Abstract
Enterotoxin produced by enterotoxigenic Bacteroides fragilis (BFT) has been associated with mucosal inflammation and diarrhoeal diseases. In this study, the anti-inflammatory molecular mechanism of 5,7-dihydroxy-3,4,6-trimethoxyflavone (eupatilin) was characterized in an HT-29 intestinal epithelial cell line stimulated with BFT. Pre-treatment of HT-29 cells with eupatilin decreased the production significantly of both interleukin (IL)-8 and prostaglandin E2 induced by BFT in a dose-dependent manner. BFT-activated nuclear factor-kappaB (NF-κB) signals in HT-29 cells and pretreatment with eupatilin suppressed NF-κB activation that resulted in the significant inhibition of IL-8 and cyclo-oxygenase-2 expression. BFT-induced phosphorylation of both IκBα and IκB kinase (IKK) signals was prevented in eupatilin-pretreated HT-29 cells. Transfection of siRNA for IKK-α and IKK-β decreased the production of IL-8 and prostaglandin E2; however, the transfection of IKK-β siRNA showed a more significant reduction of BFT-induced IκBα phosphorylation compared with that of IKK-α siRNA. In addition, herbimycin A, a specific inhibitor of heat shock protein 90 (Hsp90), decreased the BFT-induced activation of IKK and NF-κB, suggesting that Hsp90 is associated with a pathway of IKK-NF-κB-IL-8/cyclo-oxygenase-2 gene signalling. Furthermore, eupatilin dissociated the complex between Hsp90 and IKK-γ in BFT-stimulated HT-29 cells. These results suggest that eupatilin can suppress the NF-κB signalling pathway by targeting the Hsp90-IKK-γ complex in intestinal epithelial cells and may attenuate BFT-induced inflammatory responses.
Keywords: Bacteroides fragilis enterotoxin, eupatilin, Hsp90, IκB kinase, NF-κB
Introduction
Enterotoxigenic Bacteroides fragilis (ETBF) is associated with non-invasive diarrhoeal diseases. The B. fragilis enterotoxin (BFT) is an approximately 20 kDa heat-labile metalloprotease and is regarded as a virulence factor for these diarrhoeal diseases [1–4]. In addition, ETBF infection has been reported recently to be associated with inflammatory bowel diseases [5,6].
The BFT induces nuclear factor-kappaB (NF-κB) activation and interleukin (IL)-8 secretion, which are predicted to lead to mucosal transmigration of neutrophils [7–12]. BFT stimulation of intestinal epithelial cells also induces the expression of cyclo-oxygenase-2 (COX-2) and increases the release of prostaglandin E2 (PGE2) via the NF-κB pathway. Furthermore, suppression of PGE2 production by a COX-2 inhibitor prevents BFT-induced fluid secretion in a mouse model [11]. This association of NF-κB activation and BFT-induced inflammatory responses suggests that modulation of the NF-κB signalling pathway could be an important target for the prevention of BFT-induced enteric inflammation.
The NF-κB is an important transcriptional factor that controls inflammatory processes in the human intestine. NF-κB dimers are stored in the cytoplasm in an inactive state by inhibitory proteins called IκBs. IκB kinase (IKK) directly phosphorylates IκB. The IKK complex contains three subunits: two catalytic subunits, IKK-α and IKK-β, and a regulatory subunit, IKK-γ (also known as NEMO, NF-κB essential modulator) [13]. While IKK-α and IKK-β are essential for IκB phosphorylation, IKK-γ forms a tetrameric scaffold that can assemble two kinase dimers which facilitates trans-autophosphorylation [14,15]. Although IKK-γ is required for kinase activity, it seems to mediate crucial protein–protein interactions, possibly with upstream activators of the kinase complex [16]. Recently, heat shock protein 90 (Hsp90) has been found to associate stoichiometrically with the IKK complex, which may contribute to the stabilization, activation and/or shuttling of IKKs to the plasma membrane [17–20].
Flavonoids are polyphenols found in a wide variety of edible plants and are known to exert anti-inflammatory activities in several types of human cell lines [21–24]. Extracts of Artemisia asiatica Nakai (Asteraceae) are known to have anti-inflammatory activities. One of the pharmacologically active ingredients from A. asiatica extracts is 5,7-dihydroxy-3′,4′,6′-trimethoxyflavone (eupatilin) [25]. Although eupatilin has a variety of biological activities, little information is known about the molecular mechanism of eupatilin-induced attenuation of intestinal inflammation induced by BFT stimulation. In this study, we asked whether eupatilin could affect the NF-κB signal transduction pathway in BFT-stimulated human intestinal epithelial cells. Eupatilin was shown herein to reduce the activity of NF-κB and the expression of proinflammatory mediators through the dissociation of the Hsp90-IKK-γ complex in an HT-29 cell line stimulated with BFT.
Materials and methods
Purification of BFT and cell cultures
The BFT was purified from culture supernatants of a highly toxigenic strain of ETBF, as described previously [8–11]. The purity of the BFT preparations was confirmed by sodium dodecyl sulphate polyacrylamide gel electrophoresis. Typical preparations of BFT contained 0·5–1·2 mg of protein/ml as measured by the bicinchoninic acid protein assay. Buffers used in the purification were prepared using lipopolysaccharide (LPS)-free water (Gibco-Invitrogen Corporation, Carlsbad, CA, USA). The activity of LPS in BFT solutions (1 mg/ml) was less than 1 endotoxin unit/ml (Pyrosate test kit, quantitative chromogenic Limulus amebocyte lysate; Associates of Cape Cod, Inc., MA, USA). Using HEK-Blue™ LPS detection kit (InvivoGen, San Diego, CA, USA), the amount of LPS in BFT solutions (1 mg/ml) was less than 3 ng/ml. BFT was frozen in aliquots at −80°C immediately after purification.
