboratory data of investigated subjects.
| For ELISA measurements |
For RayBio cytokine array measurements |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Controls | Sarcoidosis | Löfgren's syndrome * | CXR stage I | CXR stage II | CXR stage III | Löfgren's syndrome | CXR stage III |
| n = 17 | n = 68 | n = 17 | n = 13 | n = 22 | n = 16 | n = 10 | n = 10 | |
| Age, years | 44 ± 16 | 48 ± 12 | 40 ± 10 | 52 ± 12 | 47·0 ± 10·9 | 56 ± 10 | 38 ± 11 | 52 ± 8 |
| (19−63) | (28−80) | (28−66) | (34−71) | (29·0−70·0) | (32−82) | (28−66) | (34−63) | |
| Sex (male/female) | 11/6 | 29/39 | 7/10 | 6/7 | 10/11 | 4/11 | 6/4 | 3/7 |
| Smoking (smoker/non-smoker)* | 8/9 | 16/52 | 7/10 | 2/11 | 6/15 | 1/14 | 5/5 | 1/9 |
| Treatment (yes/no)‡ | −/17 | 50/16 | 9/7 | 8/5 | 18/3 | 14/1 | 2/8 | 9/1 |
| CXR stages (I/II/III) | – | 27/25/16 | 14/3/− | – | – | – | – | – |
| BALF differential count | ||||||||
| % Macrophages | 92·7 ± 4·8 | 72·5 ± 19·0 | 76·6 ± 12·3 | 66·5 ± 23·2 | 76·1 ± 12·7 | 63·2 ± 26·1 | 73·3 ± 14·0 | 77·9 ± 16·2 |
| (83·9−99·0) | (0·0−94·0) | (59·4−91·0) | (0·0−91·1) | (40·0−90·0) | (0·0−91·4) | (49·4−91·0) | (44·4−92·1) | |
| % Lymphocytes | 5·2 ± 4·4 | 21·1 ± 12·8 | 20·8 ± 12·1 | 25·0 ± 14·1 | 20·6 ± 10·9 | 21·0 ± 15·6 | 23·01 ± 14·4 | 18·5 ± 14·3 |
| (0·0−13·6) | (0·0−54·0) | (7·0−49·0) | (0·0−47·0) | (5·0−42·0) | (0·0−54·0) | (7·0−49·0) | (7·6−54·0) | |
| % Neutrophils | 1·6 ± 2·2 | 2·9 ± 9·3 | 1·2 ± 0·9 | 0·6 ± 0·7 | 2·4 ± 4·4 | 8·2 ± 19·3 | 1·4 ± 0·7 | 3·2 ± 4·2 |
| (0·0−8·0) | (0·0−73·0) | (0·0−2·6) | (0·0−2·0) | (0·0−18) | (0·0−73·0) | (0·3−2·3) | (0·3−11·0) | |
| % Eosinophils | 0·5 ± 1·0 | 0·6 ± 2·1 | 1·4 ± 4·0 | 0·2 ± 0·3 | 0·3 ± 0·6 | 0·5 ± 1·3 | 3·0 ± 6·2 | 1·7 ± 2·2 |
| (0·0−3·6) | (0·0−17·0) | (0·0−17·0) | (0·0−1·0) | (0·0−2·5) | (0·0−5·0) | (0·3−17·0) | (0·3−5·0) | |
| % CD3+† | 78·1 ± 14·1 | 85·7 ± 12·2 | 91·4 ± 6·3 | 82·6 ± 16·1 | 86·3 ± 9·5 | 82·7 ± 15·2 | 92·7 ± 3·5 | 84·1 ± 11·0 |
| (40·0−90·0) | (43·0−98·0) | (72·0−97·0) | (45·0−97·0) | (67·0 + 98·0) | (43·0−96·0) | (87·0−97·0) | (67·0−96·0) | |
| % CD4+† | 48·2 ± 14·9 | 65·1 ± 18·4 | 77·3 ± 8·7 | 68·7 ± 17·5 | 59·5 ± 19·3 | 60·8 ± 19·8 | 77·2 ± 5·8 | 60·0 ± 15·8 |
| (22·0−68·0) | (23·0−90·0) | (57·0−89·0) | (29·0−87·0) | (23·0−86·0) | (27·0−90·0) | (64·0−83·0) | (33·0−88·0) | |
| % CD8+† | 29·1 ± 10·1 | 18·1 ± 13·6 | 10·6 ± 5·1 | 14·3 ± 9·4 | 24·5 ± 17·6 | 17·9 ± 12·4 | 9·8 ± 3·9 | 19·9 ± 10·3 |
| (14·0−51·0) | (4·0−74·0) | (4·0−23·6) | (4·0−42·0) | (4·2−74·0) | (4·7−46·0) | (6·0−19·0) | (5·0−36·0) | |
| % CD19+† | 1·0 ± 1·1 | 0·7 ± 1·1 | 0·5 ± 0·6 | 0·6 ± 1·1 | 0·7 ± 1·3 | 1·1 ± 1·4 | 0·4 ± 0·5 | 0·4 ± 0·7 |
| (0·0−3·0) | (0·0−6·0) | (0·0−2·0) | (0·0−4·0) | (0·0−6·0) | (0·0−5·0) | (0·0−1·0) | (0·0−3·0) | |
| BALF CD4+/CD8+ ratio | 1·9 ± 0·9 | 6·4 ± 5·3 | 9·0 ± 4·6 | 7·1 ± 5·6 | 4·8 ± 5·0 | 6·0 ± 5·7 | 8·9 ± 2·9 | 5·0 ± 5·1 |
| (0·5−3·7) | (0·3−22·3) | (2·9−22·3) | (1·3−21·0) | (0·3−19·8) | (0·6−19·1) | (3·4−13·3) | (1·3−17·6) | |
One ex-smoker (quit for more than 9 years) patient with Löfgren's syndrome was included in the non-smoker group.
