Abstract
Systemic inflammation can be investigated by changes in expression profiles of neutrophil receptors. Application of this technology for analysis of neutrophil phenotypes in diseased tissues is hampered by the absence of information regarding the modulation of neutrophil phenotypes after extravasation to tissues under non-inflammatory conditions. To fill this gap we measured the expression of neutrophil receptors in bronchoalveolar lavage fluid (BALF) and in the peripheral blood of healthy volunteers, which included both smokers and non-smokers. Blood and BALF neutrophils were identified by CD16bright/CD45dim cells, and triple-stained with antibodies directed against integrins, chemokine- and Fcγ-receptors. BALF neutrophils of healthy volunteers showed an activated phenotype characterized by Mac-1 (CD11b)bright, L-selectin (CD62L)dim, intrecellular adhesion molecule 1 (ICAM-1) (CD54)bright, FcγRII (CD32)bright, C5a receptor (CD88)bright and CD66bbright. A similar phenotype was observed for BALF neutrophils of patients affected by sarcoidosis. Furthermore, our results demonstrate a modulated expression of C5a receptor (CD88) and ICAM-1 (CD54) in neutrophils of sarcoidosis patients. In conclusion, our data indicate that neutrophils found in the lung exhibit an activated phenotype under both homeostatic and inflammatory conditions.
Keywords: immunophenotype, lungs, neutrophils, sarcoidosis, surface receptors
Introduction
Measurement of surface receptors on neutrophils is often used for the evaluation of systemic inflammation in peripheral blood. Changes in expression of these molecules on leucocytes is associated with an activated phenotype and correlates with an augmented chemotaxis and transendothelial migration in vitro [1,2]. Receptors defining the activation state of neutrophils have been evaluated in many chronic diseases. Up-regulation of CD11b, CD66b or down-regulation of CD181, CD182 and CD62L have been quantified in leucocytes from peripheral blood of asthmatic, chronic obstructive pulmonary disease and trauma patients [3–6]. Recruitment of neutrophils to the inflamed tissue influences the expression of integrins and CXC chemokine receptor (CXCR)1,2 [6–8]. There have been attempts to correlate disease activity with modulation of expression of activation markers between the systemic and tissue compartments, but existing reports are conflicting [9,10]. This is caused mainly by insufficient data regarding the switch in phenotype of neutrophils after homing under non-disease conditions.
The aim of this report was to investigate the modulated expression of receptors for adhesion (CD11b, CD62L, CD49d, CD54), immunoglobulins (Ig) (CD32), anaphylatoxins (CD88) and CD66b on neutrophils before and after extravasation to lung tissue in healthy volunteers and to verify the changes in expression profile in diseased neutrophils. If the phenotype switch in neutrophils is affected by inflammation and is independent of the extravasation process, then the changes in expression of surface receptors will be different between neutrophils from controls and subjects suffering from inflammatory processes. We used sarcoidosis as model of an inflammatory disease. Sarcoidosis is characterized by the formation of granulomas in many organs of the body, in particular the lung [11]. In severe sarcoidosis patients, the percentage of eosinophils and neutrophils in bronchoalveolar lavage fluid (BALF) is elevated [12–14], with a concomitant increase of the leucocyte chemoattractant interleukin (IL)-8 [15,16] and the neutrophil enzyme elastase [17]. Thus, neutrophilic alveolitis in sarcoidosis patients reflects an ongoing inflammatory process.
Material and methods
Subjects
In this study we analysed 12 healthy volunteers [seven men, mean ± standard deviation (s.d.), age 33 ± 5 years] and seven sarcoidosis patients (seven men, mean ± s.d., age 48 ± 9 years). Sixty-six per cent of the controls were smokers; none of the patients smoked. None of the subjects affected by sarcoidosis received anti-inflammatory medication. Diagnosis of sarcoidosis was established by X-rays and functional lung tests. The study was approved by the St Antonius Hospital ethical commission and written informed consent was obtained from all individuals.
The BAL and blood sampling
Bronchoscopy and BAL was performed as described by Drent et al.[13]. Briefly, 300 ml of saline (0·9% NaCl) was instilled into the left lung in 25 ml aliquots and aspirated immediately. The retrieved aliquots were pooled and collected in siliconized bottles. Cells from the BALF were recovered by low-speed centrifugation. Differential counts of cells were determined using haemocytometer and cytospin preparation. Preparations were counted by two independent operators.
Peripheral blood was collected in ethylenediamine tetraacetic acid (EDTA)-containing tubes before subjects underwent bronchoscopy.