The human colon epithelial cell line HT-29 (ATCC HTB 38) was grown in Dulbecco's minimum essential medium (Sigma, St Louis, MO, USA) with 10% fetal bovine serum, 2 mM glutamine and antibiotics (100 unit/ml of penicillin and 100 μg/ml of streptomycin). Cells were seeded at 0·5–2 × 106 cells per well onto six-well plates and allowed to attach overnight. After 12 h of serum starvation, cells were incubated with BFT for the indicated period. 5,7-dihydroxy-3′,4′,6′-trimethoxy flavone (eupatilin; Dong-A Pharmaceutical, Yong-In, Korea) was dissolved in dimethyl sulphoxide (DMSO). In some experiments, a specific inhibitor of Hsp90, herbimycin A (0·5 μM, Sigma) was used.
Quantitative reverse transcription–polymerase chain reaction (RT–PCR), real-time PCR and enzyme-linked immunosorbent assay
Total cellular RNA was extracted from HT-29 cells using Trizol (gibco BRL, Gaithersburg, MD, USA). Quantitative RT–PCR for IL-8, COX-2 and β-actin mRNA was performed using each standard RNA, as described previously [26,27]. The primers used in this study were as follows: IL-8, 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′ and 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′; COX-2, 5′-TTC AAA TGA GAT TGT GGG AAA ATT GCT-3′ and 5′-AGA TCA TCT CTG CCT GAG TAT CTT-3′; and β-actin, 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′ and 5′-CTA GAA GCA TTG CGG TGG ACG ATG GAG GG-3′. The amounts of IL-8 and PGE2 were measured by commercially available enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions [11]. Prior to performing ELISA, supernatants were filtered through a 0·22-μm filter to remove any contaminants. To measure levels of phospho-IκBα, an IκBα ELISA kit (Active Motif, Carlsbad, CA, USA) was used according to the manufacturer's instructions [28].
Electrophoretic mobility shift assay
Cells were harvested and nuclear extracts prepared as described previously [9]. Concentrations of protein in the extracts were determined using a Bradford assay (Bio-Rad, Hercules, CA, USA). Electrophoretic mobility shift assay (EMSA) was performed using an assay kit (Promega, Madison, WI), as described previously [9]. In brief, 5 μg of nuclear extract was incubated for 30 min at room temperature with a [γ32P]-labelled oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) corresponding to a consensus NF-κB binding site. After incubation, both bound and free DNA was resolved on 5% polyacrylamide gels, as described previously [9]. Supershift assays were used to identify the specific members of the NF-κB family activated by BFT stimulation. EMSA was performed as described above, except that rabbit antibodies (1 μg/reaction) against NF-κB proteins p50, p52, p65, c-Rel and Rel B (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added during the binding reaction period [9].
Transfection and reporter assays
The HA-tagged IKK-α, IKK-β, and IKK-γ constructs and a flag-tagged Hsp90 construct were kindly supplied by Dr Gang Min Hur at Chungnam National University, Korea [29]. Reporter plasmids, including pIL8-luciferase, p2x NF-κB-luciferase, pβ-actin- and pRSV-β-galactosidase-luciferase transcriptional reporters, were provided by Dr Kagnoff (University of California, San Diego, USA) [30]. A pCOX-2-luciferase (phPES2) reporter was kindly donated by Dr Inoue of the National Cardiovascular Center Research Institute, Japan [31]. Cells in six-well dishes were transfected with 1·5 μg of plasmid DNA using Lipofectamine Plus reagents (Invitrogen, Carlsbard, CA, USA), as described previously [11]. The transfected cells were incubated for 48 h at 37°C in a 5% CO2 incubator. Cells were then harvested and whole cell lysates were prepared as described previously [11]. In the reporter analysis, luciferase activity was determined in accordance with the manufacturer's instructions (Tropix Inc., Bedford, MA, USA) and luminescence was quantitated for 10 s using a luminometer (MicroLumat Plus, Berthold GmbH & Co. KG, Bad Wildbad, Germany). Luciferase activity was determined and normalized relative to β-galactosidase expression, as described previously [11,32].
Immunoblot analysis
Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 0·5 ml/well lysis buffer (150 mM NaCl, 20 mM Tris at pH 7·5, 0·1% Triton X-100, 1 mM phenylmethyl sulphonyl fluoride and 10 μg/ml aprotinin). Fifteen to 50 µg of protein per lane was size-fractionated on a 6% polyacrylamide minigel and transferred electrophoretically to a nitrocellulose membrane (0·1-μm pore size). Specific proteins were detected using mouse anti-human IκBα, IKK-α, IKK-β, IKK-γ, Hsp90 and actin (Cell Signaling Technology, Beverly, MA, USA) as primary antibodies, and peroxidase-conjugated anti-mouse IgG as a secondary antibody. Specifically bound peroxidase was detected by enhanced chemiluminescence (ECL system) and exposure to X-ray film.
Immunoprecipitation
For the immunoprecipitation assay, transfected cells were collected in a lysis buffer (50 mM HEPES at pH 7·6, 150 mM NaCl, 0·1% NP-40, 5 mM ethylenediamine tetraacetic acid, 0·5 mM phenylmethyl sulphonyl fluoride, 1 µg/ml leupeptin, 1 µg/ml aprotinin and 1 µg/ml pepstatin) after treatments indicated in the figure legends. The lysates were mixed and precipitated with the relevant antibodies (anti-HA antibodies; Santa Cruz; anti-flag antibody: Sigma) and protein G-sepharose beads by incubation at 4°C overnight. For the detection of IKK-γ protein, cell lysates were incubated with anti-IKK-γ antibody and protein G-sepharose beads at 4°C overnight. All immunoprecipitates were washed four times with lysis buffer, boiled in sodium dodecyl sulphate polyacrylamide gel electrophoresis sample buffer and resolved on an 8% polyacrylamide gel [33].