Data on T cell subpopulations were missing for one patient.
Data on treatment were absent for one patient with Löfgren's syndrome. BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay. Data are presented as mean ± standard deviation (min/max).
The treatment was according the disease course and severity of the symptoms in individual cases [2]. For LS patients non-steroidal anti-inflammatory agents (NSAIDs) were applied for symptomatic relief (four of 17 LS patients, 23%), corticosteroid therapy was required when NSAIDs was not sufficient (five of 17 LS patients, 29%) [3].
Standard BAL procedure was performed during fibreoptic bronchoscopy, as described elsewhere [14]. Briefly, during flexible fibreoptic bronchoscopy, 100 ml prewarmed saline was instilled into subsegmental bronchi in three sites: right middle lobe; right lower lobe; and lingula. The BALF recovery rate (as %) was 60·3 ± 7·3 for control subjects and 61·1 ± 12·1 for sarcoidosis patients (P > 0·05). Samples were obtained at the time of disease presentation; no patient received medication before the BAL procedure.
The control group consisted of subjects who at the time of presentation and subsequently showed no clinical signs of lung inflammation; they had no lung disease in their medical history; all had normal BALF cytology, immunology and microbiology. Patients and controls enrolled in this study were white Caucasians; all were diagnosed between 2003 and 2007 in a referral centre for interstitial lung diseases in Olomouc.
The study was approved by the Ethics Committee of the Medical Faculty of Palacky University and Faculty Hospital Olomouc. All subjects signed informed consent about the usage of an aliquot of BAL sample for the research purposes of this study.
Determination of protein levels of inflammatory molecules in BALF from patients with LS and sarcoidosis CXR stage III
The BAL samples (15 ml) were centrifuged and the BALF was separated from cells. Fluid was then aliquoted and stored at − 80°C until analysis.
The protein levels of 24 inflammatory molecules were screened using RayBio Custom cytokine antibody array (RayBiotech Inc., Norcross, GA, USA), according to the manufacturer's instructions. Briefly, after preincubation of membranes with blocking buffer for 30 min, BALF samples (1 ml) were added to membrane and incubated for 2 h. After washing with wash buffers I and II (3 × 5 min and 2 × 5 min respectively), biotin-conjugated antibody cocktail (1 ml) was added to membrane and incubated for 2 h; washed again and incubated with 2 ml of horseradish peroxidase (HRP)-conjugated streptavidin. After the final wash, chemiluminescence substrate (ECL) was added (400 µl) and intensities were recorded at 30, 60 and 90 s by Fujifilm LAS-1000 (Fuji Photo Film Co. Ltd, Tokyo, Japan). Quantification of signal intensities on recorded images was performed using ImageJ version 1·37 software (Wayne Rasband, NIH, USA; http://rsb.info.nih.gov). The data were expressed as the percentage of the integral intensity of signal (calculated as a multiple of a mean signal intensity of spot and the spot size in pixels) related to positive controls. The positive controls were run in quadruplicate, the studied molecules in duplicate. For the antibody sensitivity data see http://www.raybiotech.com/human_array_sensitivity.pdf.
Determination of protein levels of CC chemokine ligand (CCL)15, CCL16, MSP and MIF by ELISA
Concentrations of CCL15, CCL16, MSP and MIF proteins in the unconcentrated BALF samples were measured by commercially available ELISA kits, according to the manufacturer's instructions. All ELISA kits were purchased from RayBiotech except for the MIF kit, purchased from R&D Systems (Minnesota, MN, USA). The detection limits of the CCL15, CCL16, MSP and MIF ELISA were 10, 8, 8 and 0·7 pg/ml respectively.