Flow cytometric analysis of surface receptors in BALF and blood neutrophils
Cells from the BALF were recovered by low-speed centrifugation and diluted at a concentration of 106 cell/ml. Peripheral blood was collected in EDTA-containing tubes before subjects underwent bronchoscopy. Recovered BALF cells were diluted in phosphate-buffered saline buffer containing 8% (vol/vol) human isotonic pasteurized plasma-protein solution, 2 mM EDTA and 2, 5% (wt/vol) human serum albumin and kept on ice. Part of the material (100 µl of undiluted whole blood or BALF cell suspension) was stimulated with 10−6 M N-formyl-methionyl–leucyl–phenylalanine (fMLP) by incubation for 10 min at 37°C. Cells were then placed on ice during the remainder of the analysis. In the case of peripheral blood, erythrocytes were lyzed by incubation with an isotonic ice-cold NH4Cl solution followed by centrifugation at 4°C. Cells were diluted in HEPES buffer containing 20 mM HEPES, 132 mM NaCl, 6 mM KCl, 1 mM Mg2SO4, 1, 2 mM KH2PO4, 1 mM CaCl2, 0·5% (wt/vol) human serum albumin and 5 mM glucose. Thereafter, BALF and white blood cells were incubated for 30 min at 4°C with the appropriate combinations of antibodies diluted 1 : 100 times in the buffer specified above. After washing twice, the cells were analysed by flow cytometry.
The BALF and blood cells were triple-stained with antibodies directly labelled with peridinin chlorophyll protein (PerCP) and Alexa647, fluorescein isothiocyanate (FITC) or phycoerythrin (PE), to identify the expression of activation markers on the neutrophil population. Leucocyte populations were identified in a flow cytometer (FACScalibur; BD Biosciences, San Jose, CA, USA) based on forward (FCS) and side-scatter characteristics (SSC). Neutrophils were identified by CD16bright and CD45dim cells in the leucocyte population both in blood and BALF.
The following antibodies were used in this study: CD16 (clone 3G8, Alexa647- labelled), CD45 (clone 2D1, PerCP-labelled), CD62L (clone Dreg-56, FITC-labelled), CD32 (clone FL18.26, PE-labelled) and the Ig isotype controls IgG1 (clone MOPC-21, FITC-labelled) and IgG2a (clone G155–178, PE-labelled) were purchased from BD Biosciences. CD88 (clone W17/1, FITC-labelled) and Ig isotype control IgG2a (clone MRC OX-34, FITC-labelled) were from Serotec (Oxford, UK). CD181 (clone 42705, FITC-labelled) and CD182 (clone 48311, PE-labelled) were from R&D Systems (Minneapolis, USA). In addition, we used CD11b (clone 2LPM19c, PE-labelled; Dako, Glostrup, Denmark), CD66b (clone 80H3, FITC-labelled; Beckman Coulter, Fullerton, CA, USA), CD49d (clone 9F10, PE-labelled; Ebioscience, San Diego, SA, USA), CD54 (clone MEM-111 PE-labelled; Caltag, San Fancisco, CA, USA), and IgG1-PE (clone DD7; Chemicon, El Segundo, CA, USA). Directly FITC-labelled monoclonal phages antibodies (MoPhab) A17 and A27 were used to determine the activation state of FcγRII (CD32) on the surface of granulocytes (results not shown; [18]).
Data analysis and statistics
Fluorescence activated cell sorter (FACS) data were analysed by FACS express (De Novo software) and the median fluorescence intensity (MFI) of each antibody was calculated. Data were analysed by the statistical program spss version 15. The Wilcoxon non-parametric test was used in the analysis of marker expression in blood and BALF neutrophils. The Mann–Whitney U-test and the univariate statistical method were used in the analysis of data presented in Table 1 and Fig. 4 respectively. A P-value < 0·05 was regarded as significant.
Table 1.
Cellular characteristics of peripheral blood and bronchoalveolar lavage fluid (BALF).