In vitro kinase assay
The IKK activity on IκBα phosphorylation was determined using an immunocomplex kinase assay, as described previously [28]. Briefly, cells were lysed in Triton lysis buffer containing protease and phosphatase inhibitors and then cleared by centrifugation at 23 700 g for 10 min. Three hundred µg of whole cell extract was immunoprecipitated with anti-IKK-γ/protein-A beads. The kinase reaction was then performed by incubating 25 ml of kinase buffer containing 20 mM HEPES (pH 7·7), 10 mM MgCl2, 5 mM dithiothreitol, 50 mM adenosine triphosphate (ATP) and 5 mCi [γ-32P] ATP with glutathione-S-transferase (GST)-IκBα substrate (Upstate Biotechnology Inc., Lake Placid, NY, USA) for 30 min at 30°C. The substrate protein was resolved by gel electrophoresis and phosphate incorporation was assessed by autoradiography. An HTScan IKK-β kinase assay kit was obtained from Cell Signaling Technology. This kit contains GST-IKK-β kinase protein, a biotinylated peptide substrate, and a phosphoserine antibody for detection of the phosphorylated form of the substrate peptide. The assay was performed according to the manufacturer's instructions [28].
Experiments using ileal loops of mice
An animal study was performed using specific pathogen-free mice, as described previously [10,11]. Briefly, fasted (16 h) specific pathogen-free mice 20–25 g (C57BL6Cr; Orient Experimental Animal Co., Kyounggi-do, Korea) were anaesthetized by intraperitoneal injection of sodium pentobarbital (600 µg/mouse). Ileal loops (3–4 cm) were prepared and injected with buffer alone or eupatilin (300 µg) in a 200 µl volume. After 30 min, BFT (10 µg in PBS) was injected into the loops, and animals were killed 4 h later by pentobarbital overdose. Loops were cut out and tissue extracts were obtained for measurement of macrophage-inflammatory protein-2 (MIP)-2, as described previously [10,11]. All procedures were approved by the Animal Care Committee of Hanyang University College of Medicine.
Statistical analyses
Wilcoxon's rank sum test was used for statistical analyses. A P-value of less than 0·05 was considered statistically significant.
Results
Eupatilin inhibits the expression of IL-8 and COX-2 through NF-κB signals in HT-29 intestinal epithelial cells stimulated with BFT
The HT-29 cells stimulated with BFT for 24 h secreted IL-8 in higher amounts than unstimulated controls. The magnitude of the IL-8 response was dependent upon the concentration of stimulated BFT per epithelial cells. Thus, stimulation of HT-29 cells with increasing concentration of BFT was paralleled by increased IL-8 release. At concentrations of 1, 10, 100 and 500 μg/ml, IL-8 release increased 1·9-, 3·2-, 9·5- and 9·5-fold 24 h after stimulation, respectively, relative to those of unstimulated controls (mean value, n = 3). Pre-treatment of cells with eupatilin decreased significantly the release of IL-8 when induced by BFT in a dose-dependent manner (Fig. 1a). Similar results were noted for PGE2 production in eupatilin-pretreated HT-29 cells. In this experiment, DMSO alone (vehicle control) did not show any significant changes of IL-8 and PGE2 compared with untreated control. To confirm that the decrease in secretion of IL-8 and PGE2 was due to eupatilin, IL-8 and COX-2 mRNA transcript levels were measured by quantitative RT–PCR using an internal RNA standard for each. As shown in Fig. 1b, up-regulated expression of IL-8 mRNA was reduced significantly by pretreating with eupatilin. Similar results were obtained when measuring the levels of COX-2 mRNA transcripts. In this experiment, the levels of β-actin mRNA in each group remained relatively constant (–5 × 106 mRNA transcripts/μg RNA).
Fig. 1.
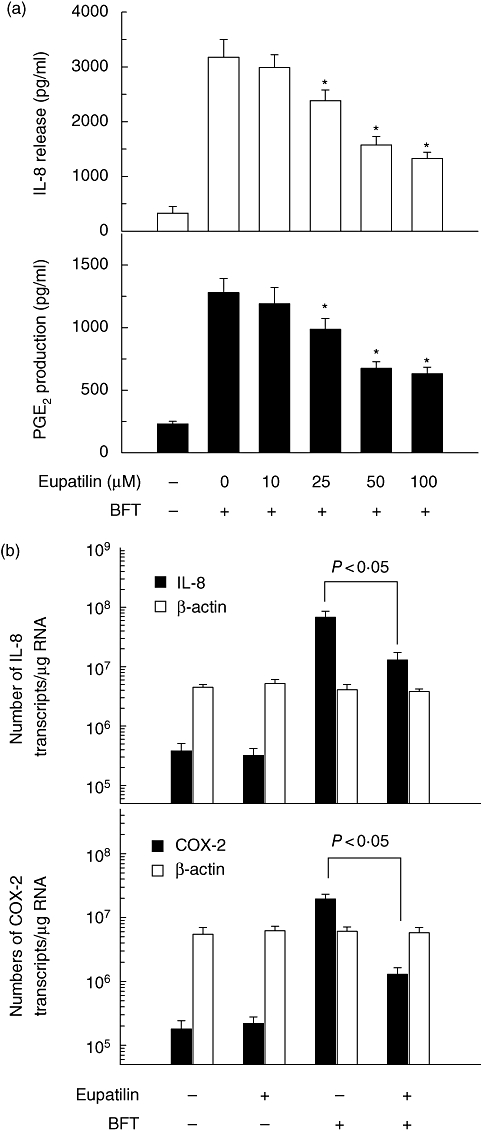
Effects of eupatilin on interleukin (IL)-8 and prostaglandin E2 (PGE2) production in HT-29 cells stimulated with Bacteroides fragilis (BFT). (a) HT-29 cells were pretreated with the indicated concentration of eupatilin for 30 min and then combined with BFT (100 ng/ml) for 24 h. The concentration of IL-8 and PGE2 was determined by enzyme-linked immunosorbent assay (ELISA) (mean ± standard error of the mean, n = 5). Asterisks indicate values that are significantly different from BFT-stimulated cells without eupatilin (P < 0·05). (b) HT-29 cells were pretreated with eupatilin (50 μM) for 30 min and then combined with BFT (100 ng/ml) for an additional 8 h. Total RNA was reverse-transcribed using an oligo(dT) primer and synthetic internal interleukin (IL)-8, cyclo-oxygenase-2 (COX-2) or β-actin RNA standards and amplified by polymerase chain reaction. Data represent the mean ± standard deviation (n = 5).