Determination of cell-associated CCL15 and MSP proteins by immunocytochemistry
The MSP and CCL15 proteins were detected on the acetone–chloroform fixed cytocentrifuge preparations (cytospins) of (1) BAL cells and (2) cells obtained by brushing during bronchoscopy. Briefly, cytospins were incubated in a 1:50 dilution of an anti-MSP alpha chain antibody or anti-CCL15 monoclonal antibody (both from R&D Systems, Abingdon, UK) respectively. Specific binding of the primary antibody was visualized by biotinylated antibody, followed by streptavidin–biotin/HRP (LSAB2 System-HRP; DakoCytomation Denmark A/S, Copenhagen, Denmark). In parallel negative control slides, the specific antibody was substituted by an irrelevant isotype-matched antibody (R&D Systems). 3,9 amino-ethyl-carbazole was used as a chromogenic substrate for HRP; the slides were counterstained with Shandon's haematoxylin.
Statistical analysis
spss version 13·0 (SPSS Inc., Chicago, IL, USA) software was used for the analyses. Data were checked for normality using the Kolmogorov–Smirnov test. Group differences in the levels of studied molecules were evaluated with Student's t-test for independent samples and analysis of variance Data are presented as mean ± standard error of the mean; P < 0·05 was considered significant and P < 0·1 was considered as trend.
Results
Screening for inflammatory molecules in BALF from patients with LS and sarcoidosis CXR stage III
In order to reveal which inflammatory mediators differentiate the polar forms of pulmonary sarcoidosis, BALF samples from patients with LS and SIII were first screened for the levels of 24 cytokines, chemokines, matrix metallo-proteinases and their inhibitors using semiquantitative methodology of protein arrays. Of 24 proteins included in the analyses, seven [CCL16, CXCL8, CXCL10, IL-16, MIF, matrix metallopeptidase 1 (MMP1) and MSP] were increased in patients with SIII compared with LS. Three further proteins (CCL15, CCL24 and CXCL9) tended to have increased levels in BALF samples of SIII patients compared with LS (Fig. 1). Altogether, 10 molecules were expressed differentially in the investigated groups.
Fig. 1.
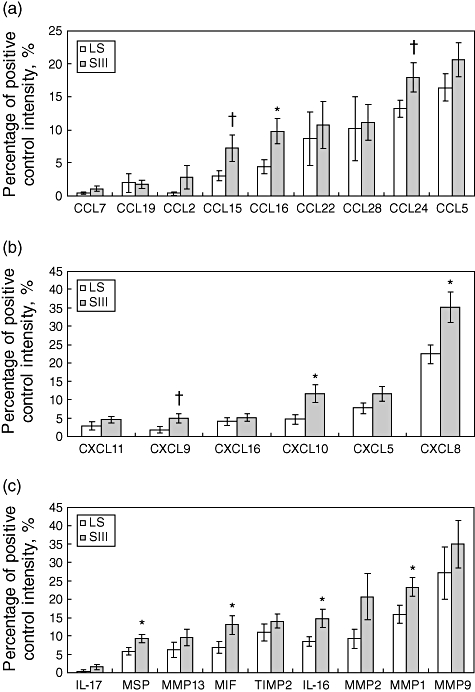
Semiquantitative levels (obtained by protein-array) of CC chemokines (a), CXC chemokines (b) and other inflammatory molecules (c) in bronchoalveolar fluid (BALF) samples of patients with Löfgren's syndrome and sarcoidosis CXR stage III. Data presented as mean ± standard error of the mean (whiskers). For more information see Subjects and methods. *P < 0·05 (significant difference); †P < 0·1 (trend).
Quantitative measurement of CCL15, CCL16, MSP and MIF proteins in BALF
Of 10 inflammatory molecules, as specified above, four (CCL15, CCL16, MSP and MIF) were detected to be expressed differentially in LS versus SIII for the first time. To confirm and extend this observation, BALF protein levels of MIF, MSP, CCL15 and CCL16 were quantified, therefore, in BALF samples from the second study cohort, which comprised a higher number of patients and controls, including those patients presenting with LS (n = 17) and stage III disease (n = 16). CCL15 and MSP levels were increased in BALF from patients with SIII compared with LS patients (P < 0·001), whereas levels of MIF and CCL16 showed no difference between the groups (Fig. 2a). Furthermore, BALF CCL15, CCL16 and MSP levels were higher in the combined group of sarcoidosis patients compared with control subjects (Fig. 2b). Because the levels of MIF did not differ between controls and patients (3·5 ± 0·4 ng/ml versus 3·9 ± 0·5 ng/ml, P > 0·05), MIF was excluded from further studies.