| Blood | Control subjects | Sarcoid patients |
|---|---|---|
| Subject numbers | 12 | 7 |
| Total cell counts 104 cells/ml | 700 ± 0·5 | 510 ± 0·4* |
| Mono% | 7·6 ± 0·4 | 11·9 ± 0·7* |
| Lympho% | 30·9 ± 1·5 | 17·9 ± 2·0* |
| Neutro% | 57·7 ± 1·9 | 62·1 ± 3·1 |
| Eosin% | 3·1 ± 0·8 | 7·5 ± 1·4* |
| Mono 104 cells/ml | 50 ± 0·0 | 61 ± 0·1 |
| Lympho 104 cells/ml | 220 ± 0·2 | 90·5 ± 0·1* |
| Neutro 104 cells/ml | 400 ± 0·3 | 318·0 ± 0·3 |
| Eosin 104 cell/sml | 24·9 ± 0·1 | 38·5 ± 0·1* |
| BALF | ||
| Total cell counts 104 cells/ml | 17·2 ± 3·5 | 22·8 ± 3·7 |
| AM% | 89·8 ± 2·2 | 67·6 ± 8·3* |
| Lympho% | 8·2 ± 2·0 | 28·2 ± 8·4* |
| Neutro% | 1·4 ± 0·3 | 1·8 ± 0·4 |
| Eosin% | 0·5 ± 0·2 | 1·8 ± 1·0* |
| AM 104 cells/ml | 17·0 ± 4·2 | 15·7 ± 2·6 |
| Lympho 104 cells/ml | 0·9 ± 0·2 | 7·5 ± 3·1* |
| Neutro 104 cells/ml | 0·2 ± 0·0 | 0·4 ± 0·1 |
| Eosin 104 cells/ml | 0·1 ± 0·0 | 0·7 ± 0·3* |
P < 0·05 versus healthy volunteers. Data are presented as mean ± standard error of the mean. AM, alveolar macrophages; Lympho, lymphocytes; Neutro, neutrophils; Mono, monocytes; Eosin, eosinophils.
Fig. 4.
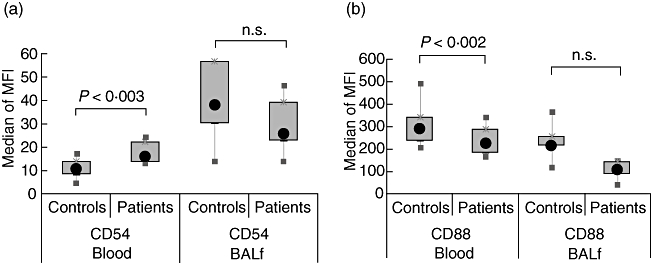
Sarcoidosis-specific changes in neutrophil surface marker expression. The plots show the modulation of surface receptor expression in blood (a) and in bronchoalveolar lavage fluid (BALF) (b) of sarcoidosis patients. The results were obtained by statistical comparison of the surface expression data reported in Tables 2 and 3. Data were analysed by univariate statistical method. The box-plots show the median of the recorded median fluorescent intensity (MFI), the 25th–75th percentiles and minimum–maximum values.
Results
Subject characteristics
Cellular characteristics of the subjects are reported in Table 1. In the control population no significant differences were found in blood and BALF cell composition when comparison was made between smokers and non-smokers (data not shown). In sarcoidosis patients the total counts and percentage of lymphocytes and eosinophils were significantly different in peripheral blood and BALF. There was a trend towards a higher percentage of neutrophils in the sarcoidosis group compared with the control group, although this was not significant (1·8 ± 0·4 versus 1·4 ± 0·3%). These data are in accordance with previously published reports [13,19] for sarcoidosis.
Blood and BALF neutrophils can be identified by a CD16bright and CD45dim phenotype
The neutrophil population in peripheral blood can be distinguished by flow cytometry based on their forward- and side-scatter characteristics. FACS identification of neutrophils in the BALF is more difficult because of the overwhelming presence of autofluorescent alveolar macrophages. To improve the recognition of BALF neutrophils we applied antibodies against receptors expressed on these cells. Several combinations of antibodies were tested. The best results were obtained with fluorescently labelled antibodies against CD16 and CD45. When cells in gate 1 (Fig. 1) were analysed according to their CD16bright and CD45dim expression, cells were highly enriched in neutrophils (Fig. 1a, b). About 90% of neutrophils present in the BALF were recovered in this gate, which was determined by cell sorting followed by cytospin identification. The same procedure was applied for analysis of blood neutrophils. The results obtained were comparable to those of BALF neutrophils. Based on these findings, we used anti-CD16 and anti-CD45 antibodies in association with antibodies against surface markers for the characterization of neutrophils in blood and in BALF.
Fig. 1.
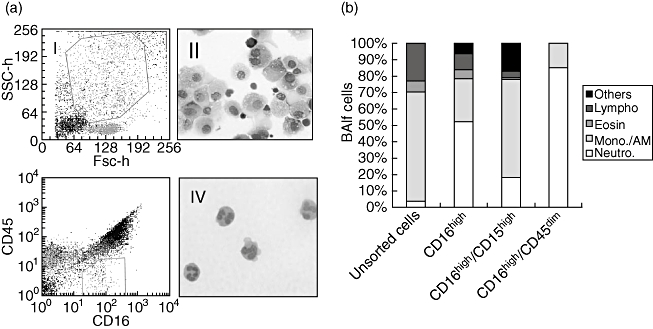
Neutrophils can be identified by their CD16bright and CD45dim phenotype in bronchoalveolar lavage fluid (BALF) and blood. BALF cells were stained with the indicated combination of fluorescent antibodies and analysed in a cell sorter (FACSvantage; BD Biosciences, San Jose, CA, USA). (a) The gates used for neutrophil identification: gate 1 contains all neutrophils and most macrophages and is referred to as gate 1; cells present in gate 1 were characterized according their CD16bright CD45dim phenotypes (see square gate 2 in Fig. 3). Cells were sorted from gate 2, cytospinned and stained with May–Grunwald–Giemsa. Pictures were taken at 400× magnification. (b) The percentage of leucocyte subpopulations counted by two independent operators. The experiment was repeated twice. Similar results were obtained with blood cells (not shown). AM, alveolar macrophages; Lympho, lymphocytes; Neutro, neutrophils; mono, monocytes; Eosi, eosinophils; Others, non-identifiable cells in the cytospin.