The transcription factor NF-κB is one component of the signalling pathway that regulates gene expression of IL-8 and COX-2 induced by BFT stimulation [8,9,11]. Thus, stimulation with BFT increased NF-κB DNA binding, as shown by EMSA (Fig. 2a). Concurrently, IκBα degradation was observed in the BFT-stimulated cells. Supershift studies demonstrated that antibodies to p65 and p50 shifted the NF-κB signals significantly. In contrast, anti-p52, anti-c-Rel or anti-Rel B antibodies did not shift the NF-κB signal (Fig. 2b). These results suggest that NF-κB activation by BFT is mediated predominantly by heterodimers of p65/p50. It was then asked whether eupatilin could prevent BFT-induced NF-κB activity in HT-29 cells. As shown in Fig. 2c, the addition of eupatilin suppressed the activated NF-κB signals induced by BFT. Similarly, BFT-induced IκBα degradation was decreased in eupatilin-pretreated HT-29 cells.
Fig. 2.

Nuclear factor-kappaB (NF-κB) activation and IκB degradation in HT-29 cells stimulated with Bacteroides fragilis (BFT). (a) HT-29 cells were stimulated with BFT (100 ng/ml) for the indicated period. NF-κB DNA binding activity was assessed by electrophoretic mobility shift assay (EMSA). Immunoblots for concurrent IκBα and actin levels in cells under the same conditions are provided beneath each EMSA. The results are representative of three repeated experiments. (b) Supershift assays using nuclear extracts from HT-29 cells stimulated with BFT for 1 h were performed using antibodies to p50, p52, p65, c-Rel and Rel B. The results are representative of three repeated experiments. (c) HT-29 cells were pretreated with eupatilin (50 μM) for 30 min and then combined with BFT (100 ng/ml) for 1 h. NF-κB DNA binding activity was assessed by EMSA. Immunoblots for concurrent IκBα and actin levels in cells under the same conditions are provided beneath each EMSA. The results are representative of five repeated experiments.
To confirm that eupatilin inhibits NF-κB activation and IL-8 and COX-2 expression, luciferase assays for NF-κB, IL-8 and COX-2 transcriptional reporters were performed. Figure 3 showed that eupatilin inhibited the activation of NF-κB, IL-8 and COX-2 transcriptional reporters significantly in BFT-stimulated HT-29 cells. For a control experiment with a known NF-κB inhibitor MG-132 (50 μM), luciferase activity was performed in HT-29 cells transfected with NF-κB or IL-8 promoter plasmid. Results showed that MG-132 decreased significantly expression of IL-8 and NF-κB in BFT-stimulated cells [IL-8 reporter activity, 7·8 ± 0·5 in BFT alone and 2·6 ± 0·4 in BFT+ MG-132; NF-κB reporter activity, 3·9 ± 0·3 in BFT alone and 1·1 ± 0·2 in BFT+ MG-132; the mean fold induction ± standard error of the mean of luciferase activity relative to unstimulated controls, n = 3]. These results indicate that eupatilin can attenuate intestinal epithelial inflammatory signals induced by stimulation with BFT, including NF-κB activation and IL-8/COX-2 expression.
Fig. 3.
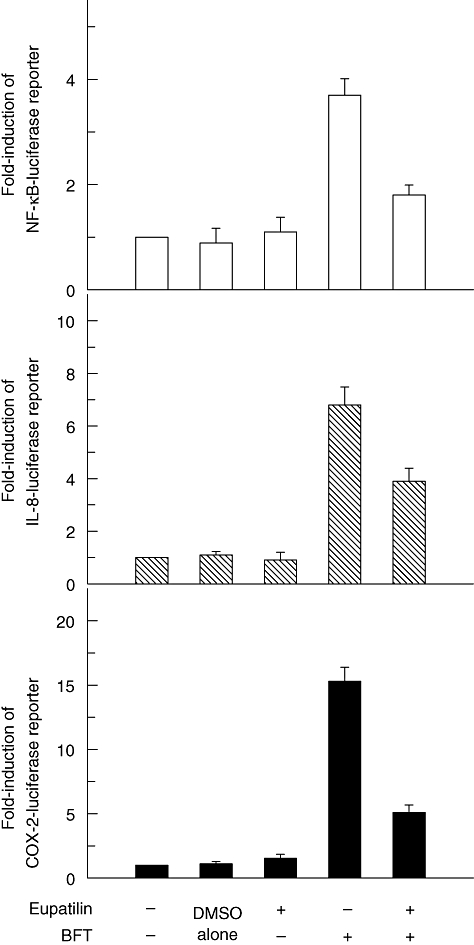
HT-29 cells were transfected with pIL8-, pCOX-2-, or p2x nuclear factor kappaB (NF-κB)-luciferase transcriptional reporters, as indicated. After 48 h, the transfected cells were pretreated with eupatilin (50 μM) for 1 h and then combined with Bacteroides fragilis (BFT) (100 ng/ml) for 1 h (NF-κB) or 8 h [interleukin (IL)-8 and cyclo-oxygenase-2 (COX-2)]. Data are expressed as the mean fold induction ± standard error of the mean of luciferase activity relative to unstimulated controls (n = 7). The mean fold induction of the β-actin reporter gene activity relative to the vehicle control unstimulated controls remained relatively constant throughout each experiment. Values of vehicle control are – 1·0. *P < 0·05 compared with BFT alone.