Fig. 2.
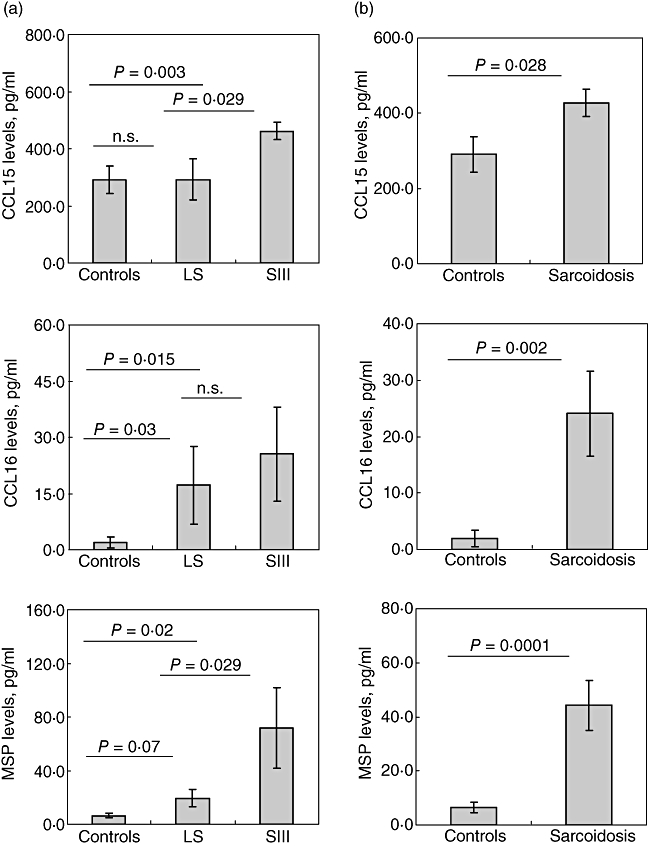
Quantitative levels (obtained by enzyme-linked immunosorbent assay) of CCL15, CCL16 and MSP in bronchoalveolar fluid (BALF) of sarcoidosis patients. (a) comparison of CCL15, CCL16 and macrophage stimulating protein (MSP) levels in BALF of Löfgren's syndrome, CXR stage III patients and healthy subjects. (b) Comparison of CCL15, CCL16 and MSP in sarcoidosis patients and healthy controls. Data presented as mean ± standard error of the mean (whiskers). For more information see Subjects and methods. *P < 0·05; n.s.: not significant.
Relationship between CCL15, CCL16 and MSP protein levels in BALF and clinical course of sarcoidosis
The levels of CCL15 and MSP were higher in patients with CXR stages II and III compared with stage I patients [CCL15 (ng/ml): SIII = 461·6 ± 29·8, SII = 541·0 ± 79·0, SI = 311·4 ± 43·9, P < 0·05; MSP (ng/ml): SIII = 71·8 ± 30·1, SII = 57·3 ± 15·3, SI = 16·8 ± 7·7, P < 0·05]. MSP levels were increased significantly in the patients requiring corticosteroid treatment (P = 0·001, Fig. 3b) and higher CCL15 levels were associated with disease progression at 2-year follow-up (P = 0·016, Fig. 3a). No differences were observed in CCL15 and MSP levels in patients subgroups divided according to the pattern of multi-organ involvement (data not shown).
Fig. 3.
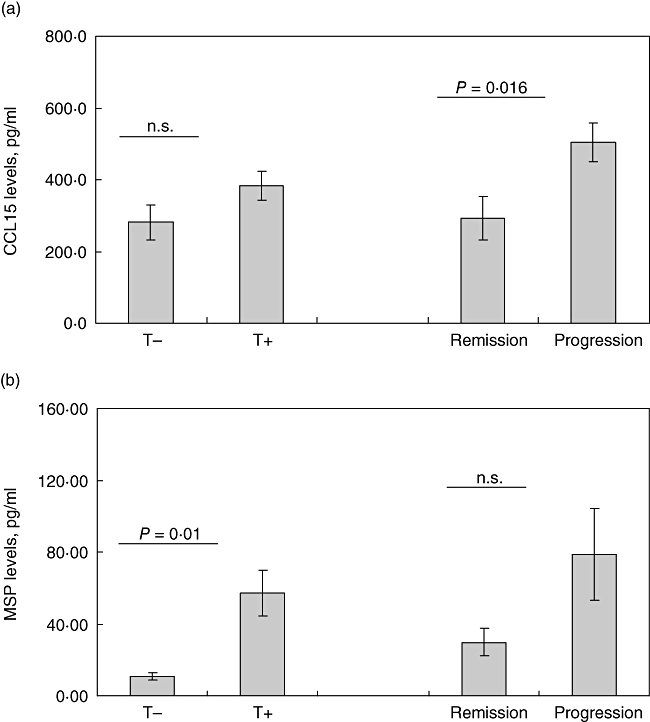
Levels of CCL15 (a) and macrophage stimulating protein (b) in patients with different treatment requirement and 2-year follow-up. Data presented as mean ± standard error of the mean (whiskers). For more information see Subjects and methods. T+: treatment needed; T–, treatment not needed; n.s.: not significant.