Activated phenotype of BALF neutrophils in healthy volunteers
In order to establish the phenotype of peripheral blood and lung neutrophils under non-disease conditions, we compared the expression of several surface receptors in blood and BALF neutrophils of healthy subjects. The results reported in Table 2 show that BALF neutrophils (CD16bright CD45dim) were characterized by a significantly higher expression of the integrin Mac-1 (CD11b) and one of its binding partners, intercellular adhesion molecule 1 (ICAM-1) (CD54) and a lower expression of l-selectin (CD62L). Furthermore, the specific granule marker CD66b and C5a-receptor (CD88) were up- and down-modulated respectively. No significant changes were observed in expression of α4 (CD49d) integrins and CXCR1, 2 (CD181, CD182) on BALF neutrophils.
Table 2.
Surface marker expression in blood and bronchoalveolar lavage fluid (BALF) neutrophils of healthy volunteers.
| Blood |
BALF |
|||||
|---|---|---|---|---|---|---|
| Median* | 25–75% | Median* | 25–75% | P-values | ||
| Adhesion receptors | CD11b | 1238·4 | (783·9; 1482·4) | 1980·9 | (1522·4; 2213·8) | 0·002 |
| CD62L | 233·7 | (203·0; 261·2) | 0·4 | (0; 6·2) | 0·002 | |
| CD49d | 0·2 | (0·0; 0·5) | 0·0 | (0; 0·1) | 0·077 | |
| CD54 | 10·8 | (8·5; 13·7) | 37·9 | (30·5; 50·3) | 0·002 | |
| IgG receptors | CD32 | 164·9 | (87·4; 237·4) | 287·8 | (133·7; 403·1) | 0·005 |
| A17 | 1031·9 | (592·7; 1482·4) | 249·4 | (37·5; 375·4) | 0·002 | |
| A27 | 1310·0 | (567·0; 1677·8) | 507·2 | (319·9; 1295·2) | 0·136 | |
| Chemokine receptors | CD181 | 65·6 | (55·1; 84·7) | 76·1 | (68·5; 94·2) | 0·075 |
| Anaphylatoxin receptor | CD88 | 292·0 | (253·5; 343·0) | 215·1 | (158·5; 256·4) | 0·004 |
| CD66b | 69·9 | (54·1; 90·0) | 162·9 | (135·5; 208·3) | 0·002 | |
Median (25th–75th percentiles) of the recorded median fluorescent intensity.
IgG, immunoglobulin G.
Immunoglobulin receptors (FcRs) are important molecules which, on neutrophils, need pre-activation for optimal interaction with pathogens opsonized with Igs. Detection of the activation state of FcRs has been shown to be useful in the discrimination of asthma phenotypes [18]. Expression of both total and activated FcγRIIa (CD32) on neutrophils was measured by CD32 antibodies and MoPhab A17 [18] respectively. The total expression of CD32 was found to be increased in BALF, whereas expression of the activated state measured by MoPhab A17 was significantly lower in BALF compared with blood neutrophils (Table 2).
In order to estimate the extent of in vivo activation of neutrophils, cells were stimulated in vitro with the bacterial-derived peptide fMLP. The activation state of CD32 and CD11b was increased further by fMLP stimulation in blood neutrophils, whereas cells in BALF failed to increase the expression of activated CD32 after stimulation. These results suggest that in contrast to blood, neutrophils in BALF are refractory in their capacity to up-regulate FcγRII functionality. Alternatively, BALF cells have reached the maximum of their activation state, albeit at much lower expression levels than found in blood after in vitro activation (Fig. 2a, b).
Fig. 2.