The BFT induces IKK activation in HT-29 cells and eupatilin inhibits activated IKK signals
Major pathways for NF-κB activation involve activation of IKK followed by IκB degradation. In BFT-stimulated HT-29 cells, phosphorylated IKK-α/β signals increased within 10 min of stimulation and continued to increase over 120 min (Fig. 4a). To determine the effects of eupatilin on IKK activity, an in vitro kinase assay for IKK was performed. As shown in Fig. 4b, stimulation of HT-29 cells with BFT induced a strong increase of IKK activity that was reduced by pre-treatment with eupatilin. To quantify the inhibition of IKK activity, an HTScan IKK-β kinase assay was performed. Pre-treatment with eupatilin prevented IKK-β activity (P < 0·01) (Fig. 4c). Furthermore, to confirm our hypothesis regarding the inhibition of the IKK-NF-κB signalling pathway by eupatilin, levels of phospho-IκBα were measured. Because detection of phospho-IκBα is difficult because of its short half-life, we used a commercial phospho-IκBα ELISA kit. The results showed that while BFT increased the levels of phospho-IκBα in HT-29 cells, the addition of eupatilin reduced significantly the levels of phospho-IκBα (Fig. 4d).
Fig. 4.
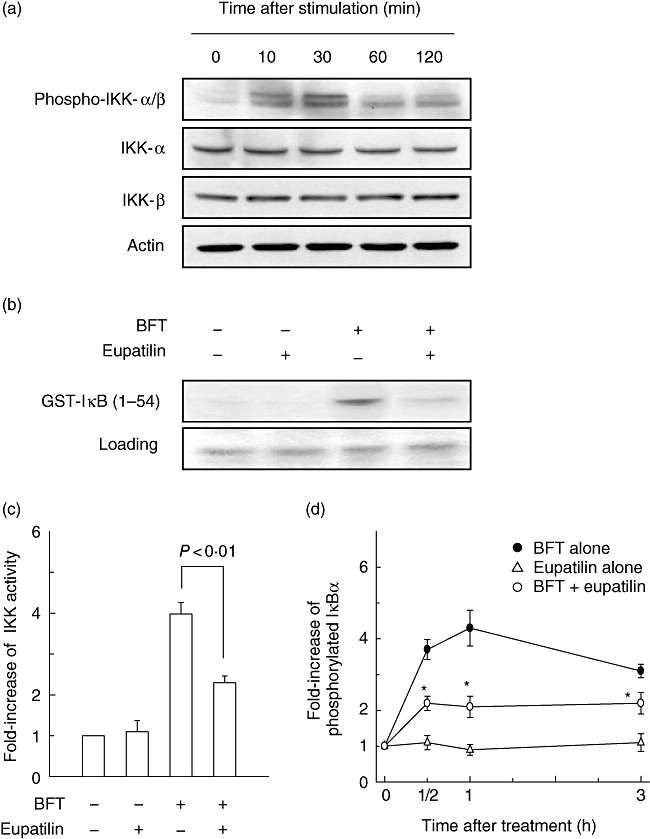
Suppression of IκB kinase (IKK) activity by eupatilin in HT-29 cells stimulated with Bacteroides fragilis (BFT). (a) HT-29 cells were stimulated with BFT (100 ng/ml) for the indicated period. Protein expression of IKK-α, IKK-β, phosphorylated IKK-α/β, and actin were assessed by immunoblot. The results are representative of three repeated experiments. (b) HT-29 cells were pretreated with eupatilin (50 μM) for 30 min and then combined with BFT (100 ng/ml) for an additional 30 min. The whole cell extract was immunoprecipitated with anti-IKK-γ/protein-A beads and kinase reactions were performed using glutathione-S-transferase-IκB as a substrate. Results shown are representative of three independent experiments. (c) IKK kinase activity was measured using an HTScan IKK-β kinase assay kit. Data are expressed as the mean fold induction ± standard error of the mean (s.e.m.) of kinase activity relative to untreated controls (n = 5). (d) Inhibition of IκBα phosphorylation in BFT-stimulated HT-29 cells by treatment with eupatilin. IκBα phosphorylation was measured using an IκBα enzyme-linked immunosorbent assay (ELISA) kit. Data are expressed as the mean fold induction ± s.e.m. of phosphorylated IκBα activity relative to untreated controls (n = 5). *P < 0·05 compared with BFT alone.
As IKK-α and IKK-β are reported to be essential for IκBα phosphorylation and NF-κB activation, it was necessary to determine which subunit of IKK is involved in IκBα phosphorylation when HT-29 cells are stimulated with BFT. As shown in Fig. 5a, siRNA for IKK-β reduced BFT-induced IκBα phosphorylation dramatically. In contrast, inhibition of IκBα phosphorylation by IKK-α siRNA was lower than that by IKK-β siRNA (Fig. 5a), although the siRNA for IKK-α decreased significantly IL-8 and PGE2 production induced by BFT (Fig. 5b and c). In this experiment, transfection efficiency for siRNA showed – 62%.
Fig. 5.

IκB kinase (IKK) is involved in the phosphorylation of IκBα induced by Bacteroides fragilis (BFT). HT-29 cells were introduced with siRNA for the control or each IKK for 48 h. (a) The siRNA-transfected cells were combined with BFT for 1 h. IκBα phosphorylation was measured using an IκBα enzyme-linked immunosorbent assay (ELISA) kit. Data are expressed as the mean fold induction ± standard error of the mean (s.e.m.) of phosphorylated activity relative to untreated controls (n = 5). (b,c) The levels of interleukin (IL)-8 and prostaglandin E2 (PGE2) in culture supernatants obtained 24 h after adding BFT were determined by ELISA. Data are expressed as mean ± s.e.m. (n = 5). Asterisks indicate values that are significantly different from BFT-stimulated cells without transfection (P < 0·05).