Determination of cell-associated MSP and CCL15 proteins by immunocytochemistry
To identify the cellular source of MSP and CCL15, cytocentrifuge preparations of BALF cells and cells were obtained by brushing from patients with sarcoidosis were immunostained for MSP and CCL15 protein respectively. The MSP protein expression was detected in alveolar macrophages, but not in alveolar lymphocytes (Fig. 4a). Moreover, MSP expression was associated with some lung tissue macrophages obtained by brushing (data not shown). CCL15 protein was not detected in alveolar lymphocytes and macrophages (Fig. 4b); it was found associated with lung cells obtained by brushing (data not shown).
Fig. 4.
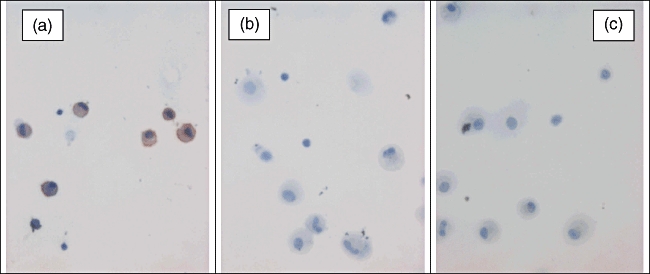
Detection of macrophage stimulating protein (MSP) (a) and CCL15 (b) proteins in bronchoalveolar cells from patients with pulmonary sarcoidosis; representative result of immunocytochemistry experiments. Positively stained cells were observed in bronchoalveolar lavage cytospin preparations incubated with anti-MSP antibody (a), but not with anti-CCL15 antibody (b) and with an irrelevant antibody (c). MSP was localized to alveolar macrophages; staining was not observed in alveolar lymphocytes. Original magnification ×400.
Discussion
The main finding from this study, which investigated differential expression of inflammatory molecules in polar clinical forms of pulmonary sarcoidosis, is up-regulation of three novel mediators: CCL15, CCL16 and MSP in bronchoalveolar fluid from sarcoidosis patients. Up-regulation of CCL15 and MSP protein levels in the bronchoalveolar space of patients with sarcoidosis, and in particular in patients with more advanced disease (CXR stage III), is reported here for the first time. Importantly, two of these novel candidate molecules were shown to be related to disease course: increased levels of CCL15 were associated with disease progression at 2 years and MSP levels were elevated in patients requiring corticosteroid treatment. Increased levels of CCL16 protein were observed in bronchoalveolar fluid from the combined group of patients in comparison with healthy controls, but no differences were observed in patient subgroups. In addition, using a semiquantitative methodology we found elevation of CXCL8, CXCL10, IL-16 and MMP1 protein in bronchoalveolar fluid from sarcoidosis patients.
Up-regulation of CXCL8, CXCL10, IL-16 and MMP1 in sarcoidosis compared with healthy subjects has been reported previously [15–19]. It has been shown that these molecules participate actively in the activation of alveolar macrophages, monocytes, in T cell attraction and differentiation and in granuloma formation [4,20,21]. As an extension of these previous studies, here we demonstrate that the protein levels of these molecules are increased in bronchoalveolar fluid from patients with a more advanced form of sarcoidosis (CXR stage III) in comparison with patients with a more favourable disease pattern (LS). These results are in line with the hypothesis that the intensity of the inflammatory process may result in a different clinical course in sarcoidosis. Also, two other studied chemoattractants, CCL2 and CCL5, have been implicated in sarcoidosis [7,22–24]. In our previous paper we reported CCL2 elevation in sarcoid BAL, where up-regulation was more apparent in patients with stage II than in stage I patients [7,23]. In this study we compared CCL2 concentration in LS and stage III patients, but we did not observe any difference between these groups in our sample set. Concerning CCL5 chemokine, we also did not detect significant elevation of this protein in SIII versus LS. A similar result was obtained by our group when CCL5 BALF concentrations were compared between stage I and stage II patients [7].