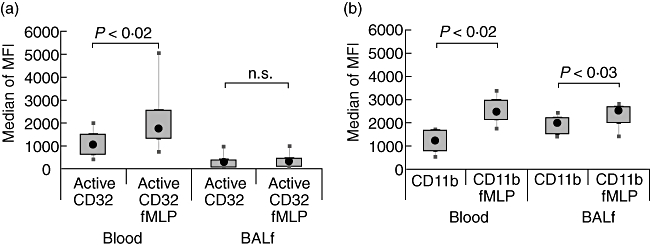
In vitro stimulation of FcγRII and CD11b on control neutrophils. Cells from blood or bronchoalveolar lavage fluid (BALF) were incubated with fMLP at 37°C for 10 min and stained with fluorescent monoclonal phages antibodies (MoPhab) A17 binding to active CD32 (a) or with a fluorescent-labelled CD11b monoclonal antibody (b). Cells were analysed by flow cytometry. The box-plots show the median of the recorded median fluorescent intensity (MFI), the 25th–75th percentiles and minimum–maximum values.
The activated phenotype of BALF neutrophils is independent of the disease state
Having characterized the expression phenotype of transmigrated neutrophils in healthy volunteers, we studied the expression profile of neutrophils from patients with inflammation in the pulmonary compartment. To this aim, we measured the modulation of surface marker expression in blood and BALF neutrophils of sarcoidosis patients. The results are shown in Table 3. Significant differences in expression were found for L-selectin (CD62L), active FcγRIIa (MoPhab A17), complement receptor C5R (CD88), ICAM-1 (CD54) and CD66b. Overall the changes in expression of surface makers overlap with those measured in healthy volunteers (compare Table 2 with Table 3), suggesting that the activated phenotype of neutrophils homed to the tissue is, at least in part, independent of a disease condition. A significant difference with the control situation is the lack of changes in CD32 expression between BALF and blood neutrophils in sarcoidosis patients (Table 3).
Table 3.
Surface marker expression in blood and bronchoalveolar lavage fluid (BALF) neutrophils of sarcoidosis patients.
| Blood |
BALF |
|||||
|---|---|---|---|---|---|---|
| Median* | 25–75% | Median* | 25–75% | P-values | ||
| Adhesion receptors | CD11b | 1313·9 | (703·8; 1482·8) | 1725·6 | (1364·0; 1993·1) | 0·091 |
| CD62L | 252·8 | (154·6; 280·4) | 2 | (0·0; 5·7) | 0·018 | |
| CD49d | 0·001 | (0·001; 0·3) | 0·001 | (0·001; 0·001) | 0·400 | |
| CD54 | 16·2 | (13·9; 22·5) | 25·7 | (23·0; 39·0) | 0·028 | |
| IgG receptors | CD32 | 189·9 | (107·5; 255·0) | 153·7 | (148·4; 209·0) | 0·735 |
| A17 | 1069·9 | (291·2; 1831·0) | 75·9 | (23·5; 177·1) | 0·018 | |
| A27 | 1498 | (213·8; 1968·5) | 741·4 | (268·1; 855·4) | 0·128 | |
| Chemokine receptor | CD181 | 54·7 | (33·0; 96·5) | 37·9 | (30·0; 52·9) | 0·128 |
| Anaphylatoxin receptor | CD88 | 222·2 | (183·4; 282·9) | 105·8 | (88·0; 142·5) | 0·018 |
| CD66b | 60·8 | (52·1; 82·6.) | 185·6 | (165·8; 251·6) | 0·018 | |
Median (25th–75th percentiles) of the recorded median fluorescent intensity.
IgG, immunoglobulin G.
Another surprising result was the functional up-regulation of FcγRIIa after in vitro stimulation with fMLP (Fig. 3a, b) in lung neutrophils of sarcoid patients. This is in contrast with the results obtained in healthy volunteers (see Fig. 2a, b), where BALF neutrophils could not be stimulated further by fMLP.
Fig. 3.
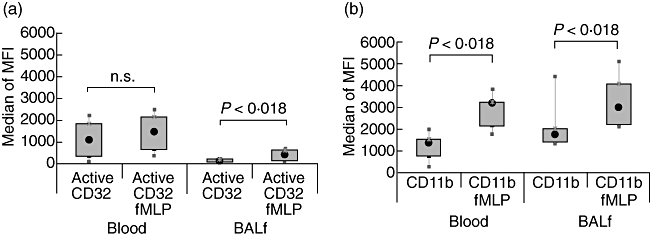
In vitro stimulation of FcγRII and CD11b on diseased neutrophils. Cells were processed as in the legend for Fig. 2. Expressions of FcγRII (CD32) and CD11b before and after in vitro stimulation with fMLP are shown in panels (a) and (b) respectively. The box-plots show the median of the recorded median fluorescent intensity (MFI), the 25th–75th percentiles and minimum–maximum values.