To determine whether Hsp90 is associated with gene expression of an IKK-NF-κB- IL-8/COX-2 pathway an inhibition assay using herbimycin A, a specific inhibitor of Hsp90, was performed. As shown in Fig. 6a, pre-treatment with herbimycin A decreased the up-regulated IKK activity induced by BFT stimulation. In addition, pre-treatment with herbimycin A resulted in a significant reduction of reporter gene activities of NF-κB, IL-8 and COX-2 in BFT-stimulated HT-29 cells (Fig. 6b–d). These results suggest that the Hsp90-IKK complex plays an important role in the activation of the NF-κB-IL-8/COX-2 gene pathway in intestinal epithelial cells in response to BFT stimulation.
Fig. 6.

Treatment with herbimycin A inhibits the activation of IκB kinase (IKK) and NF-κB, and the expression of interleukin (IL)-8 and cyclo-oxygenase-2 (COX-2) in HT-29 cells stimulated with Bacteroides fragilis (BFT). (a) HT-29 cells were incubated in the absence or presence of herbimycin A (0·5 μM) for 24 h before treatment of BFT (100 ng/ml) for 24 h. IKK kinase activity was measured using a HTScan IKK-β kinase assay kit. Data are expressed as mean fold induction ± standard error of the mean (s.e.m.) of kinase activity relative to untreated controls (n = 5). (b, c, and d) HT-29 cells were transfected with p2x nuclear factor kappaB (NF-κB)-, pIL8-, or pCOX-2-luciferase transcriptional reporters in the absence or presence of herbimycin A (0·5 μM) for 24 h. BFT (100 ng/ml) was added to the transfected cells for 1 h (NF-κB, b) or 8 h (IL-8 and COX-2, c,d). Data are expressed as mean fold induction ± s.e.m. of luciferase activity relative to untreated controls (n = 5). The mean fold induction of β-actin reporter gene activity relative to untreated controls remained relatively constant throughout each experiment. Asterisks indicate values of BFT + herbimycin A that are significantly different from those of BFT alone (P < 0·05).
The Hsp90 is associated with IKK proteins in HT-29 cells
We next sought to confirm that Hsp90 plays a role in regulating BFT-induced IKK activation in HT-29 cells. First, the interactions of Hsp90 with IKK proteins in HT-29 cells were examined when each protein of IKK-α, IKK-β and IKK-γ was overexpressed in the cells. As shown in Fig. 7a and c, IKK-α and IKK-γ interacted strongly with Hsp90 in each co-immunoprecipitation assay. However, IKK-β was unable to interact with Hsp90 (Fig. 7b), indicating that the interaction of IKK-β and Hsp90 is presumably indirect in HT-29 cells. Transfection efficiency for green fluorescent protein-expression plasmid showed – 23% in HT-29 cells.
Fig. 7.

Hsp90 is associated with IκB kinase (IKK) complex in HT-29 intestinal epithelial cells. HT-29 cells were transfected with HA-tagged IKK-α, IKK-β or IKK-γ expression plasmids and a flag-tagged Hsp90 expression plasmid. Immunoprecipitates containing Hsp90 and IKK-γ were prepared with anti-flag and anti-HA antibodies, and immunoprecipitants were analysed by Western blotting. Results shown are representative of at least three independent experiments.
Eupatilin induces dissociation of the Hsp90 and IKK-γ complex in HT-29 intestinal epithelial cells stimulated with BFT
In this study, IKK-α and -γ interacted strongly with Hsp90 in HT-29 intestinal epithelial cells (Fig. 7). To determine whether the interaction of IKK proteins with Hsp90 could be altered by eupatilin, HT-29 cells treated with eupatilin and BFT were lysed and immunoprecipitated with anti-IKK-γ and subjected to immunoblot analysis for Hsp90 and IKK proteins. As shown in the top panel of Fig. 8a, the interaction between both IKK-γ and Hsp90 was reduced after a 30-min treatment with eupatilin. When only eupatilin was added, changes of Hsp90-IKK-γ association was not able to be found. These results suggest that eupatilin may dissociate the complex of Hsp90 and IKK-γ in human intestinal epithelial cells stimulated with BFT.
Fig. 8.

Eupatilin alters IκB kinase (IKK)-γ association with Hsp90 in HT-29 cells stimulated with Bacteroides fragilis (BFT). HT-29 cells were treated with eupatilin (50 μM) and BFT (100 ng/ml) for the indicated times. Cell extracts from each sample were immunoprecipitated with anti-IKK-γ antibody. Immunoprecipitants were analysed by Western blotting using anti-heat shock protein 90 (Hsp90), anti-IKK-α, anti-IKK-β and anti-IKK-γ antibodies. Results shown are representative of three independent experiments.
Pre-treatment with eupatilin prevents BFT-induced MIP-2 secretion in mice
As described in the above, pre-treatment with eupatilin decreased IL-8 expression significantly in BFT-stimulated intestinal epithelial cells. To assess its pathophysiological relevance in an in vivo model, we investigated whether eupatilin might attenuate BFT-induced MIP-2 (CXCL2), which is a mouse homologue of IL-8, in mice. Therefore, eupatilin was injected into the lumen of a 3–4-cm ileal loop 30 min before BFT administration. As shown in Fig. 9, MIP-2 production was elevated significantly in the BFT-stimulated group compared with the control group. However, BFT-induced production of MIP-2 decreased significantly in the eupatilin-pretreated mice.
Fig. 9.