The MSP was the first mediator that we found to be expressed differentially between acute and chronic sarcoidosis in this study. MSP expression has been limited so far to hepatocytes [25]. In this study we provide evidence that alveolar macrophages as well as some lung macrophages express MSP protein. The MSP spectrum of activity is wide: it has been shown to act on mononuclear phagocytes [25], to regulate the mucociliary function [26] and to induce superoxide anion production in human alveolar macrophages in sarcoidosis and idiopathic pulmonary fibrosis [27]. Also, MSP can induce tumour necrosis factor (TNF)-α and IL-1β release from alveolar macrophages from patients with sarcoidosis and idiopathic pulmonary fibrosis via stimulation of the nuclear factor kappa B pathway [28]. Similarly, in this study we observed positive correlation between MSP and TNF-α in the BALF of CXR stage III patients (data not shown). This observation allowed us to speculate that MSP may be involved in the propagation of the inflammatory process in patients with a more progressive form of sarcoidosis. In this context, a genome-wide search for predisposing genes in sarcoidosis implicated MSP as one of the ‘candidate’ genes that contribute to the risk of sarcoidosis development [29].
The CCL15, a member of the macrophage inflammatory protein-1 family of chemokines, localized in the CC chemokine gene cluster at chromosome 17 [30], was another chemokine which was found to be elevated in our patients with stage III disease. CCL15 chemokine is synthesized in intestine, liver [30,31], adrenal gland, heart and skeletal muscles [32] and in lung leukocytes and macrophages [30]. In agreement with Pardigol et al.[30], we detected CCL15 protein in lung cells obtained by brushing. However, in our sarcoidosis patients, CCL15 protein was not expressed in alveolar macrophages and lymphocytes. Up-regulation of this chemokine has been already described in arthritis, another Th1 inflammatory disease [33]. Although reports of a role for CCL15 in immune-mediated diseases are scarce, our findings of up-regulated levels of CCL15 and of its association with 2-year disease outcome suggested to us that this chemokine may be involved in the progressive sarcoidosis. Although the exact mechanism is not yet known, the possible action for CCL15 in the lung is the attraction of neutrophils along with CXCL8. It is known that the number of neutrophils in BAL is elevated in advanced stages of disease [34,35], where we detected the highest concentration of CCL15 protein. Further studies concerning the exact function of this chemokine in inflamed lung are needed.
Finally, CCL16 protein levels were increased in sarcoidosis patients as a whole, but no difference was observed among subgroups of patients based on clinical phenotypes, 2-year outcome, treatment requirement and multi-organ involvement. CCL16 is a chemoattractant for monocytes and lymphocytes, but not for neutrophils [36]. Macrophages respond to CCL16 by enhancing their production of CCL2, IL-1β, TNF-α and IL-12 [36]. Up-regulation of CCL16 has been reported in various inflammatory diseases such as rheumatoid arthritis and osteoarthritis [33]. It is, therefore, conceivable that this chemokine may amplify inflammation non-specifically by inducing the expression of proinflammatory cytokines and chemokines.
In this study, we have chosen a two-step approach to investigate ‘candidate proteins’ involved in sarcoidosis, because of the possibility to screen for large number of proteins using semiquantitative assay, followed by quantification of differentially expressed proteins by ELISA in all stages of sarcoidosis. In the first phase, we focused on two polar presentations of sarcoidosis: LS, a clinical phenotype with good prognosis, and CXR stage III, characterized by a worse prognosis and low resolution rate. Thus, we suggested that the inflammatory markers observed in stage III may be involved in the disease progression. Of four novel candidate proteins, which were acquired from the screening phase, three proteins were confirmed by ELISA. This two-step approach is suitable for studies seeking for large numbers of proteins with limited material availability (i.e. samples from rare clinical phenotypes). We are aware that omitting analysis of BALF from CXR stage IV patients because of the limited number of available samples is a limitation of this study.
In conclusion, for the first time the results of the present study implicate chemokines CCL15, CCL16 and MSP in the pathogenesis of sarcoidosis and suggest that CCL15 and MSP may affect disease course. Hence, the exact role of these novel candidate molecules in the pathomechanisms of sarcoidosis should be elucidated in future studies.
Acknowledgments
This work was funded by a grant from the Czech Ministry of Health (IGA MZ CR NR/9037).