Neutrophil phenotype in sarcoidosis patients: up-regulation of CD54 on blood cells and down-regulation of CXCR1 and C5aR on BALF cells
Having characterized the activated phenotype of BALF neutrophils, we tested the research question of whether diseased neutrophils expressed surface markers that were associated with the disease state. We compared the expression of neutrophil surface marker expression in blood and lung compartments between healthy controls and sarcoidosis patients. As shown in Fig. 4, blood neutrophils of patients were characterized by a significantly up-regulated ICAM-1 (CD54) expression, while BALF cells showed a lower CD88 expression compared with the control situation. In addition, sarcoid neutrophils were characterized by a lower level of expression for CXCR1 (results not shown). These results are in accordance with the findings of elevated IL-8 content in the BALF of sarcoidosis patients [20] and suggest a possible role of CD54 and IL-8 receptors as disease-related markers.
Discussion
In this study we analysed and compared the expression of receptors for adhesion (CD11b, CD62L, CD49d, CD54), Igs (CD32), anaphylatoxins (CD88) and CD66b in blood and BALF neutrophils of healthy volunteers and sarcoidosis patients. We set up a flow cytometric analysis for the identification of neutrophils in the peripheral and lung compartments as CD16bright and CD45dim cells.
Our results show for the first time that an activated phenotype of neutrophils after homing of neutrophils to the lung is similar in both healthy volunteers and sarcoidosis patients. Our data confirm the suggestions of In ‘T Veen et al.[9] that the modulation of CD11b and L-selectin in sputum neutrophils is the results of tissue homing, and not specific for an inflammatory disease.
In the control population the modulated expression of Mac-1 (CD11b), ICAM-1 (CD54), C5a receptor (CD88), L-selectin (CD62L) and the integrin-associated protein CD66b [21] in BALF neutrophils are consistent with an activated phenotype. A similar phenotype was determined for lung neutrophils of sarcoidosis patients. Apparently, this phenotype is induced during homing, and interestingly mimics the phenotype of neutrophils found after transendothelial migration in vitro[9,21]. However, our finding does not prove that transendothelial movement per se is inducing this activated phenotype. It can also be explained by preferential homing of a small subpopulation of neutrophils to the lung. Buckley et al.[22] have shown that at least two populations of neutrophils identified by the expression of CXCR1 (CD181) and ICAM-1 (CD54) can be found in the blood of patients with chronic inflammatory diseases. We confirmed the data regarding ICAM-1 in our sarcoidosis patients, as ICAM-1 is increased significantly on blood neutrophils of these patients (see Fig. 4). In addition, we found an increased ICAM-1 expression on neutrophils in BALF compared with peripheral blood of normal individuals (see Table 2). Regulation of expression of ICAM-1 is at the transcriptional level (see for review [23]). The underlying mechanism of increased ICAM-1 expression on neutrophils in the lung of normal controls can be either the induction of the protein in the tissue or the homing of a small but long-lived neutrophil population high in ICAM-1. This putative population is, however, below detection level in our flow cytometric assays.
A limitation of our data is the presence of smokers in the control population. We are aware of the proinflammatory potential of tobacco smoke and of its effects on the expression of neutrophil receptors [24,25]. However, in our study the differential cell counts of BALF and blood of healthy volunteers were in range with a normal non-inflammatory situation (van den Bosch, unpublished observation; [20,26]). No statistical difference was found in the cell composition or in the expression profile of surface receptors in blood and BALF between smokers and non-smokers in the control group. These results make it less likely that a local inflammatory response is driving neutrophil extravasation in the normal lung.
It is accepted widely that leucocyte migration through the endothelium is a multi-step process [27]. Crucial phases are the so-called ‘rolling adhesion’ steps, where several adhesion molecules, including L-selectin, bind to the endothelial ligand, the ‘cell activation’ phase mediated by chemokines and the ‘firm adhesion’ where Mac-1 binds to the endothelial receptor ICAM-1 [28] After cell activation L-selectin is shed from the leucocytes surface [29]. It is not yet known whether the modulated expression of these receptors on the airway leucocytes is implicit in the process of migration or whether the modulation is associated with the disease state. Our finding of a significantly lower expression of L-selectin and higher CD11b expression in BALF neutrophils of healthy volunteers and sarcoidosis patients suggests a mechanism of regulation of these receptors that is independent of inflammation. In conclusion, the activated phenotype found in the lung of normal individuals occurs as a result of homing of neutrophils into lung tissue per se and is not the result of an ongoing inflammatory process.