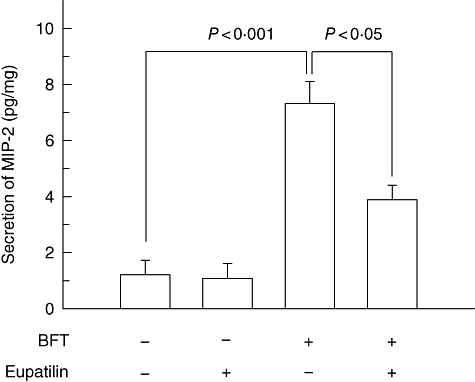
Effect of eupatilin on macrophage-inflammatory protein -2 (MIP-2) production in mouse ileum treated with Bacteroides fragilis enterotoxin. Eupatilin was injected into the lumen of an ileal loop. After 30 min, B. fragilis (BFT) enterotoxin (10 µg/loop) was administered, and mice were kiled 4 h later. MIP-2 levels were analysed by enzyme-linked immunosorbent assay. Data represent mean ± standard error of the mean (pg/mg tissue protein, seven loops per group).
Discussion
In the present study, eupatilin decreased IKK activity significantly in BFT-stimulated intestinal epithelial cells. It is possible that this effect may be due to dissociation of the Hsp90-IKK-γ complex. The dissociation of this complex led to the suppression of both IκB phosphorylation and NF-κB activation, which in turn resulted in the inhibition of IL-8 and PGE2 secretion in BFT-stimulated intestinal epithelial cells. These findings regarding the dissociation of the IKK-γ-Hsp90 complex support a novel mechanism of eupatilin function in anti-inflammatory responses to stimulation with BFT.
Intestinal epithelial cells form a tight barrier that prevents mucosal penetration by most luminal bacteria. BFT provokes signals that induce mucosal inflammation and diarrhoea in animal models [2,10]. Mucosal inflammation is mediated by several proinflammatory mediators. For example, IL-8, a member of the Cys-Xaa-Cys chemokines (where Xaa is any amino acid), is a principal mediator of inflammatory response which, with BFT stimulation, includes the infiltration of neutrophils [9]. In addition, COX-2 expression and PGE2 secretion are involved in secretory responses to stimulation with BFT [11]. Previously, we demonstrated that both the expression of IL-8 and COX-2, as well as the production of PGE2, are associated directly with NF-κB activation in intestinal epithelial cells stimulated with BFT [8,9,11]. The results presented here suggest that suppression of NF-κB by treatment with eupatilin in vivo may result in attenuation of BFT-induced clinical manifestations such as enteric inflammation and diarrhoea. Although in vivo data from an animal model would clearly strengthen our results, we have no data using in vivo animal model treated with eupatilin. Further studies using an animal model are necessary to establish clearly the role of eupatilin under ETBF-infected conditions. IL-8 expression and NF-κB activation induced by B. fragilis LPS were lower than those induced by Escherichia coli LPS. In addition, the activity of LPS in BFT solutions was not detectable. Therefore, the effect of B. fragilis LPS may be excluded on IL-8 and PGE2 secretion in HT-29 cells.
Activated IKK induces IκB phosphorylation, which is followed by NF-κB activation [13]. In this study, treatment with eupatilin blocked BFT-induced IKK activity and decreased the expression of both IL-8 and COX-2. These results suggest that eupatilin affects an IKK site in the inflammatory signalling pathway induced by BFT stimulation. Hsp90 is known to play an important role in signal transduction networks by facilitating the biosynthesis of components of the IKK complex. It also maintains the mature forms of the kinase complex in a conformation that allows for its eventual biochemical function and stability [17,18]. The present study showed that IKK-γ was associated strongly with Hsp90 in HT-29 cells. However, IKK-β was unable to interact with Hsp90, suggesting that Hsp90 may be associated indirectly with IKK-β in HT-29 cells. In this experimental condition, the interaction between IKK-γ and Hsp90 in BFT-stimulated HT-29 cells was reduced after treatment with eupatilin. The interruption of the association between IKK-γ and Hsp90 by eupatilin resulted in the decrease of both IL-8 and COX-2 mRNA expression. These results suggest that eupatilin may induce the dissociation of the Hsp90-IKK-γ complex in BFT-stimulated HT-29 cells.
Previous studies have shown that BFT can activate the mitogen-activated protein kinase (MAPK) signalling pathway to increase IL-8 production in intestinal epithelial cells. MAPK may be associated with NF-κB activation [10,12], suggesting an association between MAPK and IKK activation in BFT-stimulated intestinal epithelial cells. Considering that eupatilin diminished the extracellular-regulated kinase 1/2 (ERK1/2) activity in MCF10A-ras cells [34] and the activities of p38 and Jun N-terminal kinase (JNK) in bile acid-stimulated hepatocytes [35], it is possible that eupatilin may inhibit MAPK activity in intestinal epithelial cells. Here, it was shown that eupatilin did not inhibit completely the BFT-induced production of IL-8 and PGE2 or the activation of NF-κB. Therefore, there may be other pathways that allow BFT to induce the production of inflammatory mediators, including the MAPK and AP-1 pathways. Further investigations are required to clarify other contributing factors that can account for the eupatilin-induced inhibition of the expression of inflammatory mediators in intestinal epithelial cells stimulated with BFT.
Flavonoids do not generally eradicate all pathogenic enteric bacteria, although they decrease the density of colonization in the intestine [36]. In addition, the anti-oxidant and anti-inflammatory properties exerted by flavonoids may stabilize the intestinal barrier function and decrease mucosal inflammation. This study provides evidence that eupatilin may attenuate intestinal inflammatory responses associated with ETBF infection.
In summary, we have demonstrated that eupatilin has anti-inflammatory activity in intestinal epithelial cells stimulated with BFT through the dissociation of the Hsp90–IKK-γ complex. This novel action of eupatilin suggests that it may play a role in enhancing the host defence system.