Abbreviations
CCL2 – MCP1, monocyte chemoattractant protein 1; CCL5 – RANTES, regulation on activation, normal T cell expression and secretion; CCL7 – MCP3, monocyte chemoattractant protein 3; CCL15 – MIP-1d, macrophage inflammatory protein 5; CCL16 – HCC-4, haemofiltrate CC-chemokine-4; CCL19 – MIP-3b, macrophage inflammatory protein-3b; CCL22 – MDC, macrophage-derived chemokine; CCL24, eotaxin-2; CCL28 – MEC, mucosae-associated epithelial chemokine; CXCL5 – ENA-78, epithelial neutrophil-activating peptide-78; CXCL8 – IL-8, interleukin-8; CXCL9 – MIG, monokine induced by gamma interferon; CXCL10 – IP-10, interferon-inducible cytokine IP-10; CXCL11 – I-TAC, interferon-inducible T-cell alpha chemoattractant; CXCL16, CXC chemokine ligand 16; MMP1, matrix metallopeptidase 1, interstitial collagenase; MMP2, matrix metallopeptidase 2, gelatinase A; MMP9, matrix metallopeptidase 9, gelatinase B; MMP13, matrix metallopeptidase 13, collagenase 3; TIMP2, tissue inhibitor of metalloproteinase 2.
References
- 1.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–18. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 2.Kolek V. Sarcoidosis: known and unknown [in Czech] Prague: Grada Publishing; 1998. [Google Scholar]
- 3.American Thoracic Society, European Respiratory Society, World Association of Sarcoidosis and Other Granulomatous Disorders. Joint statement on sarcoidosis. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 4.Zissel G, Prasse A, Müller-Quernheim J. Sarcoidosis – immunopathogenetic concepts. Semin Respir Crit Care Med. 2007;28:3–14. doi: 10.1055/s-2007-970329. [DOI] [PubMed] [Google Scholar]
- 5.Agostini C, Trentin L, Facco M, et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J Immunol. 1996;157:910–18. [PubMed] [Google Scholar]
- 6.Gibejova A, Mrazek F, Subrtova D, et al. Expression of macrophage inflammatory protein-3 beta/CCL19 in pulmonary sarcoidosis. Am J Respir Crit Care Med. 2003;167:1695–703. doi: 10.1164/rccm.200205-487OC. [DOI] [PubMed] [Google Scholar]
- 7.Petrek M, Kolek V, Szotkowská J, et al. CC and C chemokine expression in pulmonary sarcoidosis. Eur Respir J. 2002;20:1206–12. doi: 10.1183/09031936.02.00289902. [DOI] [PubMed] [Google Scholar]
- 8.Kriegova E, Melle C, Kolek V, et al. Protein profiles of bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 2006;173:1145–54. doi: 10.1164/rccm.200507-1126OC. [DOI] [PubMed] [Google Scholar]
- 9.Pease JE, Williams TJ. The attraction of chemokines as a target for specific anti-inflammatory therapy. Br J Pharmacol. 2006;147(Suppl.)(1):S212–21. doi: 10.1038/sj.bjp.0706475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurrieri C, Bortoli M, Brunetta E, et al. Cytokines, chemokines and other biomolecular markers in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(Suppl.)(1):S9–14. [PubMed] [Google Scholar]
- 11.McDonnell S, Morgan M, Lynch C. Role of matrix metalloproteinases in normal and disease processes. Biochem Soc Trans. 1999;27:734–40. doi: 10.1042/bst0270734. [DOI] [PubMed] [Google Scholar]
- 12.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 13.Wang MH, Zhou YQ, Chen YQ. Macrophage-stimulating protein and RON receptor tyrosine kinase: potential regulators of macrophage inflammatory activities. Scand J Immunol. 2002;56:545–53. doi: 10.1046/j.1365-3083.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapman JT, Mehta AC. Bronchoscopy in sarcoidosis: diagnostic and therapeutic interventions. Curr Opin Pulm Med. 2003;9:402–7. doi: 10.1097/00063198-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama K, Mukae H, Ishii H, et al. Elevated levels of interferon gamma-inducible protein-10 and epithelial neutrophil-activating peptide-78 in patients with pulmonary sarcoidosis. Respirology. 2006;11:708–14. doi: 10.1111/j.1440-1843.2006.00933.x. [DOI] [PubMed] [Google Scholar]
- 16.Center DM, Berman JS, Kornfeld H, et al. The lymphocyte chemoattractant factor. J Lab Clin Med. 1995;125:167–72. [PubMed] [Google Scholar]
- 17.Ziegenhagen MW, Schrum S, Zissel G, et al. Increased expression of proinflammatory chemokines in bronchoalveolar lavage cells of patients with progressing idiopathic pulmonary fibrosis and sarcoidosis. J Invest Med. 1998;46:223–31. [PubMed] [Google Scholar]