Immunoglobulin receptors (FcRs) are interesting molecules that need pre-activation to bind optimally to targets opsonized with Igs in neutrophils. We developed the MoPhab A17 that recognizes FcγRII (CD32) only in the context of activated cells and is able to discriminate priming phenotypes in asthmatics ex vivo[18]. CD32 is a conventional type I transmembrane protein expressed on neutrophils. The intracellular signalling cascade following cross-linking of CD32 requires the activation of tyrosine kinases of both Src-family kinases and Syk, resulting in tyrosine phosphorylation of Shc, phospholipase C gamma isozymes and a [Ca2+]i transient [30]. In this paper we have shown differences in expression of active FcγRII (CD32) between BALF and blood neutrophils. These results point to changes in the control of the functionality of FcRs on neutrophils during extravasation. The regulatory mechanisms of such control remain to be elucidated. Of interest is the lack of activation of FcγRII after fMLP stimulation of BALF neutrophils in the control group, which suggests refractoriness of these cells to chemotactic stimuli to activate this receptor. It is known that under homeostatic conditions the lung environment suppresses macrophage function [31]. It is tempting to speculate that neutrophils undergo similar inhibitory mechanisms to control their local functionality, as unnecessary activation of FcRs on tissue neutrophils might lead to aspecific activation of these cells in response to some resident immune complexes. On the contrary, the acquired capacity of responding to fMLP recorded in lung neutrophils of patients is an indication of a proinflammatory role of these cells in the progression of sarcoidosis.
In the search for markers specific for sarcoidosis, we compared the changes in expression of surface receptors of lung and blood neutrophils between normal subjects and sarcoidosis patients. In patients, a significant higher expression for ICAM-1(CD54) and a lower expression of C5aR (CD88) were recorded in blood and BALF neutrophils respectively. The levels of soluble ICAM-1 and ICAM-1 expression on alveolar macrophages, as well as the level of IL-8 in BALF, have been indicated as markers of disease activity in sarcoidosis [20,32].The expression of CD54 and CD88 on neutrophils might, therefore, be considered as putative disease markers for sarcoidosis. The specificity of such markers must be investigated further.
In summary, we have demonstrated an activated neutrophil phenotype in the lung of normal individuals. This finding indicates that homing of neutrophils to the lung per se leads to this phenotype and does not necessarily reflect inflammation in the tissue.
Acknowledgments
The authors would like to thank Peter Zanen MD, PhD for statistical analysis of the data and M. Heron for his great help in the organization of the study. E. F. and L. K. designed the research and wrote the paper; E. F. and K. K. performed the experiments and analysed the data; J. G. and J. B. provided materials from normal subjects. This study is part of the research performed in St Antonius Hospital Nieuwegein aiming to characterize BALF in healthy volunteers. The authors declare no competing financial interests.
References
- 1.Schymeinsky J, Mocsai A, Walzog B. Neutrophil activation via beta2 integrins (CD11/CD18): molecular mechanisms and clinical implications. Thromb Haemost. 2007;98:262–73. [PubMed] [Google Scholar]
- 2.Warringa RA, Mengelers HJ, Raaijmakers JA, Bruijnzeel PL, Koenderman L. Upregulation of formyl-peptide and interleukin-8-induced eosinophil chemotaxis in patients with allergic asthma. J Allergy Clin Immunol. 1993;91:1198–205. doi: 10.1016/0091-6749(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata T, Sugiura H, Yokoyama T, et al. Overexpression of CD-11b and CXCR1 on circulating neutrophils: its possible role in COPD. Chest. 2007;132:890–9. doi: 10.1378/chest.07-0569. [DOI] [PubMed] [Google Scholar]
- 4.Hietbrink F, Oudijk EJ, Braams R, Koenderman L, Leenen L. Aberrant regulation of polymorphonuclear phagocyte responsiveness in multitrauma patients. Shock. 2006;26:558–64. doi: 10.1097/01.shk.0000233196.40989.78. [DOI] [PubMed] [Google Scholar]
- 5.Mann BS, Chung KF. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir Res. 2006;7:59. doi: 10.1186/1465-9921-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pignatti P, Moscato G, Casarini S, et al. Downmodulation of CXCL8/IL-8 receptors on neutrophils after recruitment in the airways. J Allergy Clin Immunol. 2005;115:88–94. doi: 10.1016/j.jaci.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Soejima K, Fujishima S, Nakamura H, et al. Downmodulation of IL-8 receptors, type A and type B, on human lung neutrophils in vivo. Am J Physiol Lung Cell Mol Physiol. 1997;273:L618–25. doi: 10.1152/ajplung.1997.273.3.L618. [DOI] [PubMed] [Google Scholar]