Acknowledgments
J. M. Kim and D. H. Lee contributed equally to this work. We thank Dr Martin F. Kagnoff and Dr Joseph A. DiDonato for providing the reporter gene plasmids and Sung-Hee Yang, Jung Sang Youn and Han Jin Lee for their expert technical assistance. This work was supported by a grant from Seoul R&BD Program and the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R11-2008-044-01004-0).
References
- 1.Marcos LA, DuPont HL. Advances in defining etiology and new therapeutic approaches in acute diarrhea. J Infect. 2007;55:385–3. doi: 10.1016/j.jinf.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Sears CL. The toxins of Bacteroides fragilis. Toxicon. 2001;39:1737–46. doi: 10.1016/s0041-0101(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 3.Sears CL, Myers LL, Lazenby A, Van Tassell RL. Enterotoxigenic Bacteroides fragilis. Clin Infect Dis. 1995;20(Suppl)(2):S142–8. doi: 10.1093/clinids/20.supplement_2.s142. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G, Svenungsson B, Karnell A, Weintraub A. Prevalence of enterotoxigenic Acteroides fragilis in adult patients with diarrhea and healthy controls. Clin Infect Dis. 1999;29:590–4. doi: 10.1086/598639. [DOI] [PubMed] [Google Scholar]
- 5.Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–32. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 6.Rabizadeh S, Rhee KJ, Wu S, Huso D, Gan CM, Sears CL. Enterotoxigenic Bacteroides fragilis: a potential instigator of colitis. Inflamm Bowel Dis. 2007;13:1475–83. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanfilippo L, Li CK, Seth R, Balwin TJ, Menozzi MG, Mahida YR. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-beta) by human colonic epithelial cells. Clin Exp Immunol. 2000;119:456–63. doi: 10.1046/j.1365-2249.2000.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-kappaB plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001;123:421–7. doi: 10.1046/j.1365-2249.2001.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JM, Cho SJ, Oh YK, Jung HY, Kim YJ, Kim N. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002;130:59–66. doi: 10.1046/j.1365-2249.2002.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol. 2005;35:2648–57. doi: 10.1002/eji.200526321. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Lee JY, Yoon YM, et al. Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-κB activation. Eur J Immunol. 2006;36:2446–56. doi: 10.1002/eji.200535808. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect Immun. 2004;72:5832–9. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–62. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 15.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 16.Yamaoka S, Courtois G, Bessia C, et al. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–40. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 17.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene. 2004;23:5378–86. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–10. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J, Devin A, Miller A, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J Biol Chem. 2000;275:10519–26. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 20.Scheibel T, Buchner J. The Hsp90 complex-a super-chaperone machine as a novel drug target. Biochem Pharmacol. 1998;56:675–82. doi: 10.1016/s0006-2952(98)00120-8. [DOI] [PubMed] [Google Scholar]
- 21.Biesalski HK. Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care. 2007;10:724–8. doi: 10.1097/MCO.0b013e3282f0cef2. [DOI] [PubMed] [Google Scholar]
- 22.González-Gallego J, Sánchez-Campos S, Tunon MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp. 2007;22:287–93. [PubMed] [Google Scholar]
- 23.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:S2993–3001. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 25.Seo HJ, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human promyelocytic leukemia cells. Mutat Res. 2001;496:191–8. doi: 10.1016/s1383-5718(01)00234-0. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Upregulated cyclooxygenase-2 inhibits apoptosis of human gastric epithelial cells infected with Helicobacter pylori. Dig Dis Sci. 2000;45:2436–43. doi: 10.1023/a:1005611613542. [DOI] [PubMed] [Google Scholar]
- 27.Kim JM, Kim JS, Lee LY, et al. Vacuolating cytotoxin in Helicobacter pylori water-soluble proteins upregulates chemokine expression in human eosinophils via Ca2+ influx, mitochondrial reactive oxygen intermediates, and NF-κB activation. Infect Immun. 2007;75:3373–81. doi: 10.1128/IAI.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JM, Kim JS, Kim YJ, et al. Conjugated linoleic acids produced by Lactobacillus dissociates IKK-gamma and Hsp90 complex in Helicobacter pylori-infected gastric epithelial cells. Lab Invest. 2008;88:541–52. doi: 10.1038/labinvest.2008.16. [DOI] [PubMed] [Google Scholar]
- 29.Park KA, Byun HS, Won M, et al. Sustained activation of protein kinase C downregulates nuclear factor-κB signaling by dissociation of IKK-γ and Hsp90 complex in human colonic epithelial cells. Carcinogenesis. 2007;28:71–80. doi: 10.1093/carcin/bgl094. [DOI] [PubMed] [Google Scholar]
- 30.Elewaut D, DiDonato JA, Kim JM, Troung F, Eckmann L, Kagnoff MF. NF-kappaB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–66. [PubMed] [Google Scholar]
- 31.Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000;275:28028–32. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Lee JY, Kim YJ. Inhibition of apoptosis in Bacteroides fragilis enterotoxin-stimulated intestinal epithelial cells through the induction of c-IAP-2. Eur J Immunol. 2008;38:2190–09. doi: 10.1002/eji.200838191. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Park HR, Oh YK, et al. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J Mol Med. 2007;85:1393–404. doi: 10.1007/s00109-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 34.Kim DH, Na HK, Oh TY, Kim WB, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem Pharmacol. 2004;68:1081–7. doi: 10.1016/j.bcp.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Park SC, Yoon JH, Kim W, et al. Eupatilin attenuates bile acid-induced hepatocyte apoptosis. J Gastroenterol. 2006;41:772–8. doi: 10.1007/s00535-006-1854-6. [DOI] [PubMed] [Google Scholar]
- 36.Puupponen-Pimiä R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. The action of berry phenolics against human intestinal pathogens. Biofactors. 2005;23:243–51. doi: 10.1002/biof.5520230410. [DOI] [PubMed] [Google Scholar]