- 18.Henry MT, McMahon K, Mackarel AJ, et al. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J. 2002;20:1220–7. doi: 10.1183/09031936.02.00022302. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor C, Odlum C, Van Breda A, et al. Collagenase and fibronectin in bronchoalveolar lavage fluid in patients with sarcoidosis. Thorax. 1988;43:393–400. doi: 10.1136/thx.43.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noor A, Knox KS. Immunopathogenesis of sarcoidosis. Clin Dermatol. 2007;25:250–8. doi: 10.1016/j.clindermatol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Ziegenhagen MW, Müller-Quernheim J. The cytokine network in sarcoidosis and its clinical relevance. J Intern Med. 2003;253:18–30. doi: 10.1046/j.1365-2796.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 22.Suga M, Iyonaga K, Ichiyasu H, et al. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J. 1999;14:376–82. doi: 10.1034/j.1399-3003.1999.14b23.x. [DOI] [PubMed] [Google Scholar]
- 23.Navratilova Z, Mrazek F, Kriegova E, et al. The MCP-1-2518 (A to G) single nucleotide polymorphism in Czech patients with pulmonary sarcoidosis: association with Löfgren's syndrome. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:33–8. [PubMed] [Google Scholar]
- 24.Keane MP, Standiford TJ, Strieter RM. Chemokines are important cytokines in the pathogenesis of interstitial lung disease. Eur Respir J. 1997;10:1199–202. doi: 10.1183/09031936.97.10061199. [DOI] [PubMed] [Google Scholar]
- 25.Skeel A, Leonard EJ. Action and target cell specificity of human macrophage-stimulating protein (MSP) J Immunol. 1994;152:4618–23. [PubMed] [Google Scholar]
- 26.Sakamoto O, Iwama A, Amitani R, et al. Role of macrophage-stimulating protein and its receptor, RON tyrosine kinase, in ciliary motility. J Clin Invest. 1997;99:701–9. doi: 10.1172/JCI119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunelleschi S, Penengo L, Lavagno L, et al. Macrophage stimulating protein (MSP) evokes superoxide anion production by human macrophages of different origin. Br J Pharmacol. 2001;134:1285–95. doi: 10.1038/sj.bjp.0704356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunella G, Bardelli C, Amoruso A, et al. Macrophage-stimulating protein differently affects human alveolar macrophages from smoker and non-smoker patients: evaluation of respiratory burst, cytokine release and NF-kappaB pathway. Br J Pharmacol. 2006;148:478–89. doi: 10.1038/sj.bjp.0706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schürmann M, Reichel P, Müller-Myhsok B, et al. Results from a genome-wide search for predisposing genes in sarcoidosis. Am J Respir Crit Care Med. 2001;164:840–6. doi: 10.1164/ajrccm.164.5.2007056. [DOI] [PubMed] [Google Scholar]
- 30.Pardigol A, Forssmann U, Zucht HD, et al. HCC-2, a human chemokine: gene structure, expression pattern, and biological activity. Proc Natl Acad Sci USA. 1998;95:6308–13. doi: 10.1073/pnas.95.11.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youn BS, Zhang S, Broxmeyer HE, et al. Isolation and characterization of LMC, a novel lymphocyte and monocyte chemoattractant human CC chemokine, with myelosuppressive activity. Biochem Biophys Res Commun. 1998;247:217–22. doi: 10.1006/bbrc.1998.8762. [DOI] [PubMed] [Google Scholar]
- 32.Youn BS, Zhang SM, Broxmeyer HE, et al. Characterization of CKbeta8 and CKbeta8-1: two alternatively spliced forms of human beta-chemokine, chemoattractants for neutrophils, monocytes, and lymphocytes, and potent agonists at CC chemokine receptor 1. Blood. 1998;91:3118–26. [PubMed] [Google Scholar]
- 33.Haringman JJ, Smeets TJ, Reinders-Blankert P, et al. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegenhagen MW, Rothe ME, Schlaak M, et al. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21:407–13. doi: 10.1183/09031936.03.00010403. [DOI] [PubMed] [Google Scholar]
- 35.Tutor-Ureta P, Citores MJ, Castejón R, et al. Prognostic value of neutrophils and NK cells in bronchoalveolar lavage of sarcoidosis. Cytometry B Clin Cytom. 2006;70:416–22. doi: 10.1002/cyto.b.20120. [DOI] [PubMed] [Google Scholar]
- 36.Cappello P, Caorsi C, Bosticardo M, et al. CCL16/LEC powerfully triggers effector and antigen-presenting functions of macrophages and enhances T cell cytotoxicity. J Leukoc Biol. 2004;75:135–42. doi: 10.1189/jlb.0403146. [DOI] [PubMed] [Google Scholar]