- 8.Warringa RA, Mengelers HJ, Raaijmakers JA, Bruijnzeel PL, Koenderman L. Upregulation of formyl-peptide and interleukin-8-induced eosinophil chemotaxis in patients with allergic asthma. J Allergy Clin Immunol. 1993;91:1198–205. doi: 10.1016/0091-6749(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 9.In't Veen JCCM, Grootendorst DC, Bel EH, et al. CD11b and L-selectin expression on eosinophils and neutrophils in blood and induced sputum of patients with asthma compared with normal subjects. Clin Exp Allergy. 1998;28:606–15. doi: 10.1046/j.1365-2222.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 10.Georas SN, Liu MC, Newman W, Beall LD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial-cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992;7:261–9. doi: 10.1165/ajrcmb/7.3.261. [DOI] [PubMed] [Google Scholar]
- 11.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl Med J. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 12.Drent M, Jacobs JA, de Vries J, Lamers RJ, Liem IH, Wouters EF. Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur Respir J. 1999;13:1338–44. doi: 10.1183/09031936.99.13613459. [DOI] [PubMed] [Google Scholar]
- 13.Ziegenhagen MW, Rothe ME, Schlaak M, Muller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21:407–13. doi: 10.1183/09031936.03.00010403. [DOI] [PubMed] [Google Scholar]
- 14.Tutor-Ureta P, Citores MJ, Castejon R, et al. Prognostic value of neutrophils and NK cells in bronchoalveolar lavage of sarcoidosis. Cytometry B Clin Cytom. 2006;70:416–22. doi: 10.1002/cyto.b.20120. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa H, Satoh M, Okazaki H, et al. Increased IL-6 and IL-8 in bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis: correlation with the clinical parameters. Clin Exp Immunol. 1997;107:175–81. doi: 10.1046/j.1365-2249.1997.d01-905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Car BD, Meloni F, Luisetti M, Semenzato G, Gialdroni-Grassi G, Walz A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994;149:655–9. doi: 10.1164/ajrccm.149.3.8118632. [DOI] [PubMed] [Google Scholar]
- 17.Peros-Golubicic T, Ivicevic A, Bekic A, Alilovic M, Tekavec-Trkanjec J, Smojver-Jezek S. Lung lavage neutrophils, neutrophil elastase and albumin in the prognosis of pulmonary sarcoidosis. Coll Antropol. 2001;25:349–55. [PubMed] [Google Scholar]
- 18.Kanters D, ten HW, Luijk B, et al. Expression of activated Fc gamma RII discriminates between multiple granulocyte-priming phenotypes in peripheral blood of allergic asthmatic subjects. J Allergy Clin Immunol. 2007;120:1073–81. doi: 10.1016/j.jaci.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Drent M, Mansour K, Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin Respir Crit Care Med. 2007;28:486–95. doi: 10.1055/s-2007-991521. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa H, Satoh M, Okazaki H, et al. Increased IL-6 and IL-8 in bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis: correlation with the clinical parameters. Clin Exp Immunol. 1997;107:175–81. doi: 10.1046/j.1365-2249.1997.d01-905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol. 2007;179:8454–62. doi: 10.4049/jimmunol.179.12.8454. [DOI] [PubMed] [Google Scholar]
- 22.Buckley CD, Ross EA, McGettrick HM, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. 2006;79:303–11. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 23.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 24.Ryder MI, Fujitaki R, Lebus S, et al. Alterations of neutrophil L-selectin and CD18 expression by tobacco smoke: implications for periodontal diseases. J Periodont Res. 1998;33:359–68. doi: 10.1111/j.1600-0765.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 25.Halvorsen B, Lund SE, Ueland T, Aukrust P, Tonstad S. Effect of smoking cessation on markers of inflammation and endothelial cell activation among individuals with high risk for cardiovascular disease. Scand J Clin Lab Invest. 2007;67:604–11. doi: 10.1080/00365510701283878. [DOI] [PubMed] [Google Scholar]
- 26.Kaseda M, Kadota J, Mukae H, et al. Possible role of L-selectin in T lymphocyte alveolitis in patients with active pulmonary sarcoidosis. Clin Exp Immunol. 2000;121:146–50. doi: 10.1046/j.1365-2249.2000.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 28.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte–endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–42. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–66. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unkeless JC, Shen Z, Lin CW, DeBeus E. Function of human Fc gamma RIIA and Fc gamma RIIIB. Semin Immunol. 1995;7:37–44. doi: 10.1016/1044-5323(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 31.Takabayshi K, Corr M, Hayashi T, et al. Induction of a homeostatic circuit in lung tissue by microbial compounds. Immunity. 2006;24:475–87. doi: 10.1016/j.immuni.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Kim DS, Paik SH, Lim CM, et al. Value of ICAM-1 expression and soluble ICAM-1 level as a marker of activity in sarcoidosis. Chest. 1999;115:1059–65. doi: 10.1378/chest.115.4.1059. [DOI] [PubMed] [Google Scholar]


